Abstract
The replication period of Escherichia coli cells grown in rich medium lasts longer than one generation. Initiation thus occurs in the ‘mother-' or ‘grandmother generation'. Sister origins in such cells were found to be colocalized for an entire generation or more, whereas sister origins in slow-growing cells were colocalized for about 0.1–0.2 generations. The role of origin inactivation (sequestration) by the SeqA protein in origin colocalization was studied by comparing sequestration-deficient mutants with wild-type cells. Cells with mutant, non-sequesterable origins showed wild-type colocalization of sister origins. In contrast, cells unable to sequester new origins due to loss of SeqA, showed aberrant localization of origins indicating a lack of organization of new origins. In these cells, aberrant replisome organization was also found. These results suggest that correct organization of sister origins and sister replisomes is dependent on the binding of SeqA protein to newly formed DNA at the replication forks, but independent of origin sequestration. In agreement, in vitro experiments indicate that SeqA is capable of pairing newly replicated DNA molecules.
Keywords: Escherichia coli, initiation of DNA replication, replication fork foci and origin foci
Introduction
The bacterium Escherichia coli has a single chromosome that is replicated from a single origin (oriC), bidirectionally to the terminus, once per division cycle (Kornberg and Baker, 1992). The cell cycle of slowly growing bacteria is quite similar to that of eukaryotic cells (Boye et al, 1996), with the G1, S and G2/M phases of bacteria termed B, C and D, respectively. E. coli (and certain other bacteria) is capable of very rapid growth in rich medium, with doubling times as short as 20 min. The replication time, however, remains long, with approximately 60–90 min required to replicate and segregate the chromosome. Therefore, the cell cycle is more complicated during rapid growth (Figure 1). If the time it takes to synthesize and segregate the daughter chromosomes (C+D) exceeds one generation time, a new round of replication must be initiated before the previous round is completed (Cooper and Helmstetter, 1968). Thus, initiation occurs at two origins in the ‘mother' cell. It can even occur in the ‘grandmother' cell at four origins if the time it takes to replicate and segregate the chromosome exceeds two generations. These initiations at two or four origins occur simultaneously, as one event per division cycle (Skarstad et al, 1986). While E. coli and Bacillus subtilis are two examples of bacteria capable of performing multifork replication, other bacteria, such as Caulobacter crescentus, are not. Eukaryotic cells do not replicate with overlapping cycles, but do initiate DNA replication at multiple replication origins, and thus perform a different kind of multifork replication, with the multiple forks on the same copy of the genome (Diffley, 2004).
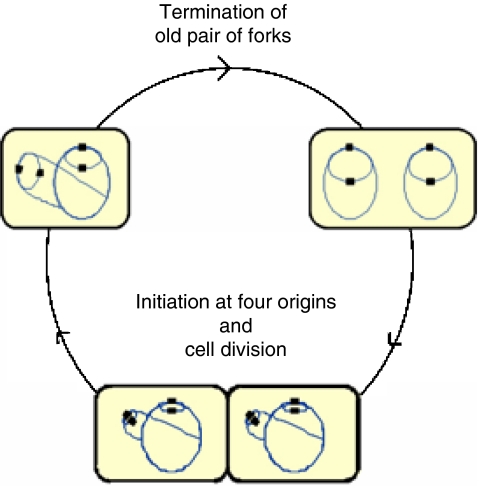
Figure 1.
Replication pattern of rapidly growing E. coli wild-type cells. Cells (yellow) with chromosomes (blue lines) and origins (black squares) are drawn schematically to show the number of replication forks and origins at different stages of the cell cycle. In this example, initiation of replication occurs at four origins at the same time as cell division (bottom). A young cell therefore contains four origins and six replication forks (upper left). As replication proceeds, the oldest pair of forks reach the terminus and the two sister chromosomes segregate. The cell then contains four origins and four replication forks (upper right). Initiation then occurs again at 4 origins and generates 8 new forks giving a total of 12 forks, as cell division approaches (bottom). Because there will be cell-to-cell variability, some cells will contain eight origins before they divide, whereas cells that divide before initiation of replication will contain only two origins (not shown). However, the majority of the cells in the culture will contain four origins.
Organization of the replication machinery into one unit, or ‘replication factory', containing both forks originating from the same origin was initially proposed by Dingman (Dingman, 1974), and was later demonstrated cytologically (Hiraga et al, 1998; Lemon and Grossman, 1998, 2000; Fossum et al, 2003; Molina and Skarstad, 2004). Presence of the replication factory unit was also recently found in the eukaryote budding yeast, where sister replication forks generated from the same origin stayed associated with each other during replication (Kitamura et al, 2006). However, others have not found evidence of associated replication forks (Brendler et al, 2000), and thus the existence of replication factories is still controversial.
Dingman also proposed that newly replicated DNA moves away from the anchored replication machinery as replication proceeds. Some researchers find support for this model (Lemon and Grossman, 2001; Wang et al, 2005; Berkmen and Grossman, 2006; Nielsen et al, 2006), whereas others find that sister chromosomes are paired during some of the replication period and then separate later in a coordinated manner (Niki et al, 2000; Sunako et al, 2001; Bates and Kleckner, 2005). Colocalization of bacterial replisomes and chromosome regions, as determined by the low resolution of light microscopy, does not necessarily imply that they are physically or functionally coupled to each other (Breier et al, 2005; Woldringh and Nanninga, 2006).
Origin sequestration is a process that prevents reinitiation of newly replicated origins through the binding of multiple hemimethylated GATC sites in oriC by the SeqA protein (Lu et al, 1994; von Freiesleben et al, 1994; Slater et al, 1995). Origin sequestration lasts for about one-third of a generation (Campbell and Kleckner, 1990). Much of the SeqA in the cell forms oligomeric nucleoprotein structures with chromosomal, hemimethylated DNA emerging from the replication factories (Hiraga et al, 1998; Onogi et al, 1999; Brendler et al, 2000; Fossum et al, 2003; Molina and Skarstad, 2004) and may play a role in organization of daughter chromosomes.
Few of the detailed studies of origin and replisome dynamics in prokaryotes have thus far been conducted on cells with overlapping replication cycles. Here, we have performed the first comparison of origin localization in cells with different growth rates. We have also examined whether SeqA is involved in origin and replisome organization during multifork DNA replication.
Results
Colocalization of origins in wild-type cells with overlapping replication cycles
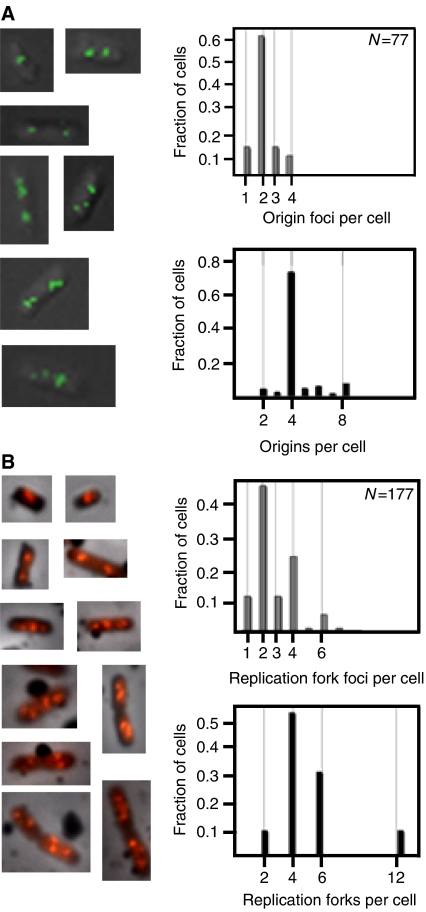
The extent to which sister origins are colocalized was investigated by measuring the distributions of origins per cell by flow cytometry, and comparing them with the distributions of separate origin foci found by microscopy of the same cells. Wild-type cells (SF72), which contain a fluorescent repressor–operator system for visualization of the origin region of the chromosome (see Materials and methods), grown in glu-CAA medium had a doubling time (Td) of 42 min, and a C+D period of 80–90 min (see Materials and methods). DNA replication cycles overlap, with initiation occurring at the very end of the ‘grandmother generation' or, in some of the cells, at the start of the ‘mother generation' (i.e. roughly coincident with cell division). Thus, these cells have four origins for most of their life-times (see Figure 1), as was demonstrated by flow cytometry (Figure 2A, bottom right panel) (Supplementary Figure S1A). Fluorescent microscopy visualization of living cells from the exponentially growing culture revealed the presence of discrete origin foci (Figure 2A, left panel). Most cells contained two, and some cells contained one, three and four origin foci (Figure 2A, top right panel), giving an average number of foci per cell of 2.2 (Table I). The average number of origins per cell was 4.4 (Table I). This twofold difference between the numbers of foci and origins can also be seen by comparing the distribution of foci (Figure 2A, top right panel) with the distribution of origins (Figure 2A, bottom right panel). Thus, sister origins in rapidly growing cells apparently are colocalized for essentially the entire cell cycle (i.e. for about 40–45 min).
Figure 2.
Origin and replication fork localization in rapidly growing wild-type cells. Cells were grown in glu-CAA medium. (A) Fluorescent micrographs of SF72 cells in which the origin region was visualized using a GFP-LacI/lac operator system (left panel). The number of origin foci per cell was tallied (top right panel), and the number of origins per cell was determined by flow cytometry analysis (bottom right panel). (B) Immunofluorescence of BrdU-labelled MG1655 cells (left panel) and distribution of replication fork foci per cell (top right panel). The number of replication forks per cell (bottom right panel) was calculated from flow cytometry histograms. The cells with origin and replication fork foci that could not be determined were 5 and 14%, respectively, of the examined population of cells.
Table 1.
Number of origins and origin foci per cell, and the period of colocalization under various growth conditions
| Medium | Td (min) | Average number of origins per cell, O | Average number of origin foci per cell, F | Origins per origin focus, O/F | Period of colocalizationa, P (min) | Period of colocalizationa, as a percentage of Td (%) |
|---|---|---|---|---|---|---|
| LB | 33 | 6.2 | 2.5b | 2.5 | 43 | 130 |
| Glu-CAA | 42 | 4.4 | 2.2b | 2.0 | 42 | 100 |
| Glu-thy | 102 | 2.5 | 2.0b | 1.3 | 33 | 32 |
| Gly-aac | 115c | 2.0c | 1.9c | 1.1 | 8 (25c) | 7 (20c) |
| aThe period of colocalization (P) was calculated from the relationship O/F=2P/Td. | ||||||
| bNumber of cells examined (N=403 in LB, N=77 in glu-CAA, N=59 in glu-thy). | ||||||
| cData obtained from Nielsen et al (2006). Numbers discussed by Nielsen et al (2006) are given in parenthesis. | ||||||
Origin organization is growth rate dependent
Most studies of origin localization have so far been performed using relatively slowly growing cells with nonoverlapping replication cycles. Results from these studies show, in contrast to our above findings, that origins are not paired for most of the cell cycle (Bates and Kleckner, 2005; Wang et al, 2005; Nielsen et al, 2006). Instead, sister origin colocalization occurs for the 10–20 min following replication, a minor fraction of the generation time. As we found extensive colocalization of origins with multifork DNA replication during rapid growth, we decided to investigate how the organization of origins may change as cellular growth rate varies.
Cells were grown in LB and glu-thy media, in addition to the glu-CAA medium as described above. Parameters from growth in glycerol medium supplemented with required amino acids were obtained by others (Nielsen et al, 2006) and are included in Table I for comparison. We found that both the average number of origins and the average number of foci per cell increased with increasing growth rate, but not to the same extent: the increase in origin numbers per cell was much higher than the increase in foci numbers (Table I). In cells growing in glu-CAA, the average number of origins in each focus was 2.0, indicating that essentially all sister origins are colocalized. For cells growing in LB, the average number of origins per focus was 2.5, suggesting that for portions of the cell cycle at this growth rate, four origins must be colocalized (for schematic illustration, see Figure 5D). Cells growing more slowly, such as those cultured in glu-thy or glycerol, showed less extensive colocalization of origins, with 1.3 and 1.1 origins per focus, respectively (Table I). This indicates that under less rich growth conditions, sister origins are colocalized for a limited period of the cell cycle and then separate.
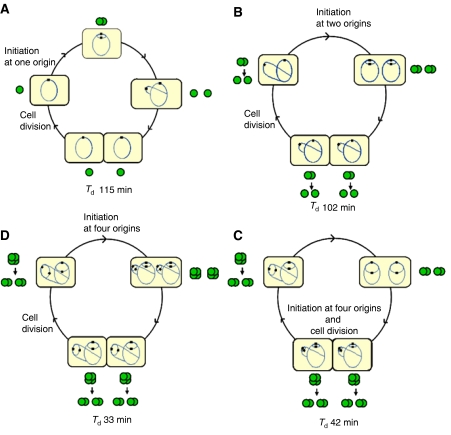
Figure 5.
Origin organization at four different growth rates. Cells (yellow) with chromosomes (blue lines) and origins (black squares) are drawn schematically to show the number of replication forks and origins at different stages of the cell cycles. Based on the observed distributions of origin foci per cell (Supplementary Table SI online), origin colocalization at these stages are indicated with green, filled circles. Each green circle represents a single copy of the origin. Two or four overlapping green circles represent foci containing two or four origins, respectively. (A) The replication and segregation period is confined within one generation time (Td>C+D). Here, a newborn cell has one chromosome with one origin and one origin focus. After replication, the two new sister origins are colocalized for 10–20 min (Wang et al, 2005; Bates and Kleckner, 2005; Nielsen et al, 2006), and then move apart. The colocalization period may correspond in duration to the sequestration period. (B) The C+D period is longer than the generation time. Initiation of replication occurs at two origins and the two new sister origins on each mother chromosome are colocalized. The pairs of sister origins in each cell half separate right before or right after cell division, so that some of the newborn cells have one focus and some have two foci. Cells with three foci (see Supplementary Table SI) probably arise, because the separation of colocalized origins in the two cell halves occurs at somewhat different times. (C) The C+D period approximately equals two generations. Initiation of replication occurs at four origins around the same time as cell division. Pairs of sister origins remain colocalized throughout an entire round of replication and segregation, until they are initiated again and become four. In some of the cells, initiation occurs at four origins at the end of the ‘grandmother' generation and in some at two origins at the beginning of the ‘mother' generation. A newborn cell contains four origins that are either colocalized or have moved apart and become two separate sister pairs right before cell division. (D) The C+D period takes about two-and–a-half generations. Initiation of replication occurs at four origins in the ‘grandmother generation'. Four origins in each cell half are then colocalized for most of the remainder of that generation and separate into foci consisting of two sister origins either before or after cell division. Most newborn cells thus have four origins in two foci, while a few have four origins colocalized in one focus.
The average number of origins per cell, O, is given by 2(C+D)/Td (Cooper and Helmstetter, 1968), and the average number of foci per cell, F, is given by 2(C+D−P)/Td, where P is the period of colocalization. The ratio O/F is then 2P/Td. The duration of the period of origin colocalization was calculated for the four different growth conditions described above using this relationship (see Materials and methods). Whereas the sister origins in cells growing in glu-thy and glycerol were colocalized for 33 and 10–20 min, respectively, the sister origins in cells growing in glu-CAA and LB were colocalized for 42 and 43 min, respectively (Table I). Thus, under the latter two growth conditions, sister origins were colocalized for the equivalent of about one and one-and-a-third doubling times, respectively (Table I).
Colocalization of replication forks in wild-type cells with overlapping replication cycles
Similar to the analysis of origin colocalization, the extent to which sister replication forks are colocalized was investigated by measuring the distributions of forks in cell cultures by flow cytometry, and comparing them with the distributions of separate replication fork foci found by microscopy of the same cells. Replication fork localization can be determined by visualizing newly replicated DNA either by immunostaining of SeqA protein or by brief 5-bromo-2-deoxyuridine (BrdU) labelling and subsequent immunostaining (Molina and Skarstad, 2004). Here, we used the latter method (see Materials and methods), and found that wild-type (MG1655) cells growing in glu-CAA medium contained mainly two or four discrete replication fork foci per cell (Figure 2B, left and top right panels). The distributions of foci detected here with BrdU staining were in agreement with the distributions of foci previously found by immunostaining of SeqA (Hiraga et al, 1998; Fossum et al, 2003; Molina and Skarstad, 2004). In these cells, when grown in glu-CAA, the C and D periods span about two generations (Td=41 min, C∼60 min and D∼25 min) (Figure 1). Thus, most of the cells had 4 or 6 replication forks, and some had 2 or 12 (Figure 2B, bottom right panel). The average number of forks per cell was 5.2. The same cells contained an average of 2.7 replication fork foci per cell (i.e., about half the number of foci compared to forks per cell). This indicates that pairs of replication forks were colocalized throughout most of the cell cycle, in accordance with previous results (Lemon and Grossman, 1998; Molina and Skarstad, 2004). The exceptions to this seems to be (i) when some of the replication fork pairs briefly and transiently move apart, and (ii) when all four newly initiated forks in each cell half stay colocalized for a while before the two replication fork pairs move in opposite directions (Molina and Skarstad, 2004).
The discovery that four newly initiated forks colocalize (Molina and Skarstad, 2004) supports the finding that sister origins stay colocalized for an entire generation. Therefore, both sets of results indicate that sister origins are colocalized before and at the time of initiation.
Organization of origins and forks in cells with mutant origins is normal, but not in cells lacking SeqA
Sequestration of newly replicated, hemimethylated origins is important to ensure that initiation of replication occurs only once per division cycle (Campbell and Kleckner, 1990). The E. coli SeqA protein binds strongly to hemimethylated GATC sites (Slater et al, 1995) and plays an important role in sequestration (Lu et al, 1994; von Freiesleben et al, 1994). Here, we investigated whether sequestration of the origin, the presence of SeqA protein on newly replicated DNA, or both, were important for the higher order organization of the origins and replisomes. To do this, two mutants were utilized. In oriCm3 mutant cells, eight of the origin GATC sites have been changed to GTTC (Bach and Skarstad, 2004). The origins of these cells cannot bind to SeqA, and the sequestration period is reduced to essentially the level found in SeqA-less cells (Bach and Skarstad, 2004). The other type of mutant cells have an in-frame deletion in the seqA gene (Slater et al, 1995). Not only are these cells unable to sequester newly replicated origins, but they are also deficient in SeqA binding of newly replicated, hemimethylated, chromosomal DNA in general (Lu et al, 1994; von Freiesleben et al, 1994; Slater et al, 1995). As expected, both types of mutant cells display reinitiation of newly replicated origins due to the lack of origin sequestration (Lu et al, 1994; Bach and Skarstad, 2004) (Supplementary Figure S1B and C). The numbers of origins per cell ranged from 4 to 8 in the oriCm3 cells and from 2 to around 15 in the ΔseqA cells.
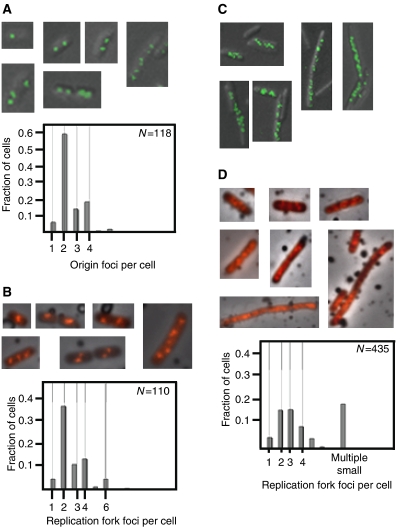
The growth, fluorescence microscopy imaging and flow cytometry analysis of the mutants were performed as for wild-type cells (Figure 2). We found essentially wild-type patterns of origin and replication fork foci in the oriCm3 mutant cells (Figure 3A and B, respectively). In contrast, most of the cells lacking functional SeqA protein contained either multiple small origin foci or no foci at all (Figure 3C). Approximately 30% of the cell population consisted of large cells with multiple small origin foci (from 5 to around 15). A few of the cells contained normal numbers of foci (2, 3 and 4), but these were aberrantly located within the cells. The distributions of number of foci per cell for the ΔseqA cells were not plotted and used for calculations because of the high frequency of cells that had lost the gene encoding GFP. The loss of the gene may be caused by plasmid DNA instability due to loss of SeqA.
Figure 3.
Origin and replication fork localization in oriCm3 and ΔseqA mutant cells. (A, C) Visualization of the origin region using the GFP-LacI/lac operator system in oriCm3 (A) and ΔseqA (C) cells (top panels). The number of origin foci per cell was counted in the oriCm3 mutant cells (A, bottom panel), but not in the ΔseqA mutant cells (C) (see Results). Immunofluorescence of BrdU-labelled oriCm3 (B) and ΔseqA (D) cells (top panels) and distribution of replication fork foci per cell (bottom panels). Of the oriCm3 and ΔseqA cells, 18 and 26%, respectively, had an undeterminable number of replication fork foci. Two percent of the oriCm3 cells examined had an undeterminable number of origin foci.
The majority of ΔseqA mutant cells also formed replication fork foci that were different in localization and distribution compared to wild-type foci (Figure 3D, top panel). The cells either contained multiple small replication fork foci (>8) or the BrdU staining was dispersed throughout the cells (scored as an undeterminable quantity of foci) (Figure 3D, bottom panel). However, in some ΔseqA cells (about 40%), we found discrete foci in quantities similar to those of wild-type cells (Figure 3D, bottom panel).
SeqA may pair sister oriC regions in vitro
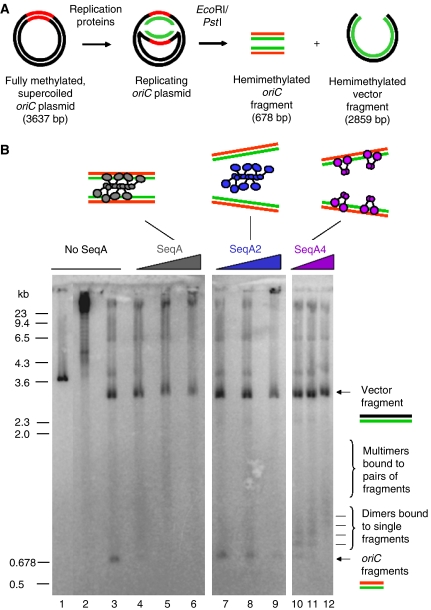
Our in vivo studies of origin localization suggest that SeqA protein may be involved, directly or indirectly, in the organization and colocalization of sister replication origins. To determine whether SeqA protein is capable of physically pairing sister origins, an oriC plasmid (3637 bp) was replicated bidirectionally in vitro using purified replication proteins with and without wild-type SeqA protein present (Figure 4A). We also analyzed the effect of two different SeqA mutants, SeqA2 or SeqA4, on sister origin pairing. The SeqA2 mutant protein (N152D) is unable to bind hemimethylated DNA (Fossum et al, 2003). The SeqA4 mutant protein (A25T) binds as a dimer with wild-type affinity to hemimethylated DNA, but is not capable of specific N-terminal multimerization (Odsbu et al, 2005).
Figure 4.
Cohesion of newly replicated origins by SeqA during bidirectional in vitro DNA replication. Supercoiled oriC plasmid (pBSoriC) was mixed with replication proteins and SeqA or SeqA mutant protein at 4°C. Replication was then performed at 30°C for 15 min, followed by restriction enzyme digestion with EcoRI and PstI (A). Native agarose (1.2%) gel electrophoresis of non-replicated pBSoriC (cut with EcoRI) (lane 1), replicated pBSoriC (supercoiled) (lane 2), replicated pBSoriC (cut with EcoRI and PstI) (lane 3), pBSoriC replicated in the presence of SeqA (lanes 4–6), SeqA2 (lanes 7–9) or SeqA4 (lanes 10–12) (cut with EcoRI and PstI), at 30, 120 and 360 nM (B).
The replication products were digested with restriction enzymes and resolved by native gel electrophoresis (Figure 4A). Linear, non-replicated oriC plasmid migrated at about 3.6 kb (Figure 4B, lane 1), whereas supercoiled, replicated oriC plasmid migrated as a high-molecular-weight molecule near the wells of the gel (Figure 4B, lane 2). Restriction enzyme digestion of the oriC plasmid replicated in the absence of SeqA yielded oriC (678 bp) and vector (2959 bp) fragments (Figure 4B, lane 3).
Addition of SeqA (30 nM) to the replication reaction resulted in a shift of the oriC fragment to a diffuse band ranging from about 800 to 2000 bp (Figure 4B, lane 4). The band presumably represents replicated oriC fragments bound by SeqA. A small portion of the oriC fragments apparently were not bound by SeqA and still migrated around 678 bp (Figure 4B, lane 4). When the concentration of SeqA was increased (120 nM), almost all oriC fragments were bound by SeqA and the 678 bp band was completely shifted to a smear ranging from around 1000 to 2500 bp (Figure 4B, lane 5). At the very high concentration of SeqA (360 nM), the DNA replication activity was inhibited to about 60% of the level obtained without SeqA (data not shown). In this case, most of the 678-bp fragment formed a complex with SeqA that seemed to be shifted to near the wells (Figure 4B, lane 6). Thus, binding of SeqA protein to a pair of newly replicated, hemimethylated origins probably results in their comigration as a complex. This indicates that SeqA is capable of tethering separate daughter chromosome origins in vitro. It cannot be excluded, although, that these large oriC-SeqA complexes contain mainly SeqA oligomer bound to only one oriC fragment. However, as the complexes are very large, we find it more likely that they contain more than one oriC fragment. We also believe that both daughter oriC fragments from the same round of replication are included because of their close proximity at the time of SeqA binding.
We also tested addition of two different SeqA mutant proteins to the replication reaction. Addition of SeqA2 mutant protein (30 and 120 nM) to the replication reaction did not shift the 678-bp fragment to a higher molecular weight molecule (Figure 4B, lanes 7 and 8, respectively). This is in agreement with previous results showing that SeqA2 is unable to bind to hemimethylated DNA in vitro (Fossum et al, 2003). At very high SeqA2 concentrations (360 nM), the oriC and plasmid fragments were shifted to the wells (Figure 4B, lane 9), probably due to unspecific DNA binding at high SeqA protein concentration (Fossum et al, 2003). Addition of the other mutant protein, SeqA4 (30, 120 and 360 nM), made a ladder-like shift of the oriC fragment, presumably representing 1, 2, 3 and more SeqA4 dimers bound to a fragment (Figure 4B, lanes 10, 11 and 12, respectively). This indicates that SeqA4 is able to bind to single hemimethylated oriC fragments, but unable to tether separate fragments or multimerize due to its N-terminal mutation (Odsbu et al, 2005).
Discussion
Extensive colocalization of sister origins in rapidly growing cells
Slowly growing E. coli cells initiate DNA replication at one origin and DNA replication is confined to a single division cycle (see schematic illustration Figure 5A). Sister origins stay colocalized for a limited fraction of the cell cycle before they separate and segregate into the future daughter cells (Figure 5A). During rapid growth, the period of DNA replication and segregation (C+D) exceeds the generation time and replication cycles overlap (Figure 5B, C and D). The number of overlapping replication cycles, and therefore number of origins, increases with increasing growth rate. We show here that origin colocalization is more extensive at higher growth rate and that the period of colocalization increases with increasing number of replication cycles. Sister origins seem to stay in relatively close proximity until the ‘appropriate generation' occurs, with colocalization ending in time for segregation into the correct cell half. In rapidly growing cells, a mechanism may exist to ensure that the ‘correct pair' of chromosomes segregate as each of the two segregating chromosomes have several replication forks and either four or two origins. It is therefore likely that the colocalization found here during rapid growth reflects chromosome organization that prevents the cell from segregating the incorrect DNA strands.
Colocalization of origins and sister replisomes is independent of origin sequestration, but dependent on functional SeqA protein
Because it was conceivable that origin sequestration might be involved in an overall organization of chromosomes that results in extensive origin and replisome colocalization, we investigated origin and replisome localization in cells that were deficient in origin sequestration (oriCm3) and cells lacking SeqA. We show here that the distributions and localization of origin and replication fork foci in the oriCm3 mutant cells were similar to those of wild-type cells. In contrast, the cells without SeqA, which were more heterogeneous in size, had numbers of origin foci ranging from 2 to around 15. Although an exact comparison was not possible, the number of foci in the ΔseqA cells seemed roughly equal to the number of origins indicating that each focus represented a single origin. Also the number and localization of replication fork foci were aberrant compared to those for wild-type cells. These results indicate that colocalization of new origins and sister replication forks does not occur, or is less extensive, in cells lacking SeqA. This effect may be direct or indirect. Although our in vitro results indicate that SeqA may be capable of pairing newly formed sister DNA copies, it is not known whether this actually occurs in vivo. One alternative possibility is that the lack of proper organization of new origins and sister replication forks in the absence of SeqA is due to the effects on DNA supercoiling levels.
Proper localization of new origins and sister replisomes was found in cells with origins that cannot be bound by SeqA, but that otherwise have normal SeqA functions. This indicates that the binding of SeqA multimers to new, hemimethylated DNA at the replication forks is important for the colocalization and organization of origins and replisomes, and also suggests that as long as continuous SeqA binding to DNA emerging from the replication forks is unperturbed, it is not a requirement that the origin is bound by SeqA. Of interest, the extra replication forks and extra origin copies that arise from re-initiation at non-sequesterable origins seem to be accommodated by this manner of chromosome organization.
The period of colocalization in rapidly growing cells is longer than the period of SeqA binding
It was previously suggested that newly replicated sister molecules are pushed apart (Dingman, 1974; Lemon and Grossman, 2000) and that SeqA aids this process by forming a separate multimeric tract on each sister DNA (Sawitzke and Austin, 2001). Recent findings indicate, however, that the newly replicated sister molecules do not immediately separate, but stay paired or colocalized at least 10 min (Bates and Kleckner, 2005; Wang et al, 2005; Nielsen et al, 2006). In vitro, SeqA seems to form a left-handed helical multimer, with the DNA wrapped around the SeqA filament (Guarne et al, 2005; Odsbu et al, 2005). Our in vitro results indicate that replicated DNA molecules can be paired by SeqA binding. These experiments do not distinguish between sister DNA molecules that are coiled around the same SeqA fiber, and sister molecules coiled around separate, but physically associated SeqA fibers. If separate SeqA filaments are formed with each sister DNA molecule, it must be assumed that the separate filaments interact by SeqA–SeqA interactions (or, in vivo, via an unknown factor). If, on the other hand, newly replicated sister DNA molecules are coiled around a single helical SeqA molecule, disassembly of the SeqA multimer must occur before the sister DNA molecules can separate. The latter scenario is also perfectly possible, as the period of sequestration seems to be shorter than, or equal to the period of colocalization.
Thus, in slowly growing cells the period of sister origin colocalization may coincide with the period of sequestration. Yet this is not the case in rapidly growing cells, where sister origins remain colocalized long after sequestration has ended. However, it is worth noting that in the studies performed here we cannot distinguish between interacting daughter DNA molecules and molecules that independently colocalize within the resolution limits of light microscopy. Thus, it is quite possible that sister origins are organized in one way during sequestration, and are then maintained in close proximity through another mechanism that prevents their migration in opposite directions for the rest of the observed colocalization period.
Materials and methods
Bacterial strains and growth conditions
All strains used are derivatives of the E. coli K-12 strain MG1655 (see Table II and Supplementary data). Cells were grown in Luria bertani medium (LB medium) or AB minimal medium (Clark and Maaløe, 1967) supplemented with 10 μg ml−1 thiamine (minimal medium). The minimal medium was also supplemented with 0.2% glucose and 0.5% casamino acids (glu-CAA medium) or 0.5% glucose and 5 μg ml−1 thymidine (glu-thy medium). Cell growth was monitored by measuring optical density at 450 or 600 nm when grown in defined or LB media, respectively.
Table 2.
Bacteria and plasmids
| Name | Description | Source |
|---|---|---|
| Bacteria | ||
| MG1655 | F- λ- | (Guyer et al, 1981; Jensen, 1993) |
| KS9921 | MG1655 ΔseqA | (Slater et al, 1995; Torheim et al, 2000) |
| oriCm3 | MG1655 oriCm3 | (Bach and Skarstad, 2004) |
| IL01 | AB1157 lacO∷kan | (Lau et al, 2003) |
| SF72 | MG1655 lacO∷kan/pSG20 | This work |
| SF75 | MG1655 oriCm3 lacO∷kan/pSG20 | This work |
| SF76 | KS9921 lacO∷kan/pSG20 | This work |
| Plasmids | ||
| pSG20 | pBAD18 gfp-lacI (Ap) | (Gordon et al, 1997) |
Live cell imaging of replication origin
MG1655 and derivatives containing a lac operator array (Lau et al, 2003; Possoz et al, 2006) near oriC and a plasmid containing lac repressor fused to green fluorescent protein (GFP-LacI) (Gordon et al, 1997) were grown in indicated media to an optical density of 0.15 (exponential phase), at which time arabinose (0.01%) was added to induce GFP-LacI expression. After 90 min, cells were prepared for flow cytometry analysis (see below) or immobilized on a glass slide with agarose (0.7% in phosphate-buffered saline) for fluorescent microscopy imaging. Fluorescent microscopy imaging was performed with an Olympus Fluoview BX61 Laser Scanning Microscope equipped with an UPlanApo × 100 objective and BA 505-525 filter. Images were captured, processed and analyzed by using Flouview 4.3, MetaMorph 6.1 and Adobe Photoshop Elements 4.0 software.
Staining of newly synthesized DNA with 5-bromo-2-deoxyuridine and immunofluorescence microscopy
MG1655 and isogenic mutants were grown in indicated media to an optical density of 0.15 (exponential phase), labelled with BrdU (20 μg ml−1; 8 min in the dark), and prepared for immunofluorescence microscopy as described previously (Molina and Skarstad, 2004) or for flow cytometry analysis (see below). Visualization of immunostained cells was performed using a Zeiss Axioplan Widefield Microscope, equipped with a × 63 objective and BP546/12 filter. Images were captured, processed and analyzed as described previously (Fossum et al, 2003).
Flow cytometry analysis
Cell cultures for flow cytometry were grown and treated the same way as the cell cultures for microscopy (see above). For origin content determination of the cells, 300 μg/ml rifampicin (Fluka) and 10 μg/ml cephalexin (Eli Lilly) was added. Incubation continued for 3–4 h to complete ongoing rounds of replication (run-out DNA histograms). Cells treated with rifampicin and cephalexin or exponentially growing cells were harvested and washed in filtered TE buffer (10 mM Tris–HCl, pH 7.5, 1 mM EDTA) and then fixed in 77% ethanol. Flow cytometry analysis was performed as described previously (Fossum et al, 2003) using FACStar+ (Becton Dickinson) or LSR II flow cytometers (BD Biosciences).
Calculation of cell-cycle parameters
Origin distributions were found directly from the run-out DNA histograms. The duration of C+D was found from the origin distribution combined with the doubling time, taking into account the exponential age distribution of the culture. Separate values for C and D periods and replication fork distributions were obtained by simulation of best fit of theoretical histograms to experimental histograms of exponentially growing cells as described previously (Skarstad et al, 1985; Molina and Skarstad, 2004). The period of colocalization (P) was found directly from the average numbers of origins per cell (O) and foci per cell (F) by the ratio O/F=2P/Td (Cooper and Helmstetter, 1968).
Reconstituted DNA replication, restriction enzyme analysis and native gel electrophoresis
DNA replication was performed as described by Wold et al (1998) with minor modifications. Primase was present at 150 nM, which supports correctly coupled bidirectional DNA replication (Hiasa and Marians, 1994). Replicated oriC plasmids were digested with EcoRI (50 U) and PstI (50 U), and the resulting fragments resolved by native agarose (1.2%) gel electrophoresis.
Supplementary Material
Supplementary Figure S1A, B and C
Supplementary data
Acknowledgments
We are grateful to the Department of Radiation Biology, The Norwegian Radium Hospital for sharing their flow cytometry facility and M Strand for expert running of the flow cytometer. The studies, in part, utilized the Microscopy and Imaging shared resource of the Lombardi Comprehensive Cancer Center, Georgetown University. We thank A Wahl for excellent technical assistance, K Boeneman for assistance with oriC visualization, and Morigen and F Molina for reading the manuscript. We thank A Wright for plasmid pSG20 and D Sherratt for strain IL01. This work was supported by The Norwegian Cancer Society (SF), The Norwegian Research Council FUGE program (KS) and NIH grant (R01GM49700) (EC).
References
- Bach T, Skarstad K (2004) Re-replication from non-sequesterable origins generates three-nucleoid cells which divide asymmetrically. Mol Microbiol 51: 1589–1600 [DOI] [PubMed] [Google Scholar]
- Bates D, Kleckner N (2005) Chromosome and replisome dynamics in E. coli: loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell 121: 899–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkmen MB, Grossman AD (2006) Spatial and temporal organization of the Bacillus subtilis replication cycle. Mol Microbiol 62: 57–71 [DOI] [PubMed] [Google Scholar]
- Boye E, Stokke T, Kleckner N, Skarstad K (1996) Coordinating DNA replication initiation with cell growth: differential roles for DnaA and SeqA proteins. Proc Natl Acad Sci USA 93: 12206–12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier AM, Weier HU, Cozzarelli NR (2005) Independence of replisomes in Escherichia coli chromosomal replication. Proc Natl Acad Sci USA 102: 3942–3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendler T, Sawitzke J, Sergueev K, Austin S (2000) A case for sliding SeqA tracts at anchored replication forks during Escherichia coli chromosome replication and segregation. EMBO J 19: 6249–6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JL, Kleckner N (1990) E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell 62: 967–979 [DOI] [PubMed] [Google Scholar]
- Clark DJ, Maaløe O (1967) DNA replication division cycle in Escherichia coli. J Mol Biol 23: 99–112 [Google Scholar]
- Cooper S, Helmstetter CE (1968) Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol 31: 519–540 [DOI] [PubMed] [Google Scholar]
- Diffley JF (2004) Regulation of early events in chromosome replication. Curr Biol 14: R778–R786 [DOI] [PubMed] [Google Scholar]
- Dingman CW (1974) Bidirectional chromosome replication: some topological considerations. J Theor Biol 43: 187–195 [DOI] [PubMed] [Google Scholar]
- Fossum S, Soreide S, Skarstad K (2003) Lack of SeqA focus formation, specific DNA binding and proper protein multimerization in the Escherichia coli sequestration mutant seqA2. Mol Microbiol 47: 619–632 [DOI] [PubMed] [Google Scholar]
- Gordon GS, Sitnikov D, Webb CD, Teleman A, Straight A, Losick R, Murray AW, Wright A (1997) Chromosome and low copy plasmid segregation in E. coli: visual evidence for distinct mechanisms. Cell 90: 1113–1121 [DOI] [PubMed] [Google Scholar]
- Guarne A, Brendler T, Zhao Q, Ghirlando R, Austin S, Yang W (2005) Crystal structure of a SeqA-N filament: implications for DNA replication and chromosome organization. EMBO J 24: 1502–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer MS, Reed RR, Steitz JA, Low KB (1981) Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb Symp Quant Biol 45 (Part 1): 135–140 [DOI] [PubMed] [Google Scholar]
- Hiasa H, Marians KJ (1994) Primase couples leading- and lagging-strand DNA synthesis from oriC. J Biol Chem 269: 6058–6063 [PubMed] [Google Scholar]
- Hiraga S, Ichinose C, Niki H, Yamazoe M (1998) Cell cycle-dependent duplication and bidirectional migration of SeqA-associated DNA-protein complexes in E. coli. Mol Cell 1: 381–387 [DOI] [PubMed] [Google Scholar]
- Jensen KF (1993) The Escherichia coli K-12 ‘wild types' W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J Bacteriol 175: 3401–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura E, Blow JJ, Tanaka TU (2006) Live-cell imaging reveals replication of individual replicons in eukaryotic replication factories. Cell 125: 1297–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A, Baker TA (1992) DNA Replication. New York: Freeman [Google Scholar]
- Lau IF, Filipe SR, Soballe B, Okstad OA, Barre FX, Sherratt DJ (2003) Spatial and temporal organization of replicating Escherichia coli chromosomes. Mol Microbiol 49: 731–743 [DOI] [PubMed] [Google Scholar]
- Lemon KP, Grossman AD (1998) Localization of bacterial DNA polymerase: evidence for a factory model of replication. Science 282: 1516–1519 [DOI] [PubMed] [Google Scholar]
- Lemon KP, Grossman AD (2000) Movement of replicating DNA through a stationary replisome. Mol Cell 6: 1321–1330 [DOI] [PubMed] [Google Scholar]
- Lemon KP, Grossman AD (2001) The extrusion-capture model for chromosome partitioning in bacteria. Genes Dev 15: 2031–2041 [DOI] [PubMed] [Google Scholar]
- Lu M, Campbell JL, Boye E, Kleckner N (1994) SeqA: a negative modulator of replication initiation in E. coli. Cell 77: 413–426 [DOI] [PubMed] [Google Scholar]
- Molina F, Skarstad K (2004) Replication fork and SeqA focus distributions in Escherichia coli suggest a replication hyperstructure dependent on nucleotide metabolism. Mol Microbiol 52: 1597–1612 [DOI] [PubMed] [Google Scholar]
- Nielsen HJ, Li Y, Youngren B, Hansen FG, Austin S (2006) Progressive segregation of the Escherichia coli chromosome. Mol Microbiol 61: 383–393 [DOI] [PubMed] [Google Scholar]
- Niki H, Yamaichi Y, Hiraga S (2000) Dynamic organization of chromosomal DNA in Escherichia coli. Genes Dev 14: 212–223 [PMC free article] [PubMed] [Google Scholar]
- Odsbu I, Klungsoyr HK, Fossum S, Skarstad K (2005) Specific N-terminal interactions of the Escherichia coli SeqA protein are required to form multimers that restrain negative supercoils and form foci. Genes Cells 10: 1039–1049 [DOI] [PubMed] [Google Scholar]
- Onogi T, Niki H, Yamazoe M, Hiraga S (1999) The assembly and migration of SeqA-Gfp fusion in living cells of Escherichia coli. Mol Microbiol 31: 1775–1782 [DOI] [PubMed] [Google Scholar]
- Possoz C, Filipe SR, Grainge I, Sherratt DJ (2006) Tracking of controlled Escherichia coli replication fork stalling and restart at repressor-bound DNA in vivo. EMBO J 25: 2596–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawitzke J, Austin S (2001) An analysis of the factory model for chromosome replication and segregation in bacteria. Mol Microbiol 40: 786–794 [DOI] [PubMed] [Google Scholar]
- Skarstad K, Boye E, Steen HB (1986) Timing of initiation of chromosome replication in individual Escherichia coli cells. EMBO J 5: 1711–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K, Steen HB, Boye E (1985) Escherichia coli DNA distributions measured by flow cytometry and compared with theoretical computer simulations. J Bacteriol 163: 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater S, Wold S, Lu M, Boye E, Skarstad K, Kleckner N (1995) E. coli SeqA protein binds oriC in two different methyl-modulated reactions appropriate to its roles in DNA replication initiation and origin sequestration. Cell 82: 927–936 [DOI] [PubMed] [Google Scholar]
- Sunako Y, Onogi T, Hiraga S (2001) Sister chromosome cohesion of Escherichia coli. Mol Microbiol 42: 1233–1241 [DOI] [PubMed] [Google Scholar]
- Torheim NK, Boye E, Lobner-Olesen A, Stokke T, Skarstad K (2000) The Escherichia coli SeqA protein destabilizes mutant DnaA204 protein. Mol Microbiol 37: 629–638 [DOI] [PubMed] [Google Scholar]
- von Freiesleben U, Rasmussen KV, Schaechter M (1994) SeqA limits DnaA activity in replication from oriC in Escherichia coli. Mol Microbiol 14: 763–772 [DOI] [PubMed] [Google Scholar]
- Wang X, Possoz C, Sherratt DJ (2005) Dancing around the divisome: asymmetric chromosome segregation in Escherichia coli. Genes Dev 19: 2367–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold S, Boye E, Slater S, Kleckner N, Skarstad K (1998) Effects of purified SeqA protein on oriC-dependent DNA replication in vitro. EMBO J 17: 4158–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh CL, Nanninga N (2006) Structural and physical aspects of bacterial chromosome segregation. J Struct Biol 156: 273–283 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1A, B and C
Supplementary data