Summary
Pulmonary hypertension is a frequent complication of sickle cell disease that is associated with haemolysis, impaired nitric oxide bioavailability and high mortality. We sought to evaluate the safety and efficacy of selective pulmonary vasodilators and antiproliferative agents in this at-risk population. After optimising sickle cell disease therapy to stabilise haemoglobin and fetal haemoglobin levels, we evaluated the safety and efficacy of sildenafil in 12 patients with sickle cell disease and pulmonary hypertension. Sildenafil therapy (mean duration 6 ± 1 months) decreased the estimated pulmonary artery systolic pressure [50 ± 4 to 41 ± 3 mmHg; difference 9 mmHg, 95% confidence interval (CI): 0.3–17, P = 0.043] and increased the 6-min walk distance (384 ± 30 to 462 ± 28 m; difference 78 m, 95% CI: 40–117, P = 0.0012). Transient headaches occurred in two patients and transient eye-lid oedema in four patients. No episodes of priapism occurred in the three men in the study; two of them were on chronic exchange transfusions and one had erectile dysfunction. In conclusion: (1) sickle cell disease patients with anaemia and pulmonary hypertension have significant exercise limitation; (2) the 6-min walk distance may be a valid endpoint in this population; (3) therapy with sildenafil appears safe and improves pulmonary hypertension and exercise capacity. Additional phase I studies in males with sickle cell disease followed by phase II/III placebo controlled trials evaluating the safety and efficacy of sildenafil therapy in sickle cell disease patients with pulmonary hypertension are warranted.
Keywords: sickle cell disease, haemolysis, pulmonary hypertension, phosphodiesterase 5 inhibitors, nitric oxide, 6-min walk test
Pulmonary hypertension is increasingly recognised as a complication of sickle cell disease, with a prevalence of approximately 30% (Gladwin et al, 2004). In spite of the mild elevation in the pulmonary artery pressure, the 2-year mortality in these patients can be as high as 50% (Castro et al, 2003). We recently demonstrated that in adult patients with sickle cell disease, pulmonary hypertension (defined as a tricuspid regurgitant jet velocity greater than or equal to 2.5 m/s) is a major independent risk factor for death [relative risk of death 10.1; 95% confidence interval (CI) 2.7–44] (Gladwin et al, 2004). It is, however, unclear whether pulmonary hypertension is a marker of disease severity or a direct cause of mortality (Hassoun & Krishnan, 2004; Klings & Farber, 2004). Nor is it known whether selective pulmonary vasodilator/antiproliferative therapies will prove efficacious in this disease because the pulmonary artery pressure is only moderately elevated, the cardiac output and pulmonary capillary wedge pressures are high, and the pulmonary vascular resistance, while increased when compared with patients without sickle cell disease is only mildly elevated (Castro et al, 2003; Gladwin et al, 2004; Hassoun & Krishnan, 2004; Klings & Farber, 2004). In this context, investigation of the response to specific therapies targeting the pulmonary hypertension could help advance our understanding of the role of pulmonary hypertension in the increased mortality seen in these patients, and potentially open the door to novel therapeutic strategies for this at-risk population.
The phosphodiesterase 5 inhibitor, sildenafil, has been shown to improve pulmonary haemodynamics and functional capacity in several forms of pulmonary arterial hypertension (Lee et al, 2005). Inhibition of cyclic guanosine monophosphate (GMP) degradation by phosphodiesterase 5 increases nitric oxide (NO)-mediated pulmonary vasodilation. This effect may be beneficial in patients with sickle cell disease and/or other haemolytic disorders, characterised by a state of relative NO deficiency. In sickle cell disease (and possibly in other haemolytic disorders), a haemolysis-associated endothelial dysfunction is caused by the release of erythrocyte haemoglobin, which scavenges NO (Reiter et al, 2002), and of erythrocyte arginase, which metabolises L-arginine, the substrate for NO synthesis (Belfiore, 1964; Azizi et al, 1970; Morris et al, 2003; Schnog et al, 2004; Morris et al, 2005) as well as a possible impairment of the pulmonary vasodilatory effects of NO (Voelkel et al, 1995, 1999).
In this open label uncontrolled pilot trial, we evaluated the safety and efficacy of sildenafil in patients with sickle cell disease and pulmonary hypertension. We hypothesised that patients with sickle cell disease and pulmonary hypertension, despite the mild nature of the pulmonary hypertension, have significant impairments in exercise capacity because of the combined effects of the anaemia and the increased pulmonary vascular resistance and that treatment with sildenafil may prove efficacious by improving the pulmonary hypertension and exercise capacity.
Methods
Patient characteristics
All patients were enrolled in a National Heart Lung and Blood Institute-approved human subjects protocol; all subjects provided written, informed consent. All patients had homozygous sickle cell disease, determined by high performance liquid chromatography of their haemoglobin. Sickle cell subjects with pulmonary hypertension were identified by a tricuspid regurgitant jet velocity of greater than or equal to 2.5 m/s (corresponding to an estimated pulmonary artery systolic pressure of greater than or equal to 30–35 mmHg). Consenting patients with tricuspid regurgitant jet velocity of greater than or equal to 2.9 m/s also underwent right heart catheterisation.
Validation of the 6-min walk test in patients with sickle cell disease
In order to validate the use of the 6-min walk test as a surrogate marker of functional impairment in patients with sickle cell disease and pulmonary hypertension, we evaluated the relationship between the 6-min walk distance and pulmonary artery pressure in a previously reported cohort that included patients enrolled in this study (Gladwin et al, 2004).
Sildenafil study design
The study was funded by the Intramural Research Division of the National Institutes of Health and medications purchased and delivered by the Clinical Center Pharmacy Development Service. Patients with tricuspid regurgitant jet velocity of greater than or equal to 2.5 m/s (corresponding to an estimated pulmonary artery systolic pressure of greater than or equal to 30–35 mmHg) were sequentially enrolled in the study. This value was selected because tricuspid regurgitant jet velocity values of greater than or equal to 2.5 m/s were associated with a significant and independent risk of death in patients with sickle cell disease (Gladwin et al, 2004). Thus a unique feature of this prospective study, was the evaluation of the treatment effect of sildenafil on patients with mild-to-moderate pulmonary hypertension. To minimise the risk of priapism, male patients were allowed to participate only if they had erectile dysfunction or were on chronic transfusion therapy. All patients had comprehensive ophthalmological evaluations prior to initiating sildenafil therapy and with each dose escalation; patients with active retinopathy or a history of retinal detachment or haemorrhage were excluded. To ensure that total haemoglobin levels and fetal haemoglobin levels were stable prior to starting sildenafil therapy, as these values can significantly affect exercise capacity, patients underwent intensification of their standard sickle cell disease therapy for 3–8 months. Such therapies included hydroxyurea, erythropoietin and/or exchange transfusion therapy, based on the current standard of care. Specific therapies used for each patient are shown in Table I.
Table I.
Baseline clinical characteristics.
| Patient | Age (years) | Gender | NYHA/WHO functional class | Haemoglobin (g/dl) | Fetal haemoglobin (%) | Final haemoglobin (g/dl)* | Therapy for sickle cell disease |
|---|---|---|---|---|---|---|---|
| 1 | 41 | F | II | 7.5 | 9.8 | 7.8 | Hydroxyurea |
| 2 | 42 | F | I | 8.8 | 3.7 | 9.5 | Hydroxyurea, erythropoietin |
| 3 | 52 | F | III | 8.7 | 15.2 | 9.6 | Hydroxyurea |
| 4 | 19 | F | III | 9.5 | 4.3 | 9.5 | Exchange transfusion |
| 5 | 49 | M | III | 8.4 | 1.4 | 8.3 | Exchange transfusion |
| 6 | 45 | F | III | 11.6 | 26.1 | 11.4 | Hydroxyurea |
| 7 | 37 | F | III | 8.4 | 31.4 | 7.3 | Hydroxyurea, erythropoietin |
| 8 | 30 | M | III | 6.2 | 9.4 | 6.9 | Hydroxyurea, erythropoietin |
| 9 | 51 | F | II | 9.0 | 18.2 | 8.1 | Hydroxyurea |
| 10 | 46 | F | I | 10.4 | 1.9 | 10.3 | Exchange transfusion |
| 11 | 49 | M | III | 11.3 | 1.6 | 11.5 | Exchange transfusion |
| 12 | 39 | F | II | 8.0 | 8.7 | 8.0 | Hydroxyurea |
| Mean ± SEM | 43 ± 3 | 8.9 ± 0.5 | 10.9 ± 2.8 | 9.0 ± 0.4 |
Haemoglobin level obtained at the time of last measured 6-min walk distance and echocardiogram.
NYHA, New York Heart Association; WHO, World Health Organisation.
When the haemoglobin and fetal haemoglobin levels had been stable for at least 4 weeks, oral sildenafil was initiated at a dose of 25 mg three times daily. The dose was increased by 25 mg three times daily every 2–4 weeks as tolerated to a maximum dose of 100 mg three times a day (100 mg four times a day was used in one patient). Pulmonary artery systolic pressure was estimated by echocardiography at baseline and at monthly intervals thereafter. The 6-min walk distance was measured at baseline and at 3 month intervals after initiation of therapy. Echocardiographic and 6-min walk distance assessments were performed sequentially and on the same day, within 1–4 h after sildenafil dosing. Assessments of estimated pulmonary artery pressure and 6-min walk distance were only performed in patients in steady state, i.e. no measurements were made within 1 week of the end of an acute vaso-occlusive crisis. For the purposes of analysis, the presildenafil treatment 6-min walk distance and tricuspid regurgitant jet velocity measurements, after stabilisation of fetal haemoglobin (Fig 1, time zero), were compared with the last measured 6-min walk distance and tricuspid regurgitant jet velocity. Because of sequential patient enrollment, these measures were obtained after 4–12 months of sildenafil therapy (mean duration sildenafil therapy 6 ± 1 months). As an additional secondary analysis, we measured the change in the tricuspid regurgitant jet velocity values over time.
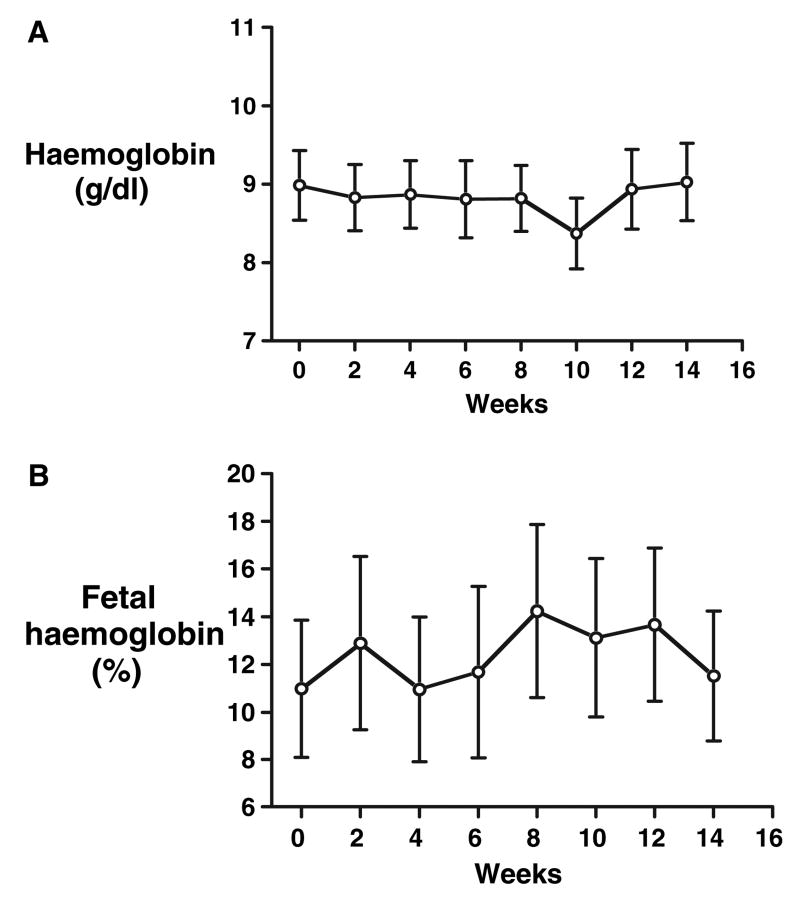
Fig 1.
Haemoglobin (A) and fetal haemoglobin levels (B) remain stable with sildenafil therapy, which was started on week 0 following maximisation of sickle cell disease therapy; data mean ± SEM.
Echocardiography
Transthoracic echocardiography was performed in all patients using the Acuson Sequoia (Siemens-Acuson Inc., Mountain-view, CA, USA) and Sonos 5500 (Philips, Inc., Andover, MA, USA). Tricuspid regurgitation was assessed in the parasternal right ventricular inflow, parasternal short-axis, and apical four-chamber views and a minimum of five sequential complexes were recorded. Continuous wave Doppler sampling of the peak regurgitant jet velocity was used to estimate the right ventricular to right atrial systolic pressure gradient using the modified Bernoulli equation [4 × (tricuspid regurgitant jet velocity) × (tricuspid regurgitant jet velocity)]. Pulmonary-artery systolic pressure was estimated by adding the Bernoulli-derived pressure gradient to the estimated mean right atrial pressure. The mean right atrial pressure was estimated according to the degree of collapse of the inferior vena cava with inspiration: 5 mmHg for a collapse of at least 50% and 15 mmHg for a collapse of less than 50% (Kircher et al, 1990). These measurements have been validated to correlate with measured pulmonary artery systolic pressure in the absence of right ventricular outflow obstruction, e.g. pulmonic stenosis (Berger et al, 1985). We recently validated this measurement in patients with sickle cell disease (Gladwin et al, 2004).
Right heart catheterisation
Right heart catheterisations were performed in nine patients in the study. Cardiac output was measured by continuous thermodilution (Sensormedics Vmax 229; Viasys Healthcare, Yorba Linda, CA, USA). The mean haemodynamics of a subgroup of these patients has been previously reported (Gladwin et al, 2004).
Acute vasodilator challenge
To evaluate vasodilator responsiveness in catheterised patients, haemodynamic measurements were performed at baseline and 10 min after inhalation of NO 40 parts per million (INO Therapeutics, Liberty Corner, NJ, USA). In a subgroup of five subjects, 50 mg of sildenafil was administered orally with hourly assessments of haemodynamic parameters.
The 6-min walk test
The 6-min walk was performed in accordance with standard practice (American Thoracic Society (ATS) Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories, 2002). Briefly, patients were asked to walk at an unhurried pace for 6 min along a premeasured path in the Clinical Center. A single practice walk was performed first.
N-terminal pro-brain natriuretic peptide measurement
Plasma N-terminal pro-brain natriuretic peptide (NT-pro-BNP) was measured by a sandwich immunoassay using polyclonal antibodies that recognise epitopes located in the N-terminal part (1–76) of pro-BNP (1–108) (Elecsys analyzer, Roche Diagnostics, Manheim, Germany) prior to introduction of sildenafil and at the end of the study. Elevated levels of brain natriuretic peptide have been demonstrated in several forms of pulmonary hypertension and correlate with the degree of elevation in pulmonary pressures and right ventricular dysfunction in this population (Leuchte et al, 2004a,b, Nagaya et al, 1998, 2000).
Statistical analysis
Differences between pre- and the most recent post-sildenafil therapy and NO responses were evaluated by Wilcoxon matched test. Comparisons of the relationship between haemoglobin, and fetal haemoglobin levels versus time were performed using one-way-analysis of variance (ANOVA). Repeated measures ANOVA was performed for the evaluation of the acute effects of sildenafil administration. Spearman correlations were performed where appropriate. The calculations were performed using GRAPHPAD PRISM (GraphPad Software Inc., San Diego, CA, USA). A P-value less than 0.05 was considered statistically significant.
Results
Validation of the 6-min walk test in patients with sickle cell disease with pulmonary hypertension
To validate the relationship between the 6-min walk distance and both the non-invasive echocardiographic estimate of pulmonary artery systolic pressure and pulmonary artery pressure measured during right heart catheterisation, we compared 11 patients without pulmonary hypertension [defined by mean pulmonary artery pressure less than 25 mmHg or tricuspid regurgitant jet velocity less than 2.4 m/s; four males, age 40 ± 3 (mean ± SEM) years, tricuspid regurgitant jet velocity 2.3 ± 0.1 m/s] and in 15 patients with pulmonary hypertension (defined by mean pulmonary artery pressures greater than 25 mmHg (n = 14) or tricuspid regurgitant jet velocity greater than or equal to 3 m/s (n = 1 patient); seven males, age 41 ± 2 years, tricuspid regurgitant jet velocity 3.2 ± 0.1 m/s). The control subjects were matched to the pulmonary hypertension subjects for total haemoglobin (8.5 ± 0.3 and 8.3 ± 0.2 g/dl, respectively; P = 0.59), fetal haemoglobin (6.9 ± 1.6 and 8.3 ± 0.2%, respectively; P = 0.9) and age (40 ± 3 and 41 ± 2 years, respectively; P = 0.74). Right heart catheterisation was performed in four of the patients without pulmonary hypertension (mean pulmonary artery pressure 22 ± 1 mmHg, pulmonary vascular resistance 73 ± 8 dynes × s × cm5) and in 14 of the patients with pulmonary hypertension (mean pulmonary artery pressure 36 ± 2 mmHg, pulmonary vascular resistance 169 ± 26 dynes × s × cm5). In these patients, the 6-min walk distance correlated inversely with the tricuspid regurgitant jet velocity (R = −0.62, P = 0.0001) and with the measured mean pulmonary artery pressure (R = −0.52, P = 0.01) (Fig 2), consistent with the 6-min walk test reflecting the functional impairment in patients with sickle cell disease and pulmonary hypertension. These data supported the use of 6-min walk distance as our primary endpoint for a phase I/II open label sildenafil trial in this population.
Fig 2.
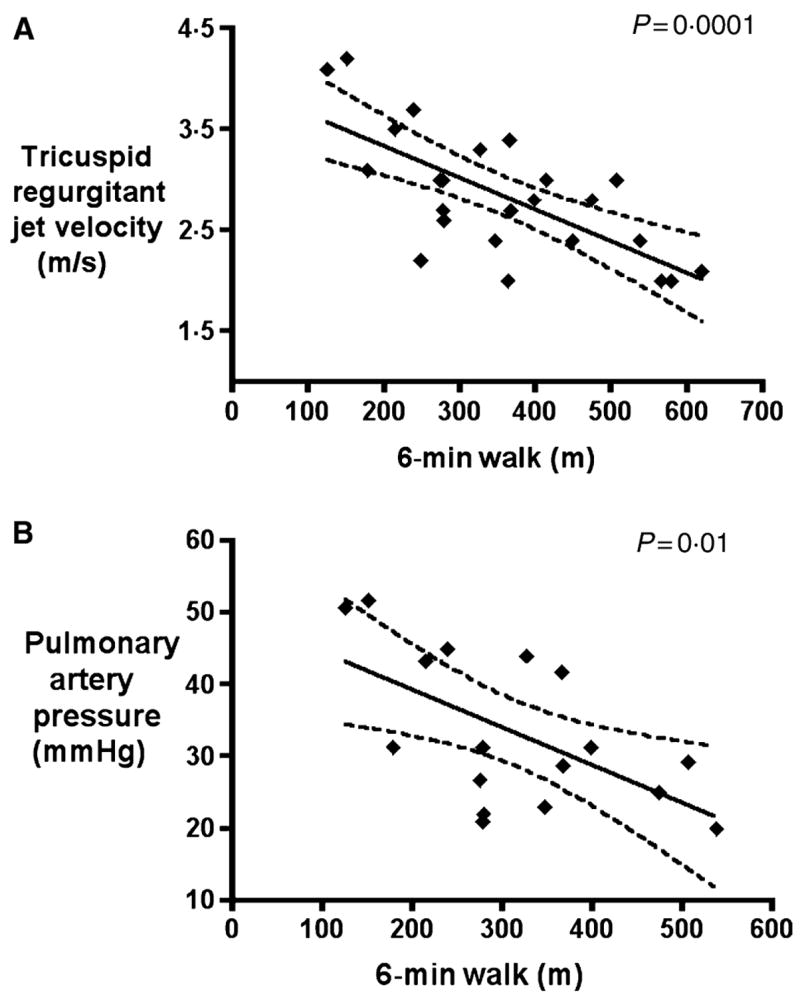
Six-minute walk distance correlated inversely with tricuspid regurgitant jet velocity (A) and mean pulmonary artery pressure (B).
Adverse events
No significant changes in systemic arterial blood pressure were observed with chronic therapy (data not shown). Transient headaches were reported in two patients upon initiation of the drug, however, with the exception of one patient who had migraine-like headaches at the initiation of therapy and could not complete the study, sildenafil was well tolerated. Transient eye-lid and/or periorbital subcutaneous oedema occurred in four patients, following initiation of the drug or at dose escalation. Comprehensive ophthalmological evaluation revealed no changes in visual acuity, ocular pressures or retinal appearance. No episodes of retinal haemorrhage occurred. No episodes of priapism occurred in the three male patients. It should be noted, however, that per inclusion criteria, at enrollment two male patients were on chronic exchange transfusion therapy and one male had pre-existing erectile dysfunction.
Acute haemodynamic effects of sildenafil
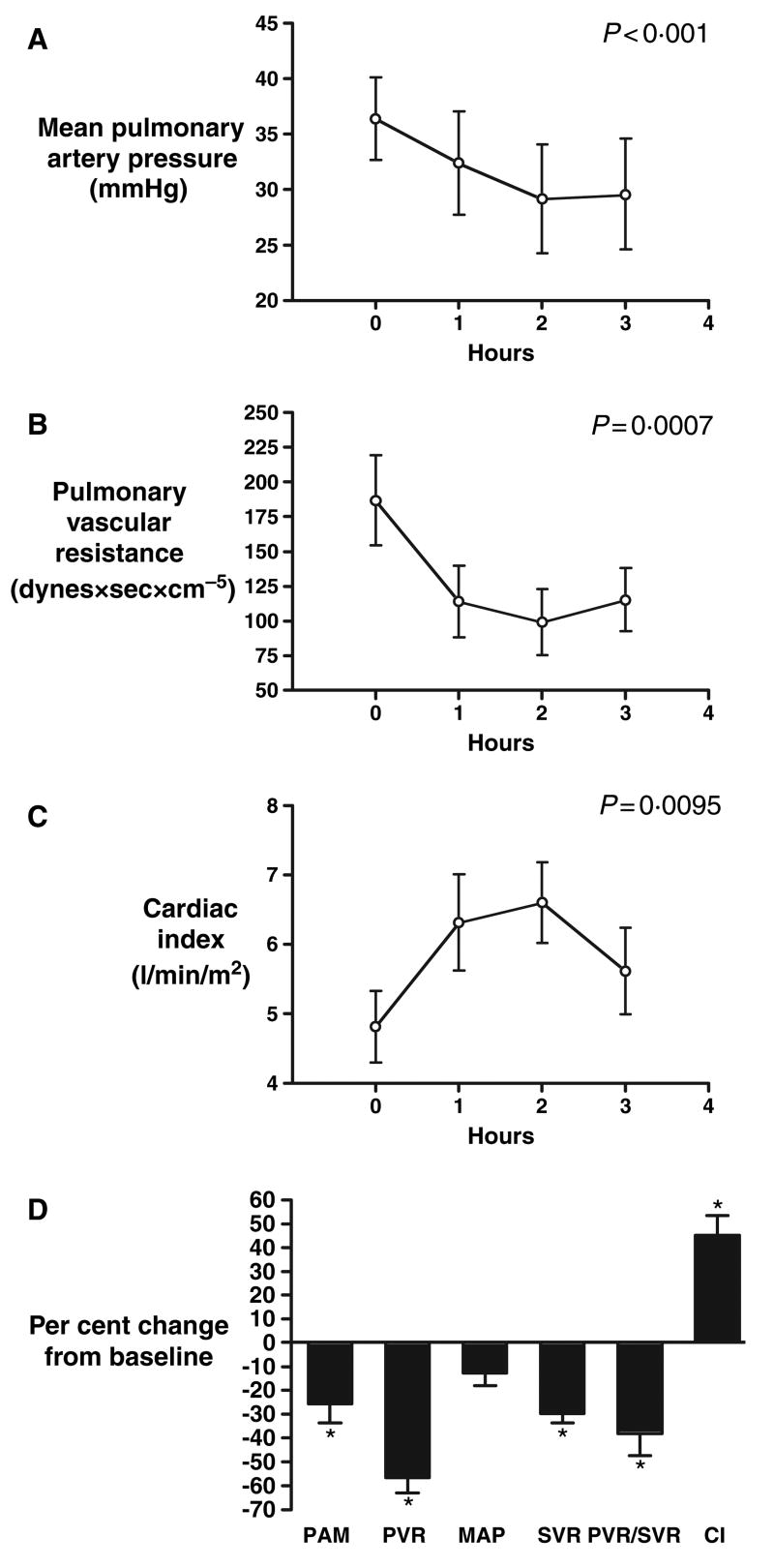
The acute pulmonary and systemic haemodynamic effects of sildenafil administration were evaluated in five patients with sickle cell disease (five females age 38 ± 8 years, mean pulmonary artery pressure 36.4 ± 4 mmHg, pulmonary vascular resistance 187 ± 32 dynes × s × cm5). Acute administration of sildenafil significantly decreased mean pulmonary artery pressure by 26% (95% CI: −47 to −4), pulmonary vascular resistance by 57% (95% CI: −74 to −39) and increased cardiac index by 45% (95% CI: 22–68). Mean arterial pressure did not statistically change (−13% change, 95% CI: 2 to −28), while both the systemic vascular resistance (−30% change, 95% CI: −41 to −18) and the ratio of pulmonary to systemic vascular resistance (−38% change, 95% CI: −64 to −12) significantly decreased, suggesting a preferential pulmonary vasodilator effect (Fig 3).
Fig 3.
Acute administration of sildenafil decreased mean pulmonary artery pressure (A); pulmonary vascular resistance (B); and increased cardiac index (C). (D) Maximal effects of sildenafil on haemodynamic parameters (n = 5), data mean ± SEM, *P < 0.05. PAM, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance; MAP, mean arterial pressure; CI, cardiac index.
Sildenafil effects on pulmonary hypertension and exercise capacity
Thirteen patients were enrolled. One patient could not tolerate sildenafil because of persistent headaches after the initiation of the drug and was discontinued from the study. Table I illustrates the clinical characteristics of the remaining 12 patients. Table II illustrates the haemodynamic and vaso-reactivity profiles of the nine study subjects who underwent cardiac catheterisation. Haemoglobin and fetal haemoglobin levels did not change after sildenafil therapy was initiated (P > 0.05, Fig 1), enabling the assessment of sildenafil-dependent changes in exercise capacity. Further, lactate dehydrogenase levels did not change with after sildenafil therapy was initiated (354 ± 52 IU/l presildenafil vs. 348 ± 53 IU/l, P = 0.5), suggesting that the haemolytic rate did not change with sildenafil therapy.
Table II.
Baseline haemodynamic parameters and response to inhaled nitric oxide.
| Baseline haemodynamic parameters* |
Nitric oxide | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | mPAP (mmHg) | PVR (dynes × s × cm5) | mCVP (mmHg) | CO (l/min) | mPCWP (mmHg) | SVR (dynes × s × cm5) | SVO2 (%) | SpO2 (%) | mPAP (mmHg) | CO (l/min) |
| 1 | 29 | 99 | 7 | 10.5 | 16 | 488 | 75 | 98 | 22 | 10.7 |
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 94 | n/a | n/a |
| 3 | 40 | 238 | 10 | 8.4 | 15 | 625 | n/a | 100 | n/a | n/a |
| 4 | 39 | 241 | 16 | 7.3 | 17 | 760 | 62 | 98 | 28 | 11 |
| 5 | 47 | 440 | 9 | 6.0 | 14 | 1107 | 62 | 97 | 36 | 7.8 |
| 6 | 26 | 114 | 10 | 7.7 | 14 | 758 | 72 | 100 | 21 | 11 |
| 7 | 30 | 169 | 12 | 7.1 | 15 | 866 | 65 | 99 | 26 | 8.6 |
| 8 | 28 | 119 | 8 | 10.1 | 13 | 418 | 70 | 96 | 25 | 12.3 |
| 9 | 23 | 83 | 4 | 10 | 12 | 568 | n/a | 98 | n/a | n/a |
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 97 | n/a | n/a |
| 11 | 50 | 285 | 11 | 10.0 | 15 | 409 | 72 | 98 | 35 | 13 |
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 96 | n/a | n/a |
| Mean ± SEM | 35 ± 3 | 199 ± 39 | 10 ± 1 | 8.6 ± 0.5 | 15 ± 1 | 667 ± 77 | 68 ± 2 | 98 ± 0.5 | 28 ± 2 | 10.6 ± 0.7 |
| P-value† | 0.015 | 0.015 | ||||||||
Right heart catheterisation was performed in consenting individuals with tricuspid regurgitant jet velocity ≥2.9 m/s.
Compared with baseline values.
mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; mCVP, mean central venous pressure; CO, cardiac output; mPCWP, mean pulmonary capillary wedge pressure; SVR, systemic vascular resistance; SVO2, mixed-venous oxygen saturation; SpO2, arterial oxygen saturation by pulse oximetry.
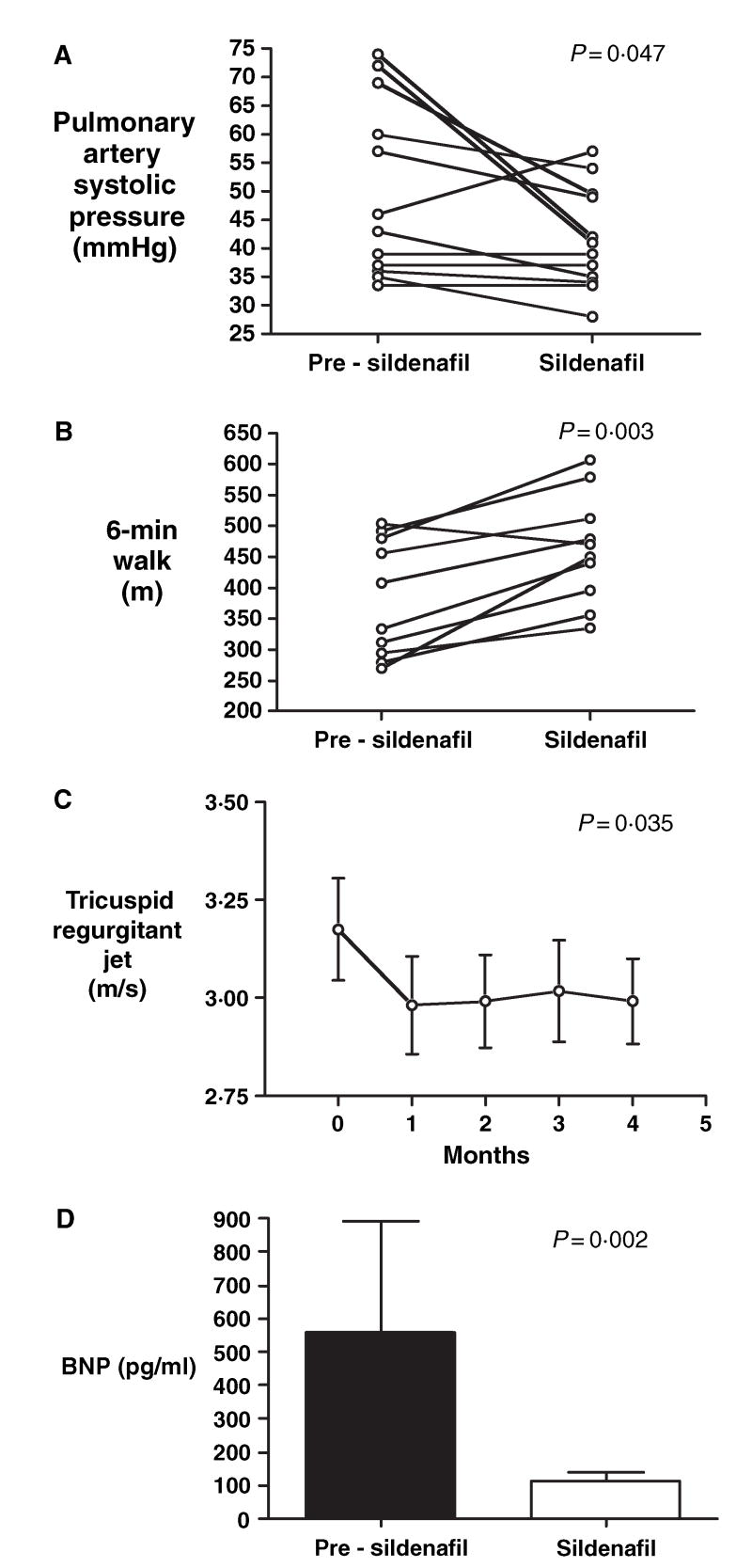
Although the pulmonary hypertension was only mild, all patients had significant impairment in exercise capacity, as evidenced by a low 6-min walk distance (Table III). Therapy with sildenafil for a mean duration of 6 ± 1 months decreased the estimated pulmonary artery systolic pressure (50 ± 4–41 ± 3 mmHg; difference −9 mmHg, 95% CI: 0.3–17, P = 0.047), and increased the 6-min walk distance (384 ± 30–462 ± 28 m; difference 78 m, 95% CI: 40–117, P = 0.003) (Table III, Fig 4). Sildenafil produced sustained reductions in pulmonary artery pressures over time, as measured by tricuspid regurgitant jet velocities (Fig 4C). In addition, sildenafil therapy significantly decreased plasma NT-pro-BNP levels [BNP (pg/ml): 559 ± 333–111 ± 28, P = 0.002], further suggesting that the improvement in exercise capacity was related to an improvement in pulmonary pressure and right ventricular function (Fig 4D).
Table III.
Effects of sildenafil on estimated pulmonary artery systolic pressure and exercise capacity.
| Duration of sickle cell disease intensification therapy (months) | Duration of sildenafil therapy (months) | Pulmonary artery systolic pressure (mmHg)
|
Six-min walk distance (m)* |
|||
|---|---|---|---|---|---|---|
| Patient | Presildenafil | Sildenafil | Presildenafil | Sildenafil | ||
| 1 | 4 | 4 | 36 | 37 | 492 | 579 |
| 2 | 6 | 4 | 35 | 28 | 408 | 479 |
| 3 | 3 | 12 | 74 | 41 | 279 | 356 |
| 4 | 3 | 12 | 69 | 49 | n/a | n/a |
| 5 | 6 | 4 | 60 | 54 | 334 | 440 |
| 6 | 3 | 12 | 43 | 34 | 312 | 396 |
| 7 | 3 | 4 | 46 | 57 | n/a | n/a |
| 8 | 8 | 6 | 57 | 49 | 456 | 512 |
| 9 | 3 | 4 | 36 | 34 | 278 | 450 |
| 10 | 6 | 4 | 34 | 34 | 487 | 607 |
| 11 | 6 | 5 | 72 | 41 | 290 | 335 |
| 12 | 3 | 5 | 39 | 39 | 504 | 470 |
| Mean ± SEM | 4.5 ± 0.5 | 6 ± 1 | 50 ± 4 | 41 ± 3 | 384 ± 30 | 462 ± 28 |
| P-value | 0.047 | 0.003 | ||||
The 6-min walk test could not be performed in two patients because of severe chronic pain and avascular necrosis of the hip.
Fig 4.
Effects of sildenafil on estimated pulmonary artery systolic pressure and exercise capacity in patients with sickle cell disease and pulmonary hypertension. Sildenafil therapy significantly decreased estimated pulmonary artery systolic pressure (A) and improved 6-min walk distance (B). (C) Time course of the effects of sildenafil on tricuspid regurgitant jet velocity. (D) Decrease in plasma NT-pro-BNP levels with sildenafil therapy.
Discussion
In patients with sickle cell disease and mild-to-moderate pulmonary hypertension, chronic therapy with sildenafil improved the estimated pulmonary arterial systolic pressure and exercise capacity. These effects were not related to changes in haemoglobin-oxygen carrying capacity since sickle cell disease therapy was intensified prior to initiation of sildenafil and haemoglobin and fetal haemoglobin levels remained unchanged throughout the study. This is the first therapeutic trial of chronic pulmonary vasodilator therapy in the sickle cell population in general, and in sickle cell patients with pulmonary hypertension in particular. The demonstration of an improvement in exercise capacity, as assessed by the 6-min walk test suggests that this test may be appropriate for future trials in this at-risk population.
In the present study, the acute haemodynamic effects of sildenafil and chronic effects on pulmonary artery pressures and exercise capacity were consistent with those seen in patients with other forms pulmonary arterial hypertension. Chronic sildenafil therapy increased the 6-min walk distance by 54 m in 12 patients with pulmonary hypertension secondary to inoperable chronic thromboembolic disease (Ghofrani et al, 2003a), by 93 m in 14 patients with severe pulmonary arterial hypertension failing inhaled iloprost therapy (Ghofrani et al, 2003b), and by 128 m in five other patients with severe pulmonary arterial hypertension (Michelakis et al, 2003). Although our patients only had mild pulmonary hypertension, these results are similar to the 78 m improvement in 6-min walk distance seen in our study, suggesting that even mild degrees of pulmonary hypertension may be poorly tolerated in chronically ill anaemic patients with sickle cell disease. Not only is the magnitude of improvement in the 6-min walk distance similar to that seen in patients with more severe pulmonary hypertension, but the baseline walk distance in our patients closely approximated that of patients enrolled in other pulmonary arterial hypertension trials (Channick et al, 2001; Rubin et al, 2002; Barst et al, 2004), again suggesting that even mild pulmonary hypertension is associated with significant functional impairment in patients with sickle cell disease. Even though the effects of sildenafil were similar to those observed in other patient populations, our results should be interpreted cautiously. This uncontrolled small open label study cannot conclusively rule out an effect of other confounders, such as patient selection bias or optimisation of supportive care. However, we used well-defined inclusion criteria, based on a known risk factor for mortality, and maximised all aspects of sickle cell specific care for 3–8 months prior to introduction of sildenafil. We specifically required that fetal haemoglobin levels were stable for at least 2 months prior to starting sildenafil. Finally, although the degree of pulmonary hypertension significantly improved in the majority of patients, tricuspid regurgitant jet velocities were mostly ≥2.5 m/s after treatment, still likely to indicate an increased risk of death.
In conclusion, pulmonary hypertension not only appears to be a marker of disease severity but also a significant cause of morbidity (i.e. poor exercise capacity) and possibly a cause of mortality in patients with sickle cell disease. Our results suggest that even mild elevations in pulmonary artery pressures are not well tolerated by patients with severe anaemia, such as those with sickle cell disease, and that treatment of the pulmonary hypertension may improve functional outcomes in this patient population. Our study was limited by the small number of patients enrolled and the open-label design. However, the data provide ‘proof of concept’ for designing a larger, multicentre randomised double-blind placebo-controlled trial evaluating the safety and efficacy of sildenafil in this patient population.
Acknowledgments
The study was funded by the Intramural Research Division of the National Institutes of Health, Bethesda, MD, USA. INO Therapeutics for the donation of nitric oxide gas and delivery system.
Footnotes
Conflict of interest
Dr Barst is a member of the scientific advisory board and a consultant for pulmonary arterial hypertension clinical drug development for Actelion, CoTherix, Encysive, and Pfizer.
References
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- Azizi E, Dror Y, Wallis K. Arginase activity in erythrocytes of healthy and ill children. Clinica Chimica Acta. 1970;28:391–396. doi: 10.1016/0009-8981(70)90063-x. [DOI] [PubMed] [Google Scholar]
- Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, McLaughlin V, Hill N, Tapson VF, Robbins IM, Zwicke D, Duncan B, Dixon RA, Frumkin LR. Sitaxsentan therapy for pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2004;169:441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- Belfiore F. Enzymatic Activities of the Blood Serum in Thalassemia and in Thalassodrepanocytosis. Riforma Medicine. 1964;78:1052–1055. [PubMed] [Google Scholar]
- Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. Journal of the American College of Cardiology. 1985;6:359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Schermuly RT, Rose F, Wiedemann R, Kohstall MG, Kreckel A, Olschewski H, Weissmann N, Enke B, Ghofrani S, Seeger W, Grimminger F. Sildenafil for long-term treatment of nonoperable chronic thromboembolic pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 2003a;167:1139–1141. doi: 10.1164/rccm.200210-1157BC. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Rose F, Schermuly RT, Olschewski H, Wiedemann R, Kreckel A, Weissmann N, Ghofrani S, Enke B, Seeger W, Grimminger F. Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension. Journal of the American College of Cardiology. 2003b;42:158–164. doi: 10.1016/s0735-1097(03)00555-2. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. New England Journal of Medicine. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- Hassoun PM, Krishnan JA. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. New England Journal of Medicine. 2004;350:2521–2522. [PubMed] [Google Scholar]
- Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. American Journal of Cardiology. 1990;66:493–496. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- Klings ES, Farber HW. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. New England Journal of Medicine. 2004;350:2521–2522. doi: 10.1056/NEJM200406103502418. [DOI] [PubMed] [Google Scholar]
- Lee AJ, Chiao TB, Tsang MP. Sildenafil for pulmonary hypertension. Annals of Pharmacotherapy. 2005;39:869–884. doi: 10.1345/aph.1E426. [DOI] [PubMed] [Google Scholar]
- Leuchte HH, Holzapfel M, Baumgartner RA, Ding I, Neurohr C, Vogeser M, Kolbe T, Schwaiblmair M, Behr J. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. Journal of the American College of Cardiology. 2004a;43:764–770. doi: 10.1016/j.jacc.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Leuchte HH, Neurohr C, Baumgartner R, Holzapfel M, Giehrl W, Vogeser M, Behr J. Brain natriuretic peptide and exercise capacity in lung fibrosis and pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 2004b;170:360–365. doi: 10.1164/rccm.200308-1142OC. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, Tymchak W, Noga M, Webster L, Wu XC, Lien D, Wang SH, Modry D, Archer SL. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation. 2003;108:2066–2069. doi: 10.1161/01.CIR.0000099502.17776.C2. [DOI] [PubMed] [Google Scholar]
- Morris CR, Morris SM, Jr, Hagar W, Van Warmerdam J, Claster S, Kepka-Lenhart D, Machado L, Kuypers FA, Vichinsky EP. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? American Journal of Respiratory and Critical Care Medicine. 2003;168:63–69. doi: 10.1164/rccm.200208-967OC. [DOI] [PubMed] [Google Scholar]
- Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Hazen SL, Vichinksy EP, Morris SM, Jr, Gladwin MT. Dysregulated arginine metabolism contributes to hemolysis-associated endothelial dysfunction, pulmonary hypertension and mortality in sickle cell disease. JAMA. 2005 doi: 10.1001/jama.294.1.81. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Nishikimi T, Okano Y, Uematsu M, Satoh T, Kyotani S, Kuribayashi S, Hamada S, Kakishita M, Nakanishi N, Takamiya M, Kunieda T, Matsuo H, Kangawa K. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. Journal of the American College of Cardiology. 1998;31:202–208. doi: 10.1016/s0735-1097(97)00452-x. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Kakishita M, Fukushima K, Okano Y, Nakanishi N, Miyatake K, Kangawa K. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. New England Journal of Medicine. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- Schnog JJ, Jager EH, van der Dijs FP, Duits AJ, Moshage H, Muskiet FD, Muskiet FA. Evidence for a metabolic shift of arginine metabolism in sickle cell disease. Annals of Hematology. 2004;83:371–375. doi: 10.1007/s00277-004-0856-9. [DOI] [PubMed] [Google Scholar]
- Voelkel NF, Lobel K, Westcott JY, Burke TJ. Nitric oxide-related vasoconstriction in lungs perfused with red cell lysate. FASEB Journal. 1995;9:379–386. doi: 10.1096/fasebj.9.5.7896007. [DOI] [PubMed] [Google Scholar]
- Voelkel NF, Allard JD, Anderson SM, Burke TJ. cGMP and cAMP cause pulmonary vasoconstriction in the presence of hemolysate. Journal of Applied Physiology. 1999;86:1715–1720. doi: 10.1152/jappl.1999.86.5.1715. [DOI] [PubMed] [Google Scholar]