Summary
Glutamate produces both fast excitation through activation of ionotropic receptors and slower actions through metabotropic receptors (mGluRs). To date, ionotropic but not metabotropic neurotransmission has been shown to undergo long-term synaptic potentiation and depression. Burst stimulation of parallel fibers releases glutamate which activates perisynaptic mGluR1 in the dendritic spines of cerebellar Purkinje cells. Here we show that the mGluR1-dependent slow EPSC and its coincident Ca transient were selectively and persistently depressed by repeated climbing fiber-evoked depolarization of Purkinje cells in brain slices. LTD(mGluR1) was also observed when slow synaptic current was evoked by exogenous application of a group I mGluR agonist, implying a postsynaptic expression mechanism. Ca imaging further revealed that LTD(mGluR1) was expressed as coincident attenuation of both limbs of mGluR1 signaling: the slow EPSC and PLC/IP3-mediated dendritic Ca mobilization. Thus, different patterns of neural activity can evoke LTD of either fast ionotropic or slow mGluR1-mediated synaptic signaling.
Introduction
Persistent, experience-dependent modulation of synaptic function, known as synaptic plasticity, is widely thought to be a major substrate for information storage in the brain. Similarly, synaptic plasticity is suggested to be an important mechanism by which experience can sculpt the form and function of neuronal circuits during later stages of brain development. It has also been hypothesized to underlie at least a portion of certain persistent pathological states such as addiction and epilepsy. Glutamate is the major excitatory neurotransmitter in the brain and most our understanding of synaptic plasticity involves the study of long-term potentiation and depression (LTP and LTD) of glutamatergic synapses. In recent years, however, it has also become clear that neural activity can persistently modulate many other aspects of synaptic function such as the strength of inhibitory GABAergic synapses (Kano et al., 1992) and the action of neuronal glutamate transporters (Shen and Linden, 2005). In addition, the intrinsic (non-synaptic) excitability of neurons can also undergo persistent use-dependent modulation (see Debanne et al., 2003 for review).
Glutamate exerts its effects on the postsynaptic membrane through activation of two classes of receptor. Ionotropic glutamate receptors rapidly open integral cation channels. It is modulation of this fast neurotransmission which has been studied as LTP and LTD. Glutamate also acts upon postsynaptic mGluRs, particularly the group I mGluRs (mGluR1 and mGluR5) which are 7-transmembrane domain G-protein coupled receptors. Activation of group I mGluRs stimulates phospholipase C and activates a slow cation conductance (the mGluR1/5 slow EPSC). Here, we have sought to test the hypothesis that neuronal activity can persistently modulate the function of postsynaptic metabotropic glutamate receptors as well.
Results
As a model system, we have used whole-cell patch clamp recording combined with laser scanning confocal Ca imaging from Purkinje cells in sagittal slices of juvenile rat cerebellum. Purkinje cells were perfused with a K-based internal saline supplemented with the Ca indicator Oregon Green BAPTA-1 (200 μM). The bath perfusion solution was also supplemented with picrotoxin (100 μM) or gabazine (5 μM) to block GABAA receptors. When the Purkinje cell was held at -70 mV, stimulation of parallel fibers with a brief burst (10 pulses at 100 Hz) gave rise to a biphasic Ca response (Figure 1). The fast component is triggered by AMPA receptors and is predominantly mediated by voltage-clamp failure-driven depolarization and consequent Ca influx through voltage-sensitive Ca channels (Hartmann et al., 2004; Tempia et al., 1996). The slow component, which overlaps the tail of the fast component, is mediated by mGluR1 (Finch and Augustine, 1998; Hartmann et al., 2004; Takechi et al., 1998). The mGluR1-mediated slow component reflects both Ca mobilization from internal stores via IP3 production (Takechi et al., 1998) and Ca influx through the activation of the plasma membrane cation channel TrpC1 (Kim et al., 2003). In Purkinje cells, functional NMDA-type glutamate receptors are not present after postnatal day 7 (Hausser and Roth, 1997).
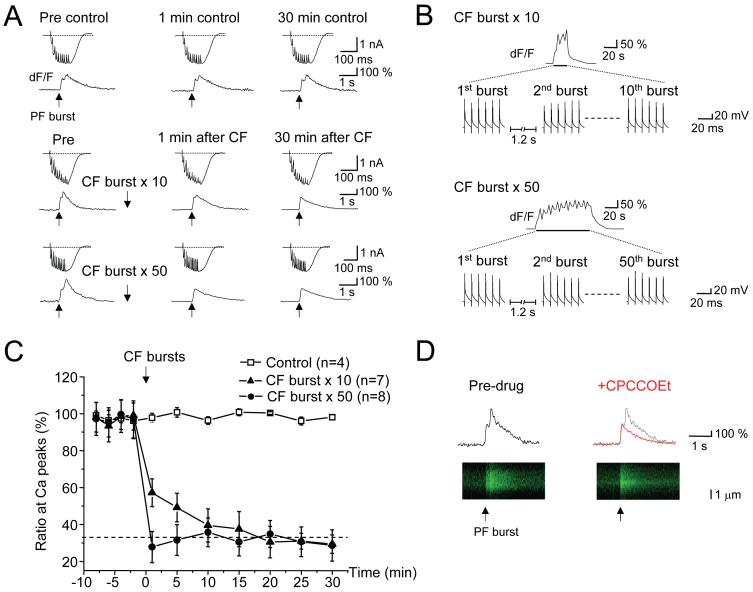
Figure 1. Climbing fiber bursts produce a persistent depression of parallel fiber-evoked mGluR1-mediated Ca transients.
A, Purkinje cells were loaded with the Ca indicator (Oregon Green BAPTA-1, 200 μM) and dendritic Ca measurements were made using the line-scan mode of a laser scanning confocal microscope. Representative single current and Ca indicator traces show responses to parallel fiber bursts (10 stimuli at 100 Hz) delivered in the presence of a maximal dose of picrotoxin (100 μM). Biphasic Ca responses with slow and fast peaks are evoked by this stimulation (expressed as dF/F). The control group shows responses without conditioning climbing fiber stimulation (n=4). Other groups show responses before (Pre), 1 min and 30 min after conditioning climbing fiber bursts (each burst consisting of 6 stimuli at 10 Hz, bursts repeated 10 × with a 1.2 sec interburst interval, middle panel, n=7; 50 bursts, bottom panel, n=8).
B, Representative Ca and membrane potential traces evoked by conditioning climbing fiber bursts are shown.
C, The ratio of the slow to the fast peak amplitude of the Ca response was calculated as an index of the mGluR1-mediated Ca transient. The time course graph plots this ratio, averaged and normalized. The dotted line indicates the mean ratio in the presence of CPCCOEt (n = 7), in which the slow peak was obliterated and only the tail of the fast peak remains. Error bars indicate the SEM in this and all subsequent figures.
D, Exemplar biphasic Ca responses evoked by parallel fibers bursts before (black trace) and after (red trace) application of the mGluR1 antagonist CPCCOEt (100 μM).
In control recordings, the biphasic Ca response to parallel fiber burst test stimuli was stable over the 30 min monitoring period (slow peak/fast peak ratio = 98.1 ± 1.4 % of baseline at t = 30 min, n = 4). When the mGluR1 antagonist CPCCOEt (100 μM) was applied, no discernable slow peak was left and the Ca transient amplitude at the former slow peak time point, solely reflected the tail of the fast peak (Figure 1D, in this case, the slow obliterated “peak” is measured using cursors located at the peak time as determined in the control condition). This finding replicates that of Hartmann et al. (2004). Following 6 min of baseline recording, conditioning stimulation was applied. This involved switching into current clamp mode (with no bias current) and delivering a series of bursts (each burst consisting of 6 pulses at 10 Hz) through a stimulating electrode that activated climbing fiber synapses (identified by a single step in the input-output relation and paired-pulse depression; data not shown) and produced complex spikes and coincident Ca transients (Figure 1B). Either 10 or 50 bursts were delivered with an interburst interval of 1.2 s. When voltage clamp recording resumed, the slow mGluR1-mediated Ca peak was abolished and remained so for the duration of the recording (peak ratio: 10 bursts, 29.4 ± 5.0% of baseline at t = 30 min, n = 7; 50 bursts, 28.6 ± 8.5 % at t = 30 min, n = 8). The depression of the slow Ca peak had a rapid onset following the 50 burst conditioning regimen (peak ratio: 27.8 ± 8.4 % of baseline at t = 1 min) and somewhat slower onset after the 10 burst regimen (peak ratio: 57.2 ± 7.6 % at t = 1 min; see Figure 1C for time-course). It should be noted that depression to ∼30% of baseline is near total: a saturating dose of mGluR1 antagonist produced a peak ratio of 33.2 ± 1.3 % of baseline (t = 10 min, n = 7). These results suggest that climbing fiber conditioning bursts produced some form of strong, persistent depression of mGluR1-mediated Ca signals at parallel fiber-Purkinje cell synapses, a phenomenon we call LTD(mGluR1).
The effect of climbing fiber conditioning bursts on the mGluR1-mediated slow Ca transient could not be mimicked by strong parallel fiber conditioning bursts. When bursts of parallel fiber activation (5 - 10 pulses at 100 Hz) were repeated 30 - 50 times with an interburst interval of 2 s, this protocol failed to induce LTD(mGluR1) as indexed by the slow mGluR1-mediated Ca peak (102.2 ± 7.1 % of baseline at t = 20 min, n= 10, p = 0.15 compared to baseline; see Supplemental Figure 1).
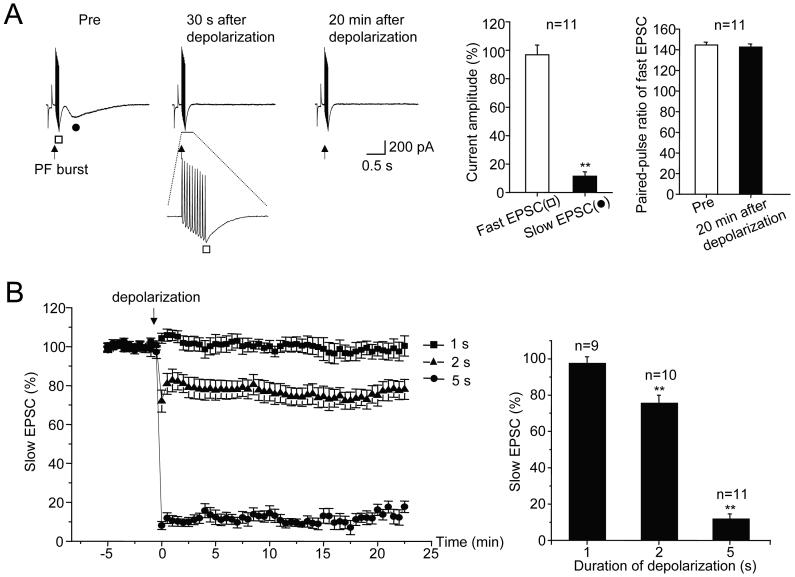
Recording with a K-based internal saline is somewhat physiological and allows for normal climbing fiber-evoked complex spikes in current clamp mode, but it has some limitations. The poor voltage clamp control in the extensive dendrite of the K-loaded Purkinje cell makes it difficult to resolve the mGluR1-mediated slow EPSC (Figure 1 and Hartmann et al., 2004). To resolve the slow EPSC, we have changed the protocol in several ways. First, we have used a Cs-based internal saline to improve voltage-clamp control. Second, we have added the AMPA/kainate receptor antagonist NBQX at a concentration which produces an ∼ 90% reduction in the amplitude of the fast EPSC (5 μM). This allowed for clearly resolvable fast and slow EPSC components (Figure 2A). Without partial antagonism by NBQX, the tail of the fast EPSC burst tends to overlap the onset of the slow EPSC. Third, we have replaced climbing fiber conditioning trains with a command depolarization designed to evoke a substantial Ca influx through dendritic voltage-sensitive Ca channels, as is produced by repeated complex spikes. Following a stable baseline recording period, a step depolarization from -70 mV to 0 mV for 5 s produced a near-complete blockade of the slow EPSC which lasted for the duration of the recording (11.4 ± 3.1 % of baseline, t = 20 min, n = 11 cells; p < 0.01 compared to pre). When a 2 s long depolarization to 0 mV was used, a small depression of the slow EPSC was observed (75.5 ± 4.5 %, t = 20 min, n = 10 cells; p < 0.01 compared to pre; Figure 2B).
Figure 2. LTD of the mGluR1-mediated slow EPSC in Purkinje cells.
A, Representative current traces show fast and slow components of the parallel fiber EPSC which were produced by bursts (10 stimuli at 100 Hz) delivered in the presence of a submaximal dose of NBQX (5 μM) and a maximal dose of picrotoxin (100 μM). The conditioning depolarization consisted of a 5 s long command to 0 mV at the soma. Current amplitudes were measured at the peak of the EPSC. Paired pulse ratios are: fast EPSC2/fast EPSC1. Right: Bar graphs show population measures at t = 20 min after depolarization
B, Population time courses of the percent changes in peak slow EPSC amplitudes induced by conditioning depolarization of different durations (1, 2, and 5 s) applied to Purkinje cells at the time indicated by the arrow. **p < 0.01 compared to pre by paired t-test.
LTD(mGluR1) evoked by a 5 s depolarization was not associated with persistent changes in either Rinput (105.5 ± 2.9 %, t = 20 min), or the amplitude of the fast EPSCs in the burst (first EPSC: 96.8 ± 6.1 %, t = 20 min, n = 11 cells; last EPSC: 96.8 ± 6.9 %, t = 20 min). These findings argue against a non-specific effect of strong depolarization on the viability of the postsynaptic Purkinje cell. Furthermore, the amplitude ratio of the second and first fast EPSCs was not changed following the depolarizing step (pre-depolarization: 1.44 ± 0.03; post-depolarization: 1.43 ± 0.03, t=20 min, n = 11 cells). This finding, together with the specific depression of the slow EPSC but not the fast EPSCs, indicates that a reduction in the probability of glutamate release from parallel fibers is unlikely to underlie LTD(mGluR1).
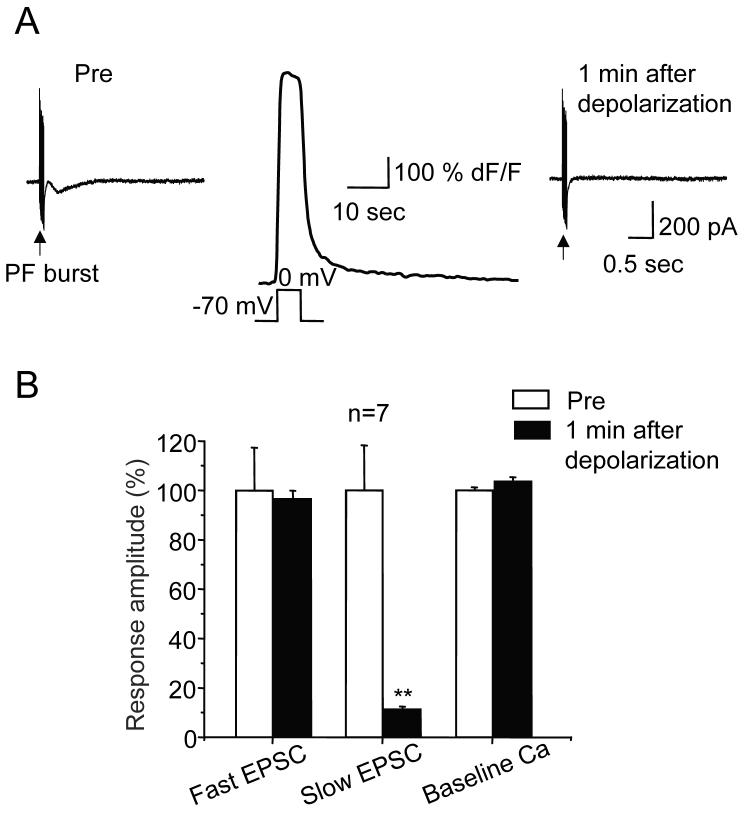
Might expression of LTD(mGluR1) require a depolarization-evoked persistent elevation in dendritic free Ca? To address this question, we repeated the experiment shown in Figure 2, but now included a Ca indicator in the internal saline to monitor dendritic free Ca concentration during and after a 5 s long conditioning depolarization. This revealed that the Ca transient evoked by the conditioning depolarization decayed to baseline values within ∼ 40 s and was completely abolished within 1 min (103.6 ± 1.8 % of baseline, n = 7 cells, see Figure 3), arguing against persistent Ca elevation as a requirement for LTD(mGluR1) expression.
Figure 3. Induction of LTD(mGluR1) does not trigger persistent alteration of dendritic basal free Ca concentration.
A, Representative current traces show fast and slow components of the parallel fiber EPSC which were produced by bursts (10 stimuli at 100 Hz) delivered in the presence of a submaximal dose of NBQX (5 μM) and a maximal dose of picrotoxin (100 μM). The conditioning depolarization consisted of a 5 s long command to 0 mV at the soma. The dendritic Ca transient evoked by the conditioning depolarization is shown between the current traces. It returns to baseline levels within ∼40 s. In fact, if anything, the high affinity of the Ca indicator used here, Oregon Green BAPTA-1, may be slowing the apparent return kinetics.
B, Population data for the manipulations shown in panels A (n = 7 for all measures).
To this point has shown that LTD(mGluR1) may be induced by climbing fiber burst conditioning stimulation delivered to K-loaded cells in current clamp mode (Figure 1), but not by strong, repeated parallel fiber burst stimulation delivered in this same configuration (Supplemental Figure 1). In addition, we have shown that LTD(mGluR1) may be induced by a depolarizing step delivered to Cs-loaded cell under voltage clamp (Figure 2). These results suggest that parallel fiber activity is not required for LTD(mGluR1) induction. To further explore this issue, we attempted to induce LTD(mGluR1) by delivering strong parallel fiber bursts to Cs-loaded voltage-clamped Purkinje cells (Supplemental Figure 2). This produced a very small depression of the sEPSC (94.3 ± 3.6 % of baseline at t = 20 min, n = 11) that was not statistically different from pre-conditioning responses (p = 0.15). We went on to perform another test for a possible associative role of parallel fibers in LTD(mGluR1). These recordings used a 5 s long step depolarization in Cs-loaded voltage-clamped cells as a conditioning stimulus, as shown in Figure 2. However, in these experiments, parallel fiber test pulses we stopped 5 min before the conditioning depolarization and were not resumed until 5 min after the depolarization (Supplemental Figure 3). When test pulses were resumed, this revealed a large LTD(mGluR1) which persisted for the duration of the recording (10.1 ± 1.9 % of baseline at t = 25 min, n = 6, p < 0.01 compared to baseline). Taken together, these experiments indicate that parallel fiber stimulation is not necessary for induction. In this way, LTD(mGluR1) is heterosynaptically expressed, but is not associative.
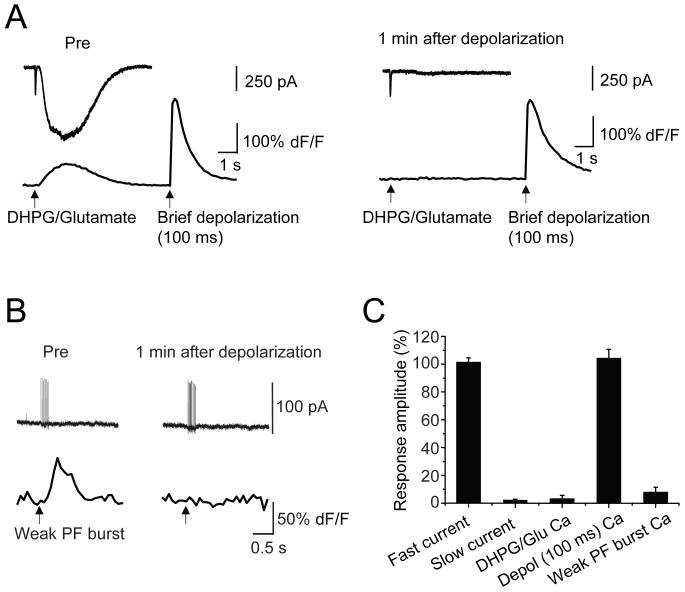
Activation of mGluR1 engages two separate G-protein-dependent signaling limbs, both of which require Gαq (Hartmann et al., 2004): one is the activation of a TrpC1-mediated slow EPSC (Kim et al., 2003) and the other is the stimulation of phospholipase C (PLC), resulting in production of diacylglycerol and IP3, and consequent mobilization of Ca from internal stores through activation of IP3 receptors (Finch and Augustine, 1998; Takechi et al., 1998). Is LTD(mGluR1) expressed only as a depression of the slow EPSC limb or are both mGluR1-activated signaling pathways affected? To begin to address this question we combined confocal Ca imaging with whole-cell patch-clamp recording in Cs-loaded Purkinje cells under partial AMPA receptor blockade.
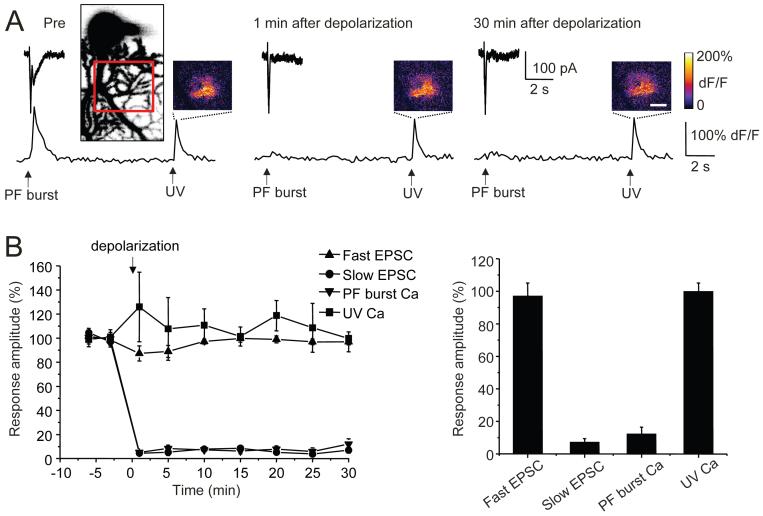
We designed an experiment to test two hypotheses 1) that the Ca transient associated with the mGluR1-mediated slow EPSC is depressed following induction of LTD(mGluR1) and 2) that LTD(mGluR1) involves attenuation of IP3 receptor function or the status of IP3-sensitive Ca stores. For this design both Ca indicator and a caged form of IP3 (NPE-IP3; 300 μM) were added to the patch pipette and allowed to perfuse the dendrites for 20 min before recording commenced. Recordings involved two different types of test pulse, a parallel fiber burst to evoke fast and slow EPSCs and, 10 s later, a UV light flash to produce IP3 uncaging (Figure 4). The diameter of the UV laser uncaging spot was set to 15 - 20 μm which produces a Ca transient that approximates that of a parallel fiber burst. A 5 msec long UV pulse activated IP3-mediated Ca release from internal stores (ΔF/F = 80.3 ± 13.1 %, n = 8 cells). A parallel fiber burst test pulse produced distinct fast and slow EPSCs. The AMPA receptors expressed in Purkinje cells are largely Ca impermeant (Tempia et al., 1996) and so the fast EPSCs were not associated with a dendritic Ca transient. However, the slow current was coincident with a local Ca transient in the dendrites (ΔF/F = 77.8 ± 14.6 %, n = 8 cells). LTD(mGluR1) was then induced using a 5 s long command depolarization to 0 mV. LTD(mGluR1) induction strongly attenuated both the mGluR1-evoked slow EPSC (7.0 ± 2.4 % of baseline, t = 30 min, n = 8 cells) and the associated change in Ca concentration (12.1 ± 4.4 %), leaving the fast EPSC unaffected (96.9 ± 8.2 %; Figure 4B). Thus, LTD(mGluR1) is manifest as a near-complete blockade of both the slow EPSC and its associated Ca transient. However, after LTD(mGluR1) induction, the UV flash test pulse produced a similar Ca transient (99.7 ± 5.5 % of baseline, t = 30 min, n = 8 cells). This finding argues that LTD(mGluR1) is not associated with an attenuation of IP3 receptor function or a depletion of IP3-sensitive Ca stores.
Figure 4. LTD(mGluR1) is not associated with attenuation of IP3-mediated signaling.
A, Purkinje cells were loaded with both caged IP3 (NPE-IP3; 300 μM) and Ca indicator (Oregon Green BAPTA-1, 200 μM). Representative current and Ca traces are shown before (Pre), 1 min, and 30 min after a 5 s long conditioning depolarization. Parallel fiber burst test pulses produced fast and slow EPSCs and accompanying Ca transients. Photolysis of caged IP3 with a UV laser spot was delivered to Purkinje cells 10 s after each PF burst test pulse. Pseudocolor images of the Purkinje cell dendrite illustrate the localized increase in Ca-dependent fluorescence (dF/F) evoked by IP3 uncaging (scale bar: 10 μm). A projected z-stack image illustrates detailed dendritic morphology of the region of interest.
B, Left panel: Population time courses of the LTD(mGluR1) experiment showing fast and slow EPSC, PF burst-evoked Ca and UV-IP3 uncaging-evoked Ca measures. Right panel: Population measures at t = 30 min (n = 8).
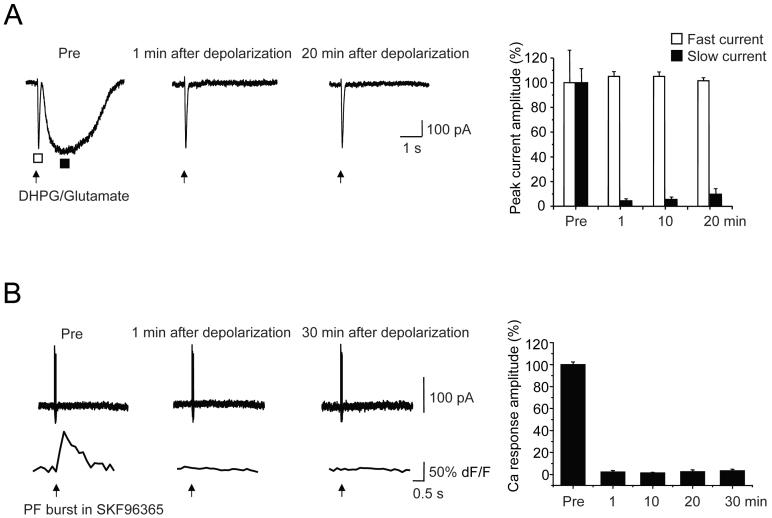
To further test the hypothesis that presynaptic changes underlie LTD(mGluR1), postsynaptic mGluR1 was activated by micropressure pulses of the selective agonist DHPG (100 μM, 10 psi, 50 msec) delivered through a patch pipette. Glutamate (40 - 100 μM) was also included in this solution to produce a fast AMPA receptor-mediated inward current, thereby serving as a control for nonspecific effects (Figure 5A). Similar to results obtained with parallel fiber bursts, a 5 s long depolarization to 0 mV produced a near-complete abolition of the DHPG-triggered slow current (9.7 ± 4.5 % of baseline, t = 20 min, n = 6 cells) leaving the fast current unaffected (101.9 ± 2.0 %).
Figure 5. LTD(mGluR1), measured using test pulses of an exogenous mGluR1 agonist.
A, Fast and slow currents were triggered by micropressure pulses (10 p.s.i., 50 ms duration) of external solution containing DHPG (100 μM) and glutamate (40 - 100 μM) delivered through a patch pipette. Representative current traces show before (Pre), 1 min and 20 min after a 5 s long conditioning depolarization. The hollow square indicates the fast current peak and the filled square, the slow current peak. Right: population measures (n = 6).
B, In the presence of the TRPC1 blocker SKF 96365 (10 μM), strong parallel fiber burst stimulation gives rise to a Ca transient but no detectable inward current. Following a 5 s long conditioning depolarization to induce LTD(mGluR1), the Ca transient is persistently abolished. Right: population measures (n = 7).
Furthermore, in a separate group of cells (Figure 6A), induction of LTD(mGluR1) by a 5 s long depolarization to 0 mV profoundly attenuated both the mGluR1-triggered slow current (2.0 ± 0.8 % of baseline, n = 6 cells) and the mGluR1-triggered Ca transient (3.1 ± 2.6 %), leaving the AMPA receptor-mediated fast current unaffected (101.1 ± 3.5 %). Bath application of the mGluR1 antagonist CPCCOEt (100 μM), abolished the DHPG-induced slow current and the associated increase in dendritic Ca concentration (current: 3.9 ± 1.9 % of baseline; Ca: 3.0 ± 1.2 % of baseline; n = 7 cells; data not shown). The coincident depression of the mGluR1-triggered slow current and Ca transient persisted for the duration of the recording (> 20 min after strong depolarization). As a control measure, global Ca transients triggered by a brief depolarizing test pulse (100 msec, 0 mV) were also measured before and after induction of LTD(mGluR1). These Ca transients were not changed (104.0 ± 6.7 % of baseline, t = 60 s, n = 6 cells), suggesting that LTD(mGluR1) is not associated with alteration in the function of postsynaptic voltage-sensitive Ca channels, or postsynaptic Ca buffering, extrusion or sequestration.
Figure 6. LTD(mGluR1) produces a selective attenuation of mGluR1-evoked Ca transients.
A, Simultaneous confocal Ca imaging and whole-cell patch clamp recording were performed on Purkinje cells loaded with Oregon Green BAPTA-1 (200 μM). Fast and slow currents and associated changes in intradendritic calcium concentrations were triggered by micropressure pulses (10 psi, 50 ms duration) of DHPG (100 μM) together with glutamate (50 μM). Representative traces are shown before (Pre) and 1 min after a 5 s long conditioning depolarization. A brief depolarization (100 ms) followed the DHPG/Glutamate pulse as a control to query the status of voltage-sensitive Ca channels.
B, Weak parallel fiber burst stimulation (6 μA, 50 Hz, 10 pulses) was used to trigger Ca mobilization without a discernable slow EPSC. Representative traces show before (Pre) and 1 min after a 5 s long conditioning depolarization.
C, Population data for the manipulations shown in panels A and B (n = 6 for all measures).
So, using either parallel fiber burst stimulation or exogenous mGluR1 agonist as a test stimulus, LTD(mGluR1) is expressed as a selective abolition of mGluR1-mediated slow current and the associated Ca transient. This argues strongly for a postsynaptic locus of expression for LTD(mGluR1). In particular, LTD(mGluR1) does not appear to be mediated by the reduction of a glutamate release triggered by depolarization-evoked endocannabinoid production that underlies depolarization-induced suppression of parallel fiber fast EPSCs (Kreitzer and Regehr, 2001). In fact, LTD(mGluR1) is unaffected by pretreatment with the CB1 receptor antagonist AM 251 (1 μM; data not shown).
To this point, the results have indicated that LTD(mGluR1) is postsynaptically expressed as a specific down regulation of mGluR1 signaling that is manifest in both the slow EPSC and its associated Ca transient. However, the mGluR1-evoked Ca transient has been shown to reflect both Ca influx (Tempia et al., 2001) through plasma membrane TrpC1 (Kim et al., 2003) and Ca mobilization from internal stores (Finch and Augustine, 1998; Takechi et al., 1998). Does LTD(mGluR1) affect the PLC/IP3 signaling limb of mGluR1 or only the TrpC1/slow EPSC signaling limb? We have employed two approaches to measure the mGluR1-evoked PLC/IP3 signals in isolation. First, we have used the drug SKF96365 (10 μM) which blocks TrpC1 channels and therefore the parallel fiber slow EPSC (Kim et al., 2003, and Figure 5B). In this situation, when parallel fibers are strongly stimulated (10 - 18 μA), a small Ca transient can be evoked in the absence of a slow EPSC. This Ca transient was completely abolished by application of CPCCOEt (100 μM; 0.7 ± 1.0 % of baseline, n = 5; see Supplementary Figure 4) or the sarcoplasmic reticulum Ca-ATPase inhibitor, cyclopiazonic acid (CPA, 50 μM; 1.4 ± 0.9 % of baseline, n = 5), indicating that it represents Ca mobilization from the mGluR1/PLC/IP3 signaling cascade. This PLC/IP3 limb Ca transient was completely abolished with induction of LTD(mGluR1) (3.3 ± 1.6 % of baseline, t = 30 min, n=7 cells).
Second, when weak burst stimulation is applied to a small number of parallel fibers, Ca mobilization may be produced without a discernable slow EPSC (Takechi et al., 1998). By reducing the stimulation strength and the frequency of the bursts (6 μA, 50 Hz, 10 pulses), we could measure parallel-fiber evoked Ca mobilization in the presumed absence of TrpC1-mediated Ca influx (Figure 6B). When LTD(mGluR1) was induced, this produced a strong attenuation of this weak parallel fiber burst Ca signal (7.8 ± 3.6 % of pre-depolarization at t = 60 s, n = 6 cells). A limitation of the second experiment is that it assumes that somatic recording will be able to detect a small slow EPSC in the dendrites. However, this concern does not apply to the first experiment. Taken together, these findings indicate that LTD(mGluR1) is expressed as a depression of both the TrpC1/slow EPSC and the PLC/IP3 signaling limbs.
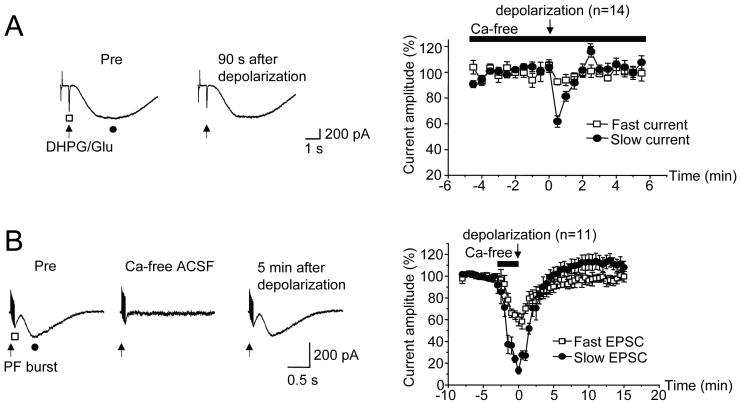
It is likely that strong depolarization triggers LTD(mGluR1) through Ca influx. To test this hypothesis, Ca was excluded from the external saline, 0.2 mM EGTA was added, and test pulses consisting of micropressure ejection of DHPG/glutamate were delivered (Figure 7A). In this configuration, a 5 s long depolarization to 0 mV failed to induce LTD(mGluR1): only a transient depression of slow mGluR1-mediated current occurred which returned to baseline within ∼ 1.5 min (102.1 ± 3.5% of baseline, t = 2 min, n = 14 cells). Fast AMPA receptor-mediated current was entirely unaffected by this manipulation (98.8 ± 2.2 %).
Figure 7. LTD(mGluR1) is prevented in Ca-free ACSF.
A, Slices were bathed in a modified ACSF with no added Ca and 0.2 mM EGTA. Left: Fast and slow currents were induced by micropressure pulses (10 psi, 20 ms duration) of DHPG (100 μM) together with glutamate (50 μM). Representative traces are shown before (Pre) and 90 s after a 5 s long conditioning depolarization. Right: Averaged, normalized time courses of the changes in fast and slow currents amplitudes evoked by conditioning depolarization (n=14).
B, Left: Representative current traces show fast and slow components of the parallel fiber EPSC which were produced by bursts (10 stimuli at 100 Hz) delivered in the presence of NBQX (5 μM) and picrotoxin (100 μM). Bath application of Ca-free ACSF totally abolished the mGluR-evoked slow EPSC. In this configuration, a 5 s long depolarization to 0 mV failed to induce LTD(mGluR1), as measured following the return of Ca-containing ACSF. Right: Averaged, normalized time courses of the percent changes in peak fast and slow EPSC amplitudes are shown. Application period of Ca-free ACSF is indicated by the horizontal bar.
Pulses of DHPG/glutamate will activate a mixture of synaptic and extrasynaptic receptors. To determine if Ca influx is necessary for LTD(mGluR1) as expressed by synaptically-activated mGluR1, an experiment using parallel fiber burst test pulses was performed (Figure 7B). In this design, parallel fiber burst evoked EPSCs were recorded for 5 min in normal external saline, following which the external saline was switched to that with 0 Ca. This produced a complete blockade of the mGluR1-mediated slow EPSC (13.3 ± 3.2 %, t = 0 min, n = 11) at which point, conditioning depolarization was delivered (5 s duration). When Ca-containing external saline was returned, the slow EPSC recovered to its pre-stimulation amplitude (101 ± 6.8 % of baseline, t = 5 min), further indicating that Ca influx is required for induction of LTD(mGluR1).
Many seven-transmembrane domain G-protein coupled receptors undergo internalization with repeated exposure to agonist. As strong depolarization of Purkinje cells is known to evoke postsynaptic glutamate release (Duguid and Smart, 2004), we wished to test the hypothesis that glutamate binding to mGluR1 might contribute to induction of LTD(mGluR1). We addressed this in two different ways. First, bath application of DHPG did indeed produce an LTD of the mGluR1-sEPSC as activated by parallel fiber burst test stimuli (19.7 ± 4.6 % of baseline at t = 30 min, n = 6; Supplemental Figure 5). It also produced a postsynaptically-expressed LTD of fast AMPA-receptor mediated EPSCs (68.3 ± 9.2 % of baseline; paired pulse ratio, 100.9 ± 7.6 % of baseline at t = 30 min), consistent with another report in Purkinje cells in brain slices (Sdrulla and Linden, 2007). It’s not exactly clear what this tells us, however. Bath application of DHPG will broadly activate group I mGluRs in many neurons in the slice in a fashion that is quite unlike our conditioning stimulation.
In a second experiment, test pulses of DHPG/glutamate were delivered and following establishment of a stable baseline response, the mGluR1 antagonist CPCCOEt (100 μM) was added to the perfusion line (Supplemental Figure 6). This rapidly produced a complete attenuation of the slow EPSC (8.5 ± 0.9 % of baseline, t = -2 min, n = 8 cells). While CPCCOEt was present, a 5 s long conditioning depolarization to 0 mV was delivered and CPCCOEt washout commenced immediately after the depolarization. The slow EPSC gradually reappeared as washout proceeded, but it only returned to 50.5 ± 4.3 % of baseline at t = 60 min. In control cells in which CPCCOEt was washed in and out but no conditioning stimulation was applied, washout was complete at t = 52 min (97.1 ± 3.3 % of baseline, n = 8 cells). In another control group, conditioning depolarization was given in the absence of CPCCOEt and LTD(mGluR1) was expressed normally and persisted for the duration of the experiment (4.1 ± 0.7 % of baseline at t = 60 min, n = 6 cells). This suggests that glutamate binding of mGluR1, while not absolutely required, contributes to the expression of LTD(mGluR1).
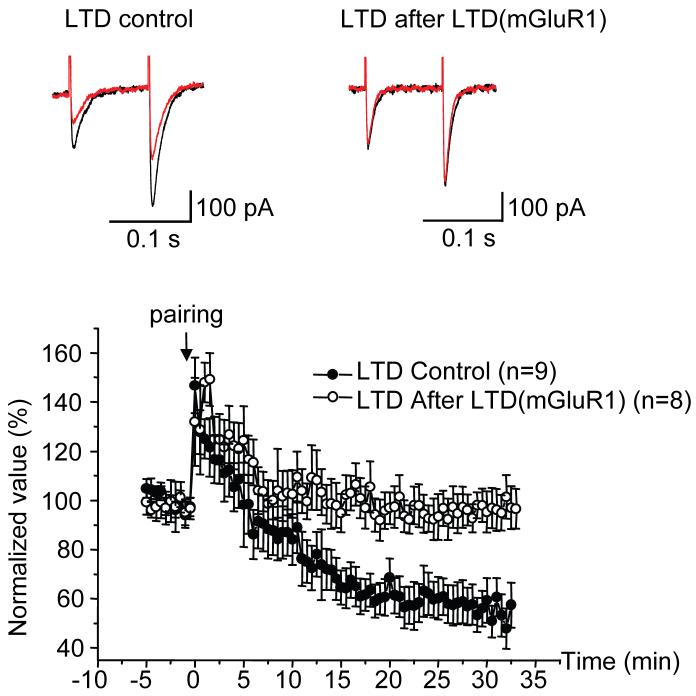
At the parallel fiber-Purkinje cell synapse, activation of mGluR1 is essential for the induction of a homosynaptic, postsynaptically-expressed form of AMPA-receptor LTD (Linden et al., 1991; Shigemoto et al., 1994), commonly called “cerebellar LTD”. Therefore, LTD(mGluR1) may have a metaplastic function, blocking the subsequent induction of cerebellar parallel fiber LTD. As a test of this hypothesis, we first induced LTD(mGluR1), and then, shortly thereafter, attempted to induce cerebellar LTD (Figure 8). A paired-pulse test stimulus (100 ms interval) was delivered every 15 s to activate parallel fibers. In the control condition, after recording baseline responses for 5 min, pairing stimulation was given to induce LTD. This consisted of a brief burst of 5 stimuli of parallel fiber at 100 Hz, the onset coincident with a step depolarization of Purkinje cell to 0 mV for 75 ms, each such paring repeated 30 times at 2 s intervals. Consistent with previous reports (Shin and Linden, 2005), this treatment induced LTD of the parallel fiber EPSC (59.4 ± 9.1 %, t = 30 min, n = 9). However, when parallel fiber/depolarization pairing was given 10 min after a 5 s depolarization to 0 mV to induce LTD(mGluR1), LTD was completely blocked (95.3 ± 8.4 %, t= 30 min, n=8), indicating that LTD(mGluR1) has a potent metaplastic effect.
Figure 8. LTD(mGluR1) blocks subsequent induction of AMPA receptor LTD at parallel fiber-Purkinje cell synapses.
Representative current traces show EPSCs evoked by parallel fiber test stimulation using paired pulses (100 ms interval). The black traces were recorded 15 s before and the red traces, 30 min after pairing stimulation designed to evoke AMPA receptor LTD. In one group pairing stimulation followed only test pulses (LTD Control) while in another, it followed 10 min after induction of LTD(mGluR1) by a 5 sec long depolarization (LTD After LTD(mGluR1)). The bottom panel shows population time course data for peak 1st EPSC amplitudes for the LTD Control group (filled circles, n=9) and the LTD After LTD(mGluR1) groups (open circles, n=8), expressed as mean ± SEM. In both groups, the pairing stimulus was given about 20 min after achieving the whole-cell configuration.
Discussion
The main finding of this study is that mGluR1 signaling at the cerebellar parallel fiber-Purkinje cell synapse undergoes selective and persistent depression following repeated climbing fiber activation and consequent strong postsynaptic depolarization and Ca influx. Therefore, different patterns of neural activity in the cerebellar cortex can produce either LTD of fast AMPA-receptor mediated signals (“conventional” cerebellar parallel fiber LTD; Figure 8) or LTD of slow mGluR1-mediated signals, LTD(mGluR1). This provides a new form of plastic computation for neural circuits.
LTD(mGluR1) can be measured as an attenuation of both of the signaling limbs triggered by mGluR1: the slow inward current mediated by TrpC1 activation and the Ca mobilization from stores mediated by PLC/IP3 signaling. LTD(mGluR1) appears to be expressed postsynaptically as 1) it is independent of changes in fast current mediated by AMPA-type glutamate receptors, 2) it may be detected using either parallel fiber burst stimulation or pulses of exogenous mGluR1 agonist, and 3) it is not associated with changes in the probability of glutamate release as indexed by the paired-pulse ratio of fast EPSCs. LTD(mGluR1) is not associated with changes in certain other postsynaptic processes such as brief depolarization-evoked Ca transients. Furthermore, dendritic Ca transients triggered by photolytic uncaging of IP3 are not decreased by the LTD(mGluR1) induction protocol, arguing against LTD(mGluR1) expression through inhibition of IP3 receptors or the status of IP3-sensitive Ca stores.
We propose that LTD(mGluR1) is expressed by attenuation of an early portion of the mGluR1 signaling complex, before it divides into separate TrpC1 and PLC/IP3 limbs. The ultimate molecular target of LTD(mGluR1) is likely to be mGluR1 itself, Gαq or a protein which functionally interacts with one or both of these. The possible role of PLCβ as a target for LTD(mGluR1) remains unclear. It has been reported that chemical inhibition of PLCβ is ineffective in blocking mGluR1-evoked slow EPSCs (Canepari et al., 2001; Tempia et al., 1998); but that genetic deletion of PLCβ4 is effective (Sugiyama et al., 1999).
At present, we do not know the second messenger(s) that transduce the depolarization-evoked Ca transient into LTD(mGluR1). One possibility involves activation of a tyrosine kinase and/or inhibition of a tyrosine phosphatase. Tyrosine phosphatase inhibitors have been shown to completely block mGluR1-triggered slow currents in Purkinje cells while tyrosine kinase inhibitors, including a specific inhibitor of src, produced a 1.5-fold potentiation (Canepari et al., 2001). Another possibility involves the protease calpain, which has recently been shown to cleave and inactivate the carboxy terminal domain of mGluR1α (Xu et al., 2007).
Strong depolarization of Purkinje cells evokes postsynaptic glutamate release which can then activate NMDA receptors on the terminals of nearby interneurons (Duguid and Smart, 2004). It is possible that this depolarization-triggered glutamate transient may also activate Purkinje cell mGluR1 in an autocrine fashion and that this event contributes to the induction of LTD(mGluR1). In heterologous expression systems, plasma membrane mGluR1α (an isoform strongly expressed in Purkinje cells), is internalized in response to sustained bath application of glutamate (0.2 - 1 mM for 10 - 60 min) or other mGluR1 agonists such as quisqualate or DHPG (Dale et al., 2001; Doherty et al., 1999; Mundell et al., 2001; Sallese et al., 2000). This internalization requires clathrin/dynamin-mediated endocytosis (Doherty et al., 1999; Mundell et al., 2001) and appears to be triggered by a signaling cascade involving β-arrestin-1 (Dale et al., 2001; Iacovelli et al., 2003) and G-protein coupled receptor kinase 4 (GRK4; (Iacovelli et al., 2003; Sallese et al., 2000)). GRK4 is expressed in Purkinje cells (Sallese et al., 2000) and its knockdown with antisense mRNA attenuates the mGluR1 internalization in cultured Purkinje cells produced by sustained bath application of agonist (Iacovelli et al., 2003). Interestingly, LTD(mGluR1) persists even when TrpC1 is blocked (Figure 5B). So, while glutamate binding to mGluR1 may be required for LTD(mGluR1) induction, mGluR1-triggered cation influx is not.
Curiously, another group has treated cultured embryonic Purkinje cells with an external saline supplemented with 50 mM KCl to produce strong depolarization (5 min duration) and this did not trigger LTD(mGluR1), but rather resulted in an increase in surface mGluR1 immunoreactivity and a corresponding increase in the Ca transient and inward current evoked by puffs of mGluR1 agonist (Minami et al., 2003). The signals evoked by bath application of KCl are many and are likely to differ from those evoked by climbing fiber activation or depolarization of single Purkinje cells in brain slices as performed herein.
What is the function of LTD(mGluR1) in Purkinje cells? While we have measured mGluR1 using parallel fiber bursts, mGluR1 is also activated by glutamate release at the climbing fiber-Purkinje cell synapse (Dzubay and Otis, 2002). At both the climbing fiber-Purkinje cell synapse (Hansel and Linden, 2000) and the parallel fiber-Purkinje cell synapse (Linden et al., 1991; Shigemoto et al., 1994), activation in mGluR1 is essential for the induction of a postsynaptically-expressed form AMPA-receptor LTD. Therefore, LTD(mGluR1) may have a metaplastic function, blocking induction of AMPA receptor LTD at both parallel fiber synapses (heterosynaptically), as shown here (Figure 8), and possibly climbing fiber synapses (homosynaptically). In addition, mGluR1 activation is an important route for the generation of endocannabinoids and the consequent short-term reduction in release of glutamate and GABA from CB1 receptor-bearing terminals impinging upon Purkinje cells (Maejima et al., 2001). LTD(mGluR1) may also have a neuroprotective function in Purkinje cells. When strong depolarization of Purkinje cells is repeated, as occurs following persistent high-frequency activation of climbing fiber synapses during stroke, this can produce excitotoxic Purkinje cell degeneration (Welsh et al., 2002). In a heterologous expression system, internalization of mGluR1α by co-expression of G-protein receptor kinases, was protective against apoptosis (Dale et al., 2000).
Looking forward, it will be particularly useful to determine whether LTD(mGluR1), seen here at an mGluR1-using synapse, is also present at synapses which utilize the related group I receptor mGluR5 either singly or in combination with mGluR1. Group I mGluRs have been implicated in both implicit and explicit memory, addiction, epilepsy, neurotoxicity and mental retardation, and so the demonstration herein that mGluR1 function is subject to persistent attenuation by neuronal activity may be central to these diverse brain functions and disease states.
Methods
Slice preparation
Parasagittal slices of the cerebellar vermis (250 μm thick) were prepared from P15-20 Sprague-Dawley rats using a vibrating tissue slicer and ice-cold standard artificial cerebrospinal fluid (ACSF) containing 124 mM NaCl, 2.5 mM KCl, 1 mM Na2HPO4, 1.3 mM MgCl2, 2.5 mM CaCl2, 26.2 mM NaHCO3 and 20 mM D-glucose, bubbled with 95% O2 and 5% CO2. After cutting, slices were kept for 30 min at 35°C and then for up to 8 hr at 25°C in ACSF.
Patch-clamp recordings
After a recovery period, the slices were placed in a submerged chamber that was perfused at a rate of 2 ml/min with ACSF supplemented with either 100 μM picrotoxin or 5 μM GABAzine to block GABAA receptors. Somatic whole-cell recording were obtained by using an Multiclamp 700B (Axon instruments, Foster City, California). The recording electrodes (resistance 2-4 MΩ) were filled with a solution containing 130 mM K-gluconate, 10 mM KCl, 10 mM HEPES, 10 mM Na2-phosphocreatine, 3 mM Mg2ATP, 0.3 mM Na3GTP and 0.2 mM Oregon Green BAPTA-1, (pH 7.25) for current clamp recording and 135 mM Cs-methanesulfonate, 10 mM CsCl, 10 mM HEPES, 4 mM Mg2ATP, 0.4 mM Na3GTP and 0.2 mM EGTA (substituted with 0.2 mM Oregon Green BAPTA-1 in Ca imaging experiments) (pH 7.25) for voltage clamp recordings. Ca-free extracellular solution was prepared by removing CaCl2 and adding 0.2 mM EGTA. Currents were filtered at 1 kHz, digitized at 5 kHz, and acquired using Pclamp 9.0 software (Axon Instruments). For parallel fiber stimulation, standard patch pipettes were used which were filled with external saline and placed in the middle third of the molecular layer. Synaptic responses were evoked every 30 s using 12 - 16 μA pulses (100 μs duration). When burst stimulation was employed, the interpulse interval was 10 ms. In some experiments, membrane currents were evoked by agonist application using a pressure application system (Picospritzer II, 10 psi, 50 ms pulse duration). Recordings were performed at 30 °C in ACSF.
Confocal fluorescence imaging and photolytic uncaging
A confocal laser-scanning head (Zeiss Pascal) attached to an upright microscope (Zeiss Axioskop 2FSmot, 40X water immersion objective, N.A. = 0.8) was used to acquire fluorescence image in parallel to the whole-cell recordings. For photolytic uncaging experiments, internal saline was supplemented with NPE-IP3 (300 μM). Uncaging was produced by directing the 355 nm output of a frequency tripled Nd:YVO4 laser operating at 100 kHz (DPSS Inc.; power at the laser head = 1.1 W; pulse duration = 50 ns) into the epifluorescence train of the confocal microscope using a 25 μm single-mode fiber and coupling optics (Rapp OptoElectronic). Light was directed to the objective by reflection from a 400 nm dichroic mirror. Uncaging test pulses (5 - 10 ms duration) were originated by Clampex 9.0 software (Axon Instruments) and controlled by a combination of switching the laser and activation of a mechanical shutter (Uniblitz, Vincent Associates).
Statistics and drugs
All group data are shown as mean ± SEM. Comparisons were made using Student’s t test. All drugs were purchased from Sigma (St. Louis, Missouri) except for CPCCOEt, GABAzine, NBQX, AM251 (Tocris Cookson, Ballwin, Missouri), and Oregon Green BAPTA-1 and NPE-IP3 (Molecular Probes).
Supplementary Material
Acknowledgements
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A040042) to S. J. Kim, and NIH MH51106 to D.J. Linden. We thank Andrei Sdrulla for help in image analysis, Roland Bock for technical assistance, and members of the Linden, Worley and S.J. Kim labs for useful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Canepari M, Papageorgiou G, Corrie JE, Watkins C, Ogden D. The conductance underlying the parallel fibre slow EPSP in rat cerebellar Purkinje neurones studied with photolytic release of L-glutamate. J Physiol. 2001;533:765–772. doi: 10.1111/j.1469-7793.2001.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LB, Bhattacharya M, Anborgh PH, Murdoch B, Bhatia M, Nakanishi S, Ferguson SS. G protein-coupled receptor kinase-mediated desensitization of metabotropic glutamate receptor 1A protects against cell death. J Biol Chem. 2000;275:38213–38220. doi: 10.1074/jbc.M006075200. [DOI] [PubMed] [Google Scholar]
- Dale LB, Bhattacharya M, Seachrist JL, Anborgh PH, Ferguson SS. Agonist-stimulated and tonic internalization of metabotropic glutamate receptor 1a in human embryonic kidney 293 cells: agonist-stimulated endocytosis is beta-arrestin1 isoform-specific. Mol Pharmacol. 2001;60:1243–1253. doi: 10.1124/mol.60.6.1243. [DOI] [PubMed] [Google Scholar]
- Debanne D, Daoudal G, Sourdet V, Russier M. Brain plasticity and ion channels. J Physiol Paris. 2003;97:403–414. doi: 10.1016/j.jphysparis.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Coutinho V, Collingridge GL, Henley JM. Rapid internalization and surface expression of a functional, fluorescently tagged G-protein-coupled glutamate receptor. Biochem J. 1999;341(Pt 2):415–422. [PMC free article] [PubMed] [Google Scholar]
- Duguid IC, Smart TG. Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron-Purkinje cell synapses. Nat Neurosci. 2004;7:525–533. doi: 10.1038/nn1227. [DOI] [PubMed] [Google Scholar]
- Dzubay JA, Otis TS. Climbing fiber activation of metabotropic glutamate receptors on cerebellar Purkinje neurons. Neuron. 2002;36:1159–1167. doi: 10.1016/s0896-6273(02)01052-8. [DOI] [PubMed] [Google Scholar]
- Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- Hansel C, Linden DJ. Long-term depression of the cerebellar climbing fiber--Purkinje neuron synapse. Neuron. 2000;26:473–482. doi: 10.1016/s0896-6273(00)81179-4. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Blum R, Kovalchuk Y, Adelsberger H, Kuner R, Durand GM, Miyata M, Kano M, Offermanns S, Konnerth A. Distinct roles of Galpha(q) and Galpha11 for Purkinje cell signaling and motor behavior. J Neurosci. 2004;24:5119–5130. doi: 10.1523/JNEUROSCI.4193-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser M, Roth A. Dendritic and somatic glutamate receptor channels in rat cerebellar Purkinje cells. J Physiol. 1997;501(Pt 1):77–95. doi: 10.1111/j.1469-7793.1997.077bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli L, Salvatore L, Capobianco L, Picascia A, Barletta E, Storto M, Mariggio S, Sallese M, Porcellini A, Nicoletti F, De Blasi A. Role of G protein-coupled receptor kinase 4 and beta-arrestin 1 in agonist-stimulated metabotropic glutamate receptor 1 internalization and activation of mitogen-activated protein kinases. J Biol Chem. 2003;278:12433–12442. doi: 10.1074/jbc.M203992200. [DOI] [PubMed] [Google Scholar]
- Kano M, Rexhausen U, Dreessen J, Konnerth A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature. 1992;356:601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TrpC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Dickinson MH, Smeyne M, Connor JA. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron. 1991;7:81–89. doi: 10.1016/0896-6273(91)90076-c. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Minami I, Kengaku M, Smitt PS, Shigemoto R, Hirano T. Long-term potentiation of mGluR1 activity by depolarization-induced Homer1a in mouse cerebellar Purkinje neurons. Eur J Neurosci. 2003;17:1023–1032. doi: 10.1046/j.1460-9568.2003.02499.x. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Mizushima A, Hirose K, Yamazawa T, Bezprozvanny I, Kurosaki T, Iino M. Ca(2+)-sensor region of IP(3) receptor controls intracellular Ca(2+) signaling. Embo J. 2001;20:1674–1680. doi: 10.1093/emboj/20.7.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell SJ, Matharu AL, Pula G, Roberts PJ, Kelly E. Agonist-induced internalization of the metabotropic glutamate receptor 1a is arrestin- and dynamin-dependent. J Neurochem. 2001;78:546–551. doi: 10.1046/j.1471-4159.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- Sallese M, Salvatore L, D’Urbano E, Sala G, Storto M, Launey T, Nicoletti F, Knopfel T, De Blasi A. The G-protein-coupled receptor kinase GRK4 mediates homologous desensitization of metabotropic glutamate receptor 1. FASEB J. 2000;14:2569–2580. doi: 10.1096/fj.00-0072com. [DOI] [PubMed] [Google Scholar]
- Sdrulla AD, Linden DJ. A double dissociation between LTD and dendritic spine morphology in cerebellar Purkinje cells. Nat Neurosci. 2007;10:546–548. doi: 10.1038/nn1889. [DOI] [PubMed] [Google Scholar]
- Shen Y, Linden DJ. Long-term potentiation of neuronal glutamate transporters. Neuron. 2005;46:715–722. doi: 10.1016/j.neuron.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Abe T, Nomura S, Nakanishi S, Hirano T. Antibodies inactivating mGluR1 metabotropic glutamate receptor block long-term depression in cultured Purkinje cells. Neuron. 1994;12:1245–1255. doi: 10.1016/0896-6273(94)90441-3. [DOI] [PubMed] [Google Scholar]
- Shin JH, Linden DJ. An NMDA receptor/nitric oxide cascade is involved in cerebellar LTD but is not localized to the parallel fiber terminal. J Neurophysiol. 2005;94:4281–4289. doi: 10.1152/jn.00661.2005. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Hirono M, Suzuki K, Nakamura Y, Aiba A, Nakamura K, Nakao K, Katsuki M, Yoshioka T. Localization of phospholipase Cbeta isozymes in the mouse cerebellum. Biochem Biophys Res Commun. 1999;265:473–478. doi: 10.1006/bbrc.1999.1628. [DOI] [PubMed] [Google Scholar]
- Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757–760. doi: 10.1038/25547. [DOI] [PubMed] [Google Scholar]
- Tempia F, Alojado ME, Strata P, Knopfel T. Characterization of the mGluR(1)-mediated electrical and calcium signaling in Purkinje cells of mouse cerebellar slices. J Neurophysiol. 2001;86:1389–1397. doi: 10.1152/jn.2001.86.3.1389. [DOI] [PubMed] [Google Scholar]
- Tempia F, Kano M, Schneggenburger R, Schirra C, Garaschuk O, Plant T, Konnerth A. Fractional calcium current through neuronal AMPA-receptor channels with a low calcium permeability. J Neurosci. 1996;16:456–466. doi: 10.1523/JNEUROSCI.16-02-00456.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol. 1998;80:520–528. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O’Hearn E, Molliver ME, Aicher SA. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol. 2002;89:331–359. [PubMed] [Google Scholar]
- Xu W, Wong TP, Chery N, Gaertner T, Wang YT, Baudry M. Calpain-mediated mGluR1alpha truncation: a key step in excitotoxicity. Neuron. 2007;53:399–412. doi: 10.1016/j.neuron.2006.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.