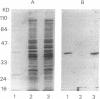
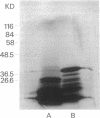
Abstract
Plasmin(ogen) receptors are expressed by many gram-positive and gram-negative bacteria. We previously isolated a plasmin receptor from a pathogenic group A streptococcal strain (C. C. Broder, R. Lottenberg, G. O. von Mering, K. H. Johnston, and M. D. P. Boyle, J. Biol. Chem. 266:4922-4928, 1991). The gene encoding this plasmin receptor, plr, was isolated from a lambda gt11 library of chromosomal DNA from group A streptococcal strain 64/14 by screening plaques with antibodies raised against the purified streptococcal plasmin receptor protein. The gene was subcloned by using a low-copy-number plasmid and stably expressed in Escherichia coli, resulting in the production of an immunoreactive and functional receptor protein. The DNA sequence of the gene contained an open reading frame encoding 335 amino acids with a predicted molecular weight of 35,787. Upstream of the open reading frame, putative promoter and ribosomal binding site sequences were identified. The experimentally derived amino acid sequences of the N terminus and three cyanogen bromide fragments of the purified streptococcal plasmin receptor protein corresponded to the predicted sequence encoded by plr. The deduced amino acid sequence for the plasmin receptor protein revealed significant similarity (39 to 54% identical amino acid residues) to glyceraldehyde 3-phosphate dehydrogenases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alefounder P. R., Perham R. N. Identification, molecular cloning and sequence analysis of a gene cluster encoding the class II fructose 1,6-bisphosphate aldolase, 3-phosphoglycerate kinase and a putative second glyceraldehyde 3-phosphate dehydrogenase of Escherichia coli. Mol Microbiol. 1989 Jun;3(6):723–732. doi: 10.1111/j.1365-2958.1989.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Allen R. W., Hoover B. A. Characterization of the processed form of a ubiquitous protein displaying a variable membrane organization in erythroid cells. Blood. 1985 May;65(5):1048–1055. [PubMed] [Google Scholar]
- Allen R. W., Trach K. A., Hoch J. A. Identification of the 37-kDa protein displaying a variable interaction with the erythroid cell membrane as glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1987 Jan 15;262(2):649–653. [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bisno A. L. Group A streptococcal infections and acute rheumatic fever. N Engl J Med. 1991 Sep 12;325(11):783–793. doi: 10.1056/NEJM199109123251106. [DOI] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant G., Branlant C. Nucleotide sequence of the Escherichia coli gap gene. Different evolutionary behavior of the NAD+-binding domain and of the catalytic domain of D-glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1985 Jul 1;150(1):61–66. doi: 10.1111/j.1432-1033.1985.tb08988.x. [DOI] [PubMed] [Google Scholar]
- Broder C. C., Lottenberg R., Boyle M. D. Mapping of the human plasmin domain recognized by the unique plasmin receptor of group A streptococci. Infect Immun. 1989 Sep;57(9):2597–2605. doi: 10.1128/iai.57.9.2597-2605.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder C. C., Lottenberg R., von Mering G. O., Johnston K. H., Boyle M. D. Isolation of a prokaryotic plasmin receptor. Relationship to a plasminogen activator produced by the same micro-organism. J Biol Chem. 1991 Mar 15;266(8):4922–4928. [PubMed] [Google Scholar]
- Broeseker T. A., Boyle M. D., Lottenberg R. Characterization of the interaction of human plasmin with its specific receptor on a group A streptococcus. Microb Pathog. 1988 Jul;5(1):19–27. doi: 10.1016/0882-4010(88)90077-0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Corbier C., Clermont S., Billard P., Skarzynski T., Branlant C., Wonacott A., Branlant G. Probing the coenzyme specificity of glyceraldehyde-3-phosphate dehydrogenases by site-directed mutagenesis. Biochemistry. 1990 Jul 31;29(30):7101–7106. doi: 10.1021/bi00482a022. [DOI] [PubMed] [Google Scholar]
- DesJardin L. E., Boyle M. D., Lottenberg R. Group A streptococci bind human plasmin but not other structurally related proteins. Thromb Res. 1989 Jul 15;55(2):187–193. doi: 10.1016/0049-3848(89)90435-0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Goudot-Crozel V., Caillol D., Djabali M., Dessein A. J. The major parasite surface antigen associated with human resistance to schistosomiasis is a 37-kD glyceraldehyde-3P-dehydrogenase. J Exp Med. 1989 Dec 1;170(6):2065–2080. doi: 10.1084/jem.170.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekman W. E., Dennis D. T., Miernyk J. A. Secretion of Ricinus communis glyceraldehyde-3-phosphate dehydrogenase by Escherichia coli. Mol Microbiol. 1990 Aug;4(8):1363–1369. doi: 10.1111/j.1365-2958.1990.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Hensel R., Zwickl P., Fabry S., Lang J., Palm P. Sequence comparison of glyceraldehyde-3-phosphate dehydrogenases from the three urkingdoms: evolutionary implication. Can J Microbiol. 1989 Jan;35(1):81–85. doi: 10.1139/m89-012. [DOI] [PubMed] [Google Scholar]
- Holland J. P., Labieniec L., Swimmer C., Holland M. J. Homologous nucleotide sequences at the 5' termini of messenger RNAs synthesized from the yeast enolase and glyceraldehyde-3-phosphate dehydrogenase gene families. The primary structure of a third yeast glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem. 1983 Apr 25;258(8):5291–5299. [PubMed] [Google Scholar]
- Hudson M. C., Curtiss R., 3rd Regulation of expression of Streptococcus mutans genes important to virulence. Infect Immun. 1990 Feb;58(2):464–470. doi: 10.1128/iai.58.2.464-470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitorel P., Pantaloni D. Bundling of microtubules by glyceraldehyde-3-phosphate dehydrogenase and its modulation by ATP. Eur J Biochem. 1985 Jul 15;150(2):265–269. doi: 10.1111/j.1432-1033.1985.tb09016.x. [DOI] [PubMed] [Google Scholar]
- Kawamoto R. M., Caswell A. H. Autophosphorylation of glyceraldehydephosphate dehydrogenase and phosphorylation of protein from skeletal muscle microsomes. Biochemistry. 1986 Feb 11;25(3):657–661. doi: 10.1021/bi00351a022. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambeir A. M., Loiseau A. M., Kuntz D. A., Vellieux F. M., Michels P. A., Opperdoes F. R. The cytosolic and glycosomal glyceraldehyde-3-phosphate dehydrogenase from Trypanosoma brucei. Kinetic properties and comparison with homologous enzymes. Eur J Biochem. 1991 Jun 1;198(2):429–435. doi: 10.1111/j.1432-1033.1991.tb16032.x. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Brundage R., Siegal G. P., Terranova V., Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981 Nov;41(11 Pt 1):4629–4636. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lottenberg R., Broder C. C., Boyle M. D. Identification of a specific receptor for plasmin on a group A streptococcus. Infect Immun. 1987 Aug;55(8):1914–1918. doi: 10.1128/iai.55.8.1914-1918.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottenberg R., Dolly F. R., Kitchens C. S. Recurring thromboembolic disease and pulmonary hypertension associated with severe hypoplasminogenemia. Am J Hematol. 1985 Jun;19(2):181–193. doi: 10.1002/ajh.2830190211. [DOI] [PubMed] [Google Scholar]
- McCoy H. E., Broder C. C., Lottenberg R. Streptokinases produced by pathogenic group C streptococci demonstrate species-specific plasminogen activation. J Infect Dis. 1991 Sep;164(3):515–521. doi: 10.1093/infdis/164.3.515. [DOI] [PubMed] [Google Scholar]
- Meyer-Siegler K., Mauro D. J., Seal G., Wurzer J., deRiel J. K., Sirover M. A. A human nuclear uracil DNA glycosylase is the 37-kDa subunit of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8460–8464. doi: 10.1073/pnas.88.19.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels P. A., Marchand M., Kohl L., Allert S., Wierenga R. K., Opperdoes F. R. The cytosolic and glycosomal isoenzymes of glyceraldehyde-3-phosphate dehydrogenase in Trypanosoma brucei have a distant evolutionary relationship. Eur J Biochem. 1991 Jun 1;198(2):421–428. doi: 10.1111/j.1432-1033.1991.tb16031.x. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Olsen K. W. Structural basis for the thermal stability of glyceraldehyde-3-phosphate dehydrogenases. Int J Pept Protein Res. 1983 Oct;22(4):469–475. doi: 10.1111/j.1399-3011.1983.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Identification of an endogenous membrane anchor-cleaving enzyme for group A streptococcal M protein. Its implication for the attachment of surface proteins in gram-positive bacteria. J Exp Med. 1989 Dec 1;170(6):2119–2133. doi: 10.1084/jem.170.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Riaad-El Sabouty S., Dani C., Marty L., Jeanteur P. Unusual abundance of vertebrate 3-phosphate dehydrogenase pseudogenes. 1984 Nov 29-Dec 5Nature. 312(5993):469–471. doi: 10.1038/312469a0. [DOI] [PubMed] [Google Scholar]
- Reis K. J., Yarnall M., Ayoub E. M., Boyle M. D. Effect of mouse passage on Fc receptor expression by group A streptococci. Scand J Immunol. 1984 Nov;20(5):433–439. doi: 10.1111/j.1365-3083.1984.tb01022.x. [DOI] [PubMed] [Google Scholar]
- Schläpfer B. S., Portmann W., Branlant C., Branlant G., Zuber H. Nucleotide sequence of the glyceraldehyde-3-phosphate dehydrogenase from Bacillus megaterium. Nucleic Acids Res. 1990 Nov 11;18(21):6422–6422. doi: 10.1093/nar/18.21.6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefring G. E., Jr, Castellino F. J. Interaction of streptokinase with plasminogen. Isolation and characterization of a streptokinase degradation product. J Biol Chem. 1976 Jul 10;251(13):3913–3920. [PubMed] [Google Scholar]
- Soukri A., Mougin A., Corbier C., Wonacott A., Branlant C., Branlant G. Role of the histidine 176 residue in glyceraldehyde-3-phosphate dehydrogenase as probed by site-directed mutagenesis. Biochemistry. 1989 Mar 21;28(6):2586–2592. doi: 10.1021/bi00432a036. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Wu R. Structure of two unlinked Drosophila melanogaster glyceraldehyde-3-phosphate dehydrogenase genes. J Biol Chem. 1985 Jul 5;260(13):8220–8228. [PubMed] [Google Scholar]
- Viaene A., Dhaese P. Sequence of the glyceraldehyde-3-phosphate dehydrogenase gene from Bacillus subtilis. Nucleic Acids Res. 1989 Feb 11;17(3):1251–1251. doi: 10.1093/nar/17.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]