Abstract
Heritability of hand preference was tested in a sample of 188 chimpanzees (Pan troglodytes). Hand preference was measured by coordinated bimanual actions, and concordance percentages were compared between parents and offspring and siblings. Among siblings, concordance percentages were compared for dyads in which both individuals were raised by chimpanzees, both were raised by humans, or 1 was raised in each environment. The results indicated population-level right hand preferences for coordinated bimanual actions. There were no significant associations in hand preference between parents and offspring. In full and maternal half siblings, concordance in hand preference was significantly greater than chance in mother- and human-reared individuals but not in cross-fostered dyads. The cumulative results suggest that the direction of hand preference is heritable in chimpanzees but the mechanism of transmission is not genetic. Several environmental explanations are proposed to explain the findings, including the potential role of maternal cradling bias and in utero fetal position.
Approximately 75% to 90% of modern humans report themselves as being right-handed (Annett, 1985; Porac & Coren, 1981). The general view is that human right-handedness evolved within the past 2 to 5 million years, as evidenced by the archeological data suggesting right-hand use in tool use and other activities in early hominids (Bradshaw & Rogers, 1993; Corballis, 1991). The pervasiveness of human right-handedness has led to numerous debates about the mechanisms involved in the expression of hand preference (B. Hopkins & Ronnqvist, 1998). Both genetic models (Annett, 1985; Corballis, 1997; Laland, Kumm, Van Horn, & Feldman, 1995; McManus, 1985; Yeo & Gangestad, 1993) and environmental models (Collins, 1985; Provins, 1997) have been proposed to explain the origin of human hand preference. The principal data in support of a genetic basis for hand preference are that it runs in families (Curt, De Agostini, Maccario, & Dellatolas, 1995; Laland et al., 1995; McGee & Cozad, 1980; McManus & Bryden, 1992) and that offspring typically exhibit patterns of hand preference more similar to their biological parents compared with offspring who have been adopted or raised by stepparents (Carter-Saltzman, 1980; Hicks & Kinsbourne, 1976).
In contrast with humans, the historical view has been that nonhuman animals, particularly nonhuman primates, do not exhibit population-level handedness (see Ettlinger, 1988; Warren, 1980). In recent years, behavioral research in a variety of nonhuman primate species has revealed that population-level handedness can be found in some species for certain measures (Bradshaw & Rogers, 1993; Fagot & Vauclair, 1991; W. D. Hopkins, 1996b; MacNeilage, Studdert-Kennedy, & Lindblom, 1987; Ward & Hopkins, 1993). Notwithstanding, considerable disagreement remains about the interpretation of the findings in nonhuman primates as they pertain to human handedness. For instance, McGrew and Marchant (1997) argued that the evidence for population-level handedness in nonhuman primates is weak and in no way resembles patterns of hand preference in human populations. In contrast, W. D. Hopkins (1996b, in press) argued that parallels between human and nonhuman primate handedness can be drawn on the basis of the existing data and that apparent differences in findings between settings may be due to methodological variation (see W. D. Hopkins & Rabinowitz, 1997).
Irrespective of whether one accepts that nonhuman primates exhibit population-level handedness, a fundamental question that has arisen is whether the potential causal mechanism in the determination of hand preference in animals is the same as that in humans. Warren (1980) proposed that human handedness is determined by genetic factors whereas nonhuman primate handedness is determined purely by environmental or experiential factors. To date, the evidence for heritability of hand preference in nonhuman primates is equivocal (Brinkman, 1984; Brooker, Lehman, Heinbuch, & Kidd, 1981; Byrne & Byrne, 1991; W. D. Hopkins, Bales, & Bennett, 1993; Kubota, 1990; Matoba, Masataka, & Tanioka, 1991; Westergaard & Suomi, 1997), and in none of these studies can potential genetic factors be isolated from environmental factors, because the offspring in most of these studies were raised by their biological mothers and, therefore, no specific manipulation of rearing environment was performed. Selective-breeding studies in mice clearly suggest no genetic basis for the determination of directional biases in paw preferences, although strength of paw preference appears to be under some genetic control (see Collins, 1985, for a review).
The purpose of this study was to examine the role of genetic and environmental factors in the expression of hand preference in chimpanzees. At the Yerkes Regional Primate Research Center (YRPRC), there is a unique opportunity to examine the role of genetics and environment in the expression of handedness in chimpanzees because there are genetically related individuals who have been raised in different environments. This rearing practice results effectively in a partial interspecies cross-fostering paradigm, which is a potentially powerful means by which to assess the effects of environmental and genetic factors on handedness (e.g., Carter-Saltzman, 1980). To date, no partial or complete cross-fostering studies have been performed in nonhuman primates, and they are necessary to test Warren's (1980) hypothesis that the mechanisms underlying hand preference differ in humans and nonhumans as well as to uncover basic information on the effects of gene–environment interactions on behavioral development in a species that is biologically very closely related to humans.
Method
Subjects
One hundred and eighty-eight captive chimpanzees (Pan troglodytes) were the subjects in this study, including 111 females and 77 males. The subjects ranged from 3 to 54 years of age (M = 16.58 years, SD = 11.43 years). Eighty-six chimpanzees were mother-reared (MR), and 102 chimpanzees were human-reared (HR). Any chimpanzee that stayed with its mother beyond 30 days of age was considered MR. Chimpanzees that entered the YRPRC nursery before 30 days of age were considered HR (see Bard, 1994, 1996, for a description of nursery-rearing practices for chimpanzees at the YRPRC).
Hand-Preference Measures and Procedure
The behavioral task used to assess hand preference in the chimpanzees is referred to as the coordinated bimanual tube task (CBTT) and has been described in detail elsewhere (W. D. Hopkins, 1995). The CBTT entailed the use of poly vinyl chloride tubes (24–31 cm long and 2.5 cm wide) with peanut butter smeared on the inside edge, approximately 2–4 cm in depth. The tubes were given to the subjects in their home cage by pushing them through the cage mesh. The digit and hand used to remove the peanut butter was recorded as either the left or the right each time the subjects inserted their finger, removed peanut butter from the tube, and placed their finger in their mouth. Observations continued until the subjects stopped showing interest in the tubes (usually when they had eaten all the peanut butter), dropped them for at least 10 s, or pushed the tubes back out of their home cage through the cage mesh. In all cases, the data were collected continuously and without interruption from other subjects housed with the focal subject. A minimum of 20 responses were collected from each subject.
Individual data were collected in the subjects' home cage, including the indoor and outdoor portions of the enclosure. The home cages varied in size and shape, depending on the housing assignment of each subject, but all cages were constructed of 5-cm wire cage mesh. In addition, the number of individuals in each home cage varied from 2 to as many as 16. Focal-animal sampling techniques were used to collect individual data. Thus, individual animals were not separated from their social group for the purpose of data collection but rather were observed in their group. To reduce competition over the tubes, all subjects were given a tube during each test session, but focal data were collected on only 1 individual per session. For all test sessions, the hand used by the experimenter in placing food items or handing test materials to the subjects was randomized across trials. This randomization was done to ensure that the subjects were not imitating the hand use of the experimenter. For each subject, a z score based on the total frequency of right- and left-hand responses was determined. On the basis of the z scores with p < .05, subjects were classified as being left-handed (z ≤ −1.96), right-handed (z ≥ 1.96), or ambiguously handed (z > −1.96 and z < 1.96). In addition to the z scores, a handedness index (HI) was derived for each subject by subtracting the number of left-hand responses (L) from the number of right-hand responses (R) and dividing by the total number of responses: (R − L)/(R + L). HI values varied from −1.0 to 1.0, with positive values reflecting right-hand biases and negative values representing left-hand biases. The absolute value of HI reflected the strength in preferred hand use (see W. D. Hopkins, in press, for a description of various statistical issues in assessing hand preference in nonhuman primates). The z-score classification scheme was used for the descriptive analyses of the sample in terms of their hand preference and any potential effects of sex, age, and rearing history. For the heritability analyses, a slightly more liberal classification criterion was used to increase the sample size and to remove the ambiguously handed category from the analyses. For these analyses, subjects with negative HI values were classified as left-handed, and subjects with positive HI values were classified as right-handed. Subjects with an HI score of zero (n = 7) were classified as left-handed.
Results
Descriptive Statistics
On the basis of the z-score classifications, the distribution of hand preference in the sample was 95 right-handed subjects, 53 left-handed subjects, and 40 ambiguously handed subjects. The number of right-handed chimpanzees differed significantly from the number of left-handed, χ2(1, N = 148) = 11.92, p < .001, and ambiguously handed, χ2(1, N = 135) = 22.41, p < .001, chimpanzees. However, the number of left-handed subjects was not significantly greater than the number of ambiguously handed subjects, χ2(1, N = 93) = 1.82, p > .05. To examine the effects of sex and rearing history on the distribution of hand preference, log-linear analyses were performed. The distribution of hand preference for each rearing condition and sex can be seen in Table 1. No significant interactions were found between either sex or rearing history and the distribution of hand preference.
Table 1.
Distribution of Hand Preference as a Function of Rearing History and Sex
| Hand preference (n) | ||||
|---|---|---|---|---|
| Sex | Rearing history | Left | Ambiguous | Right |
| Male | Mother-reared | 6 | 9 | 18 |
| Female | Mother-reared | 20 | 8 | 27 |
| Male | Nursery-reared | 13 | 6 | 25 |
| Female | Nursery-reared | 14 | 17 | 25 |
| Total | 53 | 40 | 95 | |
Heritability
Parent-offspring concordance
The distribution of offspring hand preference on the basis of parental hand preference can be seen in Table 2. For 65 offspring, hand preferences were known for both mothers and fathers. Mothers, fathers, and offspring were classified as left- or right-handed on the basis of the sign of their HI score. Offspring with two left-handed parents were classified as having familial left-handedness (FL), whereas offspring with two right-handed parents were classified as having familial right-handedness (FR). Offspring with one left-handed parent and one right-handed parent were classified as having mixed handedness (MH). In the MH group, a further distinction was made between paternal and maternal right or left hand preferences.
Table 2.
Offspring Hand Preference on the Basis of Maternal and Paternal Hand Preference
| Offspring hand preference (n) | ||
|---|---|---|
| Parental hand preference | Left | Right |
| Both parents | ||
| Group | ||
| FL: L (father and mother) | 6 | 10 |
| MH1: L (father) and R (Mother) | 4 | 9 |
| MH2: R (father) and L (mother) | 5 | 12 |
| FR: R (father and mother) | 9 | 10 |
|
| ||
| One parent | ||
| Mother | ||
| L | 12 | 31 |
| R | 29 | 53 |
| Father | ||
| L | 13 | 24 |
| R | 14 | 22 |
Note. FL = familial left-handedness; L = left hand preference; MH = mixed handedness; R = right hand preference; FR = familial right-handedness.
A chi-square test of independence analysis revealed no significant interaction between familial and offspring hand preferences, χ2(3, N = 65) = 1.52, ns. As an alternative means of testing for the effect of familial handedness on offspring hand preference, an analysis of variance (ANOVA) was used to compare the offspring HI values between the four phenotypic pairings: FL, FR, MH1 (father = left-handed, mother = right-handed), and MH2 (father = right-handed, mother = left-handed). As with the chi-square analysis, no significant effect of familial handedness was found, F(3, 62) = 1.45, ns. The mean HI values for the FL, MH1, MH2, and FR groups were .22, .38, .11, and .03, respectively.
In addition, the distribution of offspring hand preference was compared on the basis of either only the mother or only the father (see Table 2). Chi-square tests of independence failed to reveal significant interactions between parental and offspring hand preferences for both the mothers, χ2(1, N = 125) = 0.71, ns, and the fathers, χ2(1, N = 63) = 0.11, ns. As with the previous analysis, the HI data were analyzed using ANOVA for both maternal and paternal hand preference. In each analysis, parental hand preference and rearing served as between-groups variables, whereas the offspring HI score served as the dependent measure. In both analyses, no significant effects of parental hand preference or rearing were revealed.
In the next analyses, the concordance rates in hand preference between parents and offspring were examined in relation to the rearing environment. Depicted in Table 3 are the number of MR and HR offspring classified as left- or right-handed on the basis of either the mothers' or the fathers' hand preference. For offspring born to left-handed mothers, a 2 × 2 chi-square failed to reveal an effect of rearing environment on hand preference. Similarly, for offspring born to right-handed mothers, no significant interaction was found between rearing environment and the distribution of offspring hand preference. Analyses of the effects of paternal hand use and rearing were not performed because the expected values in a number of the cells of the 2 × 2 chi-square did not meet the minimum of five recommended for this statistic. Nonetheless, a cursory analysis of the data in Table 3 did not suggest that rearing had an effect on the distribution of hand preference in offspring born to either left- or right-handed fathers.
Table 3.
Concordance in Offspring and Parental Hand Preference in Mother- and Nursery-Reared Chimpanzees
| Mother-reared | Nursery-reared | |||
|---|---|---|---|---|
| Parental hand preference | Left | Right | Left | Right |
| Maternal hand preference | ||||
| Left | 2 | 9 | 12 | 23 |
| Right | 10 | 21 | 17 | 30 |
| Paternal hand preference | ||||
| Left | 5 | 12 | 3 | 6 |
| Right | 8 | 12 | 11 | 16 |
Sibling concordance
To assess whether the concordance percentages were statistically significant, expected probabilities of concordance and disconcordance were derived on the basis of the distribution of hand preferences for the entire sample. The probability of being right-handed (r), left-handed (l), or ambiguously handed (a) for the sample was .51 (95 out of 188), .28 (53 out of 188), and .21 (40 out of 188), respectively. The probability of concordance (Pconcordance) in hand preference was Σ[p(r)2 + p(l)2 + p(a)2 + 2(p(a × l))], which summed to a value of .44. The probability of disconcordance (Pdisconcordance) was equal to 1 − Pconcordance (or .56). These probability values were used to derive z scores to evaluate the significance of concordance percentages from different conditions, z scores were derived by the following formula:
where X is equal to the number of dyads with the same hand preference and N is equal to the total number of dyads. z scores were evaluated against the two-tailed normal distribution with alpha as p < .05 and a critical value of 1.96.
There were 65 full siblings and 73 maternal half siblings in the sample. For the two groups, the concordances in hand preference were 73% and 57%, respectively. To test for the effect of rearing environment on concordance rates in hand preference, full siblings and maternal half siblings were considered as one group because they did not differ significantly in concordance rates. For this sample of chimpanzees, concordance percentages were determined on the basis of whether both siblings had been MR, both siblings had been HR, or one was MR and the other was HR. The percentage of concordance in hand preference for full siblings and maternal half siblings in each rearing condition is shown in Figure 1. A 2 × 3 chi-square test of independence indicated a significant interaction between concordance percentage and rearing history, χ2(2, N = 138) = 43.99, p < .001. For both the MR (z = 5.47, p < .01) and HR (z = 4.80, p < .01) groups, the concordance percentages in sibling hand preferences were significantly greater than chance, whereas they were not significantly above chance in the cross-fostered condition (z = −1.63, ns).
Figure 1.
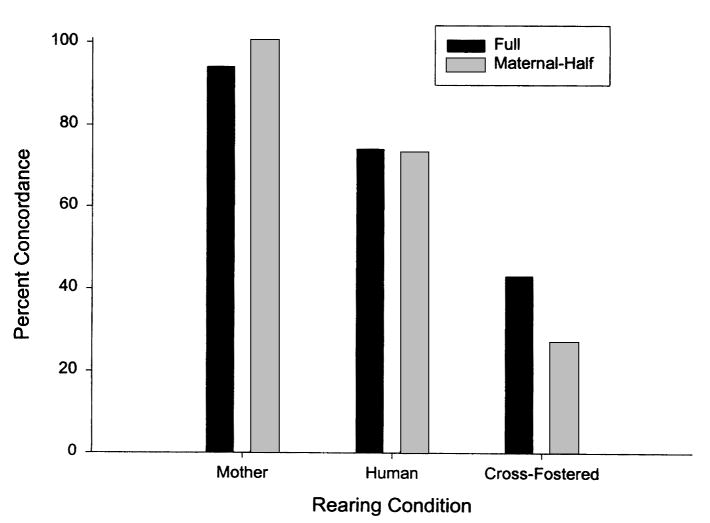
Full and maternal half sibling concordances in hand preference for mother-reared, human-reared, and cross-fostered chimpanzees.
Discussion
The results of this study indicate that chimpanzees unequivocally exhibit population-level right-handedness for coordinated bimanual actions. Furthermore, direction in hand preferences for coordinated bimanual actions is heritable to some extent, but the findings do not suggest that genetic factors are the underlying mechanism for the transmission of hand preference within families. The principal evidence in support of this argument comes from the lack of significant concordances in hand preference among cross-fostered chimpanzees. Recall that the percentage of agreement in hand preference for cross-fostered full siblings and maternal half siblings failed to reach statistical significance. If genetic factors were the mechanism for heritability of hand preference in chimpanzees, then the concordance percentages in the cross-fostered siblings should have exceeded chance levels, as they did in the MR and HR siblings. In addition, there was no evidence that offspring born to parents with either FL or FR exhibited different distributions of hand preference. In heritability studies of hand preference in humans in which a genetic mechanism has been inferred, there is typically a higher proportion of right-handed off-spring with two right-handed parents compared with either mixed-dominant or two left-handed parents (see McManus & Bryden, 1992; Porac & Coren, 1981). The only data that remotely suggest a genetic mechanism in the determination of hand preference were the higher concordances in hand preference in full siblings compared with maternal half siblings in the cross-fostered group, but this difference did not reach conventional levels of statistical significance (see Figure 1).
The lack of strong evidence for a possible genetic basis for hand preference in chimpanzees could be interpreted as support for Warren's (1980) claim that the mechanisms underlying human hand preference are fundamentally different than those observed in animals. However, it is important to emphasize several aspects of the findings that are relevant to Warren's arguments and to general genetic models of human handedness. First, the chimpanzees' hand preferences were heritable, as revealed by the significant concordance percentages in HR and MR siblings. Warren claimed that hand preferences in nonhuman species are determined by random environmental factors and therefore lead to bimodal distributions of limb preference. Hand preferences clearly were not bimodally distributed in this sample, nor were they differentially affected by rearing history. In addition, siblings reared in the same environment did show significant concordance percentages that rival, if not exceed, values that have been reported for human siblings in whom heritability of hand preferences has been reported (Carlier, Beau, Marchaland, & Michel, 1994). Therefore, the heritability of hand preference in chimpanzees appears to be determined by one or more environmental, experiential, or biological factors, but this factor does not appear to be a random event.
Second, by no means is there uniform agreement that genetic factors play the sole role in determining hand preference in humans (see above); therefore, Warren's (1980) assumptions regarding the mechanisms determining human handedness may not be warranted. Third, in heritability studies with humans, the sample sizes are typically much larger than those used in this study; therefore, significant effects can be detected despite the fact that there are significantly more right- than left-handed offspring in familial right-, familial left-, and mixed-handed groups. Perhaps with a larger sample the distribution of hand preference in familial left- and right-handed offspring would significantly differ in the chimpanzees. Finally, the degree of variation in the MR and HR environments is arguably much greater in this study compared with the typical human cross-fostering paradigm. Specifically, human cross-fostered infants are at least raised by the same species that assumes similar maternal or pedagogical stimulation, whereas the HR chimpanzees are subject to a rearing environment that is quite foreign to them. Whether genetically related human participants raised in similarly diverse environments would show high concordance rates is not clear.
In the absence of strong evidence for a genetic basis in the development of chimpanzee handedness, experiential and environmental explanations seem warranted in keeping with the goal of parsimony. As such, there are at least three possible environmental, experiential, or biological factors that may account for the heritability of direction in hand preference in chimpanzees, including (a) maternal cradling bias (Provins, 1997), (b) intrauterine fetal position (Previc, 1991), or (c) prenatal hormonal environment (Geschwind & Galaburda, 1985). The degree to which any single theory or combination of these theories explains the observed patterns of heritability in the chimpanzees varies, and at present, there are no strong data that can be used to support or challenge any of these views.
With respect to maternal cradling bias, W. D. Hopkins, Bard, Jones, and Bales (1993) previously reported that maternal cradling bias was inversely correlated with infants' hand preferences for simple reaching when tested at 3 years of age. Specifically, mothers who cradle or carry their offspring on their left side have offspring who prefer their right hand for simple reaching at 3 years of age. In contrast, mothers who cradle or carry their offspring on their right side have offspring who exhibit left hand preferences for simple reaching at 3 years of age. If individual mothers show consistent cradling or carrying biases between successive offspring, then the siblings would be predicted to have the same hand preferences. This prediction is consistent with the observed findings in the present study, particularly for MR siblings. This theory also can potentially explain the significant population-level right-handedness observed in this sample because female chimpanzees and other great apes have been reported to show population-level left-sided cradling biases for their infants (Manning & Chamberlain, 1990; but see Dienske, Hopkins, & Reid, 1995; W. D. Hopkins, Bard, et al., 1993) or the infants show left-nipple preferences (Nishida, 1993). This theory can potentially explain the observed findings in this study for the MR siblings, but it does not adequately explain the concordance percentages in the HR chimpanzees unless it is assumed that the human caretakers actively sought to cradle or carry genetically related individuals on the same side. This is highly unlikely because there was significant turnover in the care staff personnel during the period of time covered in this study (some 30 years). Unfortunately, the hand preferences of the caretakers and the manner in which they cradled or carried the offspring are not known.
An alternative possibility is that some other mechanism that is common to both MR and HR chimpanzees is governing or determining cradling or carrying bias in the human caretakers and the chimpanzee mothers. One possible mechanism is the position of the infant in utero and its effect on postural and head-orienting behavior of the off-spring. Regarding the intrauterine environment, Previc (1991) proposed that during the last trimester, the fetus is oriented such that its head is projected outward, which places constraints on the movements of the left hand of the fetus because of its proximity to the mother's pelvis and back-bone. From this position, the fetus receives different stimulation of the right otolith compared with the left because of asymmetries in acceleration and deceleration forces associated with bipedal walking by the mother. Previc proposed that about 65% of fetuses are oriented such that their right side is outwardly exposed, which results in the increased incidence in right-sided neonatal asymmetries such as head orientation. The presence of these early head-orientation asymmetries could lead to greater right hand-eye coordination during the early periods of development, which emerges as right-handedness in adults (see Michel, 1981).
The significance of Previc's (1991) theory is relevant to the findings of this study in two important ways. First, it is possible that the in utero orientation of offspring is consistent for certain female chimpanzees but varies from female to female. Second, there is some evidence in humans that the orientation of the infant's head affects the cradling bias of the mother (Ginsburg, Fling, Hope, Musgrove, & Andrews, 1979). Infants with their heads oriented to the right are cradled on the subjects' left side and vice versa. Thus, it may be that certain mothers have offspring that have their heads oriented in one direction or the other because of the in utero position, and this causes both female chimpanzees and human caretakers to cradle them on one side or the other. The subsequent effect of the maternal or caretaker cradling bias would then lead to similar hand preferences among full siblings or maternal half siblings (see above). This theory assumes that head orientation predicts hand preference in chimpanzees, and there is some support for this assumption. Neonatal chimpanzees show a population-level right-side bias for head orientation when lying in a supine posture (W. D. Hopkins & Bard, 1995), and lateral biases in head orientation were significantly correlated with hand use for the CBTT task when subjects were tested between 3 and 5 years of age (W. D. Hopkins, 1996a). Although this scenario can potentially explain the significant concordance percentages for HR and MR siblings, it does not explain the lack of significant concordance data for the cross-fostered individuals. Moreover, whether Previc's theory has any direct application to nonhuman primates remains unclear, particularly because bipedalism is not the characteristic mode of locomotion in nonhuman primates. In addition, there are no published data on intrauterine head orientation in chimpanzees, and these would seem critical in testing Previc's hypothesis.
Finally, it is possible that hormonal factors play a role in the determination of hand preference in relation to rearing history. Dahl (in press) reported that female chimpanzees who keep their offspring (i.e., the MR subjects) have significantly higher estrogen and progesterone levels during pregnancy than do female chimpanzees who reject or fail to exhibit adequate maternal care for their offspring (i.e., the HR subjects). These data have been interpreted as evidence that prenatal hormones influence maternal behavior. In terms of heritability of hand preference, it may be that chimpanzees that either consistently keep or consistently reject their offspring have stable prenatal hormonal environments that ultimately influence their offspring's hand preference. In contrast, marginal chimpanzee mothers who keep some offspring and reject others (the cross-fostered subjects of this study) may show higher variability in prenatal hormones that regulate or influence both the development of hand preference and species-typical maternal behavior. This theoretical framework would explain all of the observed sibling effects reported in Figure 1. Unfortunately, at present, there is no evidence for a relationship between prenatal hormones and hand preference in chimpanzees, and the findings in humans remain highly controversial (see Bryden, McManus, & Bulman-Fleming, 1994, and commentaries).
In conclusion, the findings of this study suggest that chimpanzee hand preferences are heritable, a result that is consistent with human findings (Porac & Coren, 1981); with one previous report in captive chimpanzees (W. D. Hopkins, Bales, et al., 1993); and, to some extent, with findings in monkeys (Brinkman, 1984; but see Brooker et al., 1981; Kubota, 1990; Westergaard & Suomi, 1997). In contrast with previous studies, this is the first heritability study in nonhuman primates in which rearing history was manipulated and allowed for a dissociation of genetic and environmental contributions to the expression of hand preference. Previous studies in nonhuman primates focused solely on parental influences on offspring hand preferences in MR individuals. Future studies in monkeys should focus on dissociating environmental and genetic factors by adopting similar cross-fostering procedures to assess whether monkeys show patterns similar to those observed in chimpanzees. In addition, in monkeys, the cross-fostering procedure could be done both within the same species as well as between species. This potentially could be important for dissociating postnatal from prenatal influences on hand preference in primates. Heritability of hand preference for additional measures of hand use in chimpanzees is warranted as well in light of the fact that not all measures significantly correlate with each other and do not necessarily induce population-level asymmetries. Finally, studies of whether similar heritability effects are observed for other functional or neuroanatomical asymmetries in chimpanzees are warranted. Cumulatively, these studies will lead to a better understanding of biological and evolutionary factors underlying the expression of hemispheric specialization in primates, including humans.
Acknowledgments
This research was supported by National Institutes of Health Grants NS29574 and RR00165 to the Yerkes Regional Primate Research Center. The Yerkes Regional Primate Research Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
I thank the animal care staff and the animal records staff for their help in executing this research. I also appreciate the helpful assistance of Samuel Fernandez, Dave Leavens, and Lisa Parr in the collection of the data.
References
- Annett M. Left, right, hand, and brain: The right-shift theory. London: Erlbaum; 1985. [Google Scholar]
- Bard KA. Evolutionary roots of intuitive parenting: Maternal competence in chimpanzees. Early Development and Parenting. 1994;1:19–28. [Google Scholar]
- Bard KA. Responsive care: Behavioral intervention for nursery-reared chimpanzees. 1996. Available from the Jane Goodall Institute, Ridgefield, CT 06877. [Google Scholar]
- Bradshaw B, Rogers L. The evolution of lateral asymmetries, language, tool-use and intellect. San Diego, CA: Academic Press; 1993. [Google Scholar]
- Brinkman C. Determinants of hand preference in Macaca fascicularis [Abstract] International Journal of Primatology. 1984;5:325. [Google Scholar]
- Brooker RJ, Lehman RAW, Heinbuch RC, Kidd KK. Hand usage in a colony of bonnett monkeys (Macaca radiata) Behavior Genetics. 1981;11:49–56. doi: 10.1007/BF01065827. [DOI] [PubMed] [Google Scholar]
- Bryden MP, McManus IC, Bulman-Fleming MB. Evaluating the empirical support for the Geschwind–Behan–Galaburda model of cerebral lateralization. Brain and Cognition. 1994;26:103–167. doi: 10.1006/brcg.1994.1045. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Byrne JM. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla gorilla berengei) Cortex. 1991;27:521–536. doi: 10.1016/s0010-9452(13)80003-2. [DOI] [PubMed] [Google Scholar]
- Carlier M, Beau J, Marchaland C, Michel F. Sibling resemblance in two manual laterality tasks. Neuropsychologia. 1994;32:741–746. doi: 10.1016/0028-3932(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Carter-Saltzman L. Biological and sociocultural effects on handedness: Comparison between biological and adoptive parents. Science. 1980 September 12;209:1263–1265. doi: 10.1126/science.7403887. [DOI] [PubMed] [Google Scholar]
- Collins RL. On the inheritance of direction and degree of asymmetry. In: Glick S, editor. Cerebral lateralization in nonhuman species. Orlando, FL: Academic Press; 1985. pp. 41–71. [Google Scholar]
- Corballis MC. The lopsided ape: Evolution of the generative mind. New York: Oxford University Press; 1991. [Google Scholar]
- Corballis MC. The genetics and evolution of handedness. Psychological Review. 1997;104:714–727. doi: 10.1037/0033-295x.104.4.714. [DOI] [PubMed] [Google Scholar]
- Curt F, De Agostini M, Maccario M, Dellatolas G. Parental hand preference and manual functional asymmetry in preschool children. Behavior Genetics. 1995;25:525–536. doi: 10.1007/BF02327576. [DOI] [PubMed] [Google Scholar]
- Dahl JF. Perineal swelling during gestation and maternal competence in chimpanzees. Journal of Medical Primatology in press. [Google Scholar]
- Dienske H, Hopkins B, Reid AK. Lateralisation of infant holding in chimpanzees: New data do not confirm previous findings. Behaviour. 1995;132:801–809. [Google Scholar]
- Ettlinger GF. Hand preference, ability and hemispheric specialization: How far are these factors related in the monkey? Cortex. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychological Bulletin. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization: Biological mechanisms, associations and pathology: I. A hypothesis and program of research. Archives of Neurology. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Ginsburg HJ, Fling S, Hope ML, Musgrove D, Andrews C. Maternal holding preferences: A consequence of newborn head-turning response. Child Development. 1979;50:280–281. [PubMed] [Google Scholar]
- Hicks R, Kinsbourne M. Genetic basis for human handedness: Evidence from a partial cross-fostering study. Science. 1976 May 28;192:908–910. doi: 10.1126/science.1273577. [DOI] [PubMed] [Google Scholar]
- Hopkins B, Ronnqvist L. Human handedness: Developmental and evolutionary perspectives. In: Simion F, Butterworth G, editors. The development of sensory, motor and cognitive capacities of early infancy: From perception to cognition. East Sussex, United Kingdom: Psychology Press; 1998. pp. 191–236. [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees (Pan troglodytes): Cross-sectional analysis. Journal of Comparative Psychology. 1995;105:178–190. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Chimpanzee handedness: Analogous or homologous processes?. Paper presented at the biennial meeting of the International Primatological Society; Madison, WI. 1996a. Aug, [Google Scholar]
- Hopkins WD. Chimpanzee handedness revisited: 55 years since Finch (1941) Psychonomic Bulletin and Review. 1996b;3:449–457. doi: 10.3758/BF03214548. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. International Journal of Primatology in press. [Google Scholar]
- Hopkins WD, Bales S, Bennett AJ. Heritability of hand preference in chimpanzees (Pan troglodytes) International Journal of Neuroscience. 1993;74:17–26. doi: 10.3109/00207459408987225. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. Evidence of asymmetries in spontaneous head turning in infant chimpanzees (Pan troglodytes) Behavioral Neuroscience. 1995;110:1212–1215. doi: 10.1037//0735-7044.109.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA, Jones A, Bales S. Chimpanzee hand preference for throwing and infant cradling: Implications for the origin of human handedness. Current Anthropology. 1993;34:786–790. [Google Scholar]
- Hopkins WD, Rabinowitz DM. Manual specialization and tool-use in captive chimpanzees (Pan troglodytes): The effect of unimanual and bimanual strategies on hand preference. Laterality. 1997;3:267–278. doi: 10.1080/713754273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K. Preferred hand use in the Japanese macaque troop, Arashiyama-R, during visually-guided reaching for food pellets. Primates. 1990;31:393–406. [Google Scholar]
- Laland KN, Kumm J, Van Horn JD, Feldman MW. A gene–culture model of human handedness. Behavior Genetics. 1995;25:433–445. doi: 10.1007/BF02253372. [DOI] [PubMed] [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–303. [Google Scholar]
- Manning JT, Chamberlain AT. The left-side cradling preference in great apes. Animal Behaviour. 1990;39:1224–1227. [Google Scholar]
- Matoba M, Masataka N, Tanioka Y. Cross-generational continuity of hand-use preference in marmosets. Behaviour. 1991;117:281–286. [Google Scholar]
- McGee MG, Cozad T. Population genetic analysis of human hand preference: Evidence for generation differences, familial resemblance and maternal effects. Behavior Genetics. 1980;10:263–275. doi: 10.1007/BF01067772. [DOI] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- McManus IC. Handedness, language dominance and aphasia: A genetic model. Psychological Medicine. 1985;8:1–40. [PubMed] [Google Scholar]
- McManus IC, Bryden MP. The genetics of handedness, cerebral dominance and lateralization. In: Rapin I, Segalowitz SJ, editors. Handbook of neuropsychology: Vol 6. Developmental neuropsychology, Pt 1. Amsterdam: Elsevier; 1992. pp. 115–144. [Google Scholar]
- Michel GF. Right-handedness: A consequence of infant supine head-orientation preference? Science. 1981 May 8;212:685–687. doi: 10.1126/science.7221558. [DOI] [PubMed] [Google Scholar]
- Nishida T. Left nipple suckling preference in wild chimpanzees. Ethology and Sociobiology. 1993;14:45–52. [Google Scholar]
- Porac C, Coren S. Lateral preferences and human behavior. New York: Springer; 1981. [Google Scholar]
- Previc FH. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychological Review. 1991;98:299–334. doi: 10.1037/0033-295x.98.3.299. [DOI] [PubMed] [Google Scholar]
- Provins KA. Handedness and speech: A critical reappraisal of the role of genetic and environmental factors in the cerebral lateralization of function. Psychological Review. 1997;104:554–571. doi: 10.1037/0033-295x.104.3.554. [DOI] [PubMed] [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Westergaard GG, Suomi SJ. Lateral bias in capuchin monkeys (Cebus apella): Concordance between parents and offspring. Developmental Psychobiology. 1997;31:143–147. [PubMed] [Google Scholar]
- Yeo RA, Gangestad SW. Developmental origins of varcation in human hand preference. Genetica. 1993;89:281–296. [Google Scholar]


