Abstract
Recent findings related to the renin-angiotensin system have provided a more elaborated understanding of the pathophysiology of hypertension and kidney diseases. These findings have led to unique concepts and issues regarding the intrarenal renin-angiotensin system. Angiotensinogen is the only known substrate for renin that is the rate-limiting enzyme of the renin-angiotensin system. Because the level of angiotensinogen in human beings is close to the Michaelis-Menten constant value for renin, changes in angiotensinogen levels can control the activity of the renin-angiotensin system, and its upregulation may lead to elevated angiotensin peptide levels and increases in blood pressure. Enhanced intrarenal angiotensinogen mRNA or protein levels or both have been observed in multiple models of hypertension including angiotensin II-dependent hypertensive rats, Dahl salt-sensitive hypertensive rats, and spontaneously hypertensive rats, as well as in kidney diseases including diabetic nephropathy, immunoglobulin A (IgA) nephropathy, and radiation nephropathy. Renal angiotensinogen is formed primarily in proximal tubular cells and is secreted into the tubular fluid. Urinary angiotensinogen excretion rates show a clear relationship to kidney angiotensin II contents and kidney angiotensinogen levels, suggesting that urinary angiotensinogen may serve as an index of the intrarenal renin-angiotensin system status. Establishment of concise and accurate methods to measure human angiotensinogen may allow clinical studies that would provide important information regarding the roles of intrarenal angiotensinogen in the development and progression of hypertension and kidney diseases.
Keywords: Angiotensinogen, kidney, hypertension, diabetic nephropathy, immunoglobulin A nephropathy, radiation nephropathy
It is a great honor to be selected to present the American Society of Hypertension 2005 Young Scholars Awards Lecture, and I greatly appreciate this recognition that has been given to our research program.
Uncontrolled hypertension induces structural and functional alterations in the kidney that can eventually lead to end-stage renal diseases.1 Effective control of blood pressure (BP) retards the progression of renal failure and reduces the morbidity and mortality rates associated with hypertensive vascular disease.2-4 Recent findings related to the renin-angiotensin system (RAS), which is one of the most important regulatory mechanisms for BP regulation and electrolyte homeostasis,5 have provided us with an improved understanding of the pathophysiology of hypertension.6-9
Angiotensinogen is the only known substrate for renin that is the rate-limiting enzyme of the RAS. Because the level of angiotensinogen is close to the Michaelis-Menten constant for renin,10,11 angiotensinogen levels can control the activity of the RAS, and its upregulation may lead to elevated angiotensin peptide levels and increases in BP. Recent studies on experimental animal models and transgenic mice have documented the involvement of angiotensinogen in the activation of the RAS and development of hypertension.12-18 Genetic manipulations that lead to overexpression of angiotensinogen have consistently been shown to cause hypertension.17 In human genetic studies, a linkage has been established between the angiotensinogen gene and hypertension.19,20 Thus angiotensinogen plays an important role in BP regulation.
Renin-Angiotensin System in the Kidney
In situ hybridization studies have demonstrated that the angiotensinogen gene is specifically present in the proximal tubules of the kidneys.21 Angiotensinogen mRNA is expressed largely in proximal convoluted tubules and proximal straight tubules, and only small amounts are present in glomeruli and vasa recta as revealed by reverse transcription-polymerase chain reaction.22 Renal angiotensinogen protein is specifically located in the proximal convoluted tubules by immunohistochemistry.23-25 There is strong positive immunostaining for angiotensinogen protein in proximal convoluted tubules and proximal straight tubules, and there is weak positive staining in glomeruli and vasa recta; however there is no staining in distal tubules or collecting ducts.26 The synthesized angiotensinogen in the kidney is secreted into the lumen, leading to angiotensin I generation and subsequent formation of angiotensin II. Renin mRNA and renin-like activity are also present in cultured proximal tubular cells.27-29 In addition low but measurable renin concentrations in proximal tubule fluid have been reported in rats.30 Abundant expression of angiotensin converting enzyme mRNA31 and protein32 have also been shown to be present in brush borders of proximal tubules of human kidneys. Angiotensin-converting enzyme has also been measured in proximal and distal tubular fluid but is more abundant in proximal tubule fluid.33 Thus conditions are present in proximal tubules for angiotensin II generation.
There are two major types of angiotensin II receptor: type 1 (AT1) receptors and type 2 (AT2) receptors. However there is much less AT2 receptor expression in adult kidneys.34,35 It has been reported that AT1 receptor mRNA has been localized to proximal convoluted and straight tubules, thick ascending limbs of the loop of Henle, cortical and medullary collecting duct cells, glomeruli, arterial vasculature, vasa recta, and juxtaglomerular cells.22 In rodents, both subtypes of AT1a receptor and AT1b receptor mRNA have been demonstrated in the vasculature and glomerulus and in all nephron segments.35 The AT1a receptor mRNA is the predominant subtype in nephron segments, whereas the AT1b receptor is more abundant than AT1a receptor in the glomerulus.36 Studies using polyclonal and monoclonal antibodies to the AT1 receptor demonstrated that AT1 receptor protein is on vascular smooth muscle cells throughout the vasculature, including the afferent and efferent arterioles and mesangial cells.37 In addition AT1 receptors are present on proximal tubule brush border and basolateral membranes, thick ascending limb epithelia, distal tubules, collecting ducts, glomerular podocytes, and macula densa cells.34,35,37 These findings suggest that the RAS in the kidney works independently of the systemic RAS.
Regulation of angiotensinogen has been extensively investigated in the liver and summarized in review articles.38,39 For example the hepatic biosynthesis of angiotensinogen is regulated by many different hormonal factors including glucocorticoid, estrogen, thyroid hormone, and insulin.38 However very little is known about intrarenal regulation of angiotensinogen.40 High-salt diet (HS) has been shown to suppress intrarenal expression of angiotensinogen in Sprague-Dawley rats41,42 and Wistar-Kyoto rats (WKY).43 In contrast a paradoxical enhancement of kidney angiotensinogen levels by HS was observed in Dahl salt-sensitive (DS) rats, but not in Dahl salt-resistant (DR) rats.44,45
Angiotensin II-Dependent Hypertension
Angiotensinogen
Angiotensin II, an extensively characterized peptide produced by successive proteolytic cleavages of its prohormone angiotensinogen, plays a critically important role in the regulation of renal hemodynamics and electrolyte homeostasis.46 It is also recognized that the tissue RAS exerts particularly important roles in several pathophysiologic conditions.47 The intrarenal RAS may be particularly significant because all components of RAS coexist in the kidney as described above and influence sodium excretion. Chronic infusion of low doses of angiotensin II provides a useful experimental model of angiotensin II-dependent hypertension and develops in association with progressive enhancement of intrarenal angiotensin II.5
Angiotensin II-infused rats have increases in renal angiotensinogen mRNA26,48 and protein49 and an enhancement of urinary excretion rate of angiotensinogen.50 Chronic angiotensin II infusion to normal rats significantly increased urinary excretion rate of angiotensinogen in a time- and dose-dependent manner. Urinary excretion rate of angiotensinogen was closely correlated with systolic BP and kidney angiotensin II content but not with plasma angiotensin II concentration. Urinary protein excretion in volume-dependent hypertensive rats was significantly increased more than in angiotensin II-dependent hypertensive rats; however urinary angiotensinogen excretion was significantly lower in volume-dependent hypertensive rats than in angiotensin II-dependent hypertensive rats.51 To determine whether circulating angiotensinogen is a source of urinary angiotensinogen, human angiotensinogen was infused in both control and hypertensive rats. Rat angiotensinogen was detected in plasma and urine before and after an acute injection of exogenous human angiotensinogen. Human angiotensinogen was detected only in the plasma collected after the acute administration of human angiotensinogen but was not detected in the urine in angiotensin II-dependent hypertensive or sham-operated normotensive rats. The failure to detect human angiotensinogen in the urine indicates limited glomerular permeability or tubular degradation or both. These findings support the hypothesis that urinary angiotensinogen originates from the angiotensinogen that is formed and secreted by the proximal tubules and not from plasma in rats.51 Moreover it was recently reported that AT1 receptor blockade prevented the enhancement of intrarenal angiotensinogen that occurs in angiotensin II-infused hypertensive rats (Fig. 1). These data suggest that the augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension is dependent on activation of AT1 receptors and that the enhanced urinary excretion rate of angiotensinogen during angiotensin II infusion is blocked by AT1 receptor blockade.52
FIG. 1.
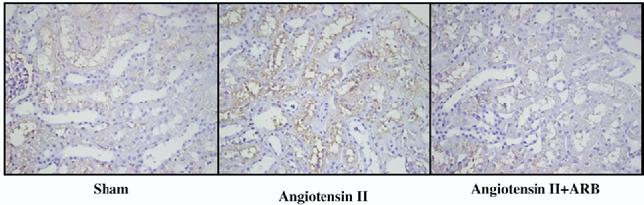
Kidney angiotensinogen immunostaining showed a significant enhancement in angiotensin II-infused rats (center panel) compared with sham-operated rats (left panel). Use of AT1 receptor blockade (ARB) prevented this augmentation (right panel). Kidney angiotensinogen immunohistochemistry was performed as previously described using an automatic robotic system (Dako autostainer) to apply the exactly same condition on all slides.
In angiotensin II-dependent hypertension, AT1 receptor blockade increased plasma angiotensin II concentrations; however it markedly limited the enhanced kidney angiotensin II contents elicited by chronic angiotensin II infusions.52 This dissociation between plasma angiotensin II and intrarenal angiotensin II may suggest a differential regulation of angiotensin II in the kidney and in the circulation. This dissociation between plasma angiotensin II and intrarenal angiotensin II has also been observed in other hypertensive models. In the Page cellophane-wrapped kidney model, it was reported that although the cellophane-wrapped group had progressive increment in BP and angiotensin II content in the kidney, the plasma levels of angiotensin II were similar in the cellophane-wrapped group and the sham-operated animals and were unchanged from baseline.53
Renin
Renin is synthesized primarily by the juxtaglomerular apparatus (JGA).54 However renin mRNA and protein have been detected in proximal and connecting tubules and in collecting duct cells of human, rat, and mouse kidneys as well as in extrarenal tissues.27,28,55,56 Although regulation of renin synthesis and secretion from JGA cells has been extensively studied,54 very little is known about the regulation of tubular renin.29,56,57 Recently it was demonstrated that chronic angiotensin II infusions to normal rats significantly increased renin mRNA and protein levels in principal cells of connecting ducts and collecting tubules.58 Moreover it was reported that this augmentation is dependent on activation of AT1 receptors.59 Although plasma renin activity and JGA renin are markedly suppressed in angiotensin II-induced hypertension, increased distal nephron renin associated with an increased proximal tubular angiotensinogen production and spillover into the distal nephron segments may collectively contribute to elevated and sustained intratubular angiotensin I and angiotensin II formation in this hypertensive model.52,59
Intratubular Renin-Angiotensin System in Hypertension
The above-mentioned experiments established that there is a quantitative relationship between urinary angiotensinogen and intrarenal angiotensinogen or angiotensin II production, and that there is both augmented angiotensinogen and distal nephron renin leading to an increased angiotensin II-mediated sodium reabsorption in distal nephron segments of angiotensin II-infused hypertension.26,49-52,58,59 Recent studies showed that angiotensin II directly stimulates epithelial sodium channel activity in cortical collecting duct cells60 and that there is intraluminal conversion of angiotensin I to angiotensin II in cortical collecting ducts.61 Thus renin in distal nephron segments may synergistically contribute to the angiotensin II-stimulatory effect on distal tubular renin and could help to explain the marked stimulation of sodium reabsorption and suppression of the pressure-natriuresis relationship observed in angiotensin II-infused hypertensive rats.62 Therefore the concomitant increases in proximal tubular angiotensinogen and distal nephron renin may play a crucial role in the sustained high intrarenal angiotensin II levels and hence may contribute to the progressive high BP observed in angiotensin II-dependent hypertension.
Importance of angiotensinogen and renin in the tubular cells to induce systemic hypertension was also reported in a transgenic mouse model.63 Lavoie et al generated mice that express human renin under the control of the kidney-specific androgen-regulated protein promoter, which is androgen responsive. One of the lines expressed the human renin transgene primarily in the kidney. Renal expression of the transgene was undetectable in females but could be induced by testosterone treatment. Because the RAS is species-specific, these investigators bred these human renin-transgenic mice with the mice expressing human angiotensinogen under the same promoter to produce offspring that expressed both transgenes. They measured mean arterial BP in the carotid artery of double-transgenic and control mice using radiotelemetry. Double-transgenic female mice had a normal baseline mean arterial BP, which increased by 15 mm Hg after 2 weeks of testosterone treatment and returned to baseline after discontinuation of the testosterone pellet. The change in arterial pressure paralleled the change in plasma testosterone. There was no mean arterial BP change in testosterone-treated control littermates. The investigators concluded that dual production of angiotensinogen and renin in the renal proximal tubules can result in a systemic increase in arterial pressure. These data support a role for a tissue-specific RAS in the renal proximal tubules that contributes to the regulation of systemic BP.
Salt-Sensitive Hypertension
Clinical studies indicate a clear linkage between salt-sensitive hypertension and a polymorphism of the angiotensinogen gene.64-66 Various epidemiologic studies have showed a correlation of dietary salt intake with the prevalence and progression of hypertension.67 Although the degree of salt sensitivity is variable, some individuals are particularly prone to develop hypertension in response to an increased dietary salt intake. Subjects with essential hypertension have a higher frequency of salt sensitivity than is found in the normotensive population.68 There is some evidence that salt sensitivity is associated with low plasma renin activity and impaired renal sodium excretion. However the mechanisms underlying this phenomenon are poorly understood.69
The DS rats have been used as a model of human salt-sensitive hypertension because salt loading exaggerates the development of hypertension in strains that are genetically predisposed to hypertension.70 Mature DS rats are reported to have low plasma renin activity, which has been interpreted as being indicative of an overall suppression of the RAS70; however few studies of angiotensinogen have been carried out in these rats. Although the animals are generally considered to be characterized by a low activity of circulating RAS, recent studies indicate that treatment with angiotensin-converting enzyme inhibitors or AT1 receptor antagonists reduces cardiac or renal dysfunction or both in DS rats fed HS.71-76 These findings suggest that the local RAS may be inappropriately activated and contribute to the development of hypertension in this animal model.
Recent studies support the concept that there is an inappropriate regulation of intrarenal angiotensinogen in DS rats fed HS. Both DR rats and DS rats were maintained on a HS or low-salt diet (LS). Systolic BP was unaltered in DR rats; however systolic BP was significantly increased in DS rats fed HS compared with DS rats fed LS. The HS suppressed plasma renin activity in both strains. Plasma angiotensinogen levels were also suppressed by HS in both strains. However kidney angiotensinogen levels were significantly increased in DS rats fed HS compared with DS rats fed LS, DR rats fed HS, and DR rats fed LS. These data indicate that DS rats fed HS experience inappropriate and paradoxical augmentation of intrarenal angiotensinogen.44
Recent studies indicate that the inappropriate augmentation of intrarenal angiotensinogen in DS by HS is caused by augmented production of reactive oxygen species. Systolic BP was significantly increased in the DS+HS group compared with the DS+LS group. Treatment with a superoxide dismutase mimetic, Tempol, or treatment with a nonspecific vasodilator, hydralazine, attenuated the hypertension to an equivalent extent. Urinary excretion of thiobarbituric acid-reactive substances, a marker of oxidative stress, was significantly increased in the DS+HS group compared with the DS+LS group. Tempol treatment prevented this effect, but hydralazine treatment only partially prevented the effect. Kidney angiotensinogen levels were significantly increased in the DS+HS group compared with the DS+LS group. Tempol but not hydralazine treatment prevented the intrarenal angiotensinogen augmentation (Fig. 2). The evidence suggests that reactive oxygen species-dependent activation of intrarenal angiotensinogen plays an important role in the development of the hypertension in DS rats fed HS.45
FIG. 2.
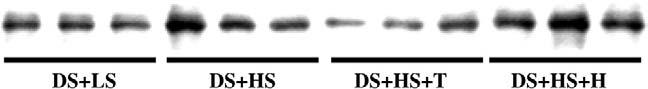
Kidney angiotensinogen protein levels were significantly increased in Dahl salt-sensitive rats (DS) on a high-salt diet (HS) compared with DS on a low-salt diet (LS). Tempol (T) but not hydralazine (H) treatment prevented the intrarenal angiotensinogen augmentation.
Genetic Hypertension
Clinical studies also indicate a clear linkage between genetic hypertension and a polymorphism of the angiotensinogen gene.77,78
Spontaneously hypertensive rats (SHR) have been used as a model of genetic hypertension.79 Although the animals are generally considered to be characterized by a low activity of circulating RAS,80,81 recent studies indicate that treatment with angiotensin-converting enzyme inhibitors or AT1 receptor blockers or both reduces cardiac or renal dysfunction or both of these dysfunctions in SHR,82-84 suggesting that the intrarenal RAS may be inappropriately activated and in turn may contribute to the development of hypertension and hypertension-induced renal damages in this animal model.
A recent study was performed to determine whether augmented intrarenal angiotensinogen may contribute to the enhanced renal angiotensin II and associated tissue injury in SHR. Both SHR and WKY were maintained on a normal diet before being killed at 7 or 14 weeks of age. Two groups of SHR received either an AT1 receptor blocker or a triple therapy, hydralazine, reserpine, and hydrochlorothiazide during weeks 7 through 14. Systolic BP and renal angiotensin II were significantly increased in SHR-14 compared with WKY-7, WKY-14, and SHR-7, and treatment with AT1 receptor blockers prevented these increases. However, although triple therapy prevented the development of hypertension in SHR, this combination therapy failed to decrease renal angiotensin II. Using urine samples or fixed renal sections, the degree of renal injury was quantified using the following parameters: urinary excretion rate of total protein, glomerular sclerosis, interstitial expansion, monocyte/macrophage infiltration in interstitium or glomeruli, and renal arterial proliferation. Angiotensinogen mRNA and protein levels in kidney cortex and all parameters of renal damage were changed in parallel, and AT1 receptor blocker treatment also prevented these increases. However triple therapy failed to prevent these increases (Fig. 3). These results indicate that SHR have enhanced intrarenal angiotensinogen production that contributes to increased angiotensin II levels, leading to the development of hypertension and renal injury in this strain.85
FIG. 3.
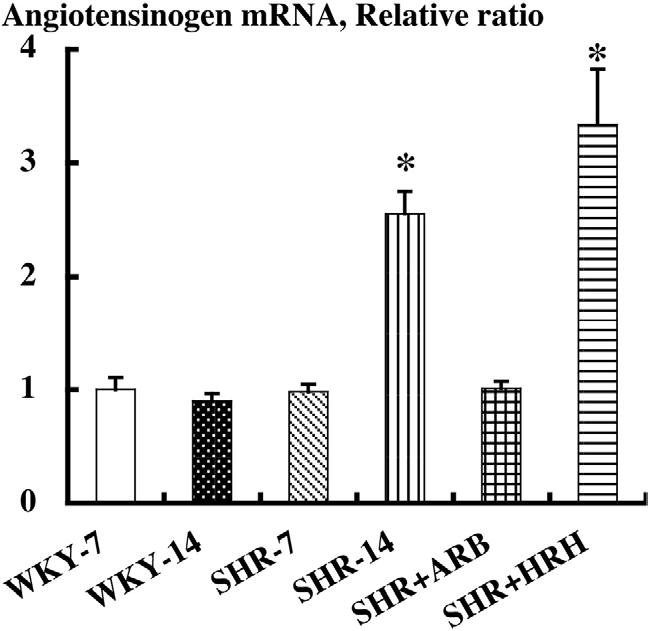
Kidney angiotensinogen mRNA levels were not changed in Wistar-Kyoto rats (WKY) at 7 weeks of age or WKY at 14 weeks of age. However angiotensinogen mRNA levels were significantly increased in spontaneously hypertensive rats (SHR) at 14 weeks of age compared with SHR at 7 weeks of age and the age-matched WKY. Treatment with AT1 receptor blockers (ARB) prevented the augmentation of angiotensinogen mRNA. However, a triple therapy of hydralazine, reserpine, and hydrochlorothiazide (HRH) failed to prevent this augmentation.
Diabetic Nephropathy
Diabetic nephropathy is one of the most common causes of end-stage renal failure in patients starting dialysis in developed countries.86 Clinical trials have demonstrated that the elevated glucose levels are closely associated to the principal cause of renal damage in both type 187 and type 288 diabetes. Until now the detailed mechanisms regarding the sequence of events leading to the development of diabetic nephropathy have remained uncertain.
High glucose induces de novo synthesis of diacylglycerol both in vivo and in vitro.89 Diacylglycerol activates the protein kinase C pathway.90 Activation of protein kinase C is one of the major mechanisms involved in high glucose-induced glomerular injury91 and produces reactive oxygen species and subsequent lipid peroxidation.92-95 High glucose generates reactive oxygen species as a result of glucose auto-oxidation, metabolism, and formation of advanced glycosylation end products.95
Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-β1 expression in rat glomerular cells, and this induction occurs via AT1 receptor-dependent mechanism.96 The cytokine transforming growth factor-β1 is known to be an important mediator of hypertrophy and fibrosis in kidney diseases via multiple pathways such as the G1 phase arrest, cell size enlargement, protein synthesis induction, inhibitory effect on proteinase activity, and extracellular matrix enhancement.97,98
Therefore high glucose-induced reactive oxygen species pathway and intrarenal RAS-dependent transforming growth factor-β1 pathway are shown to play key roles in diabetic nephropathy. Interestingly it was recently demonstrated that high glucose augments angiotensinogen gene expression in proximal tubular cells.99-105 However no in vivo studies have been performed to examine the linkage between reactive oxygen species and angiotensinogen in the kidneys of diabetic animals.
It was recently reported that temporary blockade of the RAS at the prediabetic stage attenuates renal injury in a rat model of type 2 diabetes later in life, suggesting an activated renal RAS in type 2 diabetes.106 The Zucker Diabetic Fatty (ZDF) obese rat, another model of type 2 diabetes, is well known to show progressive nephropathy; however the detailed mechanisms have remained unclear. A study was recently performed to examine the possible involvement of angiotensinogen in diabetic nephropathy of ZDF obese rats. Genetic pairs of male ZDF obese rats and ZDF lean rats were maintained on a diet containing high fat from 12 to 17 weeks of age. At the end of the protocol, ZDF obese rats showed an increased body mass compared with ZDF lean rats. Fasting blood glucose levels were also significantly higher in ZDF obese rats compared with ZDF lean rats. Urinary levels of 8-isoprostane, a marker of oxidative stress, were significantly increased in ZDF obese rats compared with ZDF lean rats. Kidney angiotensinogen protein levels were significantly increased in ZDF obese rats compared with ZDF lean rats. Considering that reactive oxygen species-associated angiotensinogen enhancement plays an important role in renal damage of salt-sensitive hypertension, as described previously here, these data may suggest that reactive oxygen species are partly involved in intrarenal angiotensinogen augmentation, leading to the development of diabetic nephropathy in ZDF obese rats.107
IgA Nephropathy
Clinical and experimental studies have demonstrated that the blockade of the RAS is successful in mitigation and therapy of IgA nephropathy,108 suggesting that the RAS is activated in the development and progression of IgA nephropathy. A clinical study was recently performed to determine whether immunoreactivity of intrarenal angiotensinogen is increased in IgA nephropathy patients. An antibody against human angiotensinogen was raised in a chicken using highly purified angiotensinogen from human plasma. The immunoreactivity of angiotensinogen was then determined by an established, semiautomatic quantification system with an immunohistochemistry robot and a computerized digital image-handling system in renal specimens from 39 patients (18 male and 21 female) with IgA nephropathy. Normal portions of surgically resected kidney served as control (four male and one female). Patients with IgA nephropathy showed higher systolic BP and lower creatinine clearance compared with the control group. The IgA nephropathy patients also showed moderate proteinuria, but the control group did not show any proteinuria. Angiotensinogen was localized pre-dominantly in proximal tubular cells, and the immunoreactivity of intrarenal angiotensinogen in IgA nephropathy was significantly increased compared with normal kidneys (Fig. 4). The IgA immunoreactivity was not correlated with clinical data including BP, creatinine clearance, or urinary protein excretion. Although these IgA nephropathy patients did not show massive renal damage, angiotensinogen immunoreactivity was increased in these patients at this point. These data suggest that the activated intrarenal angiotensinogen plays some roles in the development of IgA nephropathy patients at the early stage and may provide a supportive foundation of the effectiveness of the RAS blockade in IgA nephropathy patients.109
FIG. 4.
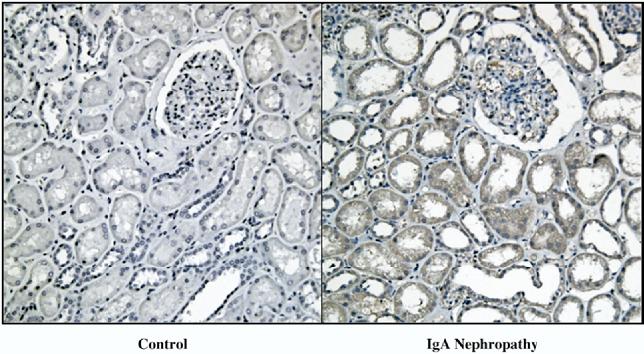
Enhanced intrarenal angiotensinogen immunoreactivity in immunoglobulin-A (IgA) nephropathy patients. Immunohistochemistry robotic system was used to apply a specimen in the exact same condition on each slide. Immunoreactivity of human angiotensinogen was significantly increased in kidneys of IgA nephropathy patients (right panel) compared with kidneys of normal subjects (left panel).
A clear linkage between the intrarenal RAS and IgA nephropathy was also recently reported using in vitro models. It was found that the glomerular AT1 receptor was reduced in IgA nephropathy, whereas there was no change in the expression of glomerular AT2 receptor.110 More recently it was demonstrated that there is constitutive expression of AT1 receptor and AT2 receptor in renal tubules with increased expression in IgA nephropathy.111
Radiation Nephropathy
Clinical radiation nephropathy is an acknowledged complication of blood stem-cell transplantation and internal radionuclide therapies. Excessive renal irradiation leads to progressive renal failure. Fractionated external beam doses of >20 Gy over 4 weeks, single doses of 10 Gy, and internal radionuclide doses of 7 Gy may cause chronic renal failure.112
Antagonists of the RAS are successful in mitigation and therapy of experimental radiation nephropathy.113 An animal model of radiation nephropathy was created in barrier-maintained rats, and angiotensinogen expression was evaluated to search for evidence of activation of the RAS in the animal. Barrier-maintained rats underwent total body irradiation in six equal fractions over 3 days and then underwent transplantation of blood stem cells from a syngeneic littermate. Control rats were not irradiated. Rats were killed on days 1, 22, 41, and 63 after total body irradiation. The apparent increase in angiotensinogen protein abundance occurred at a time when there is little or no renal injury in this model (Fig. 5). It is possible that the increase in angiotensinogen is mechanistically important and is relevant to the benefits of angiotensin-converting enzyme inhibitors or AT1 receptor blockers used in this model.114
FIG. 5.
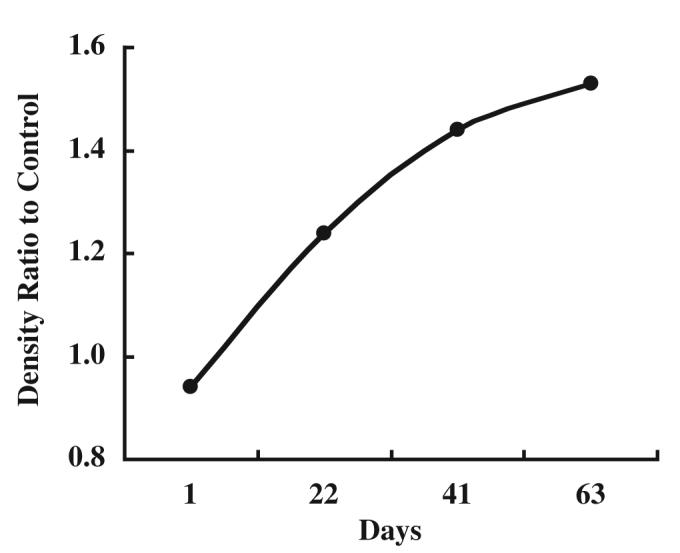
Enhanced intrarenal angiotensinogen protein levels in experimental radiation nephropathy in rats. Western blot analysis indicates that angiotensinogen protein levels significantly increased in a time-dependent manner after total body irradiation.
Human Angiotensinogen ELISA
As described in a previous section, urinary excretion rates of angiotensinogen provide a specific index of intrarenal RAS status in angiotensin II-dependent hypertensive rats.26,49-52 When this is shown to be applicable to human subjects, a diagnostic test to identify those hypertensive patients most likely to respond to blockade of the RAS could provide useful information to allow a mechanistic rationale for selecting an optimized approach to the treatment of hypertensive subjects. However concise and accurate methods to measure human angiotensinogen are unavailable at this time. To perform future human subject studies, two antibodies and a sensitive and specific quantification system using a novel microtiterplate-based sandwich enzyme-linked immunoassay (ELISA) for the measurement of human angiotensinogen have been developed. This ELISA is able to detect human angiotensinogen at range of 0.01 μg/well to 1 μg/well (R2 = 0.9945).115
Conclusion
Enhanced levels of intrarenal angiotensinogen mRNA or protein or both have been observed in multiple models of hypertension as well as in kidney diseases including diabetic nephropathy, IgA nephropathy, and radiation nephropathy.
A series of previous studies imply an augmentation of angiotensinogen by angiotensin II via reactive oxygen species pathways. Griendling et al showed that angiotensin II stimulates production of reactive oxygen species in cultured vascular smooth muscle cells.116 Nishiyama et al. also presented evidence in vivo that angiotensin II enhances oxidative stress in kidneys of rats.117 The association of renal oxidative stress, increased renal angiotensin II activity, and renal inflammation in hypertension has been emphasized recently in a review article.118 Interestingly reactive oxygen species was reported to activate angiotensinogen expression. Hsieh et al found that angiotensinogen gene expression is activated via reactive oxygen species in a proximal tubular cell line.101 In addition Kobori and Nishiyama presented in vivo evidence that reactive oxygen species stimulates angiotensinogen gene expression in kidneys of DS challenged by HS.45 These data support the concept that the enhanced expression of intrarenal angiotensinogen by angiotensin II is mediated via reactive oxygen species pathways.
Urinary angiotensinogen excretion rates show a clear relationship to kidney angiotensin II contents and kidney angiotensinogen levels, suggesting that urinary angiotensinogen may serve as an index of intrarenal RAS status. Interestingly it was recently shown that urinary angiotensinogen is a strong predictor of hypertension in women with low plasma renin and aldosterone; in contrast men did not show this correlation. Higher sodium intake may account, in part, for the lack of a similar relationship in men.119 Establishment of concise and accurate methods to measure human angiotensinogen may provide useful information regarding the roles of intrarenal angiotensinogen in the development and progression of hypertension and kidney diseases.
Acknowledgments
Research in the authors’ laboratories were supported by grants from the National Center for Research Resources (P20RR017659), the National Heart, Lung, and Blood Institute (R01HL026371), the Health Excellence Fund from Louisiana Board of Regents, and Sankyo Co. Ltd. (Tokyo, Japan). The authors acknowledge excellent technical assistance from My-Linh Rauv, Duy V. Tran, Dale M. Seth, and Mark A. Cabrera (Tulane University).
References
- 1.The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 2.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, Godwin J, Qizilbash N, Taylor JO, Hennekens CH. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiologic context. Lancet. 1990;335:827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 3.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Am Med Assoc. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 4.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) J Am Med Assoc. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell KD, Navar LG. Intrarenal actions of angiotensin II in the pathogenesis of experimental hypertension. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management. 2nd ed. Raven Press; New York: 1995. pp. 1437–1450. [Google Scholar]
- 6.Navar LG, Mitchell KD, Harrison-Bernard LM, Kobori H, Nishiyama A. Intrarenal angiotensin II levels in normal and hypertensive states. J Renin-Angiotensin-Aldosterone Syst. 2001;2:S176–S184. doi: 10.1177/14703203010020013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navar LG, Kobori H, Prieto-Carrasquero MC. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep. 2003;5:135–143. doi: 10.1007/s11906-003-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichihara A, Kobori H, Nishiyama A, Navar LG. Renal renin-angiotensin system. Contrib Nephrol. 2004;143:117–130. doi: 10.1159/000078716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res. 1971;5:86–89. doi: 10.1093/cvr/5.1.86. [DOI] [PubMed] [Google Scholar]
- 11.Brasier AR, Li JL. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27:465–475. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 12.Ding Y, Davisson RL, Hardy DO, Zhu LJ, Merrill DC, Catterall JF, Sigmund CD. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem. 1997;272:28142–28148. doi: 10.1074/jbc.272.44.28142. [DOI] [PubMed] [Google Scholar]
- 13.Kimura S, Mullins JJ, Bunnemann B, Metzger R, Hilgenfeldt U, Zimmermann F, Jacob H, Fuxe K, Ganten D, Kaling M. High blood pressure in transgenic mice carrying the rat angiotensinogen gene. EMBO J. 1992;11:821–827. doi: 10.1002/j.1460-2075.1992.tb05119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukamizu A, Sugimura K, Takimoto E, Sugiyama F, Seo MS, Takahashi S, Hatae T, Kajiwara N, Yagami K, Murakami K. Chimeric renin-angiotensin system demonstrates sustained increase in blood pressure of transgenic mice carrying both human renin and human angiotensinogen genes. J Biol Chem. 1993;268:11617–11621. [PubMed] [Google Scholar]
- 15.Bohlender J, Menard J, Ganten D, Luft FC. Angiotensinogen concentrations and renin clearance: implications for blood pressure regulation. Hypertension. 2000;35:780–786. doi: 10.1161/01.hyp.35.3.780. [DOI] [PubMed] [Google Scholar]
- 16.Smithies O. A mouse view of hypertension. Hypertension. 1997;30:1318–1324. doi: 10.1161/01.hyp.30.6.1318. [DOI] [PubMed] [Google Scholar]
- 17.Smithies O, Kim HS. Targeted gene duplication and disruption for analyzing quantitative genetic traits in mice. Proc Natl Acad Sci USA. 1994;91:3612–3615. doi: 10.1073/pnas.91.9.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merrill DC, Thompson MW, Carney CL, Granwehr BP, Schlager G, Robillard JE, Sigmund CD. Chronic hypertension and altered baroreflex responses in transgenic mice containing the human renin and human angiotensinogen genes. J Clin Invest. 1996;97:1047–1055. doi: 10.1172/JCI118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue I, Nakajima T, Williams CS, Quackenbush J, Puryear R, Powers M, Cheng T, Ludwig EH, Sharma AM, Hata A, Jeunemaitre X, Lalouel JM. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest. 1997;99:1786–1797. doi: 10.1172/JCI119343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 21.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43:1251–1259. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 23.Richoux JP, Cordonnier JL, Bouhnik J, Clauser E, Corvol P, Menard J, Grignon G. Immunocytochemical localization of angiotensinogen in rat liver and kidney. Cell Tissue Res. 1983;233:439–451. doi: 10.1007/BF00238309. [DOI] [PubMed] [Google Scholar]
- 24.Darby IA, Congiu M, Fernley RT, Sernia C, Coghlan JP. Cellular and ultrastructural location of angiotensinogen in rat and sheep kidney. Kidney Int. 1994;46:1557–1560. doi: 10.1038/ki.1994.445. [DOI] [PubMed] [Google Scholar]
- 25.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 26.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanagawa N, Capparelli AW, Jo OD, Friedal A, Barrett JD, Eggena P. Production of angiotensinogen and renin-like activity by rabbit proximal tubular cells in culture. Kidney Int. 1991;39:938–941. doi: 10.1038/ki.1991.117. [DOI] [PubMed] [Google Scholar]
- 28.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henrich WL, McAllister EA, Eskue A, Miller T, Moe OW. Renin regulation in cultured proximal tubular cells. Hypertension. 1996;27:1337–1340. doi: 10.1161/01.hyp.27.6.1337. [DOI] [PubMed] [Google Scholar]
- 30.Leyssac PP. Changes in single nephron renin release are mediated by tubular fluid flow rate. Kidney Int. 1986;30:332–339. doi: 10.1038/ki.1986.189. [DOI] [PubMed] [Google Scholar]
- 31.Sibony M, Gasc JM, Soubrier F, Alhenc-Gelas F, Corvol P. Gene expression and tissue localization of the two isoforms of angiotensin I converting enzyme. Hypertension. 1993;21:827–835. doi: 10.1161/01.hyp.21.6.827. [DOI] [PubMed] [Google Scholar]
- 32.Schulz WW, Hagler HK, Buja LM, Erdos EG. Ultrastructural localization of angiotensin I-converting enzyme (EC 3.4.15.1) and neutral metalloendopeptidase (EC 3.4.24.11) in the proximal tubule of the human kidney. Lab Invest. 1988;59:789–797. [PubMed] [Google Scholar]
- 33.Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 34.Wang ZQ, Millatt LJ, Heiderstadt NT, Siragy HM, Johns RA, Carey RM. Differential regulation of renal angiotensin subtype AT1a and AT2 receptor protein in rats with angiotensin-dependent hypertension. Hypertension. 1999;33:96–101. doi: 10.1161/01.hyp.33.1.96. [DOI] [PubMed] [Google Scholar]
- 35.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 36.Bouby N, Hus-Citharel A, Marchetti J, Bankir L, Corvol P, Llorens-Cortes C. Expression of type 1 angiotensin II receptor subtypes and angiotensin II-induced calcium mobilization along the rat nephron. J Am Soc Nephrol. 1997;8:1658–1667. doi: 10.1681/ASN.V8111658. [DOI] [PubMed] [Google Scholar]
- 37.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, El-Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol. 1997;273:F170–F177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 38.Clauser E, Gaillard I, Wei L, Corvol P. Regulation of angiotensinogen gene. Am J Hypertens. 1989;2:403–410. doi: 10.1093/ajh/2.5.403. [DOI] [PubMed] [Google Scholar]
- 39.Tewksbury D. Angiotensinogen. In: Fray JCS, editor. The Endocrine System: Endocrine Regulation of Water and Electrolyte Balance: Handbook of Physiology. 1st ed. Oxford University Press; Oxford: 2000. pp. 59–80. [Google Scholar]
- 40.Ingelfinger JR, Schunkert H, Ellison KE, Pivor M, Zuo WM, Pratt R, Dzau VJ. Intrarenal angiotensinogen: localization and regulation. Pediatr Nephrol. 1990;4:424–428. doi: 10.1007/BF00862530. [DOI] [PubMed] [Google Scholar]
- 41.Sechi LA, Griffin CA, Giacchetti G, Valentin JP, Llorens-Cortes C, Corvol P, Schambelan M. Tissue-specific regulation of type 1 angiotensin II receptor mRNA levels in the rat. Hypertension. 1996;28:403–408. doi: 10.1161/01.hyp.28.3.403. [DOI] [PubMed] [Google Scholar]
- 42.Singh I, Grams M, Wang WH, Yang T, Killen P, Smart A, Schnermann J, Briggs JP. Coordinate regulation of renal expression of nitric oxide synthase, renin, and angiotensinogen mRNA by dietary salt. Am J Physiol. 1996;270:F1027–F1037. doi: 10.1152/ajprenal.1996.270.6.F1027. [DOI] [PubMed] [Google Scholar]
- 43.Ingelfinger JR, Pratt RE, Ellison K, Dzau VJ. Sodium regulation of angiotensinogen mRNA expression in rat kidney cortex and medulla. J Clin Invest. 1986;78:1311–1315. doi: 10.1172/JCI112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobori H, Nishiyama A. Effects of Tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem Biophys Res Commun. 2004;315:746–750. doi: 10.1016/j.bbrc.2004.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navar LG. The kidney in blood pressure regulation and development of hypertension. Med Clin North Am. 1997;81:1165–1198. doi: 10.1016/s0025-7125(05)70573-3. [DOI] [PubMed] [Google Scholar]
- 47.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation. 1994;89:493–498. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- 48.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol. 1992;263:E863–E869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 49.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanegas V, Ferrebuz A, Quiroz Y, Rodriguez-Iturbe B. Hypertension in Page (cellophane-wrapped) kidney is caused by interstitial nephritis. Kidney Int. 2005;68:1161–1170. doi: 10.1111/j.1523-1755.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 54.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 55.Deschepper CF, Mellon SH, Cumin F, Baxter JD, Ganong WF. Analysis by immunocytochemistry and in situ hybridization of renin and its mRNA in kidney, testis, adrenal, and pituitary of the rat. Proc Natl Acad Sci USA. 1986;83:7552–7556. doi: 10.1073/pnas.83.19.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 57.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM. Effects of dietary sodium and genetic background on angiotensinogen and renin in mouse. Hypertension. 2002;39:1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 58.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 61.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 62.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 63.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol Renal Physiol. 2004;286:F965–F971. doi: 10.1152/ajprenal.00402.2003. [DOI] [PubMed] [Google Scholar]
- 64.Kamitani A, Rakugi H, Higaki J, Yi Z, Mikami H, Miki T, Ogihara T. Association analysis of a polymorphism of the angiotensinogen gene with essential hypertension in japanese. J Hum Hypertens. 1994;8:521–524. [PubMed] [Google Scholar]
- 65.Ishikawa K, Baba S, Katsuya T, Iwai N, Asai T, Fukuda M, Takiuchi S, Fu Y, Mannami T, Ogata J, Higaki J, Ogihara T. T+31c polymorphism of angiotensinogen gene and essential hypertension. Hypertension. 2001;37:281–285. doi: 10.1161/01.hyp.37.2.281. [DOI] [PubMed] [Google Scholar]
- 66.Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26:521–525. doi: 10.1291/hypres.26.521. [DOI] [PubMed] [Google Scholar]
- 67.Haddy FJ, Pamnani MB. Role of dietary salt in hypertension. J Am Coll Nutr. 1995;14:428–438. doi: 10.1080/07315724.1995.10718533. [DOI] [PubMed] [Google Scholar]
- 68.Luke RG. Essential hypertension: a renal disease? A review and update of the evidence. Hypertension. 1993;21:380–390. doi: 10.1161/01.hyp.21.3.380. [DOI] [PubMed] [Google Scholar]
- 69.Weinberger MH. Sodium sensitivity of blood pressure. Curr Opin Nephrol Hypertens. 1993;2:935–939. doi: 10.1097/00041552-199311000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Iwai J, Dahl LK, Knudsen KD. Genetic influence on the renin-angiotensin system: low renin activities in hypertension-prone rats. Circ Res. 1973;32:678–684. doi: 10.1161/01.res.32.6.678. [DOI] [PubMed] [Google Scholar]
- 71.Sakata Y, Masuyama T, Yamamoto K, Doi R, Mano T, Kuzuya T, Miwa T, Takeda H, Hori M. Renin angiotensin system-dependent hypertrophy as a contributor to heart failure in hypertensive rats: different characteristics from renin angiotensin system-independent hypertrophy. J Am Coll Cardiol. 2001;37:293–299. doi: 10.1016/s0735-1097(00)01064-0. [DOI] [PubMed] [Google Scholar]
- 72.Hayashida W, Kihara Y, Yasaka A, Inagaki K, Iwanaga Y, Sasayama S. Stage-specific differential activation of mitogen-activated protein kinases in hypertrophied and failing rat hearts. J Mol Cell Cardiol. 2001;33:733–744. doi: 10.1006/jmcc.2001.1341. [DOI] [PubMed] [Google Scholar]
- 73.Kodama K, Adachi H, Sonoda J. Beneficial effects of long-term enalapril treatment and low-salt intake on survival rate of Dahl salt-sensitive rats with established hypertension. J Pharmacol Exp Ther. 1997;283:625–629. [PubMed] [Google Scholar]
- 74.Otsuka F, Yamauchi T, Kataoka H, Mimura Y, Ogura T, Makino H. Effects of chronic inhibition of ACE and AT1 receptors on glomerular injury in Dahl salt-sensitive rats. Am J Physiol. 1998;274:R1797–R1806. doi: 10.1152/ajpregu.1998.274.6.R1797. [DOI] [PubMed] [Google Scholar]
- 75.Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation. 1997;96:2407–2413. doi: 10.1161/01.cir.96.7.2407. [DOI] [PubMed] [Google Scholar]
- 76.Nishikimi T, Mori Y, Kobayashi N, Tadokoro K, Wang X, Akimoto K, Yoshihara F, Kangawa K, Matsuoka H. Renoprotective effect of chronic adrenomedullin infusion in Dahl salt-sensitive rats. Hypertension. 2002;39:1077–1082. doi: 10.1161/01.hyp.0000018910.74377.93. [DOI] [PubMed] [Google Scholar]
- 77.Rudnichi A, Safar ME, Lajemi M, Benetos A. Gene polymorphisms of the renin-angiotensin system and age-related changes in systolic and diastolic blood pressure in subjects with hypertension. Am J Hypertens. 2004;17:321–327. doi: 10.1016/j.amjhyper.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 78.Nakajima T, Wooding S, Sakagami T, Emi M, Tokunaga K, Tamiya G, Ishigami T, Umemura S, Munkhbat B, Jin F, Guan-Jun J, Hayasaka I, Ishida T, Saitou N, Pavelka K, Lalouel JM, Jorde LB, Inoue I. Natural selection and population history in the human angiotensinogen gene (AGT): 736 complete AGT sequences in chromosomes from around the world. Am J Hum Genet. 2004;74:898–916. doi: 10.1086/420793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okamoto K, Tabei R, Fukushima M, Nosaka S, Yamori Y. Further observations of the development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1966;30:703–716. doi: 10.1253/jcj.30.703. [DOI] [PubMed] [Google Scholar]
- 80.Vincent M, Dupont J, Sassard J. Plasma renin activity as a function of age in two new strains of spontaneously hypertensive and normotensive rats. Clin Sci Mol Med. 1976;50:103–107. doi: 10.1042/cs0500103. [DOI] [PubMed] [Google Scholar]
- 81.Kuriyama S, Kawashima K, Sokabe H. Plasma renin activity determined by two different methods in spontaneously hypertensive rats. Jpn Heart J. 1982;23:587–592. doi: 10.1536/ihj.23.587. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura Y, Ono H, Zhou X, Frohlich ED. Angiotensin type 1 receptor antagonism and ACE inhibition produce similar renoprotection in N(omega)-nitro-l-arginine methyl ester/spontaneously hypertensive rats. Hypertension. 2001;37:1262–1267. doi: 10.1161/01.hyp.37.5.1262. [DOI] [PubMed] [Google Scholar]
- 83.Teng J, Fukuda N, Suzuki R, Takagi H, Ikeda Y, Tahira Y, Kanmatsuse K. Inhibitory effect of a novel angiotensin II type 1 receptor antagonist RNH-6270 on growth of vascular smooth muscle cells from spontaneously hypertensive rats: different anti-proliferative effect to angiotensin-converting enzyme inhibitor. J Cardiovasc Pharmacol. 2002;39:161–171. doi: 10.1097/00005344-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 84.Pu Q, Larouche I, Schiffrin EL. Effect of dual angiotensin converting enzyme/neutral endopeptidase inhibition, angiotensin converting enzyme inhibition, or AT1 antagonism on coronary microvasculature in spontaneously hypertensive rats. Am J Hypertens. 2003;16:931–937. doi: 10.1016/s0895-7061(03)01029-x. [DOI] [PubMed] [Google Scholar]
- 85.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joss N, Paterson KR, Deighan CJ, Simpson K, Boulton-Jones JM. Diabetic nephropathy: how effective is treatment in clinical practice? Q J Med. 2002;95:41–49. doi: 10.1093/qjmed/95.1.41. [DOI] [PubMed] [Google Scholar]
- 87.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 88.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 89.Haneda M, Koya D, Kikkawa R. Cellular mechanisms in the development and progression of diabetic nephropathy: activation of the DAG-PKC-ERK pathway. Am J Kidney Dis. 2001;38:S178–S181. doi: 10.1053/ajkd.2001.27438. [DOI] [PubMed] [Google Scholar]
- 90.Kikkawa R, Koya D, Haneda M. Progression of diabetic nephropathy. Am J Kidney Dis. 2003;41:S19–S21. doi: 10.1053/ajkd.2003.50077. [DOI] [PubMed] [Google Scholar]
- 91.Park JY, Ha SW, King GL. The role of protein kinase C activation in the pathogenesis of diabetic vascular complications. Peritoneal Dial Int. 1999;19(Suppl 2):S222–227. [PubMed] [Google Scholar]
- 92.Shah SV. Light emission by isolated rat glomeruli in response to phorbol myristate acetate. J Lab Clin Med. 1981;98:46–57. [PubMed] [Google Scholar]
- 93.Miyanoshita A, Takahashi T, Endou H. Inhibitory effect of cyclic AMP on phorbol ester-stimulated production of reactive oxygen metabolites in rat glomeruli. Biochem Biophys Res Commun. 1989;165:519–525. doi: 10.1016/0006-291x(89)91100-5. [DOI] [PubMed] [Google Scholar]
- 94.Ha H, Endou H. Lipid peroxidation in isolated rat nephron segments. Am J Physiol. 1992;263:F201–F207. doi: 10.1152/ajprenal.1992.263.2.F201. [DOI] [PubMed] [Google Scholar]
- 95.Ha H, Lee HB. Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int. 2000;77(Suppl):S19–S25. doi: 10.1046/j.1523-1755.2000.07704.x. [DOI] [PubMed] [Google Scholar]
- 96.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Border WA, Noble NA. Cytokines in kidney disease: the role of transforming growth factor-beta. Am J Kidney Dis. 1993;22:105–113. doi: 10.1016/s0272-6386(12)70175-0. [DOI] [PubMed] [Google Scholar]
- 99.Zhang SL, Filep JG, Hohman TC, Tang SS, Ingelfinger JR, Chan JS. Molecular mechanisms of glucose action on angiotensinogen gene expression in rat proximal tubular cells. Kidney Int. 1999;55:454–464. doi: 10.1046/j.1523-1755.1999.00271.x. [DOI] [PubMed] [Google Scholar]
- 100.Zhang SL, Tang SS, Chen X, Filep JG, Ingelfinger JR, Chan JS. High levels of glucose stimulate angiotensinogen gene expression via the p38 mitogen-activated protein kinase pathway in rat kidney proximal tubular cells. Endocrinology. 2000;141:4637–4646. doi: 10.1210/endo.141.12.7844. [DOI] [PubMed] [Google Scholar]
- 101.Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology. 2002;143:2975–2985. doi: 10.1210/endo.143.8.8931. [DOI] [PubMed] [Google Scholar]
- 102.Zhang SL, To C, Chen X, Filep JG, Tang SS, Ingelfinger JR, Chan JS. Essential role(s) of the intrarenal renin-angiotensin system in transforming growth factor-beta1 gene expression and induction of hypertrophy of rat kidney proximal tubular cells in high glucose. J Am Soc Nephrol. 2002;13:302–312. doi: 10.1681/ASN.V132302. [DOI] [PubMed] [Google Scholar]
- 103.Zhang SL, Chen X, Hsieh TJ, Leclerc M, Henley N, Allidina A, Halle JP, Brunette MG, Filep JG, Tang SS, Ingelfinger JR, Chan JS. Hyperglycemia induces insulin resistance on angiotensinogen gene expression in diabetic rat kidney proximal tubular cells. J Endocrinol. 2002;172:333–344. doi: 10.1677/joe.0.1720333. [DOI] [PubMed] [Google Scholar]
- 104.Zhang SL, Chen X, Wei CC, Filep JG, Tang SS, Ingelfinger JR, Chan JS. Insulin inhibits dexamethasone effect on angiotensinogen gene expression and induction of hypertrophy in rat kidney proximal tubular cells in high glucose. Endocrinology. 2002;143:4627–4635. doi: 10.1210/en.2002-220408. [DOI] [PubMed] [Google Scholar]
- 105.Hsieh TJ, Fustier P, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Fantus IG, Hamet P, Chan JS. High glucose stimulates angiotensinogen gene expression and cell hypertrophy via activation of the hexosamine biosynthesis pathway in rat kidney proximal tubular cells. Endocrinology. 2003;144:4338–4349. doi: 10.1210/en.2003-0220. [DOI] [PubMed] [Google Scholar]
- 106.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, Shokoji T, Kimura S, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Temporary angiotensin blockade at the prediabetic stage attenuates the development of type 2 diabetic nephropathy. J Am Soc Nephrol. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suzaki Y, Kobori H, Ozawa Y. Intrarenal angiotensinogen augmentation is precedent to diabetic nephropathy in Zucker diabetic fatty obese rats (abstract) J Am Soc Nephrol. 2005;16:202A. [Google Scholar]
- 108.Dillon JJ. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for IgA nephropathy. Semin Nephrol. 2004;24:218–224. doi: 10.1016/j.semnephrol.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 109.Kobori H, Ozawa Y, Suzaki Y, Shoji T. Enhanced intrarenal angiotensinogen in IgA nephropathy patients (abstract) J Am Soc Nephrol. 2005;16:521A. [Google Scholar]
- 110.Lai KN, Chan LY, Tang SC, Tsang AW, Li FF, Lam MF, Lui SL, Leung JC. Mesangial expression of angiotensin II receptor in IgA nephropathy and its regulation by polymeric IgA1. Kidney Int. 2004;66:1403–1416. doi: 10.1111/j.1523-1755.2004.00874.x. [DOI] [PubMed] [Google Scholar]
- 111.Chan LY, Leung JC, Tang SC, Choy CB, Lai KN. Tubular expression of angiotensin II receptors and their regulation in IgA nephropathy. J Am Soc Nephrol. 2005;16:2306–2317. doi: 10.1681/ASN.2004121117. [DOI] [PubMed] [Google Scholar]
- 112.Moulder JE, Fish BL, Cohen EP. ACE inhibitors and AII receptor antagonists in the treatment and prevention of bone marrow transplant nephropathy. Curr Pharm Des. 2003;9:737–749. doi: 10.2174/1381612033455422. [DOI] [PubMed] [Google Scholar]
- 113.Cohen EP, Fish BL, Moulder JE. The renin-angiotensin system in experimental radiation nephropathy. J Lab Clin Med. 2002;139:251–257. doi: 10.1067/mlc.2002.122279. [DOI] [PubMed] [Google Scholar]
- 114.Cohen EP, Kobori H, Moulder JE, Fish BL, Navar LG. Intrarenal angiotensinogen in radiation nephropathy (abstract) J Invest Med. 2005;53:S288. [Google Scholar]
- 115.Suzaki Y, Kobori H, Ozawa Y, Navar LG. Quantification of human angiotensinogen by a novel sandwich ELISA (abstract) Hypertension. doi: 10.1016/j.peptides.2006.05.006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 117.Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, Abe Y. Systemic and regional hemodynamic responses to Tempol in angiotensin II-infused hypertensive rats. Hypertension. 2001;37:77–83. doi: 10.1161/01.hyp.37.1.77. [DOI] [PubMed] [Google Scholar]
- 118.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol. 2004;286:F606–F616. doi: 10.1152/ajprenal.00269.2003. [DOI] [PubMed] [Google Scholar]
- 119.Lantelme P, Rohrwasser A, Vincent M, Cheng T, Gardier S, Legedz L, Bricca G, Lalouel JM, Milon H. Significance of urinary angiotensinogen in essential hypertension as a function of plasma renin and aldosterone status. J Hypertens. 2005;23:785–792. doi: 10.1097/01.hjh.0000163147.20330.f5. [DOI] [PubMed] [Google Scholar]


