Abstract
(–)-Epicatechin gallate (ECg), a component of green tea, sensitizes meticillin-resistant Staphylococcus aureus (MRSA) to β-lactam antibiotics, promotes staphylococcal cell aggregation and increases cell-wall thickness. The potentiation of β-lactam activity against MRSA by ECg was not due to decreased bacterial penicillin-binding protein (PBP) 2a expression or ECg binding to peptidoglycan. A 5-10 % reduction in peptidoglycan cross-linking was observed. Reduced cross-linking was insufficient to compromise the integrity of the cell wall and no evidence of PBP2a activity was detected in the muropeptide composition of ECg-grown cells. ECg increased the quantity of autolysins associated with the cell wall, even though the cells were less susceptible to Triton X-100-induced autolysis than cells grown in the absence of ECg. ECg promoted increased lysostaphin resistance that was not due to alteration of the pentaglycine cross-bridge configuration or inhibition of lysostaphin activity. Rather, decreased lysostaphin susceptibility was associated with structural changes to wall teichoic acid (WTA), an acid-labile component of peptidoglycan. ECg also promoted lipoteichoic acid (LTA) release from the cytoplasmic membrane. It is proposed that ECg reduces β-lactam resistance in MRSA either by binding to PBPs at sites distinct from the penicillin-binding site or by intercalation into the cytoplasmic membrane, displacing LTA from the phospholipid palisade. Thus, ECg-mediated alterations to the physical nature of the bilayer will elicit structural changes to WTA that result in modulation of the cell-surface properties necessary to maintain the β-lactam-resistant phenotype.
INTRODUCTION
Isolates of meticillin-resistant Staphylococcus aureus (MRSA) are resistant to β-lactam antibiotics due to the production of a low-β-lactam-affinity penicillin-binding protein (PBP), PBP2a, encoded by the mecA determinant. This gene was acquired by S. aureus strains from an unknown bacterial source prior to the emergence of MRSA in clinical samples (Crisóstomo et al., 2001). The capacity of S. aureus to acquire genes that confer antibiotic resistance, combined with the mutation of host genes, has facilitated the evolution of a multi-resistant pathogen for which treatment options can be severely limited. Until recently, the glycopeptide antibiotics vancomycin and teicoplanin were universally active against MRSA, but the isolation of vancomycin-intermediate-resistant S. aureus (VISA) (Hiramatsu et al., 1997) and fully vancomycin-resistant S. aureus (VRSA) isolates (Centers for Disease Control, 2002) serves to illustrate the need to maintain the search for new antimicrobial agents and to find new treatment strategies (Appelbaum, 2006). Although the utility of many, if not all, antibiotics is eroded over time due to the emergence of resistance, an opportunity exists to restore their efficacy by co-administration of compounds that compromise determinants of antibiotic resistance (Taylor et al., 2002). For example, the combination of β-lactam antibiotics and β-lactamase inhibitors, such as amoxicillin-clavulanate formulations, have for two decades proven to be remarkably effective in controlling a wide range of bacterial infections (Miller et al., 2001).
The galloylated catechins (–)-epicatechin gallate (ECg) and (–)-epigallocatechin gallate (EGCg) are structurally related constituents (Fig. 1a) of green tea (Camellia sinensis) that share a capacity to reduce PBP2a-mediated oxacillin resistance in S. aureus, rendering genotypically resistant strains susceptible to β-lactam antibiotic action (Shiota et al., 1999; Zhao et al., 2001; Stapleton et al., 2004). Non-galloylated catechins, such as (–)-epicatechin (EC), are unable to modulate β-lactam resistance but act synergistically with ECg, lowering the concentration of ECg required to eliminate β-lactam resistance in S. aureus (Stapleton et al., 2006). The process by which galloylated catechins reduce β-lactam resistance has not been elucidated. A 2% (w/v) green tea extract has been reported to reduce the expression by a MRSA isolate of PBP1, PBP2a, and to some extent PBP3 (Yam et al., 1998). However, the ability to detect the presence of PBP2a in S. aureus isolates grown in the presence of EGCg suggests that a reduction in expression of PBP2a may not account for the observed changes in β-lactam susceptibility (Zhao et al., 2001). Furthermore, the capacity of EGCg to reduce oxacillin resistance in a S. aureus isolate has been reported to be compromised by the addition of peptidoglycan to the growth medium (Zhao et al., 2001); these authors proposed that EGCg blocks PBP substrate access by binding to peptidoglycan. If steric hindrance by peptidoglycan were solely responsible, it would seem likely that the activity of all PBPs would be similarly affected and the MICs of the compounds would be low. In fact, the MICs for galloylated catechins, in the absence of a β-lactam antibiotic, are high (64-256 μg ml-1) and are similar to those for non-galloylated catechins (256-512 μg ml-1) (Stapleton et al., 2004). In addition, several studies have highlighted the interaction between catechins and lipid bilayers (Hashimoto et al., 1999; Kajiya et al., 2001, 2002; Caturla et al., 2003). Indeed, the capacity of catechins to reduce β-lactam resistance closely correlates with the degree of penetration of the molecule into membrane bilayers (Stapleton et al., 2006).
Fig. 1.
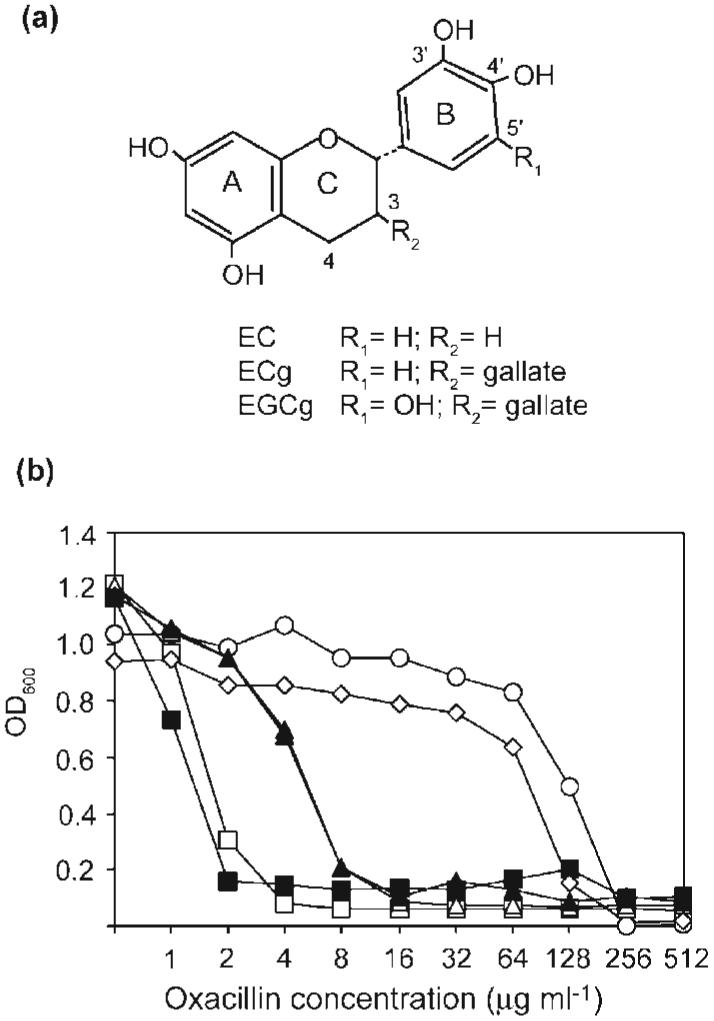
Influence of exogenous peptidoglycan on modulation of oxacillin susceptibility by ECg and EGCg. (a) Structures of the compounds used in this study. (b) Effect of exogenous peptidoglycan (32 μg ml-1; black symbols) on the capacity of ECg and EGCg to modulate oxacillin resistance in S. aureus BB568. Cells were grown in the absence (○) or presence of 12.5 μg EC ml-1 (◇), 12.5 μg ECg ml-1 (□, ■) or 12.5 μg EGCg ml-1 (△, ▲). White symbols indicate growth in the absence of exogenous peptidoglycan.
In addition to facilitating a reduction in the β-lactam resistance of S. aureus, galloylated catechins promote cell-wall thickening and cell aggregation (Hamilton-Miller & Shah, 1999), reduce slime production and inhibit the formation of biofilms in staphylococcal cell cultures (Blanco et al., 2005). In order to gain insight into the molecular basis of these phenomena, we have investigated the effect of ECg on cell-wall and cell-surface properties of S. aureus strains.
METHODS
Bacterial strains and reagents
S. aureus BB568 (a homogeneous meticillin-resistant strain that constitutively expresses PBP2a) and S. aureus BB551 (a meticillin-susceptible isolate) were kindly provided by B. Berger-Bächi, Institute of Medical Microbiology, University of Zürich, Switzerland. The epidemic MRSA isolates EMRSA-15 and EMRSA-16 were from clinical samples obtained at the Royal Free Hospital, London and were provided by J. M. T. Hamilton-Miller. ECg, EGCg and EC were obtained from Mitsui Norin. Oxacillin and Triton X-100 were purchased from Sigma-Aldrich; [2-3H]glycerol was purchased from Amersham Biosciences. Bacteria were grown in Mueller-Hinton (MH) broth (Oxoid). The MICs for EC, ECg and EGCg against the MRSA isolates used in this study were 256, 256 and 64 μg ml-1 respectively (Stapleton et al., 2004).
Preparation of purified cell-wall extracts
Peptidoglycan was extracted and purified according to the procedures described by Stranden et al. (1997), with the exception that strains were grown in MH broth.
HPLC analysis of peptidoglycan
Preparation and analysis were performed using the procedures of Stranden et al. (1997) and Roos et al. (1998). Amino acid composition was determined by Alta Bioscience.
Measurement of phosphorus content of purified cell walls
Total phosphorus was determined by the procedure of Fiske & SubbaRow as described by Leloir & Cardini (1957).
Effect of exogenous peptidoglycan on catechin activity
The capacity of ECg and EGCg to reduce oxacillin resistance in the presence of exogenous peptidoglycan was measured using the method of Zhao et al. (2001).
Effect of ECg on adherence of MRSA to glass
Cultures were grown in glass conical flasks in the presence or absence of 12.5 μg ECg ml-1 without shaking at 37 °C. Adherence was determined by visual inspection of the flasks.
Membrane extraction and PBP profiles
Cytoplasmic membrane fractions were prepared from late exponential phase cultures (OD600 0.8-0.9). Cells were recovered by centrifugation (13 000 g, 10 min), washed once in phosphate-buffered saline and suspended in 1 ml ice-cold water. The cells were disrupted with glass beads (0.1 mm) using a FastPrep cell disruption system (40 s, power setting 6) and the cell-wall debris removed by centrifugation (13 000 g, 10 min). To isolate the cytoplasmic membrane fraction, the supernatant was subjected to ultracentrifugation (Beckman Optima MAX ultracentrifuge; 130 000 g, 1 h, 4 °C), and the pellet suspended in 10 mM Tris/HCl, pH 7 containing 2% (v/v) Triton X-100. The protein content of the samples was quantified using a NanoOrange Protein Quantification kit (Invitrogen) in accordance with the manufacturer’s instructions.
PBPs in the membrane preparations were selectively labelled by pre-incubation of the samples (10 μg protein) with 25 μM Bocillin FL (Invitrogen) for 10 min at 37 °C. The proteins in the sample were separated by SDS-PAGE (SDS 10 %, w/v) and the PBPs detected by fluorography (Bio-Rad Molecular Imager FX; excitation 488 nm, emission 530 nm). To detect PBP2a by fluorography the sequential binding assay described by Hartman & Tomasz (1984) was used: meticillin (600 μg ml-1), which has low affinity for PBP2a, was pre-incubated with the sample for 10 min prior to incubation with bocillin. To evaluate its PBP-inhibiting potential, 25 μg ECg ml-1 was pre-incubated with the sample for 10 min prior to bocillin labelling.
PBP2a detection
Membrane protein preparations (10 μg) were separated by SDS-PAGE (SDS 10%, w/v) and transferred onto an Immobilon PVDF membrane (Millipore) by electroblotting (Trans Blot Cell, Bio-Rad). PBP2a was specifically labelled with a mouse anti-PBP2a antibody (Oxoid PBP2a latex agglutination kit; 1 : 10 000 dilution), and detected with a second anti-mouse IgG peroxidase-conjugated antibody (Sigma-Aldrich; 1 : 8000 dilution) using 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich) as substrate.
Lysostaphin MIC
The lowest concentration of lysostaphin to inhibit the growth of S. aureus was determined in a 96-well microtitre tray (100 μl MH broth per well) with an inoculum of approx. 104 c.f.u. The trays were incubated at 35 °C for 24 h.
Lysostaphin and mutanolysin hydrolysis assays
Purified peptidoglycan was suspended (OD600 0.5) in 50 mM Tris/HCl (pH 7.5) containing 145 mM NaCl (lysostaphin assay) or 25 mM phosphate buffer, pH 5.5 (mutanolysin assay). Peptidoglycan hydrolysis was monitored by following the decrease in OD600 of the samples incubated at 37 °C with either 4 U lysostaphin (Sigma-Aldrich) or 12 U mutanolysin. To remove teichoic acid from the samples, peptidoglycan was suspended in 10 % (v/v) TCA for 24 h at 4 °C. Peptidoglycan was recovered by centrifugation and washed several times with water before the hydrolysis assays were performed.
Bacteriolytic assay of autolysins in culture medium supernatants
The bacteriolytic assay was performed using a modification of the technique described by Fournier & Hooper (2000); 16 h cultures (37 °C, no shaking), grown in the presence and absence of ECg, were recovered by centrifugation (10 000 g, 10 min), and the supernatants filtered (0.2 μm). Supernatants were mixed with 0.1 M phosphate buffer (pH 7.0) containing heat-killed S. aureus BB568 cells (10 mg dry weight cells μl-1) and the cell suspensions incubated at 37 °C with shaking (200 r.p.m.). Bacteriolytic enzyme activity was assessed by measurement of OD600 of the cell suspensions at hourly intervals.
Triton X-100-induced autolytic assay
This assay was performed as described by de Jonge et al. (1991). Briefly, S. aureus isolates were grown to an OD600 0.3 in the presence and absence of 25 μg ECg ml-1. The cultures were chilled on ice and cells recovered by centrifugation (10 000 g, 4 °C, 10 min). Cell pellets were washed once with ice-cold water and cells suspended (OD600 1.0) in assay buffer (50 mM glycine, 0.01 %, v/v, Triton X-100, pH 8.0). Autolysis was monitored at 30 min intervals by measuring the OD600 of the cultures incubated at 37 °C with shaking (200 r.p.m.).
Preparation of autolysin extracts and bacteriolytic profiles
Cell-wall autolysin extracts were prepared from cultures grown to OD600 0.7-0.8. Cells were harvested by centrifugation, washed once with phosphate-buffered saline and autolysins extracted with 4 % (w/v) SDS for 30 min at room temperature. Cells were removed from the extracts by centrifugation. To extract autolysins from the culture medium, supernatants from 16 h cultures (37 °C, no shaking), grown in the presence or absence of 25 μg ECg ml-1, were recovered by centrifugation (10 000 g, 10 min) and concentrated 30-fold using a Centriplus YM-10 filter (Millipore; <10 kDa).
Proteins (10 μg per sample) were separated by 10 % (w/v) SDS-PAGE on gels containing heat-killed S. aureus BB568 cells (0.1 %, w/v). SDS was removed by soaking the gel in water and the lytic enzyme profile developed by incubating gels in 0.1 M phosphate buffer (pH 7.0) at 37 °C for 16 h. The profile was visualized by staining the gel with 1 % (w/v) methylene blue in 0.01 % (w/v) KOH, followed by destaining in water.
Lipoteichoic acid (LTA) release
LTA release was determined as described by Suzuki et al. (1997).
RESULTS
Capacity of ECg or EGCg to reduce oxacillin resistance is not affected by exogenous peptidoglycan
The MIC of oxacillin against BB568 in the absence of galloylated catechins was determined to be 256 μg ml-1 (Fig. 1). The non-galloylated catechin EC had no affect on the inhibitory action of oxacillin against BB568. However, 12.5 μg ECg ml-1 and 12.5 μg EGCg ml-1 reduced the oxacillin MIC to 4 and 16 μg ml-1 respectively. Exogenous peptidoglycan had no effect on the capacity of the non-galloylated compounds to reduce the oxacillin MICs against BB568 (Fig. 1) and against EMRSA-15 and EMRSA-16 (data not shown). As previously reported (Stapleton et al., 2004), ECg reduced the oxacillin MICs of 40 MRSA clinical isolates to a comparable extent. As previously reported (Stapleton et al., 2004), ECg reduced the oxacillin MICs of 40 MRSA clinical isolates to a comparable extent.
ECg promotes cell aggregation and reduces adherence to glass
ECg (Fig. 2c), but not EC (Fig. 2b), promoted cell aggregate formation in EMRSA-15, indicating that the galloyl group, found in ECg but not EC, may play a role in determining this activity. Gallic acid did not promote cell aggregation (data not shown), indicating that the gallate moiety alone did not mediate the effect. Cells grown in the presence of ECg failed to adhere to the surface of the glass flask and formed cell aggregates within the growth medium (Fig. 2d), suggesting that ECg alters the surface properties of the cell.
Fig. 2.
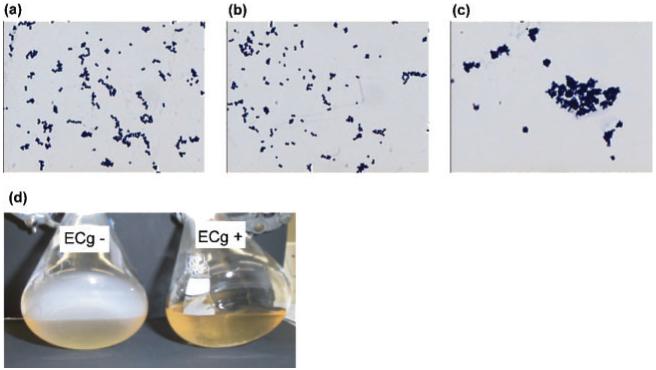
Effect of green tea catechins on cell aggregation and adherence to glass. (a-c) Gram-staining of EMRSA-15 grown in the absence (a) and presence of (b) EC (12.5 μg ml-1) or (c) ECg (12.5 μg ml-1). (d) EMRSA-15 was grown in the presence or absence of 12.5 μg ECg ml-1 for 16 h, without shaking, and the adherence to the flask wall examined by gently tilting the flask. The reduced density of the ECg-grown culture was due to flocculation as a result of cell clumping and not to inhibition of cell growth.
Reduced Triton X-100-induced autolysis in ECg-grown cells
We determined the effect of ECg on the autolytic activity of MRSA in the presence of the non-ionic detergent Triton X-100. Under standard growth conditions, the activity of cellular autolysins is tightly controlled but can be dysregulated by exposure to Triton X-100 (Raychaudhuri & Chatterjee, 1985). The rates of Triton X-100-induced autolysis of three MRSA isolates (S. aureus BB568, EMRSA-15, and EMRSA-16) and one meticillin-susceptible S. aureus (MSSA) isolate (S. aureus BB551) were assessed following growth in the presence and absence of 25 μg ECg ml-1. Growth in the presence of ECg elicited greater than 40 % reduction in the rate of Triton X-100-induced autolysis in all strains (Fig. 3); after 2.5 h, a 78 % reduction in the original OD600 was observed for the ECg-grown cells at this time interval. EC had no effect on the rate of Triton X-100-induced autolysis (data not shown). Cell walls from bacteria grown in the presence and absence of ECg were hydrolysed at the same rate by an autolysin extract obtained from the growth medium of bacteria grown in the absence of ECg, providing evidence that Triton X-100-induced autolysis was not due to altered substrate.
Fig. 3.
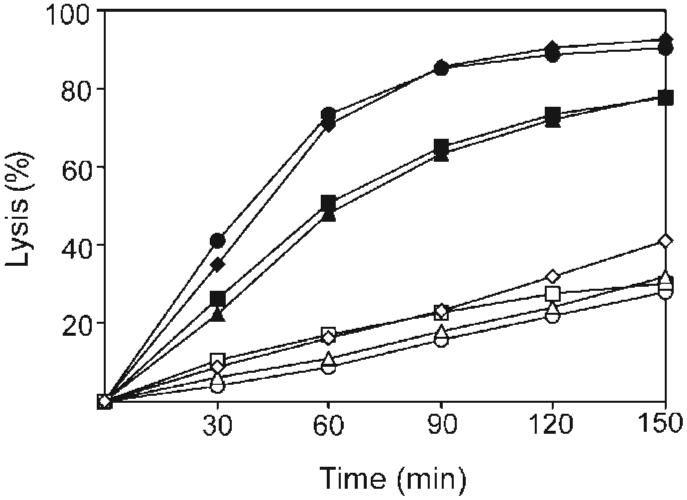
Effect of ECg on Triton X-100-induced autolysis. Triton X-100 was used to stimulate autolysis in S. aureus cells grown in the absence (black symbols) or presence (white symbols) of 12.5 μg ECg ml-1. The four S. aureus isolates investigated were: MSSA isolate BB551 (○, ●) and MRSA isolates BB568 (□, ■), EMRSA-15 (◇, ◆), and EMRSA-16 (△, ▲). Percentage lysis was determined from OD600 measurements and compared to values at time point 0.
ECg promotes the accumulation of bacteriolytic enzymes associated with the cell wall
To assess the effect of ECg on bacteriolytic enzyme production, autolysins were extracted from the cell wall of S. aureus BB568 with SDS and analysed by zymography (Fig. 4a). The profiles of cell-wall-associated bacteriolytic enzymes were broadly similar for bacteria grown in the absence of ECg and over a range of 25-100 μg ECg ml-1, although walls from ECg-grown cells elicited additional bands and concentration-dependent increases in the degree of cell lysis associated with the enzyme profiles (Fig. 4a). These observations suggested either that autolysin production was increased in ECg-grown cells or that there was a greater degree of autolysin retention within the cell wall in the presence of ECg. Therefore, culture medium supernatants from cells grown in the presence and absence of ECg were recovered by centrifugation, concentrated by filtration, and the autolytic profile determined by zymography: 25 μg ECg ml-1 reduced and ≥50 μg ECg ml-1 suppressed the release of lytic enzymes into the growth medium (Fig. 4b), a result confirmed by direct measurement of the bacteriolytic activity of autolysins within the culture medium supernatants (Fig. 4c).
Fig. 4.
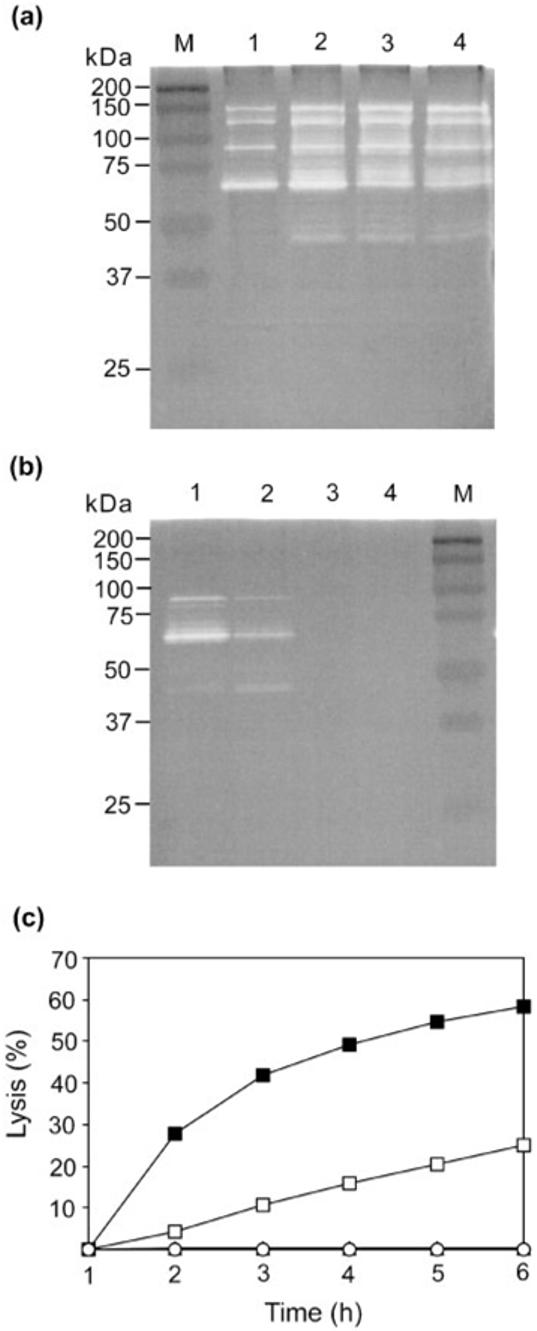
Effect of ECg on autolysin production in S. aureus BB568. (a) Autolysins were extracted with SDS from the cell walls of S. aureus BB568 grown in MH medium containing various ECg concentrations and separated on SDS-containing polyacrylamide gels containing heat-killed S. aureus cells (zymogram). M is a molecular size maker; lane 1, no ECg; lane 2, 25 μg ECg ml-1; lane 3, 50 μg ECg ml-1; and lane 4, 100 μg ECg ml-1. (b) Zymogram of extracellular autolysin production by S. aureus BB568 grown in the presence of ECg. Lane order (1 to 4) as in (a). (c) Lysis of heat-killed S. aureus cells by extracellular autolysin preparations from cells grown in the absence (■) and presence of ECg (25 μg ml-1, □; 50 μg ml-1, ○; and 100 μg ml-1, ▲).
Reduced susceptibility to lysostaphin of bacteria grown in the presence of ECg
Lysostaphin is a non-native peptidoglycan hydrolase that specifically cleaves the pentaglycine cross-bridge joining glycan strands within the peptidoglycan of S. aureus. To determine the effect of ECg on the susceptibility of the cell wall to peptidoglycan hydrolase, the minimum concentration of lysostaphin required to inhibit S. aureus growth was determined. Growth in the presence of ECg resulted in a >100-fold increase in the MIC of lysostaphin: values increased from 0.06 to >8 μg ml-1 for all the isolates investigated in this study. Consistent with this increase, the rate of lysostaphin-mediated cell lysis was severely reduced in ECg-grown BB568 and EMRSA-16. Pre-incubation of lysostaphin with 25 μg ECg ml-1 for 20 min prior to addition to the cell suspension had no effect on the capacity of the enzyme to hydrolyse cells grown in the absence of ECg, indicating that ECg is unlikely to function as a lysostaphin inhibitor. Pre-incubation of cells (grown in ECg-free medium) with ECg 20 min prior to testing also had no effect on the rate of lysostaphin-mediated cell lysis. Since a reduction in the number of glycine residues in the peptidoglycan cross-bridge can severely reduce the capacity of lysostaphin to hydrolyse S. aureus, the peptide composition of peptidoglycan preparations was determined by HPLC. No changes were revealed in the amino acid composition of peptidoglycan extracted from ECg-grown cells. Growth in the presence of EC had no effect on cell susceptibility to lysostaphin-mediated cell lysis.
Teichoic acid reduces lysostaphin activity in ECg-grown cells
To further investigate the nature of the ECg-mediated reduced susceptibility to lysostaphin, peptidoglycan with covalently bound wall teichoic acid (WTA; a polymer consisting of ribitol residues linked by phosphodiester linkages) was extracted and purified from S. aureus BB568 cells grown in the presence or absence of 12.5 μg ECg ml-1. Consistent with the whole-cell studies, peptidoglycan with covalently bound WTA extracted from ECg-grown cells had reduced susceptibility to lysostaphin hydrolysis. Removal of WTA from the peptidoglycan by acid treatment restored the capacity of lysostaphin to hydrolyse peptidoglycan from ECg-grown cells. Therefore, ECg-induced alterations to teichoic acid, either conformational or structural, are likely to account for the reduced lysostaphin susceptibility. Pre-incubation of peptidoglycan and covalently bound WTA (extracted from non-ECg-grown cells) with 12.5 μg ECg ml-1 had no effect on the capacity of lysostaphin to hydrolyse peptidoglycan, indicating it is unlikely that a conformational change induced by direct binding of ECg to WTA or peptidoglycan could account for the observed inhibition. Measurement of the phosphorus content of WTA, an indication of the relative quantities of teichoic acid in the samples, revealed no differences between ECg-grown and control cells: the phosphorus content was 750 and 718 mmol per mg cell wall for non-ECg-grown and ECg-grown BB568 cells respectively.
Increased mutanolysin hydrolysis of peptidoglycan from ECg-grown cells
In order to determine if reduced susceptibility of peptidoglycan to hydrolytic activity was restricted to lysostaphin, the activity of a second peptidoglycan hydrolase, mutanolysin, was investigated. Mutanolysin is an enzyme that cleaves the glycosidic bonds between disaccharide residues in peptidoglycan and thus acts at a site distinct from lysostaphin. In contrast to lysostaphin, a small increase in the rate of mutanolysin hydrolysis of peptidoglycan extracted from ECg-grown cells was observed (Fig. 5a) which was enhanced in the absence of WTA (Fig. 5b). Furthermore, the difference in the rate of hydrolysis between cells grown in the absence and presence of ECg was not dependent on the degree of O-acetylation of the peptidoglycan; removal of O-acetyl groups through exposure to strongly alkaline conditions had no effect on the hydrolytic capacity of mutanolysin (data not shown).
Fig. 5.
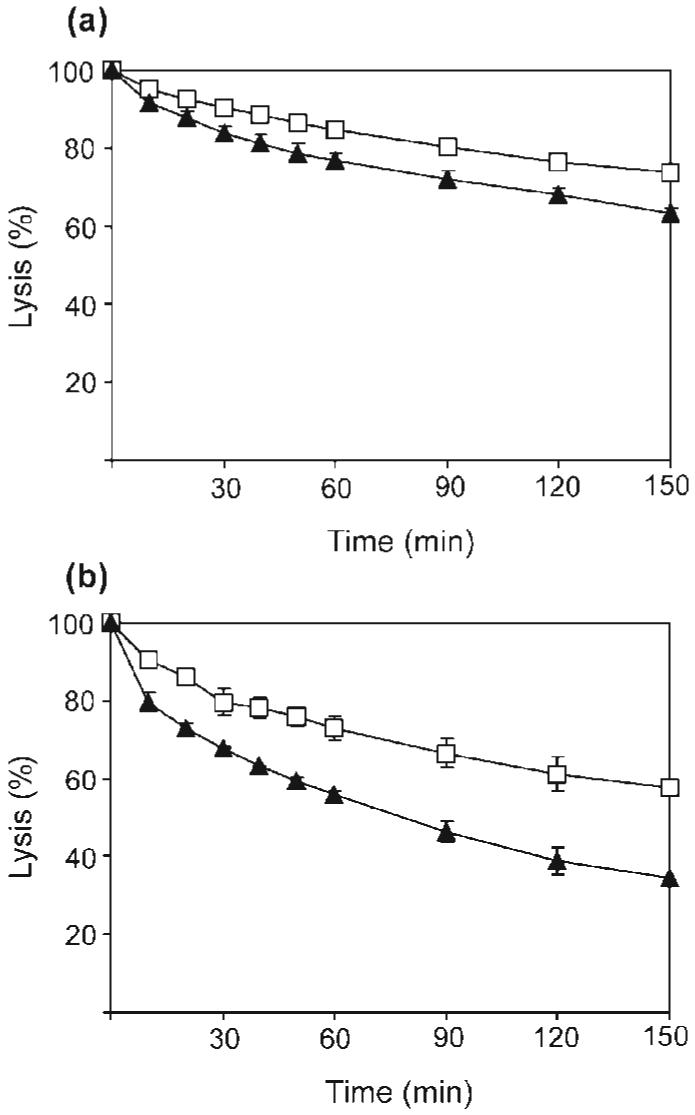
Effect of WTA on the capacity of mutanolysin to hydrolyse peptidoglycan extracted from BB568. Cells were grown in the absence (□) or presence (▲) of 12.5 μg ECg ml-1. (a) Peptidoglycan hydrolysis in the presence of covalently bound WTA. (b) Peptidoglycan hydrolysis after removal of WTA with acid.
ECg promotes the release of LTA
Teichoic acid is found in two forms in S. aureus, one covalently bound to peptidoglycan (WTA) and the other anchored to the cytoplasmic membrane in the form of LTA. To investigate the possibility that ECg promoted LTA release, LTA was labelled with [2-3H]glycerol and the rate of release determined. ECg, but not EC, promoted LTA release from S. aureus BB568, although the quantity released over a 3 h period was less than for Triton X-100, a detergent known to release LTA from S. aureus (Fig. 6).
Fig. 6.
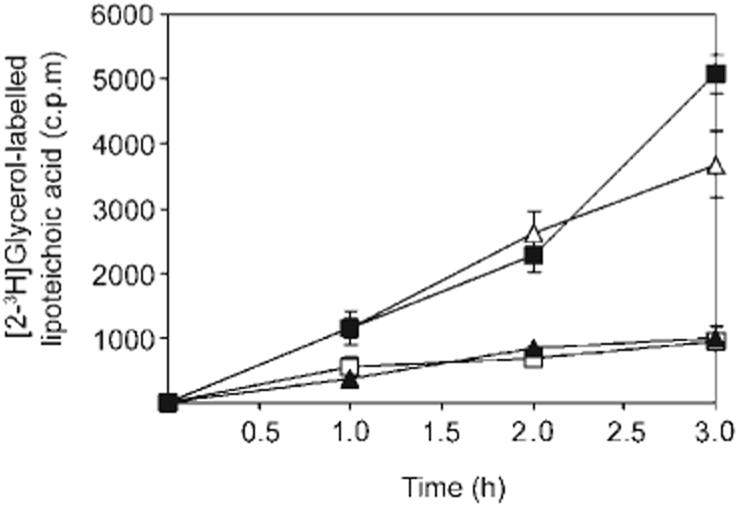
Effect of ECg on LTA release. Release of [2-3H]glycerol-labelled LTA from S. aureus BB568 grown in the absence (□) or presence of EC (12.5 μg ml-1; ▲), ECg (12.5 μg ml-1; ■), and Triton X-100 (0.02 %, v/v; △).
Reduced cell wall cross-linking in ECg-grown cells
To determine the composition and degree of cross-linking of the cell wall of ECg-grown cells, peptidoglycan was digested with M1 muramidase and the fragments analysed by HPLC. Examination of two MRSA isolates, BB568 and EMRSA-16, grown in the presence or absence of ECg, revealed no major changes to the biochemical composition of the peptidoglycan. However, differences were noted in the degree of cross-linking between glycan chains within the samples (Fig. 7). In BB568 and EMRSA-16, the degree of cross-linking was reduced by 5-10 % (indicated by a reduction in the quantities of high molecular mass muropeptides detected and reflected in a decrease in area under the curve for fragments eluting between 120 and 180 min) when grown in the presence of 12.5 μg ECg ml-1, suggesting that ECg interfered with PBP production and/or PBP activity.
Fig. 7.
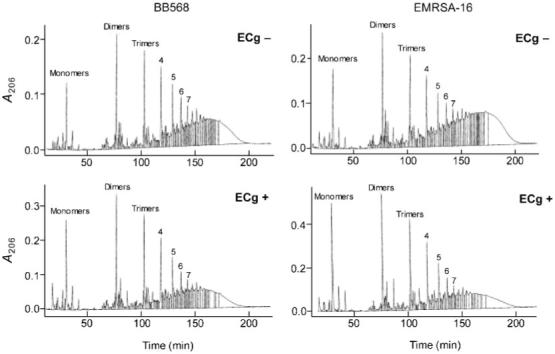
Effect of ECg on the muropeptide composition and degree of cross-linking of peptidoglycan extracted from S. aureus BB568 and EMRSA-16. Purified peptidoglycan preparations from cells grown in the presence or absence of ECg were digested with muramidase and the digestion products separated by HPLC.
Reduced binding of bocillin to PBP1 in ECg-grown cells
Bocillin FL, a BODIPY FL fluorescent dye-labelled penicillin V derivative, was used to detect PBPs, separated according to molecular size by SDS-PAGE (Fig. 8a). Growth of BB568 in the presence of ECg led to a reduction in the quantities of PBP1 (reduced by 40 %) and PBP3 (reduced by 33 %) detected (Fig. 8b). In order to distinguish between reduced PBP expression and PBP inhibition, PBP preparations were pre-incubated with ECg prior to bocillin labelling. This procedure revealed that PBP3 labelling was unaffected by the presence of ECg and, consequently, the reduction in bocillin binding observed was most likely due to reduced PBP3 production (Fig. 8b). In contrast, pre-incubation with ECg resulted in a reduction of bocillin binding to PBP1 of 36-48 % (Fig. 8b). EC had no effect on bocillin-labelling of PBPs (data not shown).
Fig. 8.
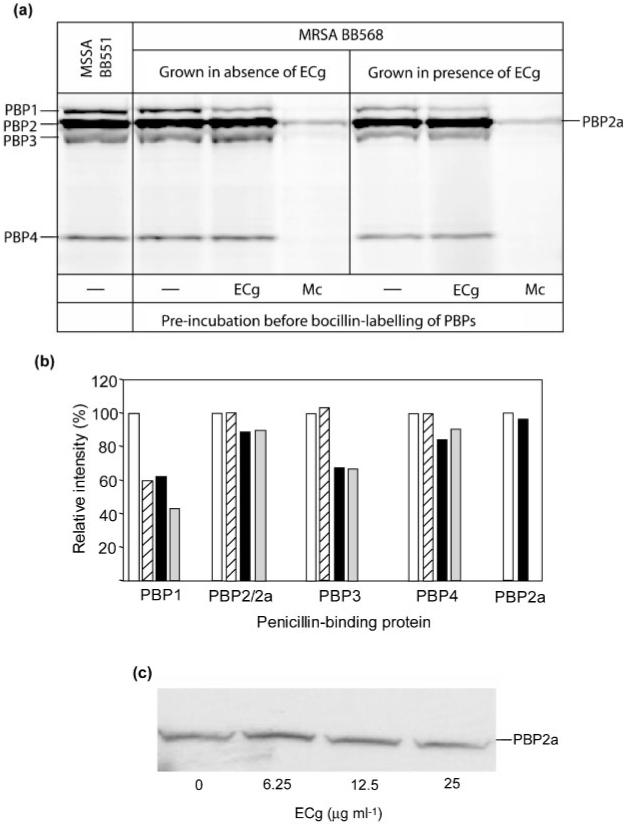
Effect of ECg on PBPs from S. aureus BB568. (a) PBPs were extracted from cells grown in the absence or presence of 12.5 μg ECg ml-1 and pre-incubated with 12.5 μg ECg ml-1, 600 μg meticillin ml-1, or assay buffer, before PBP labelling with biocillin. Proteins were separated by SDS-PAGE and the PBPs visualized by fluorography. The PBP profile for meticillin-susceptible isolate BB551 is also shown. (b) Relative intensities of the PBP bands in (a); all values are given relative to the intensity of each PBP band grown in the absence of ECg (100 %). Key: white bars, grown in the absence of ECg; hatched bars, grown in the absence of ECg and pre-incubated with ECg before bocillin labelling; black bars, grown in the presence of ECg; grey bars, grown in the presence of ECg and pre-incubated with ECg before bocillin labelling. (c) Membrane proteins extracted from cells grown in ECg were separated on SDS-polyacrylamide gels and PBP2a detected with anti-PBP2a antibody.
ECg does not affect PBP2a production
To facilitate the detection of PBP2a in the PBP profile, which is masked by PBP2 due to its molecular size, samples were pre-incubated with meticillin prior to bocillin labelling. PBP2a has a low affinity for meticillin. Thus, at appropriate meticillin concentrations, the other four PBPs are saturated with meticillin, do not bind bocillin and remain undetected (Fig. 8a). With this technique, no differences in the quantity of PBP2a were detected when BB568 cells were grown in the presence of ECg (Fig. 8a). No differences in bocillin binding to PBP2 and PBP2a were found following pre-incubation with ECg. Furthermore, PBP2a was detected using anti-PBP2a immunoblotting; the intensity of the bands appeared very similar (except at 6.25 μg ECg ml-1, where a slight increase in intensity was observed) in preparations from BB568 cells grown in medium containing a wide range of ECg concentrations (Fig. 8c).
DISCUSSION
ECg is one of a small number of structurally diverse compounds that modulate meticillin resistance in S. aureus: others include the triazine dye Cibacron blue F3GA and the non-ionic detergent Triton X-100 (Suzuki et al., 1997; Shirai et al., 1998; Stapleton & Taylor, 2002). Our earlier studies established that the gallate moiety, found in ECg but not EC, is an essential requirement for modulation of β-lactam resistance in S. aureus by catechins (Stapleton et al., 2004). The conclusions of the present study, that cell aggregation, Triton X-100-induced autolysis, lysostaphin susceptibility and LTA release were promoted by ECg but not EC, are consistent with this observation.
In contrast to a previous report (Zhao et al., 2001), we were unable to demonstrate that the presence of exogenous peptidoglycan compromised the capacity of ECg or EGCg to reduce PBP2a-mediated β-lactam resistance and we believe it unlikely that ECg and EGCg exert their resistance-modifying effects by binding directly to peptidoglycan. We were able to demonstrate reduced binding of penicillin to PBP1 and PBP3 in the presence of ECg, an observation that is consistent with the study of Hamilton-Miller & Shah (1999). However, in agreement with Zhao et al. (2001), we were unable to demonstrate a reduced expression of PBP2a, although we cannot rule out that direct binding of ECg to PBP2a may account for the observed reductions in β-lactam resistance.
Growth of MRSA in ECg-containing medium resulted in a small reduction in the degree of peptidoglycan cross-linking, presumably reflecting the capacity of ECg to reduce the activities of PBP2 and PBP4; in contrast to other PBPs, inhibition of the activity of these two proteins leads to a reduction in peptidoglycan cross-linking observed by HPLC muropeptide profiling. As growth in the presence of ECg neither reduced the binding of bocillin to PBP2 and PBP4 nor reduced the expression of these proteins, ECg is likely to affect their activity indirectly. The activities of PBP2, PBP4 and PBP2a are linked in an interdependent fashion (Łęski & Tomasz, 2005) and, as ECg may interfere with the interaction of PBP2 (and/or PBP4) and PBP2a, interruption of functional cooperation may account for ECg-mediated reductions in oxacillin resistance. Reduced PBP cooperation could be due to reduced mobility of proteins in the cytoplasmic membrane resulting from insertion of ECg into the bilayer, or to inhibition of protein-protein interactions following binding of ECg to PBPs.
PBP3 is not essential for staphylococcal growth and its inactivation appears to have few consequences other than a small decrease in autolysis (Pinho et al., 2000). On the other hand, PBP1 is essential for the viability of MSSA (Pinho et al., 2000). It remains to be determined if this is also the case for MRSA, but presumably PBP2a could substitute for the transpeptidase activity of PBP1 in MRSA if the activity of this protein was severely compromised by ECg. However, the muropeptide profiles obtained in this study provided no evidence for PBP2a enzymic activity in ECg-grown cells: PBP2a activity in the presence of low concentrations of meticillin results in an abnormal muropeptide profile (de Jonge & Tomasz, 1993) and such changes were not found in this study.
Like Cibracon blue F3GA (Shirai et al., 1998), ECg inhibits cell separation. Cibacron blue F3GA promotes cell aggregation by directly inhibiting endo-β-N-acetylglucosaminidase and N-acetylmuramyl-l-alanine amidase; these bacteriolytic enzymes are involved in cell separation (Sugai et al., 1990, 1995). Importantly, studies with Cibracon blue have shown that secretion of the autolysins into the growth medium is a prerequisite for cell separation (Sugai et al., 1995). We found that ECg-grown cells also retained autolysins within the cell wall, and autolysin release into the growth medium was markedly reduced; these observations are consistent with the report by Hamilton-Miller & Shah (1999) that tea polyphenols reduce cell separation and result in formation of pseudomulticellular forms. However, bacteriolytic enzyme inhibition by Cibacron blue F3GA does not account for its capacity to reduce oxacillin resistance in S. aureus (Shirai et al., 1998) and by analogy this may also be the case for ECg.
Although autolysins accumulated within the cell wall of ECg-grown cells, the degree of Triton X-100-induced autolysis was markedly reduced. This apparent contradiction indicated that cell-wall-associated autolysin activity was compromised, a contention supported by the observation that lysostaphin had a reduced capacity to hydrolyse whole cells and peptidoglycan extracted from ECg-grown bacteria. This reduced lysostaphin activity was not due to alterations in the formation of peptidoglycan pentaglycine cross-bridges (the substrate for lysostaphin) but was associated with the acid-labile component, teichoic acid. Interestingly, teichoic acid-mediated lysostaphin resistance is associated with the VISA isolates VM, JH-9 and JH-14; these also exhibit greatly reduced β-lactam resistance (Sieradzki et al., 1999; Sieradzki & Tomasz, 1996, 2003). In addition, these VISA isolates have, in a similar fashion to ECg-grown cells, an increased autolysin cell-wall content, reduced Triton X-100-induced autolysis and the tendency to grow as aggregates (Sieradzki & Tomasz, 2003). In this study we have established that ECg does not directly interfere with lysostaphin activity by binding to peptidoglycan, teichoic acid or lysostaphin. Unlike the ‘JH-series’ VISA isolates, which have increased quantities of teichoic acid associated with the cell wall (Sieradzki & Tomasz, 2003), no difference in teichoic acid content was observed in ECg-grown cells compared to cells grown in the absence of ECg, making it probable that changes to the structure of teichoic acid that confer reduced lysostaphin resistance occur during growth in ECg-containing medium. Variations in the d-alanine content of teichoic acid are known to significantly alter the properties of the cell wall; increases in the d-alanine content of WTA increase lysostaphin susceptibility but have only a minor affect on β-lactam susceptibility (Peschel et al., 2000). We are currently attempting to determine if ECg-grown cells possess modified teichoic acid.
Peptidoglycan extracted from ECg-grown cells had an increased susceptibility to mutanolysin and was similar in this regard to the VISA isolate VM described by Sieradzki et al. (1999). The increase in mutanolysin susceptibility of ECg-grown cells was not due to changes in O-acetylation, a modification known to affect lysozyme and mutanolysin activity (Bera et al., 2005), but probably resulted from reduced peptidoglycan cross-linking, as proposed for the VISA isolates (Sieradzki et al., 1999).
Although the VISA phenotype is heterogeneous, reduced Triton X-100-induced autolysis, lysostaphin susceptibility and increased cell-wall thickness are frequently associated with reduced vancomycin susceptibility (Boyle-Vavra et al., 2001; Koehl et al., 2004; Wootton et al., 2005). ECg-grown cells share these characteristics but we found no evidence that they possess decreased vancomycin susceptibility (unpublished data). This may be related to the expression of PBP4 in ECg-grown cells; reduced PBP4 expression is known to contribute to the VISA phenotype (Sieradzki et al., 1999; Finan et al., 2001).
In addition to its effects on WTA, ECg also stimulated LTA release. LTA is normally released from the cytoplasmic membrane in small quantities during staphylococcal cell growth, but its release can be stimulated by incubation with β-lactam antibiotics at concentrations equal to or above the MIC (Raynor et al., 1979; van Langevelde et al., 1998). ECg has only minimal impact on the degree of peptidoglycan cross-linking and it is unlikely that ECg-related modulation of cell-wall structure can account for the high levels of LTA that are released following growth in the presence of this catechin. Triton X-100 has, like ECg, the capacity to reduce β-lactam resistance in S. aureus and to promote LTA displacement and release from the cytoplasmic membrane (Raychaudhuri & Chatterjee, 1985; Suzuki et al., 1997). Indeed, it has been proposed that Triton X-100 sensitizes MRSA to oxacillin by virtue of its capacity to engender the release of LTA (Ohta et al., 2000). Like Triton X-100, non-galloylated and galloylated catechins interact with lipid bilayers (Hashimoto et al., 1999; Caturla et al., 2003; Kajiya et al., 2001, 2002) and their degree of penetration into lipid bilayers mirrors their capacity to modulate oxacillin resistance. Thus, active galloylated catechins such as ECg penetrate deep into the phospholipid palisade whereas non-active non-galloylated catechins such as EC occupy a more superficial location (Caturla et al., 2003; Stapleton et al., 2006). EC was unable to stimulate LTA release and its degree of penetration into the bilayer is insufficient to displace LTA. Conversely, the deeper location afforded to ECg facilitated LTA displacement.
In this study, we have examined the consequences of growth in ECg-containing medium on the staphylococcal cell in relation to the capacity of this naturally occurring polyphenol to sensitize MRSA isolates to β-lactam antibiotics. We have been able to discount ECg-mediated reduction in PBP2a expression and ECg binding to peptidoglycan as factors affecting ECg action, although we cannot yet exclude the possibility that modulation of antibiotic resistance may be related to inhibition of the enzymic activity of PBP2a. ECg inserts into the cytoplasmic membrane, releases LTA and is likely to effect changes to the structure of WTA; these events affect the surface properties of the cell and probably account for the retention of autolysins within the cell wall, altered autolysin activity and reduced adherence. We are currently investigating the interrelationships between these factors in the context of modulation of β-lactam susceptibility.
ACKNOWLEDGEMENTS
This work was funded by the Medical Research Council through Strategic Grant G0000996.
Abbreviations
- EC
(–)-epicatechin
- ECg
(–)-epicatechin gallate
- EGCg
(–)-epigallocatechin gallate
- LTA
lipoteichoic acid
- MRSA
meticillin-resistant Staphylococcus aureus
- MSSA
meticillin-susceptible S. aureus
- PBP
penicillin-binding protein
- VISA
vancomycin-intermediate-resistant S. aureus
- WTA
wall teichoic acid
REFERENCES
- Appelbaum PC. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2006;12(Suppl. 1):16–23. doi: 10.1111/j.1469-0691.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- Bera A, Herbert S, Jakob A, Vollmer W, Götz F. Why are pathogenic staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferase OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol Microbiol. 2005;55:778–787. doi: 10.1111/j.1365-2958.2004.04446.x. [DOI] [PubMed] [Google Scholar]
- Blanco AR, Sudano-Roccaro A, Spoto GC, Nostro A, Rusciano D. Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates. Antimicrob Agents Chemother. 2005;49:4339–4343. doi: 10.1128/AAC.49.10.4339-4343.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle-Vavra S, Carey RB, Daum RS. Development of vancomycin and lysostaphin resistance in a methicillin-resistant Staphylococcus aureus isolate. J Antimicrob Chemother. 2001;48:617–625. doi: 10.1093/jac/48.5.617. [DOI] [PubMed] [Google Scholar]
- Caturla N, Vera-Samper E, Villalaín J, Mateo CR, Micol V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic Biol Med. 2003;34:648–662. doi: 10.1016/s0891-5849(02)01366-7. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Staphylococcus aureus resistant to vancomycin - United States, 2002. MMWR Morbid Mortal Wkly Rep. 2002;51:565–567. [PubMed] [Google Scholar]
- Crisóstomo MI, Westh H, Tomasz A, Chung M, Oliveira DC, de Lencastre H. The evolution of methicillin resistance in Staphylococcus aureus: similarity of genetic backgrounds in historically early methicillin-susceptible and -resistant isolates and contemporary epidemic clones. Proc Natl Acad Sci U S A. 2001;98:9865–9870. doi: 10.1073/pnas.161272898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge BLM, Tomasz A. Abnormal peptidoglycan produced in a methicillin-resistant strain of Staphylococcus aureus grown in the presence of methicillin: functional role for penicillin-binding protein 2A in cell wall synthesis. Antimicrob Agents Chemother. 1993;37:342–346. doi: 10.1128/aac.37.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge BLM, de Lancastre H, Tomasz T. Suppression of autolysis and cell wall turnover in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J Bacteriol. 1991;173:1105–1110. doi: 10.1128/jb.173.3.1105-1110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan JE, Archer GL, Pucci MJ, Climo MW. Role of penicillin-binding protein 4 in expression of vancomycin resistance among clinical isolates of oxacillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:3070–3075. doi: 10.1128/AAC.45.11.3070-3075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier B, Hooper DC. A new two-component regulatory system involved in adhesion, autolysis, and extracellular proteolytic activity of Staphylococcus aureus. J Bacteriol. 2000;182:3955–3964. doi: 10.1128/jb.182.14.3955-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller JMT, Shah S. Disorganization of cell division of methicillin-resistant Staphylococcus aureus by a component of tea (Camellia sinensis): a study by electron microscopy. FEMS Microbiol Lett. 1999;176:463–469. doi: 10.1111/j.1574-6968.1999.tb13698.x. [DOI] [PubMed] [Google Scholar]
- Hartman BJ, Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984;158:513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Kumazawa S, Nanjo F, Hara Y, Nakayama T. Interaction of tea catechins with lipid bilayers investigated with liposome systems. Biosci Biotechnol Biochem. 1999;63:2252–2255. doi: 10.1271/bbb.63.2252. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- Kajiya K, Kumazawa S, Nakayama T. Steric effects on interaction of tea catechins with lipid bilayers. Biosci Biotechnol Biochem. 2001;65:2638–2643. doi: 10.1271/bbb.65.2638. [DOI] [PubMed] [Google Scholar]
- Kajiya K, Kumazawa S, Nakayama T. Effects of external factors on the interaction of tea catechins with lipid bilayers. Biosci Biotechnol Biochem. 2002;66:2330–2335. doi: 10.1271/bbb.66.2330. [DOI] [PubMed] [Google Scholar]
- Koehl JL, Muthaiyan A, Jayaswal RK, Ehlert K, Labischinski H, Wilkinson B. Cell wall composition and decreased autolytic activity and lysostaphin susceptibility of glycopeptide-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:3749–3757. doi: 10.1128/AAC.48.10.3749-3757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloir LF, Cardini CE. Characterization of phosphorus compound by acid lability. Methods Enzymol. 1957;3:840–850. [Google Scholar]
- Łęski TA, Tomasz A. Role of penicillin-binding protein 2 (PBP2) in the antibiotic susceptibility and cell wall cross-linking of Staphylococcus aureus: evidence for the cooperative functioning of PBP2, PBP4 and PBP2A. J Bacteriol. 2005;187:1815–1824. doi: 10.1128/JB.187.5.1815-1824.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Ratnam K, Payne DJ. Beta-lactamase-inhibitor combinations in the 21st century: current agents and new developments. Curr Opin Pharmacol. 2001;1:451–458. doi: 10.1016/s1471-4892(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Ohta K, Komatsuzawa H, Sugai M, Suginaka H. Triton X-100-induced lipoteichoic acid release is correlated with the methicillin resistance in Staphylococcus aureus. FEMS Microbiol Lett. 2000;182:77–79. doi: 10.1111/j.1574-6968.2000.tb08877.x. [DOI] [PubMed] [Google Scholar]
- Peschel A, Vuong C, Otto M, Götz F. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob Agents Chemother. 2000;44:2845–2847. doi: 10.1128/aac.44.10.2845-2847.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho MG, de Lencastre H, Tomasz A. Cloning, characterization, and inactivation of the gene pbpC, encoding penicillin-binding protein 3 of Staphylococcus aureus. J Bacteriol. 2000;182:1074–1079. doi: 10.1128/jb.182.4.1074-1079.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri D, Chatterjee AN. Use of resistant mutants to study the interaction of Triton X-100 with Staphylococcus aureus. J Bacteriol. 1985;164:1337–1349. doi: 10.1128/jb.164.3.1337-1349.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor RH, Scott DF, Best GK. Oxacillin-induced lysis of Staphylococcus aureus. Antimicrob Agents Chemother. 1979;16:134–140. doi: 10.1128/aac.16.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos M, Pittenauer E, Schmid E, Beyer M, Reinike B, Allmaier G, Labischinski H. Improved high-performance liquid chromatographic separation of peptidoglycan isolated from various Staphylococcus aureus strains for mass spectrometric characterization. J Chromatogr B Biomed Sci Appl. 1998;705:183–192. doi: 10.1016/s0378-4347(97)00506-9. [DOI] [PubMed] [Google Scholar]
- Shiota S, Shimizu M, Mizushima T, Ito H, Hatano T, Yoshida T, Tsuchiya T. Marked reduction in the minimum inhibitory concentration (MIC) of beta-lactams in methicillin-resistant Staphylococcus aureus produced by epicatechin gallate, an ingredient of green tea (Camellia sinensis) Biol Pharm Bull. 1999;22:1388–1390. doi: 10.1248/bpb.22.1388. [DOI] [PubMed] [Google Scholar]
- Shirai C, Sugai M, Komatsuzawa H, Ohta K, Yamakido M, Suginaka H. A triazine dye, cibacron blue F3GA, decreases oxacillin resistance levels in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:1278–1280. doi: 10.1128/aac.42.5.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieradzki K, Tomasz A. A highly vancomycin-resistant laboratory mutant of Staphylococcus aureus. FEMS Microbiol Lett. 1996;142:161–166. doi: 10.1111/j.1574-6968.1996.tb08424.x. [DOI] [PubMed] [Google Scholar]
- Sieradzki K, Tomasz A. Alterations of cell wall structure and metabolism accompany reduced susceptibility to vancomycin in an isogenic series of clinical isolates of Staphylococcus aureus. J Bacteriol. 2003;185:7103–7110. doi: 10.1128/JB.185.24.7103-7110.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieradzki K, Pinho MG, Tomasz A. Inactivated pbp4 in highly glycopeptide-resistant laboratory mutants of Staphylococcus aureus. J Biol Chem. 1999;274:18942–18946. doi: 10.1074/jbc.274.27.18942. [DOI] [PubMed] [Google Scholar]
- Stapleton PD, Taylor PW. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci Prog. 2002;85:57–72. doi: 10.3184/003685002783238870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton PD, Shah S, Anderson JC, Hara Y, Hamilton-Miller JMT, Taylor PW. Modulation of beta-lactam resistance in Staphylococcus aureus by catechins and gallates. Int J Antimicrob Agents. 2004;23:462–467. doi: 10.1016/j.ijantimicag.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Stapleton PD, Shah S, Hara Y, Taylor PW. Potentiation of catechin gallate-mediated sensitization of Staphylococcus aureus to oxacillin by nongalloylated catechins. Antimicrob Agents Chemother. 2006;50:752–755. doi: 10.1128/AAC.50.2.752-755.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranden AM, Ehlert K, Labischinski H, Berger-Bächi B. Cell wall monoglycine cross-bridges and methicillin hyper-susceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J Bacteriol. 1997;179:9–16. doi: 10.1128/jb.179.1.9-16.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai M, Akiyama T, Komatsuzawa H, Miyake Y, Suginaka H. Characterization of sodium dodecyl sulfate-stable Staphylococcus aureus bacteriolytic enzymes by polyacrylamide gel electrophoresis. J Bacteriol. 1990;172:6494–6498. doi: 10.1128/jb.172.11.6494-6498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai M, Komatsuzawa H, Akiyama T, Hong Y-M, Oshida T, Miyake Y, Yamaguchi T, Suginaka H. Identification of endo-β-N-acetylglucosaminidase and N-acetylmuramyl-l-alanine amidase as cluster-dispersing enzymes in Staphylococcus aureus. J Bacteriol. 1995;177:1491–1496. doi: 10.1128/jb.177.6.1491-1496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Komatsuzawa H, Sugai M, Ohta K, Kozai K, Nagasaka N, Suginaka H. Effects of various types of triton X on the susceptibilities of methicillin-resistant staphylococci to oxacillin. FEMS Microbiol Lett. 1997;153:327–331. doi: 10.1111/j.1574-6968.1997.tb12592.x. [DOI] [PubMed] [Google Scholar]
- Taylor PW, Stapleton PD, Paul Luzio J. New ways to treat bacterial infections. Drug Discov Today. 2002;7:1086–1091. doi: 10.1016/s1359-6446(02)02498-4. [DOI] [PubMed] [Google Scholar]
- van Langevelde P, van Dissel JT, Ravensbergen E, Applemelk BJ, Schrijver IA, Groeneveld PHP. Antibiotic-induced release of lipoteichoic acid and peptidoglycan from Staphylococcus aureus: quantitative measurements and biological reactivities. Antimicrob Agents Chemother. 1998;42:3073–3078. doi: 10.1128/aac.42.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton M, Bennett PM, MacGowan AP, Walsh TR. Reduced expression of the atl autolysin gene and susceptibility to autolysis in clinical heterogeneous glycopeptide-intermediate Staphylococcus aureus (hGISA) and GISA strains. J Antimicrob Chemother. 2005;56:944–947. doi: 10.1093/jac/dki289. [DOI] [PubMed] [Google Scholar]
- Yam TS, Hamilton-Miller JMT, Shah S. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2′ synthesis, and β-lactamase production in Staphylococcus aureus. J Antimicrob Chemother. 1998;42:211–216. doi: 10.1093/jac/42.2.211. [DOI] [PubMed] [Google Scholar]
- Zhao W-H, Hu Z-Q, Okubo S, Hara Y, Shimamura T. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1737–1742. doi: 10.1128/AAC.45.6.1737-1742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


