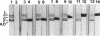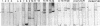Abstract
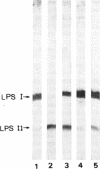
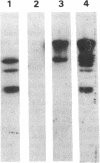
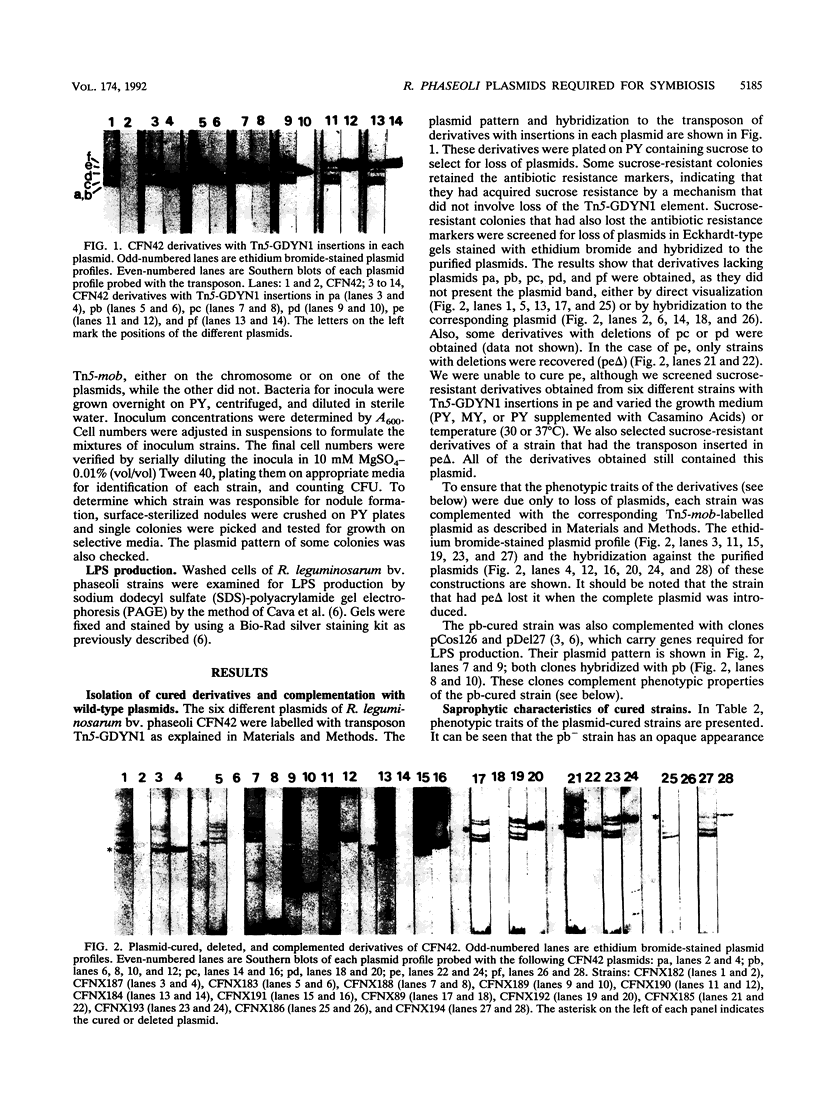
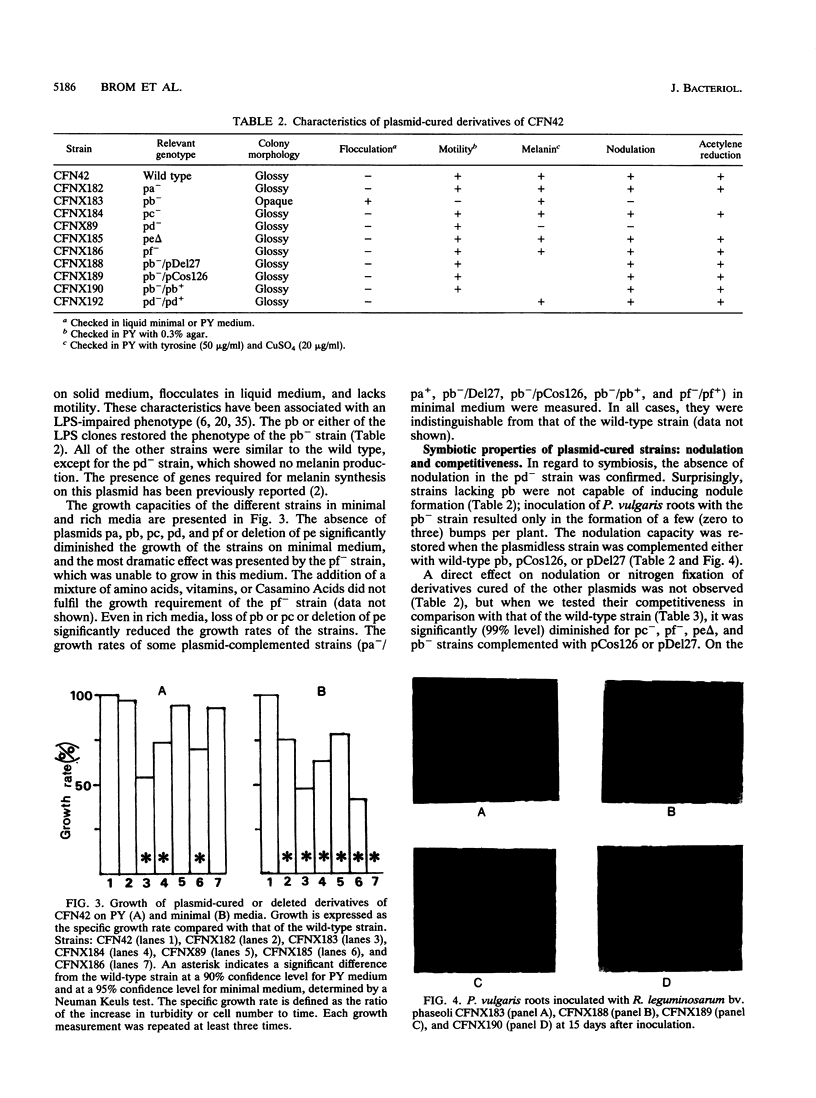
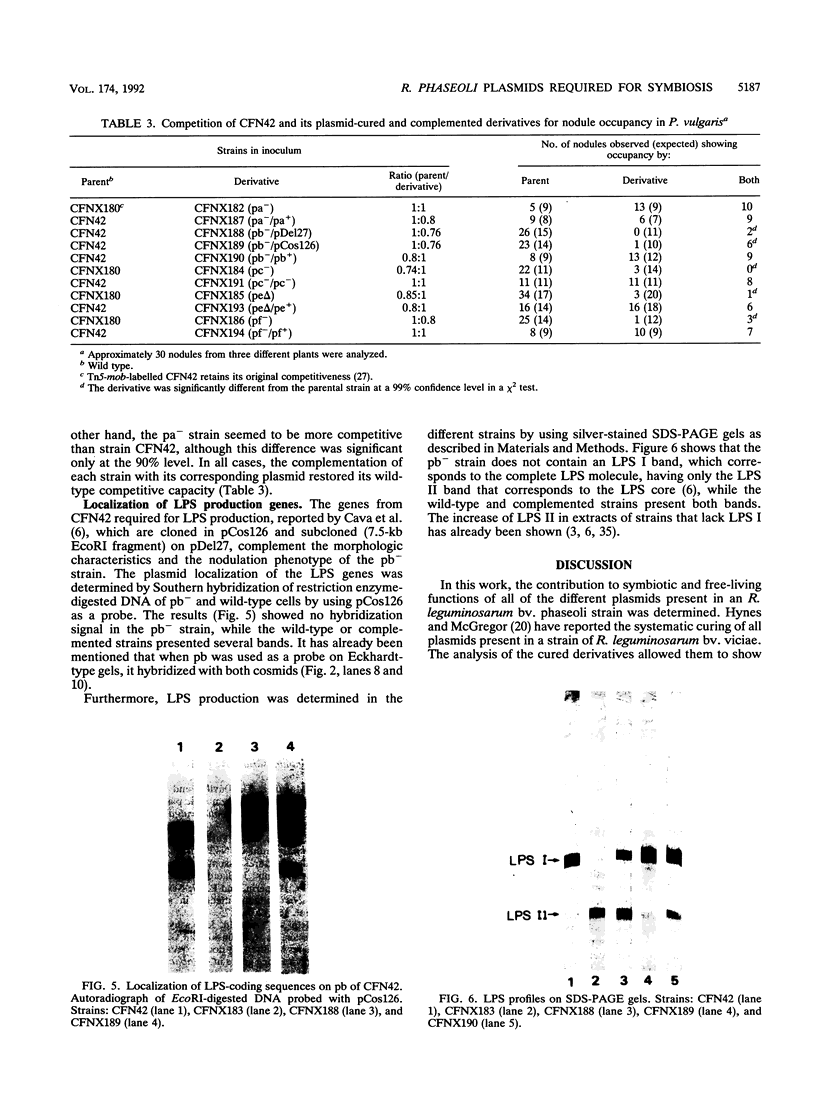
Rhizobium leguminosarum bv. phaseoli CFN42 contains six plasmids (pa to pf), and pd has been shown to be the symbiotic plasmid. To determine the participation of the other plasmids in cellular functions, we used a positive selection scheme to isolate derivatives cured of each plasmid. These were obtained for all except one (pe), of which only deleted derivatives were recovered. In regard to symbiosis, we found that in addition to pd, pb is also indispensable for nodulation, partly owing to the presence of genes involved in lipopolysaccharide synthesis. The positive contribution of pb, pc, pe, and pf to the symbiotic capacity of the strain was revealed in competition experiments. The strains that were cured (or deleted for pe) were significantly less competitive than the wild type. Analysis of the growth capacity of the cured strains showed the participation of the plasmids in free-living conditions: the pf- strain was unable to grow on minimal medium, while strains cured of any other plasmid had significantly reduced growth capacity in this medium. Even on rich medium, strains lacking pb or pc or deleted for pe had a diminished growth rate compared with the wild type. Complementation of the cured strains with the corresponding wild-type plasmid restored their original phenotypes, thus confirming that the effects seen were due only to loss of plasmids. The results indicate global participation of the Rhizobium genome in symbiotic and free-living functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Borthakur D., Lamb J. W., Johnston A. W. Identification of two classes of Rhizobium phaseoli genes required for melanin synthesis, one of which is required for nitrogen fixation and activates the transcription of the other. Mol Gen Genet. 1987 Apr;207(1):155–160. doi: 10.1007/BF00331503. [DOI] [PubMed] [Google Scholar]
- Brink B. A., Miller J., Carlson R. W., Noel K. D. Expression of Rhizobium leguminosarum CFN42 genes for lipopolysaccharide in strains derived from different R. leguminosarum soil isolates. J Bacteriol. 1990 Feb;172(2):548–555. doi: 10.1128/jb.172.2.548-555.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom S., García de los Santos A., de Lourdes Girard M., Dávila G., Palacios R., Romero D. High-frequency rearrangements in Rhizobium leguminosarum bv. phaseoli plasmids. J Bacteriol. 1991 Feb;173(3):1344–1346. doi: 10.1128/jb.173.3.1344-1346.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom S., Martinez E., Dávila G., Palacios R. Narrow- and Broad-Host-Range Symbiotic Plasmids of Rhizobium spp. Strains That Nodulate Phaseolus vulgaris. Appl Environ Microbiol. 1988 May;54(5):1280–1283. doi: 10.1128/aem.54.5.1280-1283.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cava J. R., Elias P. M., Turowski D. A., Noel K. D. Rhizobium leguminosarum CFN42 genetic regions encoding lipopolysaccharide structures essential for complete nodule development on bean plants. J Bacteriol. 1989 Jan;171(1):8–15. doi: 10.1128/jb.171.1.8-15.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Halperin W., Nester E. W. Agrobacterium tumefaciens mutants affected in attachment to plant cells. J Bacteriol. 1982 Dec;152(3):1265–1275. doi: 10.1128/jb.152.3.1265-1275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylan T., Ielpi L., Stanfield S., Kashyap L., Douglas C., Yanofsky M., Nester E., Helinski D. R., Ditta G. Rhizobium meliloti genes required for nodule development are related to chromosomal virulence genes in Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4403–4407. doi: 10.1073/pnas.83.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Oresnik I., Bottacin A. Mutants of Rhizobium meliloti defective in succinate metabolism. J Bacteriol. 1988 Aug;170(8):3396–3403. doi: 10.1128/jb.170.8.3396-3403.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores M., González V., Brom S., Martínez E., Piñero D., Romero D., Dávila G., Palacios R. Reiterated DNA sequences in Rhizobium and Agrobacterium spp. J Bacteriol. 1987 Dec;169(12):5782–5788. doi: 10.1128/jb.169.12.5782-5788.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores M., González V., Pardo M. A., Leija A., Martínez E., Romero D., Piñero D., Dávila G., Palacios R. Genomic instability in Rhizobium phaseoli. J Bacteriol. 1988 Mar;170(3):1191–1196. doi: 10.1128/jb.170.3.1191-1196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrai T., Vincze E., Bánfalvi Z., Kiss G. B., Randhawa G. S., Kondorosi A. Localization of symbiotic mutations in Rhizobium meliloti. J Bacteriol. 1983 Feb;153(2):635–643. doi: 10.1128/jb.153.2.635-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M. L., Flores M., Brom S., Romero D., Palacios R., Dávila G. Structural complexity of the symbiotic plasmid of Rhizobium leguminosarum bv. phaseoli. J Bacteriol. 1991 Apr;173(8):2411–2419. doi: 10.1128/jb.173.8.2411-2419.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. F., McGregor N. F. Two plasmids other than the nodulation plasmid are necessary for formation of nitrogen-fixing nodules by Rhizobium leguminosarum. Mol Microbiol. 1990 Apr;4(4):567–574. doi: 10.1111/j.1365-2958.1990.tb00625.x. [DOI] [PubMed] [Google Scholar]
- Hynes M. F., Quandt J., O'Connell M. P., Pühler A. Direct selection for curing and deletion of Rhizobium plasmids using transposons carrying the Bacillus subtilis sacB gene. Gene. 1989 May 15;78(1):111–120. doi: 10.1016/0378-1119(89)90319-3. [DOI] [PubMed] [Google Scholar]
- Long S., McCune S., Walker G. C. Symbiotic loci of Rhizobium meliloti identified by random TnphoA mutagenesis. J Bacteriol. 1988 Sep;170(9):4257–4265. doi: 10.1128/jb.170.9.4257-4265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Romero Esperanza, Rosenblueth Monica. Increased Bean (Phaseolus vulgaris L.) Nodulation Competitiveness of Genetically Modified Rhizobium Strains. Appl Environ Microbiol. 1990 Aug;56(8):2384–2388. doi: 10.1128/aem.56.8.2384-2388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metts J., West J., Doares S. H., Matthysse A. G. Characterization of three Agrobacterium tumefaciens avirulent mutants with chromosomal mutations that affect induction of vir genes. J Bacteriol. 1991 Feb;173(3):1080–1087. doi: 10.1128/jb.173.3.1080-1087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Sanchez A., Fernandez L., Leemans J., Cevallos M. A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984 Apr;158(1):148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Vandenbosch K. A., Kulpaca B. Mutations in Rhizobium phaseoli that lead to arrested development of infection threads. J Bacteriol. 1986 Dec;168(3):1392–1401. doi: 10.1128/jb.168.3.1392-1401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priefer U. B. Genes involved in lipopolysaccharide production and symbiosis are clustered on the chromosome of Rhizobium leguminosarum biovar viciae VF39. J Bacteriol. 1989 Nov;171(11):6161–6168. doi: 10.1128/jb.171.11.6161-6168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto C., De La Vega H., Flores M., Leemans J., Cevallos M. A., Pardo M. A., Azpiroz R., De Lourdes Girard M., Calva E., Palacios R. Nitrogenase reductase: A functional multigene family in Rhizobium phaseoli. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1170–1174. doi: 10.1073/pnas.82.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Romero D., Brom S., Martínez-Salazar J., Girard M. L., Palacios R., Dávila G. Amplification and deletion of a nod-nif region in the symbiotic plasmid of Rhizobium phaseoli. J Bacteriol. 1991 Apr;173(8):2435–2441. doi: 10.1128/jb.173.8.2435-2441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Chan Y. K., Wheatcroft R., Yang A. F., Han S. H. Rhizobium meliloti genes required for C4-dicarboxylate transport and symbiotic nitrogen fixation are located on a megaplasmid. J Bacteriol. 1988 Feb;170(2):927–934. doi: 10.1128/jb.170.2.927-934.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley M. H., Bodwin J. S., Lippincott B. B., Lippincott J. A. Role of Agrobacterium cell envelope lipopolysaccharide in infection site attachment. Infect Immun. 1976 Apr;13(4):1080–1083. doi: 10.1128/iai.13.4.1080-1083.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]