Abstract
We compared the gene expression pattern of thymic tumors from precursor T-cell lymphoblastic lymphoma/leukemia (pre-T LBL) that arose in transgenic mice which over-expressed SCL, LMO1, or NUP98-HOXD13 (NHD13) with that of thymocytes from normal littermates. Only two genes, Ccl8 and Mrpl38, were consistently more than 4-fold over-expressed in pre-T LBL from all three genotypes analyzed, and a single gene, Prss16 was consistently under-expressed. However, we identified a number of genes, such as Cfl1, Tcra, Tcrb, Pbx3, Eif4a, Eif4b, and Cox8b that were over or under-expressed in pre-T LBL that arose in specific transgenic lines. Similar to the situation seen with human pre-T LBL, the SCL/LMO1 leukemias displayed an expression profile consistent with mature, late cortical thymocytes, whereas the NHD13 leukemias displayed an expression profile more consistent with immature thymocytes. We evaluated two of the most differentially regulated genes as potential therapeutic targets. Cfl1 was specifically over-expressed in SCL-LMO1 tumors; inactivation of Cfl1 using Okadaic acid resulted in suppression of leukemic cell growth. Overexpression of Ccl8 was a consistent finding in all 3 transgenic lines, and an antagonist for the Ccl8 receptor induced death of leukemic cell lines, suggesting a novel therapeutic approach.
Keywords: T-cell leukemia, Scl, Nup98, chemokine, cofilin, Pbx3
Introduction
It has been shown that gene expression profiling is a useful technique for classification, subtype discovery, and prognosis in patients with precursor T-cell lymphoblastic leukemia/lymphoma (pre-T LBL) 1, 2. Many pre-T LBL patients show chromosomal aberrations that result in the generation of fusion genes and/or the aberrant expression of proto-oncogenes. In addition, gene expression profiling and quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) has been shown to detect aberrantly expressed proto-oncogenes in the absence of chromosomal abnormalities. SCL (TAL1), HOX11, LYL1, LMO1, and LMO2 are frequently over-expressed in patients with pre-T LBL 1, 2. SCL was shown to be up-regulated in 49% of the cases with pre-T LBL and considered to be associated with relatively unfavorable prognosis. Additional studies have been employed to identify the important alterations in gene expression, as well as to identify mechanism(s) that lead to altered gene expression 3, 4.
One advantage of studying genetically engineered mice is that the inciting event is known, which makes classification more straightforward. In order to identify candidate genes that might contribute to T-cell leukemogenesis in the context of aberrant expression of known T-cell oncogenes, we compared the gene expression profile of thymic tumors from pre-T LBL that arose in transgenic mice that over-expressed SCL, LMO1, and NUP98-HOXD13 (NHD13) with that of thymocytes from normal littermates. We have proceeded to evaluate several of the candidate genes as potential therapeutic targets for treatment of pre-T LBL.
Materials and Methods
Mouse models for human pre-T LBL
LMO1 transgenic mice that over-express LMO1 driven by the lck promoter were obtained from Dr. Stanley Korsmeyer5. SCL transgenic mice- that over-expresses SCL under control of the SIL promoter has been described previously6. SCL-LMO1 double transgenic mice were generated by crossing the SIL-SCL mice with lck- LMO1 mice6, 7.
NHD13 transgenic mice that expresses a NUP98-HOXD13 fusion from vav regulatory elements have been previously described8. Thymic tumors were harvested from clinically ill transgenic mice. Normal thymi were harvested from 40 non-transgenic littermates. Both thymic tumors and normal thymi were immediately frozen on dry ice and transferred to liquid nitrogen. In some cases, single cell suspensions of the thymic tumors were cultured in Iscove’s Modified Durbecco’s Medium, with 15% FBS9 in order to establish pre-T LBL cell lines.
Microarray analysis
RNA was isolated from cryopreserved thymic tumors and normal thymocytes with Trizol reagent (Invitrogen, Carlsbad, CA) and purified with RNeasy MiniElute Cleanup kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The purified RNA was assessed by electrophoresis through denaturing agarose gels to verify that it was not degraded. The RNA from 40 normal thymi were pooled and used as a reference RNA. First strand cDNA was synthesized and dye-coupled using a FairPlay Microarray labeling kit (Stratagene, La Jolla, CA). The experimental cDNA probe was labeled with Cy3 and the reference cDNA probe was labeled with Cy5. Dye-coupled cDNA was purified with a Qiagen Mini Elute PCR purification kit. The Cy3 labeled experimental probe was combined with the Cy5 labeled reference probe and the mixture was hybridized to an NCI production oligonucleotide DNA microarray containing 22272 long oligonucleotide (70 mer) features (Compugen, San Jose, CA). The microarray was scanned using an Axon GenePix scanner. The fluorescence ratio was quantified for each transcript and reflected the relative abundance of the gene in the experimental mRNA sample compared with the reference mRNA. Statistical analyses, hierarchical clustering, and gene ontology of the differentially-regulated genes were analyzed according to the NCI mAdb web site (http://nciarray.nci.nih.gov/). Briefly, the signal intensity was defined as by the mean pixel intensity minus median background pixel intensity. A log base 2 ratio of tumor to normal signal was calculated by dividing the tumor signal intensity by the normal thymus signal intensity. A raw gene list of those genes either 4 fold up-regulated or 4 fold down-regulated was obtained, and then curated by hand to eliminate duplicate genes. Any multiple occurrences of features were reduced to a single instance by selecting the feature with the strongest signal (Channel A + Channel B). In some cases, the identity of anonymous (such as the Riken collection) genes could be ascertained by comparing to the most recent Genbank relaease.
Okadaic acid and Ccr3 antagonist treatment
Leukemic cell lines established from the thymic tumors of SCL-LMO1 and NHD13 transgenic mice were cultured in the presence of the CCR3 antagonist, SB328437 (Sigma-Aldrich, St. Louis, MO), at the concentration of 69 µM for 72 hours, or vehicle (DMSO) alone. In separate experiments, SCL-LMO1 or NHD13 cell lines were treated with 1 µM okadaic acid (Sigma-Aldrich, St. Louis, MO) or vehicle (dH2O) alone for 4 hours. Viable cells were evaluated by Trypan blue exclusion.
Western blot analysis
Extracts of leukemic cell lines were prepared by lysing cells in HNTG buffer supplemented with Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO) for one hour. Forty µg of whole cell lysate was separated on an 8% Tris-Glycine Gel (Invitrogen, Carlsbad, CA) at 125V for 90 minutes and then transferred to a nitrocellulose membrane. Membranes were blocked for one hour in SuperBlock Blocking buffer (Pierce, Rockford, IL) and placed in appropriate primary antibody dilution (in TBS, 5% Blotto, 2.5% Tween 20). Primary antibody (anti Cfl1, #3311, and anti phospho-Cfl1, #3312, Cell Signaling, Danvers, MA) was detected using horseradish peroxidase-linked goat anti-rabbit IgG antibodies and visualized using the chemiluminescent detection system (SuperSignal; Pierce, Rockford, IL).
Transfections of SCL-LMO1 pre-T LBL cell line
We transfected a NHD13 expression vector into a pre-T LBL cell line (#6812) established from SCL-LMO1 transgenic-mice using DMRIE-C reagent and the manufacturer’s recommended protocol (Invitrogen, Carlsbad, CA). The NHD13 expression vector used EF1alpha regulatory sequences and the NHD13 cassette was identical to that previously reported8.
RT-PCR
RNAs were extracted with Trizol reagent, and 1.0 µg of RNA was reverse transcribed using Superscript II reverse transcriptase with an oligo (dT) primer (Invitrogen, Carlsbad, CA). The first strand cDNAs were amplified with mouse Pbx3F primers 5’-CAATTATAGAGCAGGCAAGGTTCACCCTG-3’ and mouse Pbx3R primers 5’-GTTCAGAGGTAGTAAGTGAGCGCTCTA-3’ in the volume of 20 µl. After a “hot start” at 94°C, 35 cycles of 94°C for 1 min., 62°C for 1 min., and 72°C for 1 min. was used, followed by a terminal 10 min. extension at 72°C. PCR products were analyzed by agarose gel electrophoresis.
Results
Hierarchical Clustering of tumors obtained from different lines of transgenic mice
We used two-color hybridization of high density oligonucleotide microarrays containing 22272 features to compare the gene expression profiles of individual mouse tumors to a reference of normal thymus RNA. In order to produce a large supply of normal thymus reference RNA, 40 mice aged (3–6 months) were euthanized and the thymi harvested. We assayed 12 SCL/LMO1, 10 NHD13, and 4 LMO1 only pre-T LBL samples, as well as 2 normal thymi. Hierarchical clustering of the arrays using a classical Pearson analysis revealed four major groups: SCL/LMO1 tumors, normal thymus, NHD13 tumors, and a fourth group consisting primarily of LMO1 only tumors (Figure 1). All tumors of the same genotype clustered together, with the exception of two NHD13 tumors (arrays ma18–40C and –43C, mouse # 1149 and 1901).
Figure 1. Hierarchical clustering of murine pre-T LBL gene expression profiles.
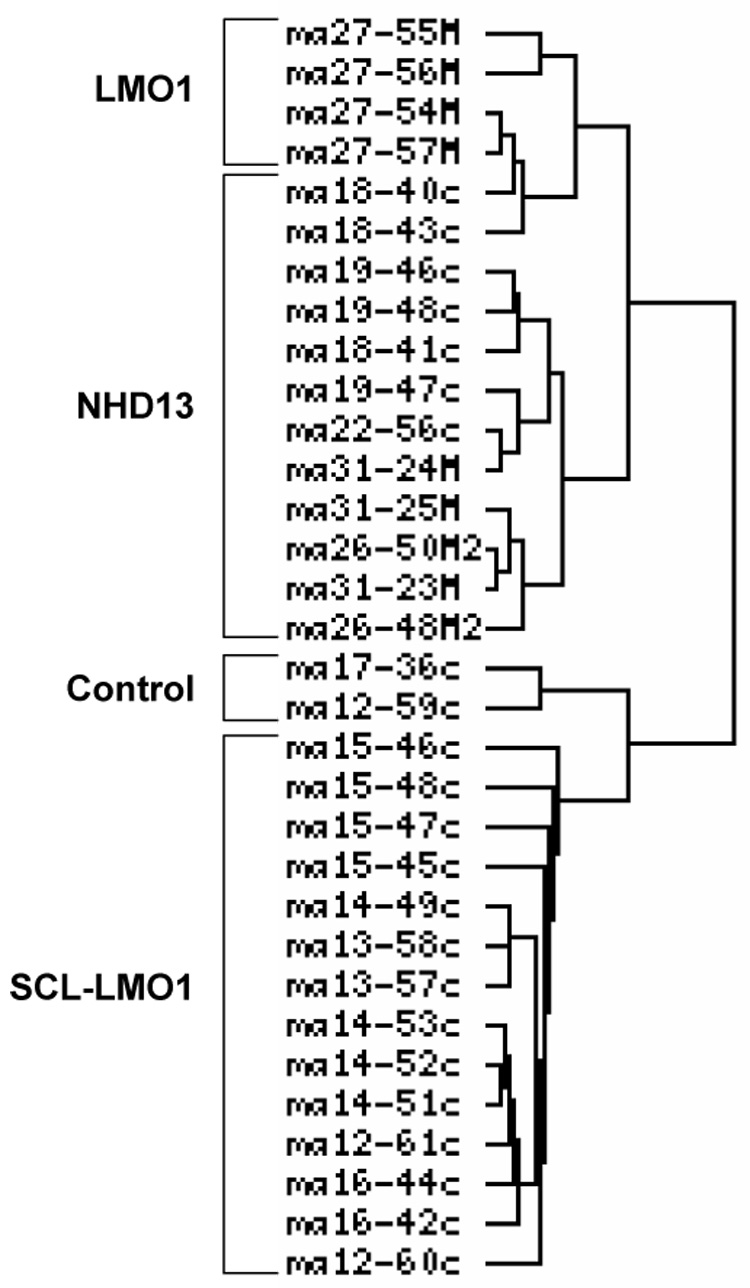
Hierarchical clustering was determined using the classical Pearson method. The first hierarchical break distinguished a group of the LMO1 and NHD13 tumors from the group of the SCL-LMO1 tumors and normal thymus.
Differentially expressed genes and gene ontology (Table 1–Table 4)
Table 1.
Genes most differentially expressed in T-cell tumors compared to normal thymus
| Fold increase | Gene | Description |
|---|---|---|
| 7.5 | Iltifb | interleukin 10-related T cell-derived inducible factor beta (Iltifb), |
| 7.2 | Dtx1 | deltex 1 homolog (Drosophila) (Dtx1), mRNA. |
| 6.7 | Igh-6 | Immunoglobulin heavy chain 6 (heavy chain of IgM) |
| 5.8 | Ccl8 | chemokine (C-C motif) ligand 8 (Ccl8), mRNA. |
| 5.5 | Tcrb | T-cell receptor beta-1 chain C region (LOC669847), mRNA. |
| 5.3 | Mrpl38 | mitochondrial ribosomal protein L38 (Mrpl38), mRNA. |
| 4.3 | Gapdh | glyceraldehyde-3-phosphate dehydrogenase (LOC14433),. |
| 4.3 | Eef2 | eukaryotic translation elongation factor 2 (Eef2), mRNA. |
| 4.3 | Lmo1 | LIM domain only 1 (Lmo1), mRNA. |
| 4.3 | Tubb5 | tubulin, beta 5 (Tubb5), mRNA. |
| 4.2 | eEF-Tu | Mouse eEF-Tu gene encoding elongation factor Tu, 5′ end. |
| 4.2 | Ftl1 | ferritin light chain 1 (Ftl1), mRNA. |
| | ||
| | ||
| Fold decrease | Gene | Description |
| | ||
| 4.0 | Arpp21 | cyclic AMP-regulated phosphoprotein, 21 (Arpp21) |
| 4.1 | Ly49n | Mus musculus natural killer cell receptor (Ly49n) gene |
| 4.2 | Cbr2 | carbonyl reductase 2 (Cbr2), mRNA. |
| 4.2 | Bop1 | block of proliferation 1 (Bop1), mRNA. |
| 4.3 | Tcrd | T-cell receptor delta |
| 4.3 | Fabp3 | fatty acid binding protein 3, muscle and heart (Fabp3), mRNA. |
| 4.3 | Lcn5 | lipocalin 5 (Lcn5), mRNA. |
| 4.4 | 1700084E18Rik | RIKEN cDNA 1700084E18 gene (1700084E18Rik) |
| 4.4 | Rb1cc1 | RB1-inducible coiled-coil 1 (Rb1cc1), mRNA. |
| 4.5 | Scnn1a | sodium channel, nonvoltage-gated, type I, alpha (Scnn1a) |
| 4.6 | Fkbp6 | FK506 binding protein 6 (Fkbp6), mRNA. |
| 4.7 | Hba-a1 | hemoglobin alpha, adult chain 1 (Hba-a1), mRNA. |
| 4.7 | Ctsl | cathepsin L (Ctsl), mRNA. |
| 4.8 | Cstb | cystatin B (Cstb), mRNA. |
| 4.8 | Slc18a3 | solute carrier family 18, member 3 (Slc18a3) |
| 4.9 | LOC674927 | PREDICTED: similar to melanoma antigen (LOC674927) |
| 5.4 | Erf1 | Mus musculus ETS-related transcription factor ERF (Erf1) |
| 5.4 | Cdo1 | cysteine dioxygenase 1, cytosolic (Cdo1), mRNA. |
| 5.7 | Diras2 | DIRAS family, GTP-binding RAS-like 2 (Diras2), mRNA. |
| 5.7 | Tctex1d1 | Tctex1 domain containing 1 (Tctex1d1), mRNA. |
| 5.8 | Spatial | Mus musculus fetal thymus spatial protein mRNA |
| 6.1 | Dgat2 | diacylglycerol O-acyltransferase 2 (Dgat2), mRNA. |
| 6.4 | Cidea | cell death-inducing DNA fragmentation factor, alpha subunit |
| 6.5 | Fabp9 | Fatty acid binding protein 9, testis |
| 6.6 | Cd83 | CD83 antigen (Cd83), mRNA. |
| 7.0 | Krt2-8 | keratin complex 2, basic, gene 8 (Krt2-8), mRNA. |
| 7.3 | Fabp4 | Fatty acid binding protein 4, adipocyte |
| 7.3 | Cox8b | cytochrome c oxidase, subunit VIIIb (Cox8b), mRNA. |
| 14.5 | Ccl25 | Chemokine (C-C motif) ligand 25 |
| 22.4 | Prss16 | protease, serine, 16 (thymus) (Prss16), mRNA. |
Table 4.
Genes most differentially regulated in NHD13 tumors
| Fold increase | Gene | Description |
|---|---|---|
| 12.9 | Pbx3 | pre B-cell leukemia transcription factor 3 (Pbx3), mRNA. |
| 6.4 | Cox6a2 | cytochrome c oxidase, subunit VI a, polypeptide 2 (Cox6a2), mRNA. |
| 5.6 | Mrpl38 | mitochondrial ribosomal protein L38 (Mrpl38), mRNA. |
| 5.6 | Eef2 | eukaryotic translation elongation factor 2 (Eef2), mRNA. |
| 5.4 | Ccl8 | chemokine (C-C motif) ligand 8 (Ccl8), mRNA. |
| 5.3 | Gapdh | glyceraldehyde-3-phosphate dehydrogenase (LOC14433), mRNA. |
| 5.2 | Ctse | cathepsin E (Ctse), mRNA. |
| 4.8 | Tfrc | transferrin receptor (Tfrc), mRNA. |
| 4.4 | Eif3s9 | eukaryotic translation initiation factor 3, subunit 9 (eta) (Eif3s9), mRNA. |
| 4.2 | Mpo | myeloperoxidase (Mpo), mRNA. |
| 4.1 | H2-D1 | Histocompatibility 2, D region locus 1 |
| 4.0 | Farsla | phenylalanine-tRNA synthetase-like, alpha subunit (Farsla), mRNA. |
| Fold decrease | Gene | Description |
| 4.0 | Spo11 | sporulation protein, meiosis-specific, SPO11 homolog (S. cerevisiae) |
| 4.1 | Ubd | ubiquitin D (Ubd), mRNA. |
| 4.1 | Tcra | T-cell receptor alpha. |
| 4.2 | Gtf2h4 | general transcription factor II H, polypeptide 4 (Gtf2h4), mRNA. |
| 4.5 | Cfd | complement factor D (adipsin) (Cfd), mRNA. |
| 4.5 | 1700084E18Rik | PREDICTED: RIKEN cDNA 1700084E18 gene (1700084E18Rik), mRNA. |
| 4.6 | Fabp9 | Fatty acid binding protein 9, testis |
| 4.6 | Ly49n | Mus musculus natural killer cell receptor (Ly49n) gene |
| 4.7 | Vamp1 | vesicle-associated membrane protein 1 (Vamp1), mRNA. |
| 4.7 | Diras2 | DIRAS family, GTP-binding RAS-like 2 (Diras2), mRNA. |
| 4.9 | Ccni | Cyclin I |
| 4.9 | 9130430L19Rik | RIKEN cDNA 9130430L19 gene |
| 5.8 | H2-Aa | histocompatibility 2, class II antigen A, alpha (H2-Aa), mRNA. |
| 6.0 | Erf1 | Mus musculus ETS-related transcription factor ERF (Erf1) mRNA. |
| 6.3 | Fabp4 | Fatty acid binding protein 4, adipocyte |
| 6.5 | 5830406J20Rik | RIKEN cDNA 5830406J20 gene (5830406J20Rik), mRNA. |
| 6.6 | Cd8a | CD8 antigen, alpha chain, transcript variant 1 (Cd8a), mRNA. |
| 6.8 | Arpp21 | cyclic AMP-regulated phosphoprotein, 21 (Arpp21) |
| 6.9 | Crip3 | cysteine-rich protein 3 (Crip3), transcript variant TLP-B, mRNA. |
| 7.2 | Cd74 | CD74 antigen |
| 7.4 | Dntt | deoxynucleotidyltransferase, terminal (Dntt), mRNA. |
| 8.8 | Igh-6 | Immunoglobulin heavy chain 6 (heavy chain of IgM) |
| 8.9 | Cd83 | CD83 antigen (Cd83), mRNA. |
| 9.5 | Tctex1d1 | Tctex1 domain containing 1 (Tctex1d1), mRNA. |
| 11.4 | Tcrb-V13 | T-cell receptor beta, variable 13 |
| 11.7 | H2-Eb1 | histocompatibility 2, class II antigen E beta (H2-Eb1), mRNA. |
| 12.2 | LOC545854 | Immunoglobulin kappa chain |
| 16.3 | LOC674927 | PREDICTED: similar to melanoma antigen (LOC674927), mRNA. |
| 17.6 | Ccl25 | Chemokine (C-C motif) ligand 25 |
| 32.8 | Prss16 | protease, serine, 16 (thymus) (Prss16), mRNA. |
Table 1 shows the genes that were most highly over- or under-expressed relative to normal thymus. There were only 12 genes whose mean expression levels were more than 4-fold increased when all three groups of tumors (LMO1, SCL/LMO1, and NHD13) were analyzed, and none of these were more than 7.5-fold increased. These represented a diverse group of genes including notch-signaling pathway (Dtx1), immune-system genes (Iltifb, Igh, Ccl8, Tcrb), polypeptide elongation factors (Eef2, Eef-tu, and Mrpl38), Lmo1, and Ftl1. Genes with the highest fold -decrease included 3 related fatty acid binding proteins (Fabp4, Fabp9, and Fabp3), apoptosis pathway genes (Cox8b, Cidea) and CD83. These results suggested that there were relatively few genes consistently over-expressed in tumors arising in all three transgenic lines compared to normal thymus.
Since tumors derived from mice with the same genotypes clustered closely together, we repeated the analysis of the most differentially expressed genes using tumors from each genotype individually. Table 2 shows genes that are more than 4-fold increased in the SCL-LMO1 tumors compared to normal thymus. The two the most highly over-expressed genes were ferritin light chain (Ftl1) and cofilin 1 (Cfl1). Additional over-expressed genes included a large number that encoded proteins involved in the immune response (Tcra, Tcrb, Igh, Iltifb, Ccl8, Tcf7), cell proliferation and division (Pin1, Cdk4, Map2k4, Cdc37, and Ctnnb1), protein translation (Eef1a1, Eef-tu, Eif4a1, Eef2, Eif5a, Tceal8, Eif4b, Mrpl38, and L13), and Notch signaling (Notch1 and Dtx1). Genes with the most significantly decreased expression again included apoptotic pathway genes (Cox8b, Cox7a1, Ucp1, and Cidea), genes encoding fatty acid binding proteins (Fabp3, 4, 9), and CD83.
Table 2.
Genes most differentially expressed in SCL-LMO1 T-cell tumors
| Fold increase | Gene | Description |
|---|---|---|
| 15.6 | Ftl1 | ferritin light chain 1 (Ftl1), mRNA. |
| 15.0 | Cfl1 | cofilin 1, non-muscle (Cfl1), mRNA. |
| 11.5 | Tcrb | T cell receptor beta chain mRNA, partial cds. |
| 11.0 | Hspa8 | heat shock protein 8 (Hspa8), mRNA. |
| 11.0 | Lmo1 | LIM domain only 1 (Lmo1), mRNA. |
| 10.9 | Eef1a1 | Eukaryotic translation elongation factor 1 alpha 1 |
| 10.7 | Tuba7 | tubulin, alpha 7 (Tuba7), mRNA. |
| 10.4 | Eef-tu | Mouse eEF-Tu gene encoding elongation factor Tu |
| 9.4 | Ef2 | Elongation factor 2 (EF-2) (LOC673429), mRNA. |
| 9.2 | Igh-6 | Immunoglobulin heavy chain 6 (heavy chain of IgM) |
| 8.9 | Gapdh | glyceraldehyde-3-phosphate dehydrogenase |
| 8.4 | Dtx1 | deltex 1 homolog (Drosophila) (Dtx1), mRNA. |
| 8.4 | Cnbp2 | cellular nucleic acid binding protein 2 (Cnbp2), mRNA. |
| 8.2 | Tubb5 | tubulin, beta 5 (Tubb5), mRNA. |
| 8.0 | Zmym1 | zinc finger, MYM domain containing 1 (Zmym1), mRNA. |
| 7.9 | Siglece | sialic acid binding Ig-like lectin E (Siglece), mRNA. |
| 7.8 | Eif4a1 | eukaryotic translation initiation factor 4A1 (Eif4a1), mRNA. |
| 7.6 | Bzw2 | basic leucine zipper and W2 domains 2 (Bzw2), mRNA. |
| 7.5 | Eef2 | eukaryotic translation elongation factor 2 (Eef2), mRNA. |
| 7.2 | Eif5a | eukaryotic translation initiation factor 5A (Eif5a), mRNA. |
| 6.8 | Tcf7 | transcription factor 7, T-cell specific (Tcf7), mRNA. |
| 6.3 | Aes | amino-terminal enhancer of split (Aes), mRNA. |
| 6.3 | Iltifb | interleukin 10-related T cell-derived inducible factor beta (Iltifb) |
| 6.3 | Tcra | T-cell receptor alpha chain (TCRA) |
| 6.0 | Coro1a | coronin, actin binding protein 1A (Coro1a), mRNA. |
| 5.5 | Pin1 | protein (peptidyl-prolyl cis/trans isomerase) NIMA-interacting 1 (Pin1), mRNA. |
| 5.4 | Cdk4 | cyclin-dependent kinase 4 (Cdk4), mRNA. |
| 5.4 | Tceal8 | Transcription elongation factor A (SII)-like 8 |
| 5.3 | Map2k4 | mitogen activated protein kinase kinase 4 (Map2k4), mRNA. |
| 5.1 | Eif4b | eukaryotic translation initiation factor 4B (Eif4b), mRNA. |
| 5.0 | Ccl8 | chemokine (C-C motif) ligand 8 (Ccl8), mRNA. |
| 4.8 | Dap | death-associated protein (Dap), mRNA. |
| 4.7 | Dennd2d | DENN/MADD domain containing 2D (Dennd2d), mRNA. |
| 4.5 | Mrpl38 | mitochondrial ribosomal protein L38 (Mrpl38), mRNA. |
| 4.3 | Rpl13 | Ribosomal protein L13 |
| 4.3 | Hnrpu | heterogeneous nuclear ribonucleoprotein U (Hnrpu), mRNA. |
| 4.3 | Notch1 | Notch gene homolog 1 (Drosophila) |
| 4.2 | Hnrpl | heterogeneous nuclear ribonucleoprotein L (Hnrpl), mRNA. |
| Fold decrease | Gene | Description |
| 6.1 | Cd83 | CD83 antigen (Cd83), mRNA. |
| 6.1 | Prr6 | Proline-rich polypeptide 6 |
| 6.4 | Erf1 | Mus musculus ETS-related transcription factor ERF (Erf1) mRNA |
| 6.5 | Krt2-8 | keratin complex 2, basic, gene 8 (Krt2-8), mRNA. |
| 6.6 | Mgst1 | microsomal glutathione S-transferase 1 (Mgst1), mRNA. |
| 6.6 | Ucp1 | uncoupling protein 1 (mitochondrial, proton carrier) (Ucp1), mRNA. |
| 6.8 | Fabp3 | fatty acid binding protein 3, muscle and heart (Fabp3), mRNA. |
| 6.9 | Diras2 | DIRAS family, GTP-binding RAS-like 2 (Diras2), mRNA. |
| 7.0 | Cox7a1 | cytochrome c oxidase, subunit VIIa 1 (Cox7a1), mRNA. |
| 8.5 | Slc18a3 | solute carrier family 18 (vesicular monoamine), |
| 10.7 | Fabp4 | Fatty acid binding protein 4, adipocyte |
| 11.3 | Cdo1 | cysteine dioxygenase 1, cytosolic (Cdo1), mRNA. |
| 11.3 | Cox8b | cytochrome c oxidase, subunit VIIIb (Cox8b), mRNA. |
| 11.6 | Fabp9 | Fatty acid binding protein 9, testis |
| 12.1 | Prss16 | protease, serine, 16 (thymus) (Prss16), mRNA. |
| 12.3 | Cidea | cell death-inducing DNA fragmentation factor, alpha subunit |
| 14.3 | Dgat2 | diacylglycerol O-acyltransferase 2 (Dgat2), mRNA. |
| 15.7 | Ccl25 | Chemokine (C-C motif) ligand 25 |
| 20.3 | Snap25 | Synaptosomal-associated protein 25 |
some genes between 4 and 6-fold upregulated not listed due to space limitations
Somewhat surprisingly, there was relatively little overlap between the most highly over-expressed genes in the SCL-LMO1 set and the LMO1 only set (Table 3). Of the 22 genes that were 4-fold increased in the LMO1 tumors compared to normal thymus, only 5 (Igh, Dtx1, Iltifb, Ccl8, and Mrpl38) were also at least 4-fold increased in the SCL-LMO1 tumors. There also was relatively little overlap between the SCL-LMO1 group and the LMO1 only group among the genes with the highest fold decrease compared to normal thymus. Prss16, Tctex1, Cidea, Dstb, and Diras2 were 5 of the only genes that showed decreased expression in both groups. Parodoxically, several genes that were at least 4-fold increased in the SCL-LMO1 set were at least 4-fold decreased in the LMO1 set. These genes included Tcrb and Tcra.
Table 3.
Genes most differentially regulated in LMO1 tumors
| Fold increase | Gene | Description |
|---|---|---|
| 107.8 | Iltifb | interleukin 10-related T cell-derived inducible factor beta (Iltifb), mRNA. |
| 24.7 | Igh-6 | Immunoglobulin heavy chain 6 (heavy chain of IgM) |
| 17.4 | Bst1 | bone marrow stromal cell antigen 1 (Bst1), mRNA. |
| 9.9 | Ccl8 | chemokine (C-C motif) ligand 8 (Ccl8), mRNA. |
| 8.0 | Gzma | granzyme A (Gzma), mRNA. |
| 7.5 | Mrpl38 | mitochondrial ribosomal protein L38 (Mrpl38), mRNA. |
| 6.8 | Dennd2d | DENN/MADD domain containing 2D (Dennd2d), mRNA. |
| 6.7 | Il9 | interleukin 9 (Il9), mRNA. |
| 5.4 | Ccdc18 | coiled-coil domain containing 18 (Ccdc18), mRNA. |
| 5.0 | Dtx1 | deltex 1 homolog (Drosophila) (Dtx1), mRNA. |
| 4.7 | Adam19 | a disintegrin and metallopeptidase domain 19 (meltrin beta) (Adam19) |
| 4.6 | Il12rb2 | interleukin 12 receptor, beta 2 (Il12rb2), mRNA. |
| 4.4 | Hdgfrp3 | hepatoma-derived growth factor, related protein 3 (Hdgfrp3), mRNA. |
| 4.2 | Aldh1b1 | aldehyde dehydrogenase 1 family, member B1 (Aldh1b1), mRNA. |
| 4.2 | Pabpc1 | poly A binding protein, cytoplasmic 1 (Pabpc1), mRNA. |
| 4.2 | Ck2 | Mus musculus casein kinase 2 beta subunit (gMCK2) gene |
| 4.2 | Dpp4 | dipeptidylpeptidase 4 (Dpp4), mRNA. |
| 4.1 | Flnc | PREDICTED: filamin C, gamma (actin binding protein 280), transcript variant 3 (Flnc), mRNA. |
| 4.1 | Igk-V23 | Immunoglobulin kappa chain variable 23 (V23) |
| 4.1 | Ifi205 | interferon activated gene 205 (Ifi205), mRNA. |
| 4.0 | Hes1 | hairy and enhancer of split 1 (Drosophila) (Hes1), mRNA. |
| 4.0 | Tfrc | transferrin receptor (Tfrc), mRNA. |
| Fold decrease | Gene | Description |
| 4.0 | Anxa2 | annexin A2 (Anxa2), mRNA. |
| 4.0 | Laptm4b | lysosomal-associated protein transmembrane 4B (Laptm4b), mRNA. |
| 4.0 | Nrbp | nuclear receptor binding protein (Nrbp), mRNA. |
| 4.1 | Cstb | cystatin B (Cstb), mRNA. |
| 4.1 | Igfbpl1 | Insulin-like growth factor binding protein-like 1 |
| 4.1 | Ctdsp2 | CTD (carboxy-terminal domain, RNA polymerase II, polypeptide A) small phosphatase 2 |
| 4.2 | Krt2-6a | Keratin complex 2, basic, gene 6a |
| 4.2 | Gtf2h4 | general transcription factor II H, polypeptide 4 (Gtf2h4), mRNA. |
| 4.3 | Rb1cc1 | RB1-inducible coiled-coil 1 (Rb1cc1), mRNA. |
| 4.4 | Plxdc2 | plexin domain containing 2 (Plxdc2), mRNA. |
| 4.5 | Cidea | cell death-inducing DNA fragmentation factor, alpha subunit-like effector A (Cidea), mRNA. |
| 4.9 | Arhgef1 | Rho guanine nucleotide exchange factor (GEF) 1 (Arhgef1), mRNA. |
| 4.9 | Cxcl11 | chemokine (C-X-C motif) ligand 11 (Cxcl11), mRNA. |
| 4.9 | Diras2 | DIRAS family, GTP-binding RAS-like 2 (Diras2), mRNA. |
| 5.0 | H2-Ab1 | histocompatibility 2, class II antigen A, beta 1 (H2-Ab1), mRNA. |
| 5.2 | Zfp236 | Zinc finger protein 236 |
| 5.2 | S100a16 | S100 calcium binding protein A16 (S100a16), mRNA. |
| 5.4 | Plxnd1 | Plexin D1 |
| 5.5 | LOC674927 | PREDICTED: similar to melanoma antigen (LOC674927), mRNA. |
| 5.5 | Ubd | ubiquitin D (Ubd), mRNA. |
| 5.8 | Serinc3 | serine incorporator 3 (Serinc3), mRNA. |
| 6.0 | Tcrd | T-cell receptor delta |
| 6.2 | 5830406J20Rik | RIKEN cDNA 5830406J20 gene (5830406J20Rik), mRNA. |
| 6.2 | Cox6a2 | cytochrome c oxidase, subunit VI a, polypeptide 2 (Cox6a2), mRNA. |
| 6.7 | A430035B10Rik | RIKEN cDNA A430035B10 gene |
| 7.0 | Coro1a | coronin, actin binding protein 1A (Coro1a), mRNA. |
| 7.1 | Cbr2 | carbonyl reductase 2 (Cbr2), mRNA. |
| 7.2 | Itk | IL2-inducible T-cell kinase |
| 7.3 | Tcra | T-cell receptor alpha chain V region CTL-F3 precursor |
| 7.6 | Tceal8 | Transcription elongation factor A (SII)-like 8 |
| 7.9 | LOC14433 | similar to glyceraldehyde-3-phosphate dehydrogenase (LOC14433), mRNA. |
| 10.4 | Tcrb | T-cell receptor beta, variable 13 |
| 10.7 | Crip3 | cysteine-rich protein 3 (Crip3), transcript variant TLP-B, mRNA. |
| 10.9 | Tctex1d1 | Tctex1 domain containing 1 (Tctex1d1), mRNA. |
| 55.2 | Prss16 | protease, serine, 16 (thymus) (Prss16), mRNA. |
The most highly over-expressed gene in the NHD13 tumors was Pbx3, which was not over-expressed in any of the other groups of tumors. Pbx3 overexpression was of interest since Pbx3 is known to bind to Hox genes. Additional genes that were at least 4-fold over-expressed included Ccl8, Tfrc, Mpo, Ctse, and several genes involved in protein synthesis (Mrpl38, Eef2, and Eif3s9).
Ccl8 is important for growth of leukemic cells. (Fig. 2)
Figure 2. Chemokine ligand 8 is important for leukemic cell survival.
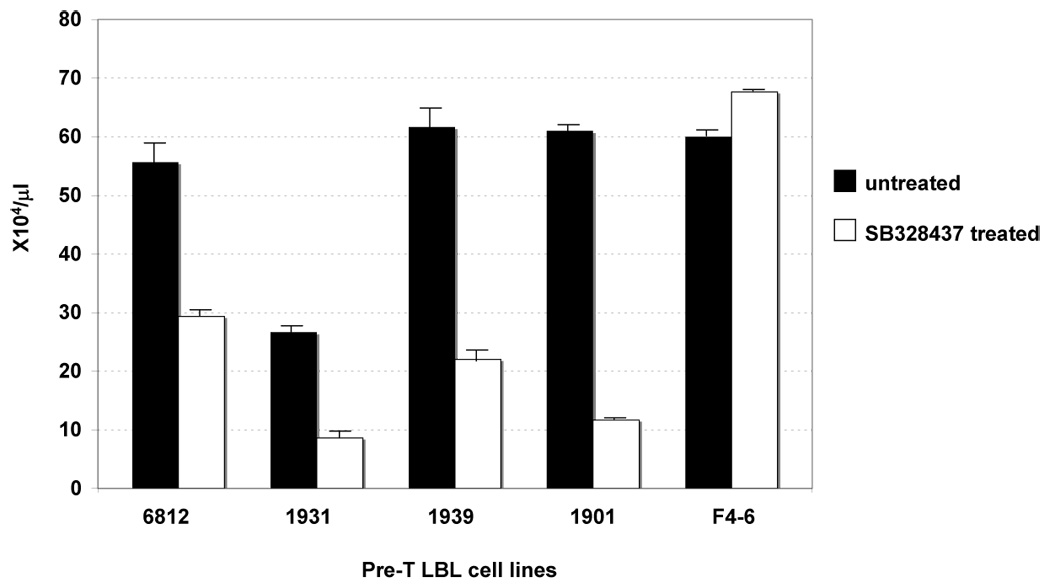
Murine pre-T LBL cell lines were treated with the Ccr3 antagonist, SB328437, at the concentration of 69 µM for 72 hours. #6812 and 1901 are pre-T LBL cell lines established from SCL-LMO1 and NHD13 mice, respectively. #1931 and 1939 are pre-T LBL cell lines established from OLIG2-LMO1 mice. F4–6 is a Friend virus-induced murine erythroleukemia cell line.
We focused on Ccl8 since it was over-expressed in all three series of tumors [SCL-LMO1 (5.0-fold), LMO1 (10-fold), and the NHD13 (5.4-fold) as well as OLIG2-LMO1 tumors (8.2-fold) 10], and therefore a candidate for a gene that was generally important for malignant transformation of thymocytes. Since Ccl8 functions through the chemokine receptor (Ccr3), we investigated whether Ccr3 signaling was important for growth of the Ccl8-expressing cell lines. Murine pre-T LBL cell lines established from SCL-LMO1 mice, OLIG2-LMO1 mice, and NHD13 mice were treated with the Ccr3 antagonist SB328437. As shown in Figure 2, SB328437 inhibits the growth of the SCL-LMO1, OLIG2-LMO1, and NHD13 cell lines, whereas growth of a non-T cell control (murine erythroleukemic) cell line (F4–6) was unaffected.
Cfl1 phosporylation is associated with growth inhibition of SCL-LMO1 tumors. (Fig. 3)
Figure 3. Cofilin1 is important for growth of SCL-LMO1 pre-T LBL.
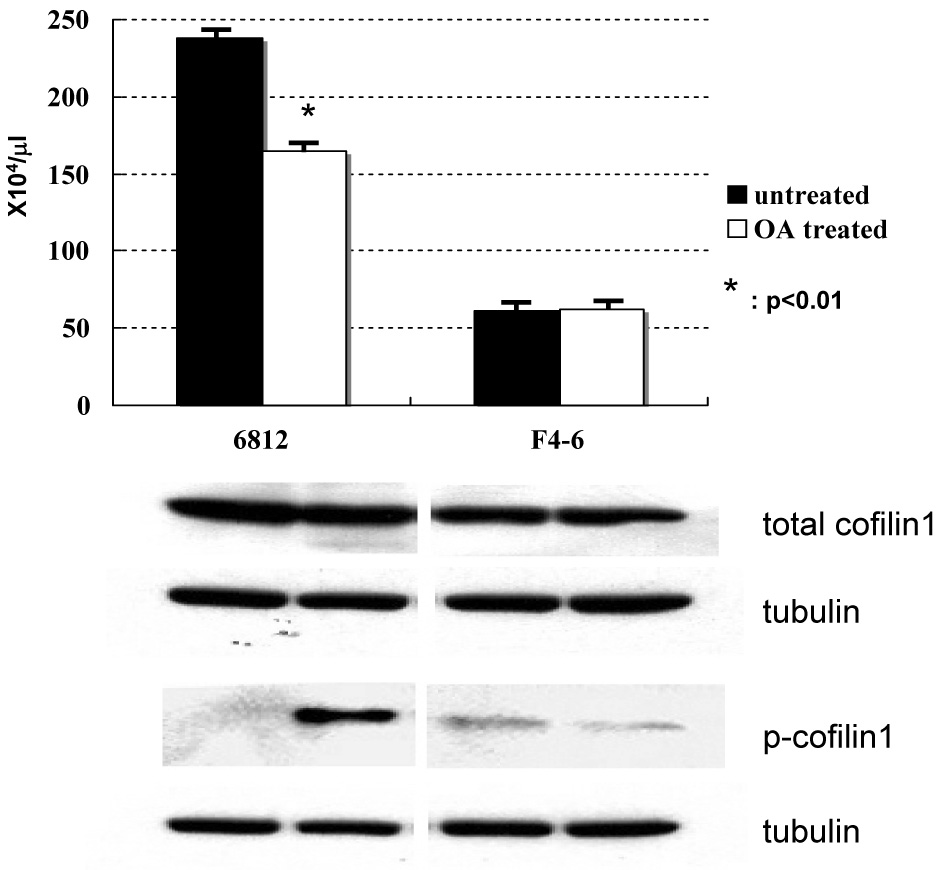
Treatment of the SCL-LMO1 pre-T LBL cell line #6812 with Okadaic acid resulted in the suppression of cell growth and an increased proportion of inactivate phospho-cofilin1.
Cfl1 was 10.9-fold up-regulated specifically in the SCL-LMO1 tumors. Cfl1 has been shown to be phosphorylated and inactivated in peripheral T-lymphocytes. Activation through an accessory receptor leads to dephosphorylation of Cfl1 by PPA211. Dephosphorylated Cfl1 can bind to polymeric F-actin and process it to mono- or oligo-meric G-actin, which can then gain access into the nucleus. In the nucleus, G-actin contributes to transcription via inhibition of DNAse I and activation of RNA polymerase II12, 13. Okadaic acid (OA) has been shown to specifically inhibit PPA2 so that Cfl1 is phosphorylated and inactivated14. In order to investigate whether overexpression of Cfl1 is important for cell growth in SCL-LMO1 tumors, we treated leukemic cell lines with OA at the concentration of 1 µM for 4 hours. A murine pre-T LBL cell line (6812), that was established from a SCL-LMO1 thymic tumor, showed activated (dephosphorylated) Cfl1. OA treatment led to simultaneous suppression of cell growth and dephosphorylation of Cfl1 in the 6812 cell line. By way of comparison, a murine erythroleukemic cell line (F4–6), which expresses less Cfl1 than the 6812 cell line, was unaffected by OA treatment, either in terms of cell viability or Cfl1 dephosphorylation.
Pbx3 is a down-stream target of NHD13 (Fig. 4)
Figure 4. Pbx3 is a downstream target of NHD13.
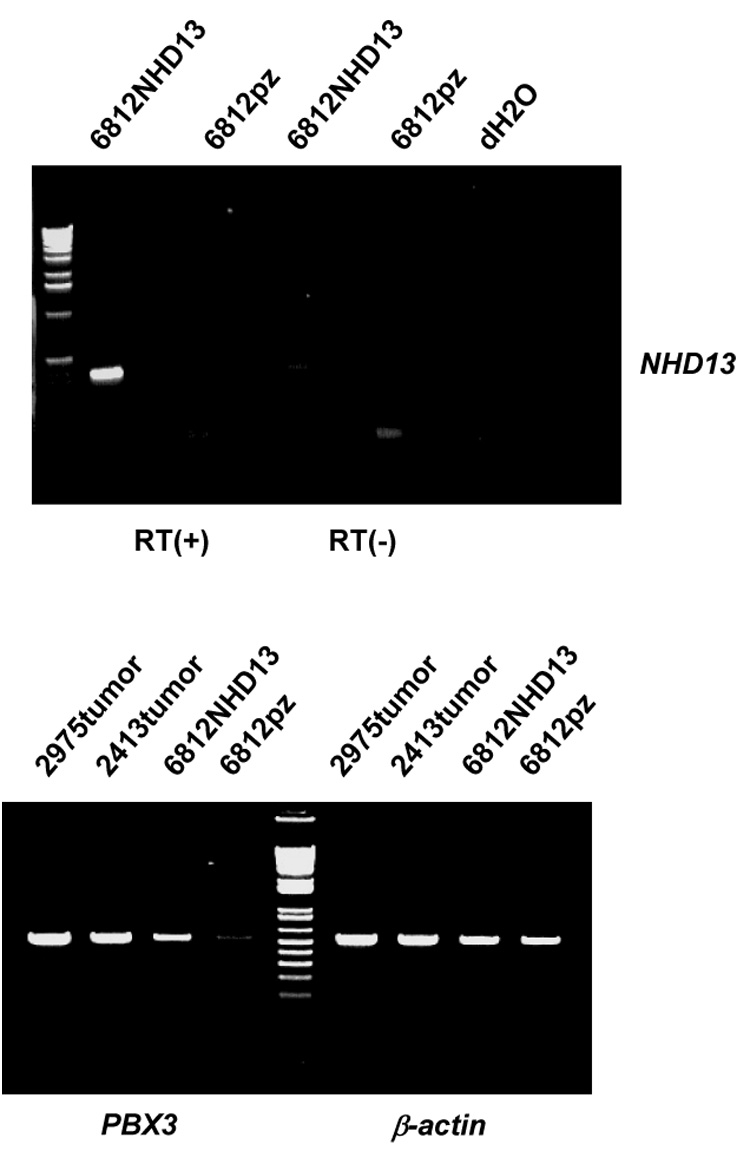
Upper panel. A pre-T LBL cell line established from an SCL-LMO1 mouse (#6812) was transfected with an NHD13 expression vector or empty vector (lanes 6812NHD13 and 6812pz respectively). Expression of NHD13 was detected by RT-PCR; RT(+) and RT (−) indicate presence or absence or reverse transcriptase respectively. Lower panel. PBX3 is up-regulated in the sample transfected with the NHD13 expression vector. 2975 and 2413 are two primary NHD13 tumors used as positive controls for PBX3 expression. β-actin amplification is used as an RNA quality control.
Pre-B cell leukemia transcription factors (PBXs) are important co-factors for the transcriptional regulation mediated by a number of Hox proteins15. PBX1 was first identified in chromosomal translocations in B-lineage leukemia and is required for normal hematopoiesis. PBX2 and PBX3 were later identified as members of this highly conserved family by their strong homology to PBX1. We found that Pbx3 was 8.3-fold up-regulated exclusively in the NHD13 tumors. To investigate whether Pbx3 is a downstream target of NHD13, we transfected an NHD13 expression vector into a murine pre-T LBL cell line, 6812, which was established from an SCL-LMO1 tumor that did not overexpress Pbx3. Two NHD13 pre-T LBL primary tumors, 2975 and the 2413 expressed Pbx3 and were used as controls. 6812 cells transfected with the NHD13 expression vector showed an expression of Pbx3 comparable to the NHD13 primary thymic tumors, whereas 6812 cell transfected with the empty vector showed only low level Pbx3 expression, supporting the hypothesis that expression of NHD13 leads to up-regulation of Pbx3.
Discussion
In order to gain insight into the molecular events that lead to pre-T LBL, we compared the gene expression profile of murine pre-T LBL samples to that of the normal murine thymus. We chose to analyze tumors that were initiated by defined mutations (SCL and/or LMO1 over-expression, or expression of a NUP98-HOXD13 fusion gene). Our experimental approach was designed to identify two groups of differentially expressed genes. The first group of genes were those that were differentially expressed in all pre-T LBL samples, irrespective of the initiating mutation; these should be genes that are universally important for the malignant transformation of thymocytes. The second group of genes we were interested in identifying were those that were differentially expressed only in a specific subgroup of pre-T LBL (ie, only in SCL/LMO1 mice, only in NHD13 mice, etc). Genes identified in this manner should point to genes and pathways that might not be generally important for malignant transformation of thymocytes, but that are important for genetically defined subsets. Hierarchical clustering using a classical Pearson analysis indicated that the 3 groups of pre-T LBL tumors (SCL/LMO1, LMO1 only, and NHD13) could be distinguished from one another, as well as from normal thymus.
We identified 12 genes whose mean expression levels were at least 4-fold over-expressed and 30 genes that were at least 4-fold under-expressed when sample from all of the different genotype groups were analyzed together. The over-expressed genes included several known to be involved in protein synthesis (Eef2, eEF-Tu, Mrpl38), Notch signaling (Dtx1), and Ccl8. Several classes of genes were commonly under-expressed in the pre-T LBL samples, including apoptotic pathway genes (Cox8b, Cidea)16, genes involved in T-cell differentiation (Prss16, Ccl25, CD83, Spatial, Fkbp6, and Tcrd) 17–20, a group of fatty acid binding proteins (Fabp3, Fabp4, and Fabp9)21, and Bop122 and Rb1cc123, two genes whose over-expression is linked to decreased cell proliferation. The Fabp proteins are typically expressed in adipocytes21, and their under-expression in pre-T LBL relative to normal thymus may reflect a decreased proportion of adipocytes in the pre-T LBL tumor samples compared to normal thymus. Given the rapid rate at which the malignant thymocytes proliferate, it is not surprising that several of the over-expressed genes are known to be involved in new protein synthesis. Moreover, several of the genes under-expressed in pre-T LBL with respect to normal thymus were pro-apoptotic or associated with decreased cell proliferation. However, since we used the mean expression level to generate Table 1, it was possible that some of the genes were identified because they were highly up-regulated in one subset only. To investigate this possibility, we analyzed differential gene expression in specific genotypes. When each genotype was analyzed separately, we were surprised to find that only two genes (Ccl8 and Mrpl38) were at least 4-fold over-expressed in all three genotypes, and a single gene (Prss16) was at least 4-fold under-expressed in all three genotypes.
We identified a number of genes that were over-expressed in SCL/LMO1 tumors. Several of these genes were found to be over-expressed only in the SCL/LMO1 tumors. These included Ftl1, Cfl1, Tcrb, Tcra, Pin1, and a large number of genes involved in protein synthesis (Eef1a1, Eef-tu, Ef2, Eif4a1, Eif5a, Eif4b, Tceal8, and Rpl13). Of note, TCRA and TCRB were two of the most differentially up-regulated genes in human leukemias that activated SCL1; these findings likely reflect a differentiation arrest at the late cortical stage of thymic development1, 24. Also, the identification of a large number of genes involved in protein synthesis, particularly Eif4a1 and Eif4b, was of interest given recent studies showing activation of the EIF4 complex through mTOR signaling25. In addition, over-expression of Eif5a was of interest, as this is the only eukaryotic protein known to be activated by post-translational hypusination, and hypusination inhibitors have demonstrated an anti-proliferative effect on leukemic cell lines in vitro26. Several other genes, including Dtx1, Iltifb, Igh, were found to be over-expressed in both the SCL/LMO1 and LMO1 only tumors. Although we had predicted that the SCL/LMO1 and LMO1 only tumors would be fairly similar, the list of commonly over-expressed genes was relatively small. Moreover, Tcra and Tcrb, among the most highly over-expressed genes in the SCL/LMO1 tumors were more than 4-fold under-expressed in the LMO1 only tumors; this difference may reflect an earlier stage of thymocyte differentiation arrest in the LMO1 tumors, prior to the expression of Tcra and Tcrb. Of note, this finding is consistent with prior observations that overexpression of a closely related gene (LMO2), blocks thymocyte differentiation at the CD4-/CD8- (DN) stage of differentiation27, 28.
The genes most highly over-expressed in the NHD13 pre-T LBL had little overlap with those most highly over-expressed in the SCL/LMO1 and LMO1 only tumors, except for Mrpl38 and Ccl8, discussed above, and transferrin receptor (Tfrc). There were several genes over 4-fold under-expressed in both the NHD13 and LMO1, but no the SCL/LMO1 tumors. These genes included Tcra, Tcrb, H2-A, Crip3, Ubd, and Diras2, and, similar to the case with human pre-T LBL that overexpress HOX111, 3, likely reflect differentiation arrest at an earlier stage of thymocyte differentiation, prior to expression of Tcra and Tcrb24. The overexpression of Mpo, a gene typically expressed in myeloid cells, in the NHD13 tumors may reflect a biphenotypic differentiation potential in NHD13 pre-T LBL, a possibility consistent with the finding that NHD13 mice typically develop a MDS, which often progresses to an AML. Alternatively, the Mpo expression might be explained by the contamination of small number of myeloid leukemic cells that were seeded from the co-incident MDS present in the NHD13 mice.
We were intrigued by the observation that Ccl8 was consistently over-expressed, and hypothesized that Ccl8 might be an autocrine growth factor for pre-T LBL. Since Ccl8 exerts its effects through the Ccr3 receptor, we reasoned that inhibition of the Ccr3 receptor might suppress the growth of pre-T LBL cell lines. We verified that the Ccr3 receptor was expressed in pre-T LBL cell lines, and treated four pre-T LBL cell lines, derived from SCL-LMO1, OLIG2-LMO1, or NHD13 tumors with a Ccr3 antagonist (SB328437). All four of these pre-T LBL cell lines demonstrated growth inhibition, by as much as 85%, suggesting that treatment with a Ccr3 antagonist might be an effective anti-leukemic therapy.
Genes whose expression levels were altered specifically in SCL-LMO1 tumors represent candidates for genes important in malignant transformation of these cells. Of those candidates, Cfl1 was 10.9-fold up-regulated. Since Cfl1 has been shown to be an inhibitor of glucocorticoid receptor that is consistent with the relative resistance of pre-T LBL overexpressing SCL compared to pre-T LBL with other genetic alterations1, 29, 30, we investigated whether the up-regulation of the Cfl11 is important for proliferation of leukemic cell lines derived from SCL-LMO1 mice. It has been shown that both Ras and a costimulation of TCR/CD3 and CD28 activate MAPK/ERK kinase and PI3K, which induces the dephosphorylation of cofilin1. An activation of PI3K by stimulation through CD28 also down-regulates a cyclin-dependent-kinase inhibitor p27kip131. Those cascades lead to the production of cytokines such as IL-2 and subsequent proliferation of T-lymphocytes31, 32. OA, an inhibitor for the serine/threonine phosphatase type2A that phosphorylates cofilin1, treatment demonstrated inhibition of leukemic cell growth, suggesting that activation of Cfl1 is important for growth of leukemic cell lines that overexpress SCL and LMO1. Similarly, Pbx3 was specifically up-regulated in NHD13 pre-T LBL; transfection of T-cell lines with an NHD13 expression vector led to upregulation of Pbx3, suggesting that Pbx3 may be a direct downstream target of NHD13.
We have used gene expression profiling to identify genes and pathways that may be important for malignant transformation of T-cells in general, as well as those genes and pathways that may be important for transformation of T-cells that express known oncoproteins. We found relatively few genes consistently over or under-expressed in pre-T LBL compared to normal thymus, and a larger number of genes that may be important for transformation of thymocytes that express known oncoproteins. Similar to findings with human pre-T LBL, we suspect that the differences in gene expression profile seen in different genetically defined subsets of pre-T LBL may reflect different stages of thymocyte maturation arrest. We tested and confirmed several of the genes that were candidates for general or specific involvement in the malignant transformation of thymocytes, and have identified Ccr3, the Ccl8 receptor, as a novel potential target for treatment of pre-T LBL.
Acknowledgment
We would like to thank our colleague, Drs. Li Li, Christopher Slape, Zhenhua Zhang, and Helge Hartung for technical advices. We also greatly appreciate Dr. Masue Hayashi’s significant discussion. This research was supported by the Intramural Research Program of the NIH, NCI.
References
- 1.Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1:75–87. doi: 10.1016/s1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 2.Yeoh EJ, Ross ME, Shurtleff SA, Williams WK, Patel D, Mahfouz R, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1:133–143. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 3.Ferrando AA, Herblot S, Palomero T, Hansen M, Hoang T, Fox EA, et al. Biallelic transcriptional activation of oncogenic transcription factors in T-cell acute lymphoblastic leukemia. Blood. 2004;103:1909–1911. doi: 10.1182/blood-2003-07-2577. [DOI] [PubMed] [Google Scholar]
- 4.Palomero T, Odom DT, O'Neil J, Ferrando AA, Margolin A, Neuberg DS, et al. Transcriptional regulatory networks downstream of TAL1/SCL in T-cell acute lymphoblastic leukemia. Blood. 2006;108:986–992. doi: 10.1182/blood-2005-08-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuire EA, Rintoul CE, Sclar GM, Korsmeyer SJ. Thymic overexpression of Ttg-1 in transgenic mice results in T-cell acute lymphoblastic leukemia/lymphoma. Mol Cell Biol. 1992;12:4186–4196. doi: 10.1128/mcb.12.9.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aplan PD, Jones CA, Chervinsky DS, Zhao X, Ellsworth M, Wu C, et al. An scl gene product lacking the transactivation domain induces bony abnormalities and cooperates with LMO1 to generate T-cell malignancies in transgenic mice. Embo J. 1997;16:2408–2419. doi: 10.1093/emboj/16.9.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chervinsky DS, Zhao XF, Lam DH, Ellsworth M, Gross KW, Aplan PD. Disordered T-cell development and T-cell malignancies in SCL LMO1 double-transgenic mice: parallels with E2A-deficient mice. Mol Cell Biol. 1999;19:5025–5035. doi: 10.1128/mcb.19.7.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin YW, Slape C, Zhang Z, Aplan PD. NUP98-HOXD13 transgenic mice develop a highly penetrant, severe myelodysplastic syndrome that progresses to acute leukemia. Blood. 2005;106:287–295. doi: 10.1182/blood-2004-12-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chervinsky DS, Lam DH, Zhao XF, Melman MP, Aplan PD. Development and characterization of T cell leukemia cell lines established from SCL/LMO1 double transgenic mice. Leukemia. 2001;15:141–147. doi: 10.1038/sj.leu.2401997. [DOI] [PubMed] [Google Scholar]
- 10.Lin YW, Deveney R, Barbara M, Iscove NN, Nimer SD, Slape C, et al. OLIG2 (BHLHB1), a bHLH transcription factor, contributes to leukemogenesis in concert with LMO1. Cancer Res. 2005;65:7151–7158. doi: 10.1158/0008-5472.CAN-05-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambach A, Saunus J, Konstandin M, Wesselborg S, Meuer SC, Samstag Y. The serine phosphatases PP1 and PP2A associate with and activate the actin-binding protein cofilin in human T lymphocytes. Eur J Immunol. 2000;30:3422–3431. doi: 10.1002/1521-4141(2000012)30:12<3422::AID-IMMU3422>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.Samstag Y, Bader A, Meuer SC. A serine phosphatase is involved in CD2-mediated activation of human T lymphocytes and natural killer cells. J Immunol. 1991;147:788–794. [PubMed] [Google Scholar]
- 13.Samstag Y, Eckerskorn C, Wesselborg S, Henning S, Wallich R, Meuer SC. Costimulatory signals for human T-cell activation induce nuclear translocation of pp19/cofilin. Proc Natl Acad Sci U S A. 1994;91:4494–4498. doi: 10.1073/pnas.91.10.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samstag Y, Dreizler EM, Ambach A, Sczakiel G, Meuer SC. Inhibition of constitutive serine phosphatase activity in T lymphoma cells results in phosphorylation of pp19/cofilin and induces apoptosis. J Immunol. 1996;156:4167–4173. [PubMed] [Google Scholar]
- 15.Chang CP, Shen WF, Rozenfeld S, Lawrence HJ, Largman C, Cleary ML. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 1995;9:663–674. doi: 10.1101/gad.9.6.663. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S, et al. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet. 35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
- 17.Cheunsuk S, Lian ZX, Yang GX, Gershwin ME, Gruen JR, Bowlus CL. Prss16 is not required for T-cell development. Mol Cell Biol. 2005;25:789–796. doi: 10.1128/MCB.25.2.789-796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Saito F, Liu Z, Lei Y, Uehara S, Love P, et al. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 2006;108:2531–2539. doi: 10.1182/blood-2006-05-024190. [DOI] [PubMed] [Google Scholar]
- 19.Zinser E, Lechmann M, Golka A, Hock B, Steinkasserer A. Determination of the inhibitory activity and biological half-live of soluble CD83: comparison of wild type and mutant isoforms. Immunobiology. 2006;211:449–453. doi: 10.1016/j.imbio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Scholler N, Hayden-Ledbetter M, Dahlin A, Hellstrom I, Hellstrom KE, Ledbetter JA. Cutting edge: CD83 regulates the development of cellular immunity. J Immunol. 2002;168:2599–2602. doi: 10.4049/jimmunol.168.6.2599. [DOI] [PubMed] [Google Scholar]
- 21.Rolph MS, Young TR, Shum BO, Gorgun CZ, Schmitz-Peiffer C, Ramshaw IA, et al. Regulation of dendritic cell function and T cell priming by the Fatty Acid-binding protein AP2. J Immunol. 2006;177:7794–7801. doi: 10.4049/jimmunol.177.11.7794. [DOI] [PubMed] [Google Scholar]
- 22.Strezoska Z, Pestov DG, Lau LF. Functional inactivation of the mouse nucleolar protein Bop1 inhibits multiple steps in pre-rRNA processing and blocks cell cycle progression. J Biol Chem. 2002;277:29617–29625. doi: 10.1074/jbc.M204381200. [DOI] [PubMed] [Google Scholar]
- 23.Chano T, Ikegawa S, Saito-Ohara F, Inazawa J, Mabuchi A, Saeki Y, et al. Isolation, characterization and mapping of the mouse and human RB1CC1 genes. Gene. 2002;291:29–34. doi: 10.1016/s0378-1119(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 24.Rodewald HR, Fehling HJ. Molecular and cellular events in early thymocyte development. Adv Immunol. 1998;69:1–112. doi: 10.1016/s0065-2776(08)60606-9. [DOI] [PubMed] [Google Scholar]
- 25.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 26.Balabanov S, Gontarewicz A, Ziegler P, Hartmann U, Kammer W, Copland M, et al. Hypusination of eukaryotic initiation factor 5A (EIF-5A): a novel therapeutic target in BCR-ABL-positive leukemias identified by a proteomics approach. Blood. 2006;109:1701–1711. doi: 10.1182/blood-2005-03-037648. [DOI] [PubMed] [Google Scholar]
- 27.Neale GA, Rehg JE, Goorha RM. Ectopic expression of rhombotin-2 causes selective expansion of CD4-CD8- lymphocytes in the thymus and T-cell tumors in transgenic mice. Blood. 1995;86:3060–3071. [PubMed] [Google Scholar]
- 28.Larson RC, Osada H, Larson TA, Lavenir I, Rabbitts TH. The oncogenic LIM protein Rbtn2 causes thymic developmental aberrations that precede malignancy in transgenic mice. Oncogene. 1995;11:853–862. [PubMed] [Google Scholar]
- 29.Bernard M, Delabesse E, Novault S, Hermine O, Macintyre EA. Antiapoptotic effect of ectopic TAL1/SCL expression in a human leukemic T-cell line. Cancer Res. 1998;58:2680–2687. [PubMed] [Google Scholar]
- 30.Ruegg J, Holsboer F, Turck C, Rein T. Cofilin 1 is revealed as an inhibitor of glucocorticoid receptor by analysis of hormone-resistant cells. Mol Cell Biol. 2004;24:9371–9382. doi: 10.1128/MCB.24.21.9371-9382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 32.Wabnitz GH, Nebl G, Klemke M, Schroder AJ, Samstag Y. Phosphatidylinositol 3-kinase functions as a Ras effector in the signaling cascade that regulates dephosphorylation of the actin-remodeling protein cofilin after costimulation of untransformed human T lymphocytes. J Immunol. 2006;176:1668–1674. doi: 10.4049/jimmunol.176.3.1668. [DOI] [PubMed] [Google Scholar]


