Abstract
Historically, population-level handedness has been considered a hallmark of human evolution. Whether nonhuman primates exhibit population-level handedness remains a topic of considerable debate. This paper summarizes published data on handedness in great apes. Comparative analysis indicated that chimpanzees and bonobos show population-level right handedness, whereas gorillas and orangutans do not. All ape species showed evidence of population-level handedness when considering specific tasks. Familial analyses in chimpanzees indicated that offspring and maternal (but not paternal) handedness was significantly positively correlated, but this finding was contingent upon the classification criteria used to evaluate hand preference. Overall, the proportion of right handedness is lower in great apes compared with humans, and various methodological and theoretical explanations for this discrepancy are discussed.
Keywords: handedness, great apes, laterality, behavior genetics
Approximately 85% to 90% of humans report themselves as being right handed (Annett, 1985, 2002; Perelle & Ehrman, 1994; Porac & Coren, 1981; Raymond & Pontier, 2004). Right handedness presumably reflects a left-hemisphere specialization for motor skills and is thought to be an indirect marker of lateralization for language functions. For example, 96% of right-handed individuals are left-hemisphere dominant for language in contrast to about 70% in left-handed individuals (Knecht et al., 2000; Rasmussen & Milner, 1977). The association between hand preference and language dominance has led to a number of evolutionary and biological theories proposing that hemispheric specialization and language (or perhaps other higher cognitive functions) coevolved and are unique characteristics of the human brain (see Annett, 1985; Corballis, 1992, 2002; Crow, 1998; Yeo, Thoma, & Gangestad, 2002, for reviews). The historical lack of evidence for functional asymmetries in nonhuman species, with the exception of birds, was taken as evidence in support of the uniqueness of hemispheric specialization to humans (Bradshaw & Rogers, 1993). However, based on research carried out in the past 10 to 15 years, good evidence exists of population-level hemispheric specialization in nonhuman species for various cognitive, perceptual, and to a lesser extent, motor functions (Rogers & Andrew, 2002; Ward & Hopkins, 1993; Vallortigara & Rogers, 2005). For example, toads prefer to the use their right paw when removing materials from their bodies and orienting themselves when floating upside-down (Bisazza, Cantalupo, Robins, Rogers, & Vallortigara, 1996). Chicks and pigeons show a host of behavioral asymmetries including left-hemisphere biases in local discrimination of food items (Andrew, 1991; Güntürkün, 1997). Chicks also show and right-hemisphere biases in predator detection (Vallortigara, 1992), as has also been reported in certain species of fish (Bisazza, Cantalupo, Capocchiano, & Vallortigara, 2000). These data clearly challenge the long held belief that hemispheric specialization is a uniquely human trait and, at a minimum, indicate that language is not a sufficient condition for the expression of hemispheric specialization in vertebrates.
Despite the recent significant evidence of behavioral asymmetries in animals, one area that continues to be a source of considerable theoretical and empirical debate is whether population-level hand preferences are evident in nonhuman primates. Evidence of population-level hand preference has been reported in some species for specific measures of hand use (see Hook-Costigan & Rogers, 1997; Hopkins, 1996; MacNeilage, Studdert-Kennedy, & Lindblom, 1987; Ward & Hopkins, 1993) but many remain skeptical of these findings (Ettlinger, 1988; McGrew & Marchant, 1993, 1997; Palmer, 2002, 2003). In the specific case of the human's closest living relatives, the great apes, which include the genus Pan (chimpanzees, bonobos), Pongo (orangutans) and Gorilla (gorillas), very few studies have been conducted until recently, and most had very small sample sizes. However, a number of studies on handedness in captive and wild apes have recently been reported, and the data have been inconsistent, further fueling the debate over the existence of population-level handedness in nonhuman primates. In captivity, evidence of population-level right handedness has been reported, but the data are less clear in wild apes (see Hopkins & Cantalupo, 2005, for review). Some have suggested that population-level handedness is restricted to captive apes because they are modeling the right handedness of humans they are exposed to or their hand preferences are shaped by living in a right-handed human world (see McGrew & Marchant, 1997; Palmer, 2003). Others have argued that differences in handedness are not the result of rearing per se but reflect methodological and statistical issues (Hopkins & Cantalupo, 2005).
In addition, previous attempts to characterize handedness in nonhuman primates have used arbitrary criteria for inclusion and exclusion of certain studies (McGrew & Marchant, 1997), and this has resulted in the omission of many important and significant studies in the literature. For example, McGrew and Marchant (1997) did not include any studies that did not maintain independence of data points during handedness data collection. Independence of data points refers the probability of using one hand for a response based on the occurrence of the same hand being used on the previous response, in a sequence or bout of actions (see Hopkins, 1999b, for discussion). Recent empirical studies have shown that hand use is consistent whether discrete or nonindependent data collection techniques are used (Damerose & Hopkins, 2002; Hopkins et al., 2005); thus independence of data points is not a reasonable criterion for inclusion (or exclusion) of certain studies (Lehman, 1993). Moreover, conceptually no reason exists to assume that a lack of independence of individual data points would skew the distribution in hand use uniformly to the left or right. Presumably, lack of independence of data points in hand use would be randomly distributed across subjects. Last, other attempts to summarize ape handedness across studies have not evaluated hand use independently for different measures or have used limited data sets (Palmer, 2002).
To address the limitations of previous summary studies of handedness in great apes, one aim of this paper is to summarize and evaluate the distribution of handedness in great apes using a common index of hand use across studies. Rather than arbitrarily define which studies should or should not be included, as many studies as possible on handedness in great apes since 1900 are included in this paper. In as much as is possible, individual data were extracted from the reports and empirical tests of population-level and species differences in handedness were performed using a variety of approaches. A second aim of this paper is to evaluate the influence of rearing history on hand preference in great apes. This variable is very important because, as suggested by Palmer (2002) and others (McGrew & Marchant, 1997), the absence of population-level handedness in wild apes in the face of contradictory findings in captive apes would raise questions about the validity of any evolutionary models of cerebral dominance, as they pertain to handedness, in nonhuman primates when based primarily on findings from captive apes. To date, little evidence exists of a significant influence of rearing history on handedness in great apes, but this remains an important variable for consideration. To address the significance of rearing history, subjects from as many studies as possible were characterized as being tested in the wild or captivity, and the influence of settings on handedness was empirically tested on the existing published data. If living or being reared in captivity has a significant effect on handedness in great apes, then captive-born apes should be significantly more right handed than wild apes.
A third aim of this paper is to evaluate whether handedness runs in families of apes. The pervasiveness of human right handedness, and the presumed lack of population-level handedness in nonhuman species, has also led to numerous debates about the mechanisms involved in the development and evolution of hand preference (Hopkins & Ronnqvist, 1998; Yeo, Thoma, & Gangestad, 2002). Some genetic models propose one or two loci coding for handedness (or related lateralized brain functions [i.e., language]) (Annett, 1985; Corballis, 1997; Klar, 1999; McManus, 1985), whereas other genetic models rely on invoking general levels of heterozygosity where high levels of homozygosity are associated with higher levels of developmental instability and deviations in modal right handedness (Laland, Kumm, Van Horn, & Feldman, 1995; Yeo & Gangestad, 1993). Some non-genetic models also exist for the development of hand preference in humans, such as social learning and pressure, postnatal infant head position, or in utero fetal position (Collins, 1985; Michel, 1981; Previc, 1991; Provins, 1997).
The principal data in support of the genetic models for human hand preference are that it runs in families (Curt, De Agostini, Maccario, & Dellatolas, 1995; Laland et al., 1995; McGee & Cozad, 1980; McManus & Bryden, 1992), and that offspring typically exhibit patterns of hand preference more similar to their biological parents compared with offspring who have been either adopted (Carter-Saltzman, 1980) or raised by step-parents (Hicks & Kinsbourne, 1976). Studies in twins also indicate that concordance rates in hand preference are higher in monozygotic compared with dizygotic individuals, further supporting a genetic basis for handedness (Sicotte, Woods, & Mazziota, 1999). Genetic models are heuristically good, but it should be emphasized that at this time, no candidate gene or set of genes has been identified for the expression of handedness, although ongoing programs seek potential candidate genes (e.g., Francks et al., 2002; Geschwind & Miller, 2001; Kim et al., 1999).
Warren (1980) was a strong proponent of the view that population-level handedness was uniquely human and that genetic factors determined human handedness, whereas nongenetic factors determined nonhuman limb preferences. Approximately 20 years ago very few studies existed on heritability of hand preference in nonhuman primates, and the results were equivocal (see Hopkins, 1999a, for review). Furthermore, paw preference studies failed to reveal any evidence that directional biases in paw use could be selectively bred for in certain strains of mice, although it should pointed out that strength in paw preference could be selectively bred for (Collins, 1985). In contrast, paw preferences in mice easily conform to biased environments and can be acquired through observational learning (Collins, 1975). These findings reinforced the view that nongenetic mechanisms explain the preponderance of limb biases in nonhuman animals. However, more recent comparative studies of paw preference in different strains of selectively bred and genetic knock-out mice have revealed some evidence for a possible genetic basis for paw preference (Biddle, Coffaro, Ziehr, & Eales, 1993; Signore, Chaoui, Nosten-Bertrand, Perez-Diaz, & Marchaland, 1991; Waters & Denenberg, 1994). In addition, laterality in eye preferences for viewing predators can be selectively bred for in fish (Bisazza, Facchin, & Vallortigara, 2000). Selective breeding on eye preference resulted in concordant separation in laterality for other behavioral measures in these fish (Bisazza, Sovrano, & Vallortiagara, 2001). The recent success in selective breeding for laterality in fish and phenotypic variation in preferences in different strains of mice raises questions about both the proposed uniqueness of laterality to humans and the mechanisms that influence hand preferences in human and nonhuman primates.
In summarizing all the published raw data on hand preference in great apes, pedigree information (i.e., maternity, paternity, or both) was provided for subjects in a number of studies. The pedigree information was used to evaluate heritability in hand preference in great apes. In particular, the degree of phenotypic variation in hand preference was compared in the offspring born to parents with similar or different hand preferences. This analysis focused on great ape handedness, because the apes share as much as 96% of the same DNA proteins as humans; therefore if handedness has a genetic basis and the gene or set of genes was present in other nonhuman species, then great apes would be a good species for testing heritability given their close phylogenetic relationship to humans.
The results are broken down into two parts. In part I, data on the distribution of handedness in great apes and the effects of sex and rearing history are presented. In part II, the data on heritability of handedness are presented. Following the presentation of the results, the potential role of genetic and nongenetic factors in explaining individual and species difference in handedness are discussed. In addition, methodological and statistical issues in the assessment of handedness are discussed.
Method
Selection of Studies
Data were obtained from published articles or book chapters, theses or dissertations, and published abstracts or unpublished reports since 1900 (see Tables 1 & 2). Searches from several databases (i.e., Medline, PubMed, Psychlit, Dissertation Abstracts International) were performed to identify studies on handedness in great apes. The search results were combined with an assessment of reference sections of extant papers, and any unknown reports were identified and obtained through interlibrary loans, if the journals were not readily available. One set of analyses was conducted from reports that provided individual raw data for the various handedness measures used in the study (see Table 1). Critical for inclusion in this analysis was the ability to derive or obtain individual data for each subject and measure(s) used to assess handedness in the report. Studies that focused on asymmetries in neonatal or infant apes were not included in the analysis (Chorazyna, 1976; Fagot & Bard, 1995; Hopkins & Bard, 1993, 1995; Hopkins, Bard, & Griner, 1997; Itakura, 1996; Nishida, 1993). In addition, studies that only examined laterality in maternal cradling bias were not included, because theoretical and empirical reasons exist to predict different patterns of asymmetry for this behavior (Dienske, Hopkins, & Reid, 1995; Manning, Heaton, & Chamberlain, 1994).
Table 1.
List of Published Studies, Theses, and Abstracts on Handedness in Great Apes
| Authors | Testing environment | Task(s) | |
|---|---|---|---|
| 1. | Albrecht & Dunnett (1971) | W | Bimanual feeding |
| 2. | Annett & Annett (1991) | C | Simple reaching |
| 3. | Aruguete et al. (1992)NRD | C | Object manipulation, self-touching |
| 4. | Boesch (1991) | W | Simple reaching, grooming, wadge-dipping, nut cracking |
| 5. | Brenot (1992) | C | Simple reaching |
| 6. | Bresard & Bresson (1983) | C | Unimanual and bimanual hand use tasks |
| 7. | Byrne & Byrne (1991) | W | Bimanual feeding |
| 8. | Butterworth & Itakura (1998)NRD | C | Simple reaching |
| 9. | Carpenter (1937) | W | Grooming |
| 10. | Christel (1994) | C | Simple reaching |
| 11. | Christel et al. (1998) | C | Simple reaching |
| 12. | Colell et al. (1995a, b) | C | Food reaching, hand drinking, current making, object throwing |
| 13. | Colell et al. (1995a, b) | C | Vertical panel, horizontal panel, latch box |
| 14. | Corp & Byrne (2004) | W | Bimanual feeding |
| 15. | Cunningham et al. (1989) | C | Leading limb, nonfood reaching, food reaching, baby touches body, baby touches head, baby touches mother, mother touches baby |
| 16. | Dimond & Harries (1984) | C | Face touching |
| 17. | Fagot & Vauclair (1988) | C | Simple reaching, coordinated visual alignment task |
| 18. | Fernandez-Carriba & Loeches (2001) | C | Fruit smearing |
| 19. | Fernandez-Carriba et al. (2001) | C | Numerous observational and experimental tasks |
| 20. | Ferster (1957)NRD | C | Lever press |
| 21. | Finch (1941) | C | Simple reaching |
| 22. | Fischer et al. (1982)NRD | C | Object manipulation, simple reaching |
| 23. | Fletcher & Weghorst (2005) | C | Groom, scratch, pick up, eat, touch face, hold, nose wipe, pull, cross arms, tool use |
| 24. | Foucart et al. (in press)NRD | C | Tool use |
| 25. | Glaser (1971)NRD | C | Scribbling |
| 26. | Goodall (1963)NRD | W | Tool use |
| 27. | Grzimek (1949)NRD | C | Numerous behaviors |
| 28. | Haas (1958)NRD | C | Simple reaching |
| 29. | Harrison & Nystrom (2001)NRD | C | Carry, gesture, object manipulation, tool use, reach |
| 30. | Heestand (1986) | C | Walk/run, support sitting, climb, eat, manipulate with fingers, manipulate with whole hand, leading limb |
| 31. | Holder (2000) | W | Groom, leading limb, feeding |
| 32. | Hopkins (1991) | C | Joystick manipulation |
| 33. | Hopkins (1993) | C | Simple and bipedal reaching |
| 34. | Hopkins (1994) | C | Bimanual feeding |
| 35. | Hopkins (1995a) | C | Coordinated bimanual hand use |
| 36. | Hopkins (1995b) | C | Simple reaching (infants only) |
| 37. | Hopkins et al. (2001) | C | Coordinated bimanual hand use |
| 38. | Hopkins & Cantalupo (2003) | C | Coordinated bimanual hand use |
| 39. | Hopkins and deWaal (1995) | C | Gesture, self-touch, leading limb, carry, face touch, eat |
| 40. | Hopkins & Rabinowitz (1997) | C | Tool use |
| 41. | Hopkins et al. (1993) | C | Bipedal reaching, tripedal reaching, reaching, gesture, self-touch, leading, limb, carry |
| 42. | Hopkins et al. (in press) | C | Throwing |
| 43. | Hopkins, Hook, et al. (2003) | C | Coordinated bimanual hand use |
| 44. | Hopkins, Stoinski, et al. (2003) | C | Coordinated bimanual hand use |
| 45. | Hopkins et al. (2004) | C | Coordinated bimanual hand use |
| 46. | Hopkins et al. (2002, 2005) | C | Simple reaching |
| 47. | Hopkins et al. (2005) | C | Manual gestures |
| 48. | Ingmanson (1996, 1998) | W | Simple reaching |
| 49. | Jones-Engel & Bard (1996) | C | Simple reaching |
| 50. | Kellogg & Kellogg (1933)NRD | C | Simple reaching |
| 51. | Krause & Fouts (1997) | C | Manual gestures |
| 52. | Leavens et al. (2001) | C | Scratch |
| 53. | Lockard (1984)NRD | C | Simple reaching, social and nonsocial activities |
| 54. | Lonsdorf & Hopkins (2005) | W | Tool use |
| 55. | Lutz-Maki & MacNeilage (1991)NRD | C | Pulling task |
| 56. | Marchant (1983) | C | Feeding, nonsocial reach, social reach, simple reaching, hold, carry, groom, throw |
| 57. | Marchant & Steklis (1986) | C | Unimanual and bimanual structured tasks |
| 58. | Marchant and McGrew (1996) | W | Numerous natural behaviors |
| 59. | Matsuzawa et al. (2001) | W | Tool use |
| 60. | McGrew and Marchant (1992) | W | Tool use |
| 61. | McGrew & Marchant (1996) | W | Tool use |
| 62. | McGrew and Marchant (1999)NRD | W | Tool use |
| 63. | McGrew and Marchant (2001) | W | Numerous natural behaviors |
| 64. | McGrew et al. (1999) | W | Anvil use |
| 65. | Miles (1990)NRD | C | Sign language |
| 66. | Milliken et al. (1994) | C | Self-touching |
| 67. | Morange (1994) | C | Tool use |
| 68. | Moricello et al. (in press) | C | Leading limb in locomotion and posture |
| 69. | Morris et al. (1993) | C | Numerous unimanual and bimanual structured tasks |
| 70. | Nishida & Hiraiwa (1982) | W | Tool use |
| 71. | Olson et al. (1990) | C | Bipedal and tripedal reaching |
| 72. | O'Neil et al. (1978)NRD | C | Simple reaching |
| 73. | Parnell (2001) | W | Feeding |
| 74. | Peters (2005) | W | Leading limb, feeding, simple reaching |
| 75. | Redshaw (1993)NRD | C | Simple reaching |
| 76. | Rensch & Ducker (1966)NRD | C | Complex motor tasks |
| 77. | Reiss et al. (1949) | C | Simple reaching |
| 78. | Robinson (1979) | C | Simple reaching |
| 79. | Rogers & Kaplan (1995)NRD | W | Self-touching, carry, feed |
| 80. | Schaller (1963)NRD | W | Chest beating |
| 81. | Schiller (1951)NRD | C | Drawing |
| 82. | Shafer (1988)NRD | C | Sign language |
| 83. | Shafer (1993) | C | Touch self, touch others, hit/slap, throw, eat, manipulate large object, manipulate small object, dig/sift, gesture |
| 84. | Shafer (1997) | C | Touch self, touch others, hit/slap, throw, eat, manipulate large object, manipulate small object, dig/sift, gesture |
| 85. | Steiner (1990) | C | Groom self, groom other, eat/hand, eat/object, manipulate, reach, hold, carry, hang, hit, throw, sign language |
| 86. | Sugiyama et al. (1993) | W | Simple reaching, tool use |
| 87. | Sugiyama (1995)NRD | W | Tool use |
| 88. | Toback (1999) | C | Carry, slap, manipulate, hold, suck, grab, stomp, cradle, hand to mouth, pick up, extend, touch, self-touch, catch, thrust |
| 89. | Tonooka & Matsuzawa (1995) | C | Simple reaching |
| 90. | Vleeschouwer et al. (1994) | C | Simple reaching |
| 91. | Vleeschouwer et al. (1995) | C | Simple reaching |
| 92. | Weidauer (1972) | C | Simple reaching |
| 93. | Welles (1976)NRD | C | Small item reaching |
| 94. | Yanez-Gonzalez et al. (2004) | C | Feeding, grooming, scratching |
| 95. | Yeager (1991)NRD | W | Feeding |
| 96. | Yerkes (1927) | W | Numerous natural behaviors |
Note. NRD = no raw data provided by the authors. For the testing environment variable, W = wild; C = captive.
Table 2.
Studies Reporting Hand Preferences in Great Apes but for Which No Individual Data Were Reported
| Investigator | #L | #A | #R | Species |
|---|---|---|---|---|
| Harrison & Nystrom (2001) | 6 | 3 | 10 | Bonobo |
| Aruguete et al. (1992) | 1 | 19 | 7 | Pan |
| Butterworth & Itakura (1998) | 3 | 7 | 1 | Pan |
| Ferster (1957) | 0 | 0 | 1 | Pan |
| Foucart et al. (in press) | 0 | 0 | 1 | Pan |
| Glaser (1971) | 1 | 0 | 0 | Pan |
| Goodall (1963) | 1 | 7 | 0 | Pan |
| Grzimek (1949) | 0 | 2 | 0 | Pan |
| Kellogg & Kellogg (1933) | 0 | 1 | 0 | Pan |
| Lutz-Maki & MacNeilage (1991) | 2 | 1 | 11 | Pan |
| McGrew et al. (1999) | 8 | 3 | 2 | Pan |
| O'Neil et al. (1978) | 4 | 0 | 4 | Pan |
| Schiller (1951) | 0 | 0 | 1 | Pan |
| Sugiyama (1995) | 2 | 2 | 4 | Pan |
| Welles (1976)* | 1 | 5 | 4 | Pan |
| Carpenter (1937) | 0 | 0 | 2 | Gorilla |
| Haas (1958) | 0 | 0 | 3 | Gorilla |
| Lockard (1984) | 2 | 1 | 5 | Gorilla |
| Redshaw (1993) | 0 | 0 | 2 | Gorilla |
| Rensch & Ducker (1966) | 0 | 1 | 0 | Gorilla |
| Schaller (1963) | 0 | 0 | 8 | Gorilla |
| Shafer (1988) | 1 | 1 | 0 | Gorilla |
| Grzimek (1949) | 0 | 1 | 0 | Pongo |
| Miles (1990) | 1 | 0 | 0 | Pongo |
| Rensch & Ducker (1966) | 0 | 1 | 0 | Pongo |
| Rogers & Kaplan (1995) | 16 | 12 | 1 | Pongo |
| Yeager (1991) | 0 | 3 | 4 | Pongo |
| Total | 49 | 70 | 71 |
For the Welles (1976) paper, only the data presented for the center position responses were considered for this analysis.
A number of reports of hand use in apes also existed that did not include individual raw data (see Table 1, indicated by NRD after the name of the author[s]). In these cases, the authors provided hand preference classifications of their subjects but without raw data. Rather than completely ignore these results, the descriptive hand preference classifications are reported in Table 2 along with the name of the author(s) and the species studied.
Data Coding and Analysis
For this study, a spreadsheet was configured that coded for the following subject information including (a) species, (b) subject name or identification code, (c) sex, (d) testing environment, (e) number of tasks used to assess hand preference, and (f) percent of right-hand use for each of the behaviors measured within a study. Sex was coded as male, female, or unknown. Testing environment was coded as wild or captivity. The number of tasks used to assess hand preference was entered for each subject and study and could vary within and between studies. For example, some studies may have recorded hand preferences on three measures, whereas other studies may have used more or fewer measures. Additionally, within a study, not all subjects may have been assessed on every measure of hand use.
Within each study, individual handedness was calculated several ways. First, the total number of left- and right-hand responses was calculated across all measures. Based on the total number of responses, a percentage of right-hand use was calculated by dividing the total number of right-hand responses by the total number of response [SUM%R = (#R/#R + #L)]. Individual SUM%R scores were derived only for subjects where at least six observations of the behavior were reported in the paper. In addition, z-scores were calculated based on the total frequency of right- and left-hand use for each individual subject. Based on the individual z-scores, subjects were classified as strongly left handed (SL), mildly left handed (ML), ambiguously handed (AH), mildly right handed (MR) and strongly right handed (SR). Subjects with z-scores < = −1.96 were classified as SLH, −1.95 to −1.0 (ML), −0.99 to 0.99 (AH), 1.0 to 1.95 (ML), and = > 1.96 (SR), respectively. The cut points were chosen based on two criteria. First, for the SL and SR categories, subjects with z-scores greater than 1.95 or less than −1.95 would have hand preferences that exceed chance at p < .05. This has historically been a common practice on the assessment of handedness in nonhuman primates, despite the relative conservative basis of this statistic. Second, the cut points of −1.0 and 1.0 for MR and ML categories were selected because they represent one standard deviation from a mean of zero in a normally distributed population.
One problem with the SUM%R and individual z-scores is that the total number of observations of left- and right-hand use will be influenced by the frequency of occurrence of different behaviors recorded within a study. For example, within a study, the total number of responses observed for grooming might be 400, whereas only 125 observations were made for reaching. In this case, grooming would contribute more to the total number of left- and right-hand responses than reaching, and this would be particularly problematic if there were strong task-specific variation in directional biases in hand use. Thus a mean percentage right-hand (MEAN%R) use was entered for each subject using the following criteria. For this analysis, for each subject and measure, a percentage right-hand use was calculated based on the number right-handed responses divided by the total number of responses. The MEAN%R was determined by calculating the average percentage right-hand use for each subject across all measures within a study. Thus each subject had a MEAN%R based on all measures of hand use.
Lastly, some consistency was noted in the behaviors assessed for handedness when compared across different studies. To assess the potential influence of the type of behavior on the expression of handedness, a percentage right-hand use was derived following the formula %R = [(#R)/(#R + #L)∗100]. The individual %R scores were calculated so that handedness could be evaluated for specific behaviors or tasks across studies in which the same measures were obtained in the apes. For example, handedness for grooming was collected in nine studies, and %R scores were derived from each individual in each study and combined to evaluate whether the %R score for the combined grooming data differed significantly or not from chance (50%).
In some previous summaries of great ape handedness, summary values were derived from all studies, although the same subjects were represented in different studies (McGrew & Marchant, 1997; Palmer, 2002). This factor is problematic because the data points are not independent of each other. For example, Corp and Byrne (2004) examined hand preferences for bimanual feeding in the Mahale chimpanzees, whereas McGrew and Marchant (2001) studied spontaneous hand use in the Mahale chimpanzees. Because they studied the same cohort of chimpanzees, some overlap exists in the subjects making up these two separate data sets. In this review, all measures of hand use, independent of the investigator, were combined to derive a single SUM%R and MEAN%R for each subject. This was done to ensure independence of data points such that one SUM%R or MEAN%R value went with one and only one subject.
Part 1: Hand Preferences in Great Apes
Descriptive Statistics
Individual raw hand preference data were reported in 1,524 great apes including 1,044 chimpanzees, 97 bonobos, 280 gorillas, and 103 orangutans, respectively. Of the total sample, there were 840 females, 600 males, and 84 individuals with an unknown sex. In terms of rearing histories of the sample, there were 681 captive-born apes, 670 wild-born apes, and 173 with unknown rearing histories. In addition, hand preferences were reported in an additional 25 studies comprising 190 apes for which no individual raw data accompanied the report (see Table 2).
Population Effects and Hand Preference Distribution
The SUM%R and MEAN%R percentages were 52.74% t(1520) = 4.73, p < .001 and 54.80%, t(1523) = 4.66, p < .001, values that differ significantly from 50%, as would be predicted if the values were normally distributed. The number of measures used in determining each SUM%R and MEAN%R score (range = 1 to 25) did not significantly correlate with the SUM%R (r = −.014, df = 1522, ns) or MEAN%R values (r = −.013, df = 1522, ns). Thus the SUM%R and MEAN%R scores indicated significant population-level right handedness for the entire sample. This finding is not surprising because the correlation between the two measures was strongly positive and significant (r = .96, df = 1522, p < .001). Because the two scores were so strongly correlated, for subsequent analyses, the SUM%R scores were used rather than both measures. Because there were a number of related individuals in the sample, one additional t-test was performed on the SUM%R scores for the unrelated individuals in the sample. This analysis also revealed a significant population-level right-hand bias (Mean = 52.08) t(1047) = 2.88, p < .004.
The distribution of hand preferences based on the classification cut points applied to the individual z-scores is shown in Table 3. A chi-square goodness-of-fit test indicated that there were significantly more strongly right than strongly left-handed subjects χ2(1, N = 935) = 27.72, p < .01 but not significantly more mildly right-than mildly left-handed subjects χ2(1, N = 199) = 1.13, ns.
Table 3.
Distribution of Handedness as a Function of Species, Sex, and Rearing History
| Hand preference classification | |||||
|---|---|---|---|---|---|
| SL | ML | AH | MR | SR | |
| Species | |||||
| Bonobo | 5 | 4 | 22 | 21 | 40 |
| Chimpanzee | 281 | 68 | 171 | 71 | 375 |
| Gorilla | 70 | 14 | 46 | 11 | 100 |
| Orangutan | 31 | 6 | 14 | 4 | 33 |
| Sex | |||||
| Females | 217 | 52 | 146 | 51 | 308 |
| Males | 157 | 35 | 87 | 45 | 222 |
| Unknown | 13 | 5 | 20 | 11 | 18 |
| Testing Environment | |||||
| Captivity | 286 | 68 | 183 | 68 | 442 |
| Wild | 101 | 24 | 70 | 39 | 106 |
| Total | 388 | 91 | 253 | 107 | 548 |
Note. Values represent raw frequencies.
Meta-Analysis
The significant population-level right handedness found in the previous analysis was confirmed when meta-analytic statistics were performed on the results from the existing independent studies. Specifically, for each individual data set with eight or more subjects, a SUM%R was calculated for each of the behavioral measures using the procedure described previously (see Data Coding and Analysis). Recall that some subjects may have been studied on multiple measures and presented in different studies. The individual studies that were combined to derive each individual data set are indicated in Table 4 after the name of the author(s). The SUM%R scores for each study were then analyzed using a one-sample t-test and the resulting t-value, significance probability, sample size, and effect size are shown in Table 4. Each statistical probability was converted to a z-score, and the average z-score was derived for the entire sample and divided by the appropriate error term. This analysis revealed that, overall, great apes showed population-level handedness (z = 6.06, p < .0001) but the effect size was relatively small (d = .156). A funnel plot depicting the SUM%R for each study against the sample size (N) is shown in Figure 1.
Table 4.
Effect Sizes of One Sample T-tests for Handedness in Different Samples of Great Apes
| Authors | t | N | p | Cohen's d |
|---|---|---|---|---|
| Albrecht & Dunnett (1971) | 0.259 | 11 | .801 | 0.163 |
| Annett & Annett (1991) | 0.503 | 31 | .619 | 0.183 |
| Boesch (1991) | 1.568 | 66 | .112 | 0.389 |
| Brenot (1992) | −3.340 | 9 | .010 | 2.227 |
| Byrne & Byrne (1991)* | −0.178 | 38 | .860 | 0.058 |
| Christel (1994)10,11 | 2.379 | 25 | .026 | 0.971 |
| Colell et al. (1995a, b)12,13 | 1.290 | 36 | .206 | 0.436 |
| Dimond & Harris (1984) | −5.085 | 20 | .000 | 2.331 |
| Fagot & Vauclair (1988) | −0.702 | 10 | .501 | 0.467 |
| Fernanadez-Carriba et al. (2001)18,19,68 | 0.822 | 9 | .433 | 0.306 |
| Finch (1941) | −0.425 | 30 | .674 | 0.158 |
| Fletcher & Weghorst (2005) | −0.166 | 26 | .870 | 0.066 |
| Gijzen (1972) | 0.095 | 18 | .926 | 0.041 |
| Heestand (1986) | 3.822 | 57 | .000 | 1.201 |
| Holder (2000) | −0.621 | 26 | .540 | 0.248 |
| Hopkins (1993–2003)33–38,42,46 | 6.383 | 218 | .000 | 0.866 |
| Hopkins et al. (2003)43, 86 | 1.610 | 159 | .109 | 0.256 |
| Hopkins et al. (2004)45 | 2.140 | 147 | .034 | 0.354 |
| Hopkins et al. (2003) (Gorilla)44 | 1.420 | 38 | .164 | 0.631 |
| Hopkins et al. (2003) (Pongo)44 | −2.541 | 19 | .021 | 1.197 |
| Hopkins & de Waal (1995)39,41 | 2.179 | 21 | .041 | 0.974 |
| Ingmanson (1996, 1998) | 5.339 | 44 | .000 | 1.647 |
| Jones-Engel & Bard (1996) | −1.385 | 13 | .191 | 0.799 |
| Marchant (1983)56,57 | −0.618 | 26 | .542 | 0.131 |
| Marchant et al. (Gombe)58,60,61 | 0.029 | 56 | .977 | 0.008 |
| McGrew et al. (Mahale)14,63 | −0.454 | 49 | .652 | 0.131 |
| Milliken et al. (1994) | −1.426 | 8 | .197 | 1.078 |
| Olson et al. (1990) | 2.092 | 25 | .047 | 0.834 |
| Parnell (2001) | −0.887 | 32 | .382 | 0.318 |
| Peters (2005) | 3.620 | 21 | .001 | 1.619 |
| Shafer (1997) | 1.629 | 14 | .127 | 0.904 |
| Shafer (1993) | 2.864 | 47 | .007 | 0.839 |
| Steiner (1990) | 0.145 | 12 | .887 | 0.087 |
| Sugiyama et al. (1993)59,86 | 0.646 | 20 | .526 | 0.296 |
| Tonooka & Matsuzawa (1995) | −0.822 | 80 | .414 | 0.185 |
| Vleeschower et al. (1994, 1995)90,91 | 0.415 | 20 | .683 | 0.190 |
| Weidauer (1972) | 0.592 | 8 | .572 | 0.448 |
| Yanez-Gonzalez et al. (2004) | 7.085 | 8 | .000 | 5.356 |
Note. Superscripted values indicate papers from which raw data were combined to derive a single set of data for the apes (see Table 1).
To be consistent with other reports of hand use for bimanual actions, the data from this study were reverse scored from the reported results so that dominant hand use reflected the hand used for feeding.
Figure 1.
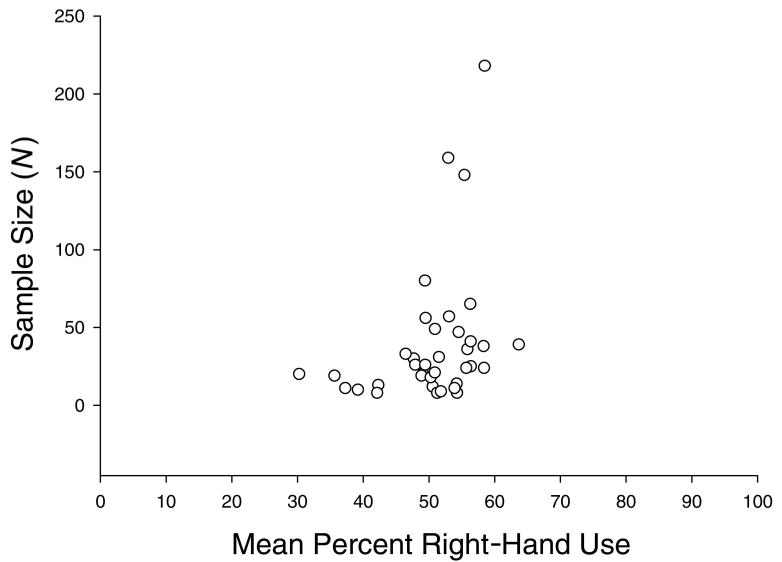
Funnel plot of SUM%R scores and N for each study in the meta-analysis.
Species Differences
Species differences in handedness were compared using an analysis of covariance (ANCOVA). Species was the between group factor and the SUM%R score was the dependent variable. The number of tasks used to derive each individual SUM%R score served as the covariate. A significant effect of species on hand preference was found F(3, 1519) = 4.15, p < .01. The SUM%R scores for each species can be seen in Figure 2. Posthoc analysis using Tukey's Honestly Significant Difference (HSD) indicated that bonobos, gorillas, and chimpanzees had significantly higher SUM%R scores than orangutans. In addition, the bonobos had significantly higher SUM%R scores than chimpanzees and gorillas. One sample t-test within each species revealed population-level right handedness for chimpanzees t(1043) = 3.72, p < .001 and bonobos t(96) = 6.17, p < .01. No population-level handedness was found for gorillas t(279) = 1.71, p < .08 or orangutans t(102) = −0.983, ns. A chi-square test of independence comparing the distribution of hand preference in great apes revealed a similar association χ2(12, N = 1387) = 54.75, p < .001. The distribution of hand preferences as a function of species can be seen in Table 3.
Figure 2.
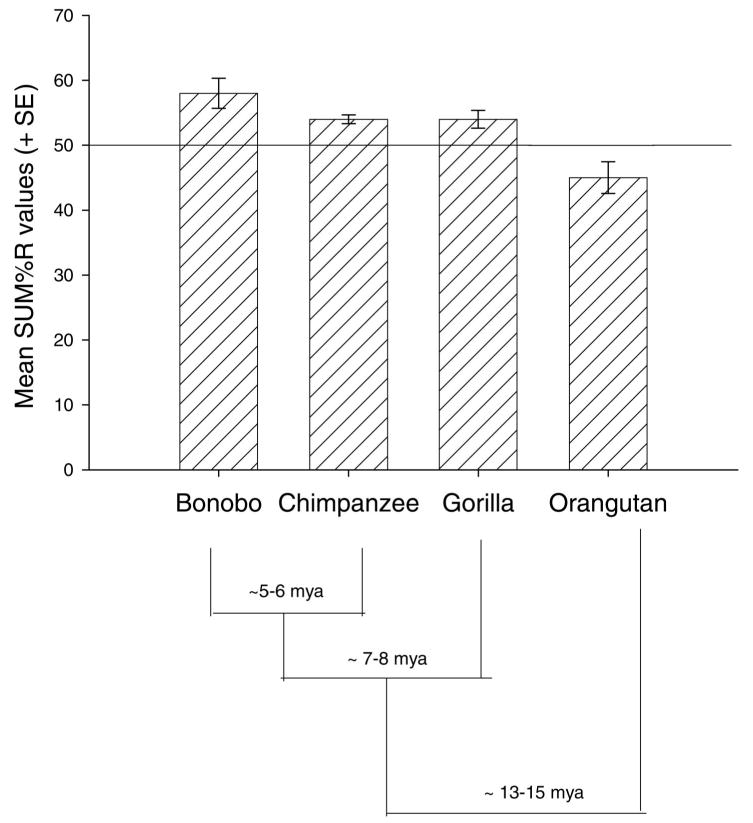
Mean SUM%R score (+ SE) for each species of great ape. Lines indicate evolutionary divergence of different great apes species.
The pattern of hand preference classification data presented in Table 2 are similar to those based on the raw data reported previously. Recall that the authors reported the hand preference data presented in Table 2, but no specific individual data were presented in the study. A chi-square test of independence indicated a significant association between hand preference classification and species χ2(6, N = 190) = 35.37, p < .01. There were more right- than left-handed bonobos, chimpanzees, and gorillas, whereas there were more left- than right-handed orangutans.
Sex and Rearing Effects
For these analyses, subjects with an unknown testing environment or sexes were excluded from the analyses. A two-factor ANCOVA on the SUM%R scores was performed with sex and testing environment serving as between group factors, whereas the number of tasks served as the covariate. Neither sex F(1, 1435) = 0.022, ns; testing environment F(1, 1432) = 1.27, ns; or the interaction between these two variables F(1, 1432) = 0.609, ns reached conventional levels of statistical significance. Because differences were found in the overall handedness scores between species (see previous discussion), separate two-way ANCOVAs were performed for each species. For all four species, no significant main effects or interactions were found.
Rather than use only the SUM%R scores, the effect of rearing and sex on handedness was further analyzed using the classification data. Loglinear analysis was conducted with sex, testing environment, and the interaction term serving as predictor variables for hand preference classification. The higher-order or saturated model was not significant, and subsequent conditional loglinear analyses evaluating each two-way interaction were also not significant. However, chi-square tests of independence revealed a significant association between testing environment and hand preference χ2(4, N = 1,387) = 18.16, p < .001 (see Table 3). To further evaluate this association, the number of strongly right- and mildly right-handed subjects was compared with the number of strongly left- and mildly left-handed subjects that were tested in either captivity or the wild. For the wild apes, no significant difference was found in the number of strongly right- compared with strongly left-handed subjects χ2(1, N = 207) = 1.21, ns; however, a borderline significant difference was found in the number of mildly right- compared with mildly left-handed subjects χ2(1, N = 63) = 3.57, p < .06. For apes tested in captivity, significantly more strongly right-than strongly left-handed apes χ2(1, N = 728) = 33.43, p < .001 existed, but no significant difference in the number of mildly right- compared with mildly left-handed apes χ2(1, N = 136) = 0.00, ns. Thus both great apes tested in captivity and the wild showed trends toward population-level right handedness, but apes tested in captivity were shifted more to the right than the apes tested in the wild.
Task-Specific Handedness
The previous analyses were based on SUM%R scores derived from multiple measures. As an alternative means of analyzing great ape handedness, separate percentage right-hand use and z-scores were derived for specific measures of hand use that were common in at least three separate studies. The behaviors measured, MEAN%R scores, standard errors, one-sample t-test values, and distribution of hand preferences are shown in Table 5. Hand preference classification used the same criteria as applied to the z-scores based on overall frequencies in left- and right-hand use (see previous discussion). Population-level right handedness was evident for leading limb in locomotion, grooming, manual gestures, bimanual feeding, throwing, simple reaching and the Tube task. The Tube task is a measure that requires the subjects to hold a polyvinyl chloride (PVC) pipe with one hand and remove food contained inside the pipe with the opposite hand. The dominant hand is designated as the one extracting the food. Population-level left handedness was evident for carrying, holding, and termite fishing, a form of tool use observed in wild chimpanzees. No population-level biases were found for self-touch, nose wipe, pick up, pluck, hit/slap, object manipulation, scratching, eat, and nut cracking (another form of tool use observed in wild chimpanzees).
Table 5.
Mean Percentage Right-Hand Use, Standard Errors, T-value, and Hand Preference Classifications for Different Behavioral Measures of Hand Use in Great Apes
| Quantitative | Hand preference classification | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | %R | SE | t-value | #SL | #ML | #AH | #MR | #SR | R:L Odds |
| Throw42, 56, 83, 84, 85 | 63 | 3.67 | 3.50 | 28 | 3 | 15 | 6 | 58 | 2.07 |
| Gesture19, 39, 41, 47, 51, 83, 84, 85 | 62 | 1.38 | 8.81 | 32 | 24 | 70 | 28 | 128 | 2.79 |
| Leading limb15, 30, 31, 39, 41, 68, 74 | 60 | 1.63 | 6.16 | 18 | 3 | 12 | 21 | 76 | 4.62 |
| Tube task19, 35, 37, 38, 43, 44, 45 | 56 | 1.22 | 4.89 | 162 | 30 | 50 | 32 | 263 | 1.54 |
| Bimanual feeding1, 7, 14, 19, 34, 39, 41 | 54 | 1.67 | 2.11 | 94 | 19 | 50 | 33 | 122 | 1.37 |
| Simple reach2, 4–5, 8, 10–12, 15, 17, 18–19, 23, 33, 48–49, 56, 58, 63, 69, 71, 83–85, 89–93 | 53 | 0.78 | 4.35 | 177 | 52 | 163 | 65 | 278 | 1.50 |
| Hit/slap83, 84, 88 | 52 | 1.63 | 0.96 | 14 | 22 | 51 | 18 | 23 | 1.14 |
| Object manipulation69, 83, 84, 85, 88 | 51 | 1.00 | 1.28 | 31 | 14 | 55 | 23 | 35 | 1.29 |
| Eat19, 23, 30, 31, 39, 41, 56, 57, 63, 69, 73, 74, 83, 84, 85 | 50 | 0.85 | 0.76 | 55 | 40 | 116 | 31 | 72 | 1.08 |
| Touch other15, 56, 83, 84, 88 | 49 | 0.99 | 1.19 | 15 | 16 | 77 | 17 | 12 | 0.61 |
| Self-touch15, 16, 23, 39, 41, 66, 83, 84, 88 | 48 | 0.81 | 2.31 | 36 | 31 | 105 | 21 | 34 | 0.82 |
| Carry39, 41, 55, 58, 69, 85, 88 | 42 | 1.67 | 4.96 | 36 | 19 | 44 | 11 | 8 | 0.35 |
| Pan Only | |||||||||
| Nut cracking4, 59, 86 | 56 | 3.76 | 1.63 | 16 | 16 | 13 | 14 | 30 | 1.38 |
| Groom other4, 19, 23, 31, 55, 57, 61, 83, 94 | 54 | 1.47 | 2.67 | 7 | 11 | 63 | 21 | 16 | 2.06 |
| Scratch19, 23, 52, 55, 57, 61, 94 | 51 | 0.95 | 1.31 | 8 | 16 | 70 | 19 | 17 | 1.50 |
| Nosewipe25, 57, 61 | 51 | 1.39 | 0.44 | 7 | 11 | 41 | 15 | 6 | 1.17 |
| Pick up25, 57, 67, 86 | 49 | 1.27 | 1.18 | 24 | 20 | 37 | 14 | 17 | 0.70 |
| Pluck25, 57, 67 | 49 | 1.84 | 0.55 | 10 | 18 | 48 | 13 | 8 | 0.75 |
| Hold23, 55, 57, 61, 67 85 | 44 | 1.17 | 5.19 | 42 | 23 | 53 | 14 | 7 | 0.32 |
| Termite fishing23, 54, 60, 61, 67, 70, 85 | 39 | 4.85 | 2.32 | 44 | 4 | 10 | 2 | 22 | 0.50 |
Note. Superscripted values indicate papers from which raw data were obtained for analysis (See Table 1). Bolded behaviors indicate population-level right-handedness. Underlined behaviors indicate population-level left-handedness.
For each of the individual measures in Table 5, MEAN%R scores were compared between species to assess whether the apes differed with respect to hand preferences. Significant species differences were found for only three measures including simple reaching F(3, 733) = 4.31, p < .001, the Tube task F(2, 535) = 5.19, p < .001 and self-touching F(3, 219) = 7.17, p < .001. Shown in Table 6 are the MEAN%R values and standard errors for these three measures in each species. For the Tube task, chimpanzees showed population-level right handedness, whereas orangutans showed population-level left handedness. With respect to reaching, the bonobos and orangutans showed population-level right handedness. For self-touching, orangutans showed population-level left-handedness (see also Rogers & Kaplan, 1995, and Table 2). Lastly, for leading limb in locomotion, both bonobos and orangutans showed population-level right handedness.
Table 6.
Comparative Analysis of Handedness Index Scores in Great Apes Handedness for Identical Measures of Hand Use
| Behaviors | Orangutan | Gorilla | Chimpanzee | Bonobo |
|---|---|---|---|---|
| Tube task | 35 | 57 | 57 | NA |
| SE | 1.67 | 4.54 | 1.28 | |
| N | 19 | 38 | 467 | |
| Self-touch | 36 | 50 | 48 | 50 |
| SE | 2.83 | 1.57 | 1.08 | 1.97 |
| N | 17 | 55 | 116 | 35 |
| Simple reaching | 55 | 54 | 52 | 61 |
| SE | 2.11 | 2.50 | 0.92 | 2.39 |
| N | 55 | 74 | 547 | 81 |
| Leading limb | 73 | 56 | 55 | 63 |
| SE | 2.36 | 3.24 | 2.95 | 2.29 |
| N | 26 | 35 | 48 | 21 |
Note. Bold numbers indicate significant population-level right-handedness at p < .05. Underlined numbers indicate population-level left-handedness at p < .05. SE = standard error; N = sample size. See Table 5 for references cited for each behavioral measure.
Results II: Heritability of Hand Use in Pan
These analyses were restricted to data from the subjects representing the genus Pan, including chimpanzees (Pan troglodytes) and bonobos (Pan paniscus). This was done because nearly 85% of the pedigree data came from this genus. Therefore the analyses provided a more controlled assessment of heritability in handedness, without the potential confound of genus differences in hand use.
Parent-Offspring Associations
In the initial analysis, offspring SUM%R hand preference scores were correlated with maternal, paternal, and midparent SUM%R handedness scores. Midparent handedness scores were derived by averaging the sire and dam SUM%R handedness scores. For directional biases in hand preference, a significant positive correlation was found between the offspring and dam handedness values (see Table 7). Studies in rodents have convincingly shown that strength of laterality can be selectively bred for (see Collins, 1985). To evaluate whether strength rather than direction of hand preference ran in families, the absolute value of the SUM%R was calculated for all offspring, dams, and sires following the formula [ABS(SUM%R-50)], and the resulting values were correlated with each other. Significant positive correlation coefficients were found between the offspring and the sire, dam, and midparent values (see Table 7).
Table 7.
Correlation Coefficients between Offspring Hand Preference and Dam, Sire, and Midparent Hand Preference
| Sire | Dam | Midparent | |
|---|---|---|---|
| All Subjects | |||
| Direction | .115 (198) | .140** (399) | .114 (190) |
| Strength | .228** (198) | .420** (399) | .317* (190) |
Note. Values in parentheses indicate sample size.
p < .05.
p < .01.
In the next set of analyses, the hand preference classifications of the offspring were evaluated in relation to the hand preferences of the dam and sire. To increase statistical power, strongly left- and mildly left-handed subjects were classified as left handed, and strongly right- and mildly right-handed subjects were classified as right handed. Subjects previously classified as ambidextrous remained classified in this category. To further enhance statistical power, parental hand preference classifications were characterized as familial left handed (i.e., both parents were left handed, or one parent was left handed and the other ambidextrous), familial right handed (i.e., both parents were right handed, or one parent was right handed and the other ambidextrous), or mixed handed (i.e., one parent was right handed, and one parent was left handed). The hand preferences of the offspring were compared as a function of these three parental groups and the results are shown in Table 8. The ratios of right- to left-handed offspring in each parental handedness group are also shown in Table 8. Although the proportion of right-handed offspring born to familial right- compared with familial left-handed parents was substantially higher, this analysis failed to reveal a significant association between parent and offspring hand preferences χ2(4, N = 183) = 3.93, ns.
Table 8.
Distribution of Parental and Offspring Hand Preferences
| Offspring hand preference | ||||
|---|---|---|---|---|
| Left | Ambidextrous | Right | R:L ratio | |
| Both Parent Handedness | ||||
| Familial left-handed | 14 | 13 | 20 | 1.43 |
| Mixed handedness | 14 | 10 | 26 | 1.86 |
| Familial right-handed | 17 | 20 | 49 | 2.88 |
| Maternal Hand Preference | ||||
| Left | 30 | 33 | 40 | 1.33 |
| Ambidextrous | 29 | 41 | 35 | 1.21 |
| Right | 41 | 40 | 92 | 2.24 |
| Paternal Hand Preference | ||||
| Left | 11 | 9 | 20 | 1.82 |
| Ambidextrous | 16 | 19 | 40 | 2.50 |
| Right | 18 | 18 | 39 | 2.17 |
Note. Values represent raw frequencies. R:L ratio calculated by dividing the number of right-handed offspring by the number of left-handed offspring.
Rather than consider the handedness of both parents, separate chi-square tests of independence were also performed comparing the distribution of offspring hand preference to maternal and paternal hand preferences. Maternal χ2(4, N = 381) = 13.26, p < .01 but not paternal χ2(4, N = 190) = 0.57, ns hand preferences were significantly associated with offspring hand preference distributions (see Table 8). For maternal hand preference, offspring were 2.24 times more likely to be right handed if born to a right-handed compared with a left-handed dam (odds ratio = 1.68).
Because of the large number of ambidextrous individuals, the association between offspring and parental handedness was evaluated several other ways. First, the chi-square tests of independence were run without the inclusion of the ambidextrous parents and offspring. For this analysis, no significant associations between parental and offspring handedness were found. Thus the previously reported association between maternal and offspring handedness is removed when ambidextrous subjects are excluded. As an alternative approach, left-handed and ambidextrous parents and offspring were combined to create a category of non–right-handed individuals. Using this criteria, chi-square tests of independence between parent and offspring handedness revealed a significant association between maternal and offspring handedness χ2(2, N = 381) = 33.67, p < .001. In summary, the results of this analysis suggest that maternal and offspring handedness are positively associated, but this conclusion is tempered by the manner in which subjects' handedness is classified.
Discussion
Five significant findings were revealed in the analyses performed on the extant hand preference data in great apes. First, when considered as a collective group, great apes exhibit population-level right handedness. These results are evident when considering either the handedness index scores or based on a hand preference classification criteria. Second, species differences in the distribution of handedness were evident with bonobos and chimpanzees showing population-level right handedness, whereas gorillas and orangutans did not. Third, handedness was more pronounced for some measures compared with others, suggesting that hand preferences in apes are not unidimensional but rather are task specific. Fourth, captive apes were more right handed than wild apes, although both cohorts showed population-level right handedness at some level. Lastly, in the genus Pan, both strength and direction of hand preferences ran in families and offspring handedness was primarily influenced by the handedness of the mother, although this finding was contingent upon the manner in which handedness classifications were made between parents and offspring.
Although the great apes showed population-level handedness, the distribution of hand preferences in the great apes differ from human hand preferences in at least two important ways. First, the ratio of right- to left-handed subjects is much lower in the apes compared with humans. Specifically, there was about a 2:1 ratio of right to left handedness in the great ape sample. This is substantially lower than the typical 8:1 or 9:1 ratio of right to left handedness reported in human populations (Annett, 1985). Second, many more ambidextrous subjects exist in the great ape sample compared with humans. Whether this reflects a weaker manifestation of handedness in great apes or is due to other factors is also discussed below. Several possible explanations exist for these discrepancies, and these are now discussed within the context of various evolutionary models of cerebral dominance.
Potential Explanations for Discrepancies Between Studies and Settings
Numerous reports exist of nonsignificant findings of population-level handedness in great apes, particularly among wild subjects (see Table 4). In contrast, significant results have been reported in studies of captive-born apes, and this has led to the suggestion that the rearing environment has a significant impact on handedness in great apes (McGrew & Marchant, 1997; Palmer, 2003). The results from this analysis partially support this interpretation, but some caveats remain. Hopkins and Cantalupo (2005) have argued that variability in findings between captive and wild apes has little to do with the setting but rather reflects (a) differences in sample size that influence statistical power, (b) the types of measures used to assess handedness in captive and wild apes, and (c) statistical methods used to evaluate handedness. These three points, as they relate to settings differences in handedness, are addressed following.
Effect Size and Statistical Power
Statistical power is an important consideration when comparing human and nonhuman primate handedness, as well as for comparison between studies in wild and captive populations of nonhuman primates. Hopkins and Cantalupo (2005) have previously argued that population-level handedness is not a large effect in nonhuman primates, and this argument is supported by the relative small proportion of right- to left-handed subjects reported in many studies. If handedness in nonhuman primates is a small effect, as suggested by the results in this study, then larger sample sizes are needed to detect the effect with any level of confidence. The importance of statistical power can be demonstrated by comparing the individual results from many of the papers reviewed in this paper (see Table 4). As previously noted, the one sample t-test conducted on the entire data (see Results) yields an effect size of .35, based on Cohen's d (Cohen, 1988). Assuming this effect size and specifying a desired statistical power of .75, the number of subjects needed to detect population-level handedness in any individual study is 59 individuals. If the effect size is reduced to a small effect (∼.20, Cohen, 1988) (and likely is based on the meta-analysis results), then the number of subjects needed to detect the effect with a desired statistical power set to .75 is 176 individuals.
As can be seen in Table 4, nearly all individual studies of handedness fail to obtain adequate sample sizes to have a reasonable amount of statistical power. Of the studies reviewed in this paper, only one set of data (see entry for Hopkins 1993-2003 in Table 4) has a sample size large enough to detect a small effect with a desired power of .75 (i.e., n ≥ 176). Considering the exact effect size derived for all apes (.35), only five studies had sample sizes exceeding 59 individuals of the same species. Of these five, only one was obtained from a sample of wild apes (Boesch, 1991). It is of note that in the Boesch (1991) paper, two measures including grooming and wadge-dipping, showed population-level right handedness, although the handedness for all measures did not reach conventional levels of significance. In addition, if the data from the youngest apes are omitted, then the adult wild chimpanzees in the Boesch (1991) study show population-level right handedness across all measures. In short, most studies of handedness in great apes have very little statistical power, and it is difficult to argue that differences in findings between wild and captive apes are attributable to the settings when only one study in wild apes had enough statistical power to detect a small-to-moderate effect size. When the collective data are combined for all apes, or relatively large samples of apes are tested (as has been the case in some captive populations), population-level handedness appears evident because much greater statistical power exists to detect the relatively small-to-moderate effect. The best example of the role of sample size and statistical power comes from data on handedness for termite fishing in wild chimpanzees at Gombe. In two separate reports with samples sizes less than 20 individuals, McGrew and Marchant (1992, 1996) failed to report population-level handedness; however, when their data are combined with a third report by Lonsdorf and Hopkins (2005) with a sample of 17 individuals, the entire sample reached a total of 56 individuals and population-level left handedness was found for this behavior.
Handedness Measurement
Another problem in comparing handedness in wild and captive apes is with the types of measures used to assess hand use. In the majority of studies with low power and small effect sizes, the primary measure of handedness has been simple reaching. It is well known that situational factors, posture, and grip morphology all significantly influence handedness for simple reaching in great apes and other primates (see Christel, 1994; Lehman, 1993; Westergaard, Kuhn, & Suomi, 1998) but in many studies, these extraneous variables are not controlled for or considered in the analyses. Another indicator of the problem with simple reaching as the primary measure of handedness in nonhuman primates is that often many ambiguously handed or nonpreferent animals exist (Papademetriou, Sheu, & Michel, 2005). This likely reflects the lack of complex motor skill necessary for grasping a piece of food and the vulnerability of simple reaching to situational factors. In contrast to simple reaching, more complex manual actions, particularly those requiring coordinated bimanual actions of the hands, often elicit strong hand preferences at the individual level and results in fewer nonpreferent subjects. A comparison of some of the results presented here support this argument. For example, 38% of the subjects in the category eat were ambiguously handed in contrast to only 16% for bimanual feeding (see Table 5). For both behaviors, the subjects are eating food but in the eat category, subjects are eating with one hand in a unspecific situational context, whereas bimanual feeding was recorded when one hand maintained a supportive role in foraging and the opposite hand was involved in the action of feeding. Thus the apes were more lateralized for bimanual compared with unimanual feeding.
The problem of measurement validity in nonhuman primate handedness is not restricted to simple reaching, and this has further contributed to problems of comparing findings in wild and captive apes. For example, in the two studies of handedness in wild chimpanzees with the largest sample sizes (Marchant & McGrew, 1996; McGrew & Marchant, 2001), hand use for daily actions was measured. Hand use in each study was based on the 10 or 15 most frequent behaviors, all of which were behaviors that did not induce significant preferences at the individual level (see also Fletcher & Weghorst, 2005). These behaviors included scratch, groom, eat, pluck, pull, nose wipe, pincer, pick up, hold, and cradle. Indeed, in the McGrew and Marchant (2001) study, there were significantly more nonlateralized than lateralized subjects for the 15 most frequent behaviors. This is quite different from the pattern of results reported in most studies with captive apes and, in some cases, wild apes (see Byrne & Byrne, 1991). It seems difficult to argue that wild apes do not show population-level handedness when the bulk of the measures do not elicit strong individual preferences. This issue is at least one potential source of the large number of ambidextrous animals reported in this meta-analysis.
Last, the most direct means to assess setting effects on handedness is to compare findings in wild and captive apes on which comparable measures have been used to evaluate hand use. This has not frequently occurred (but see Lonsdorf & Hopkins, 2005); however, within the assembled data set for this study, t-test comparisons in percentage right-hand use scores for all behaviors in which population-level handedness was reported and available in both wild and captive apes (see Table 5) revealed no significant differences. Thus when comparable behaviors have been examined between wild and captive apes, similar results have been obtained. Notwithstanding, further research is needed in this area of investigation.
Quantifying Handedness
In nearly every published study on handedness in great apes (and other primates), individual hand preferences are based on the use of z-scores with alpha set to p < .05. In the typical study, z-scores are derived based on the number of left- and right-hand responses for all or some specific set of behaviors. Subjects with z-scores exceeding 1.96 or −1.96 are classified as right and left handed. All others are characterized as ambiguously handed or nonpreferent. The use of z-scores is a very conservative approach to estimating individual handedness in nonhuman primates, and serious limitations exist in using them (see Hopkins, 1999b, for review). For example, with alpha set to p < .05 and a two-tailed test, a 95% chance exists that no significant hand preference will be found. This is very high; if the same standard for determining human handedness was based on a similar criterion, then there would be a much larger proportion of ambidextrous subjects than is typically reported in the literature.
Z-scores are also influenced by sample size. For instance, a subject that uses the right hand to pick up a grape on 7 of 10 trials (z = 1.27) would be classified as nonpreferent based on these frequencies, whereas a subject that uses the right hand 35 out of 50 responses would be classified as right handed (z = 2.83, p < .01). The percentage right-hand use is identical between the two sets of data, yet the interpretation of their handedness differs for arbitrary statistical reasons. Certainly it could be argued that the experimenter has more confidence in the handedness of the latter subject because it is based on a larger sample of observations, but the importance of increased sample size would depend on how much emphasis the experimenter places on the internal validity of the measure rather than the external validity of the findings. In short, as is the case in many psychological studies, a trade-off exists between increased observations per subjects contrasted with fewer observations in larger samples of subjects. Are 10 responses from 200 subjects better or worse than 50 responses from 50 subjects? The answer depends entirely on the experimenter's choice of method and approach, and many factors influence these decisions.
Last, the rationale for using z-scores is rooted in the concept that handedness is a discrete trait and subjects need to be classified as left handed, right handed, or ambidextrous, yet no basis exists to assume that handedness is a discrete trait in nonhuman or human handedness. Moreover, the use of z-scores also results in many investigators adopting nonparametric statistics to evaluate population-level handedness. This is also a questionable practice because nonparametric statistics are less powerful. Further, if handedness is a relatively small effect in nonhuman primates (as previously shown), then transforming an initially continuous scale of measurements into a discrete scale of measurement reduces the sensitivity of the measure and forces the use of less powerful statistics. As done in this paper, one sample t-test on handedness indices derived from the raw frequencies resolves the scales of measurement and analysis problems described previously. At a minimum, it offers an alternative means of considering population-level handedness in nonhuman primates. Finally, z-scores cannot be applied to other kinds of measures of asymmetry in hand use that do not record discrete responses. For example, asymmetries in the duration or rate of occurrence of specific kinds of behaviors do not lend themselves to z-scores but are nonetheless potentially valuable measures of functional differences in hand use.
Potential Explanations for Species Differences in the Ratio of Right to Left Handedness
As previously noted, the relative proportion of right- to left-handed subjects was lower in apes compared with the ratio typically reported in human populations. Moreover, within the great apes, there were species differences in handedness. What explains these differences is not entirely clear but some explanations are offered below.
Ecological and Morphological Factors
With respect to the species differences in handedness between the great apes, it might be argued that population-level right handedness evolved recently and is unique to the genus Pan compared with Gorilla and Pongo. This would explain the prevalence of right handedness in the genus Pan and not in Gorilla or Pongo. Alternatively, species differences in handedness preference is the result of ecological and biomechanical factors that have selected for the differential expression of handedness in these three species. In terms of ecological variables, Pongo, Gorilla, and Pan differ significantly in terms of their positional and locomotor behavior, diet, and foraging behavior, all of which may have an effect on handedness (see Tuttle, 1986). For example, orangutans spend significantly more time in the trees than other apes and primarily locomote through the trees by arm-over-arm locomotion. In contrast, gorillas primarily locomote on the ground (see Byrne & Byrne, 1991). Posture and positional behavior have been reported to have significant transient effects on handedness in great apes (see Hopkins, 1993; Olson, Ellis, & Nadler, 1990; Peters, 2005), and it may be that endogenous differences in positional and locomotor behavior have overt effects on the distribution of handedness in apes (see Peters, 2005). Similarly, endogenous differences between species in the use of the forelimb(s) during feeding could explain the observed findings on hand preference. For example, both mountain gorillas, and to a lesser extent lowland gorillas, feed while seated and use their hands in a coordinated manner (Byrne & Byrne, 1991; Parnell, 2001; Remis, 1999). In contrast, orangutans, and to a lesser extent chimpanzees, forage in the trees with one hand while posturally supporting themselves with the opposite hand (Cant, 1992; Doran, 1993; Peters, 2005). In terms of bimanual feeding, studies indicate that chimpanzees and gorillas are right handed but only under conditions where the hands are involved in the independent actions of holding and feeding (Hopkins & de Waal, 1995). When one of the hands is involved in either postural or forelimb substrate support, no population-level handedness is evident in both wild and captive apes (see Hopkins & de Waal, 1995; Marchant & McGrew, 1996, for examples). Little data exist on bimanual feeding in orangutans but, based on our findings, it would be predicted that they would posturally support themselves with the right handed and feed with the left. This explanation is largely consistent with the “postural origins” theory of handedness proposed by MacNeilage et al. (1987).
Genetic Explanations
One explanation for the difference in distributions of right and left handedness between humans and great apes may reflect genetic factors. Corballis (1997) has suggested that mutations in the human genome, after the split from chimpanzees, might explain the greater preponderance of right handedness in humans. Alternatively, great apes and humans may share a common genetic basis for handedness, but the relative frequency or representation of this gene or genes may be more pronounced in human populations compared with great apes.
Unfortunately, different genetic explanations cannot be adequately addressed at this point in time, because no candidate genes have been identified for the expression of handedness; therefore neither of these potential genetic explanations can be empirically tested at present (see Franck et al., 2002; Kim et al., 1999). Crow (1998) has suggested that functional and neuroanatomical asymmetries are unique to humans and associated with selection for genes associated with language. The data reported here provide no support for this view because population-level handedness was found, and it possibly runs in families. Additionally, evidence of robust neuroanatomical asymmetries in the planum temporale, inferior frontal lobe, and cerebral torquing in great apes has been reported, and none of these findings support this theory (Cantalupo & Hopkins, 2001; Cantalupo, Pilcher, & Hopkins, 2003; Cantalupo, Freeman, & Hopkins, in press; Gannon, Holloway, Broadfield, & Braun, 1998; Hopkins, Pilcher, & Cantalupo, 2003). Indeed, the evidence of left-hemisphere asymmetries in the planum temporale of apes has been independently reported in three different laboratories using both cadaver brains and in vivo imaging techniques (Gannon et al., 1998; Cantalupo et al., 2003), suggesting that neither observer bias nor the method of assessment have a strong influence on the expression of these asymmetries.
Social Learning
Another possible explanation for differences in the distribution of hand preference between humans and great apes may be influenced by cultural and pedagogical systems unique to human evolution compared with great apes. When considering measures of hand preference in humans, defining a culturally unbiased task operationally is nearly impossible. For instance, in humans, some of the more commonly used items on a handedness questionnaire are, With which hand do you write, throw, open a bottle, or shake hands? Clearly cultural, pedagogical, and mechanical influences exist on the expression of hand use for these types of tasks. Presumably, pedagogical or cultural pressures to conform to a particular hand preference are not present in great apes, although they cannot be ruled out (e.g., Whiten et al., 2001). From this perspective, what is manifest in great ape handedness is the biological expression of a functional asymmetry. The difference in distributions of hand preference between apes and humans may reflect the additive effect of cultural and pedagogical conformity to human handedness over and above the biological asymmetric expression.
Alternatively, apes learn their hand use by modeling the lateralized behaviors around them. Social learning of paw preference has been demonstrated in rats (Collins, 1988) and McGrew and Marchant (1997) have suggested that observational learning of humans modeling right-hand use may explain the greater prevalence of right handedness found in captive compared with wild chimpanzees. The positive association between offspring and maternal hand preferences reported here clearly support a potential social learning explanation for handedness in chimpanzees (see Tables 7 & 8). The best example of potential social learning of hand use comes from data in wild chimpanzees on tool use (Boesch, 1991; Lonsdorf & Hopkins, 2005; McGrew & Marchant, 1996). Geographical differences exist in the types of tool use observed in wild chimpanzees (Whiten et al., 2001), suggesting that variation is acquired through social learning. Based on the published data on tool use in wild chimpanzees, offspring hand preference vary in accordance with the hand preference of the mother (see Figure 3). Thus offspring appear to learn which hand to use by modeling the handedness of their mother, although it should noted that genetic mechanisms could similarly explain the phenotypic variation in offspring handedness. However, it is difficult to isolate the potential role of genetic and social learning factors on the development of handedness in wild chimpanzees.
Figure 3.
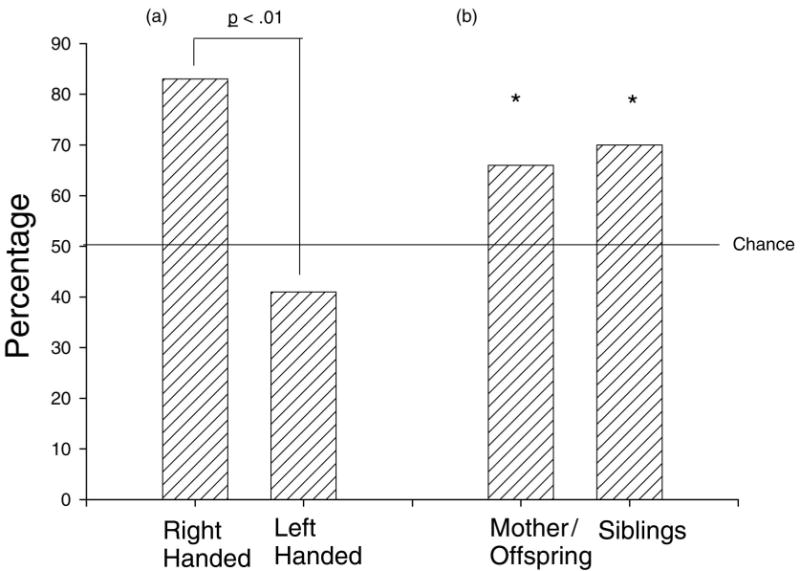
(a) Percentage of right-handed offspring born to right- and left-handed females for all the combined tool use data in wild chimpanzees. (b) Percent concordance in hand use between mothers and offspring, as well as between maternal half-siblings. Asterisks indicate that the probabilities are significantly different from chance.
In an attempt to isolate these factors, a recent study in chimpanzees by Hopkins, Wesley, Russell, and Schapiro (in press) compared hand preferences for a task measuring coordinated bimanual actions (the TUBE task) in related and unrelated individuals living in the same or different social groups. Specifically, in socially housed chimpanzees of six or more individuals, the handedness index (HI) values of each focal chimpanzee in the group was compared with the average HI for all chimpanzees (a) related to them and living in the same group, (b) all chimpanzees unrelated to them and living in the same group, and (c) chimpanzees related to them but not living in the same group. The mean HI for each cohort is shown in Figure 4. The mean HI scores of individual chimpanzees did not differ significantly from related individuals living in the same group t(110) = 0.61, ns; they did not differ significantly from related individuals living outside the group t(88) = 1.167, ns; but they did differ significantly from unrelated individuals living in the same group t(111) = 2.05, p < .04. In addition, individuals related to the focal subject living in the same group had significantly higher HI scores than unrelated individuals living in the same group t(111) = 2.48, p < .02, but they did not differ from related individuals living outside the group t(97) = 0.41, ns. Thus chimpanzee HI scores for the TUBE task were more similar to related individuals compared with unrelated individuals living in the same social groups. This suggests that social learning is not the most likely explanation for the development and heritability of hand use in chimpanzees, although as stated previously, this explanation cannot be entirely ruled out.
Figure 4.
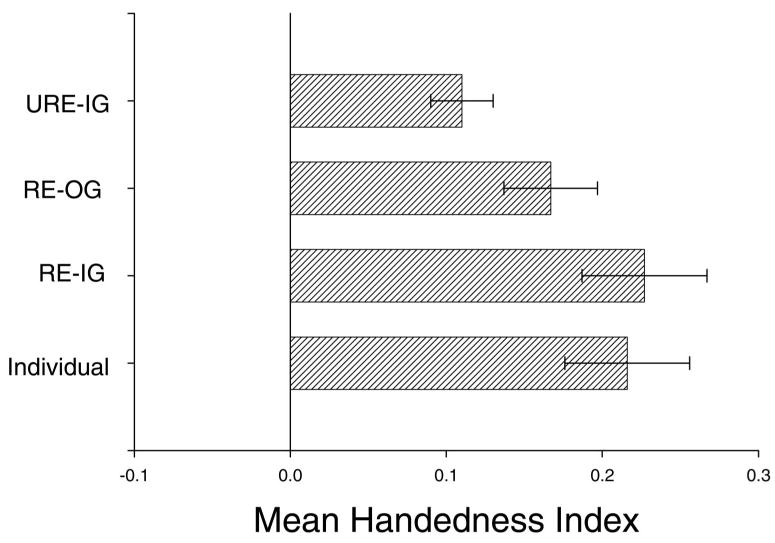
Mean HI scores on the TUBE task for focal individuals, related and unrelated chimpanzees living in the same or different social groups. RE-IG = related individuals living in same group, RE-OG = related individuals living outside the group, URE-IG = unrelated individuals living in the same group. HI scores were derived following formula [HI = (#R−#L)/#R + #L)].
Early Postural and Mother-Infant Interactions
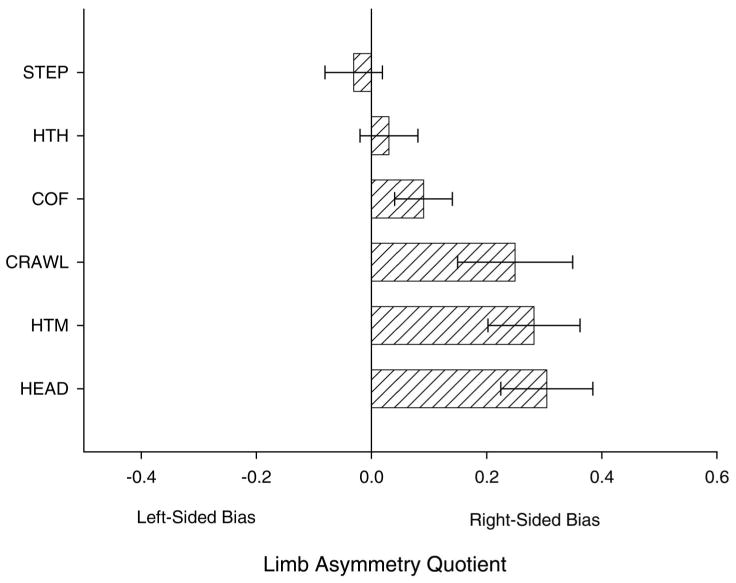
Several authors have proposed that early orienting and postural asymmetries such as in utero position (Previc, 1991) and head orientation (Michel, 1981) may lead to the development of motor asymmetries in hand use (see Hopkins & Ronnqvist, 1998). Several studies in neonatal chimpanzees less than 3 months of age have reported population-level postural and motor asymmetries including right-sided biases for grasping (Fagot & Bard, 1995), head orientation (Hopkins & Bard, 1995), hand-to-mouth (Hopkins & Bard, 1993), and leading limb in locomotion (Hopkins, Bard, & Griner, 1997). Shown in Figure 5 are the mean HI scores for each of seven neonatal behaviors measured in captive born, human-raised chimpanzees. It has further been reported that head orientation asymmetries within the first 3 months of life predict handedness for coordinated bimanual actions at 4 to 5 years of age (Hopkins & Bard, 2000). These results support the view that early motor asymmetries are present in chimpanzees and predict subsequent handedness at later periods of life.
Figure 5.
Mean laterality scores for six measures of asymmetries in neonatal chimpanzees. HEAD = head orientation; HTM = hand-to-mouth; CRAWL = forelimb used in locomotion; COF = removal of cloth from face; HTH = hand-to-hand; STEP = foot used during stepping response. Limb asymmetries quotients were determined following the formula (#R−#L)/#R + #L). The data used to derive the LQ scores are from “Hemispheric Specialization in Infant Chimpanzees (Pan troglodytes): Evidence for a Relation with Gender and Arousal,” by W. D. Hopkins & K. A. Bard, 1993, Developmental Psychobiology, 26, 219–235; “Evidence of Asymmetries in Spontaneous Head Turning in Infant Chimpanzees,” by W. D. Hopkins & K. A. Bard, 1995, Behavioral Neuroscience, 109, 808–812; and “Locomotor Adaptation and Leading Limb Asymmetries in Neonatal Chimpanzees (Pan troglodytes),” by W. D. Hopkins, K. A. Bard, and K. M. Griner, 1997, International Journal of Primatology, 18, 104–114.
The presence of early motor asymmetries is not restricted to captive chimpanzees being raised by humans. Several studies in wild and captive chimpanzees have shown evidence of population-level left-sided nipple preferences, and these results might be influenced by left-sided cradling preferences reported in adult female great apes (see Hopkins, 2004). Of specific interest may be the influence of maternal cradling biases, infant nipple preferences, or both on the subsequent development of handedness in primates. For example, in chimpanzees, Hopkins et al. (1993) reported a negative association between maternal cradling biases and infant hand preferences for simple reaching. Depicted in Figure 6 are the mean handedness scores for 15 offspring born to 10 females who preferred to cradle their offspring on the left and five that preferred to cradle on the right (Hopkins et al., 1993). Maternal cradling bias was assessed for the first 2 weeks of life. Infant hand preferences for simple reaching were assessed when the offspring were approximately 3 years of age. As can be seen, with the exception of one dyad, an inverse relationship is found between maternal cradling bias and infant hand preference. In other words, females who cradle their infants on the left side have right-handed offspring for simple reaching. In contrast, females who cradle their offspring on the right side have left-handed offspring for simple reaching.
Figure 6.
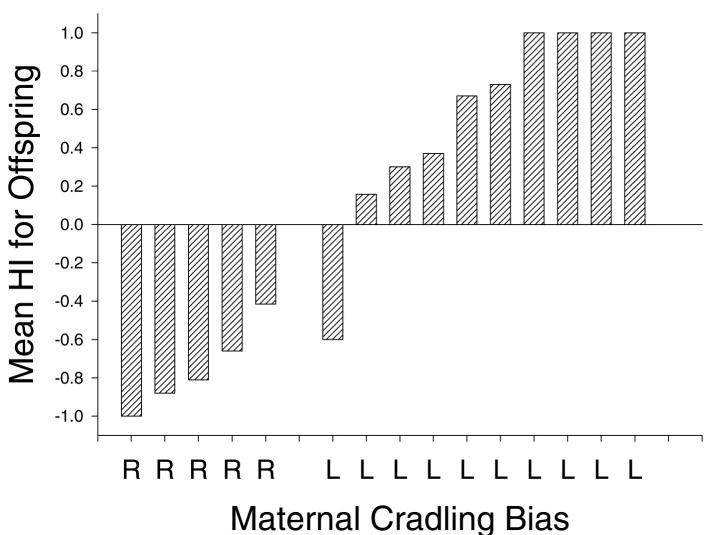
Mean handedness index for offspring born to females that cradled them on the left (L) or right side (R).
The possible effect of early cradling biases on heritability in hand preference could conceivably work in the following manner. Female great apes with offspring may cradle them either on the left or right, because they may want to keep their dominant hand free for locomotion or other noncradling activities. The asymmetries in cradling bias may lead to differential stimulation of the right or left hand for the offspring, which, in turn, leads to the development of hand preferences. This simple relationship would explain the association between maternal and offspring hand preference. Consistency in cradling biases by a given female between offspring would produce high rates of concordance in full and maternal half-siblings.
Laterality in maternal cradling may also explain the 2:1 ratio in right to left handedness in great apes. In two recent reviews of the data on laterality in maternal cradling and infant nipple preferences, great apes, particularly chimpanzees and gorillas, show a left-sided maternal cradling bias and infants show a left nipple preference (Damerose & Vauclair, 2002; Hopkins, 2004). About twice as many females cradle on the left compared with right side. If it is assumed that left-sided cradling results in the development of right-handed offspring, as reported by Hopkins et al. (1993) in chimpanzees, then this would explain the roughly 65% to 70% degree of right handedness found in the great ape summarized in this paper. Between the apes, it is interesting to note that chimpanzees and gorillas cradle much more on the left side compared with orangutans, albeit data were available only in few orangutans. From the handedness perspective, the chimpanzees and gorillas are more right handed than the orangutans.
Evolutionary Stable Strategy
Finally, Vallortigara and Rogers (2005) have recently suggested that population-level asymmetries may have evolved as an evolutionary stable strategy when asymmetrical organisms must coordinate their behavior with other asymmetrical organisms (see also, Ghirlanda & Vallortigara, 2004). This perspective stands in contrast to the prevailing view that argues hemispheric specialization evolved in the context of selection for increasing cognitive functions that were differentially represented in the two halves of the brain (e.g., Gazzaniga, 2000). Vallortigara and Rogers (2005) argue that selection for efficient processing of perceptual and cognitive processes via duality of function did not necessitate that any species show the same pattern of laterality of function. Rather, these authors argue that social conformity, in the form of population-level behavioral asymmetries, offers evolutionary advantages for social species in the context of predator-prey relations. How this theory specifically applies to the expression of manual asymmetries in primates is not clear; however, it is of note that throwing and manual gestures are behaviors emitted in social contexts, and these were the two most lateralized behaviors in the great ape sample (see Table 5).
Conclusions
In conclusion, the results of this meta-analysis suggest that great apes show population-level handedness. When each genus and species is considered independently, bonobos and chimpanzees show population-level handedness, whereas orangutans and gorillas do not. Handedness in great apes is task-specific and some measures are more sensitive than others in detecting population-level right and left handedness in all for species. Although captive-born apes are more right handed than wild-born individuals, both cohorts show population-level biases. In addition, within the genus Pan, handedness is heritable, although the mechanism by which handedness runs in families is not clear, and this result is subject to different interpretations depending on how offspring and parental hand preference is classified.
The previous explanations offered to clarify the discrepancy in the distribution of human and ape handedness are not meant to be exhaustive but represent some feasible alternatives—testable hypotheses that hopefully provide some direction for future studies. In terms of the factors that influence handedness, in all likelihood it will be some combination of genetic and nongenetic factors that explain variation in hand preferences in apes and humans (Hopkins, Dahl, & Pilcher, 2001).
Clearly, much more data are needed in wild apes and greater consideration of the types of behaviors observed and the quantification of handedness needs to be examined in these studies. Studies in captive apes need to try and focus on studying behaviors that parallel those recorded in wild apes so that the question of rearing or setting differences in handedness can be resolved more definitively. Selection of better handedness tasks, in both captive and wild apes, is needed, and greater emphasis should be placed on selecting behaviors or tasks that have some theoretical relevance and are sensitive enough to detect individual hand preferences. In addition, every effort should be made to evaluate handedness for behaviors that require coordinated actions of the hands, because they are less sensitive to situational factors, elicit strong individual hand preferences, and have been shown to correlate with neuroanatomcial asymmetries (see Hopkins & Cantalupo, 2004). The best evidence of population-level handedness in wild (Byrne & Byrne, 1991; Corp & Byrne, 2004; Lonsdorf & Hopkins, 2005) and captive apes (Hopkins et al., 2001, 2004) has been obtained using measures that required coordinated bimanual actions. Measures of hand skill rather than preference per se are also needed to increase understanding of the potential link between motor skill, hand preference, and hemispheric specialization (see Hopkins & Russell, 2004). Lastly, from a statistical standpoint, more flexible approaches in evaluating individual and population-level handedness are needed.
Orangutans and bonobos have been the least studied of the great apes, and the sample sizes are woefully low, particularly among wild individuals. The fact that the orangutans show a different pattern of handedness should be interpreted with some caution because of the small sample size and the focus of many studies on behaviors that were quite different from those studied in chimpanzees and gorillas. At a minimum, the evidence of population-level handedness in the genus Pan suggest that hemispheric specialization for motor skill can be dated back to 6 or 7 million years ago when the Pan and Homo genuses split.
The exact mechanisms that selected for increased preferential use of one hand or another remain unclear, but several existing hypotheses can be ruled out. Notably absent in this paper is any explicit discussion of language origins and the evolution of handedness, because arguably too much emphasis has been placed on this association; therefore it may not be warranted. Recent studies in chimpanzees have shown that handedness for a coordinated bimanual task correlates with a region of the precentral gyrus where the hand is represented and does not correlate with the traditional language areas such as the planum temporale or frontal operculum (Hopkins & Cantalupo, 2004). The de-emphasis of language as a necessary condition for the expression of handedness is further supported by evidence of population-level behavioral asymmetries in lower vertebrates (Rogers & Andrew, 2002). The theory that handedness evolved in the context of tool use is also not strongly supported by the findings reported here (see Bradshaw & Rogers, 1993). Great apes showed population-level handedness for numerous behaviors, none of which involved the use of tools. This is not to suggest that tool use may not have enhanced or promoted increased preferential use of one hand or another; rather it simply implies that tool use was and is not a necessary condition for the expression of population-level handedness. Some have suggested that lateralization for throwing (Calvin, 1983) and gestural communication (Corballis, 2002) may have facilitated the evolution of handedness and hemispheric specialization. In principle, the data presented here support these theories, because population-level right handedness was found for both throwing and manual gestural communication. However, more data are needed because the ways in which these behaviors relate to other aspects of hand use is not clear. Finally, this study focuses on handedness in great apes. Meta-analyses on handedness in other taxonomic families would be very fruitful for constructing an evolutionary tree on the origins of hand use in primates. Further studies at both the behavioral and neurophysiological level in great apes should provide important insight into the evolution and development of handedness and other manifestations of hemispheric specialization in primates, including humans.
Acknowledgments
This work was supported in part by NIH Grants RR-00165, NS-36605, NS-42867 and HD-38051. I am grateful to Hani Freeman, Anna Hopkins, Autumn Hostetter, and Ashley Watson for helping with data entry. I also appreciate the many scientists including Drs. Marianne Christel, Monseratt Colell, Samuel Fernandez-Carriba, Alison Fletcher, Rebecca Harrison, David Leavens, Linda Marchant, William McGrew, and Erna Toback for providing raw data and pedigree information for many of the analyses. Lastly, the helpful comments of Dr. Victor Bissonnette on data analyses techniques are greatly appreciated. Some of the meta-analyses were performed on shareware software provided from Dr. Ralf Schwarzer.
References
- Albrecht H, Dunnett SC. Chimpanzees in Western Africa. Munich, Germany: R. Piper & Company; 1971. [Google Scholar]
- Amunts K, Schlaug G, Schleicher A, Steinmutz H, Drabinghaus A, Roland P, et al. Asymmetry in the human motor cortex and handedness. NeuroImage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- Andrew RJ. The nature of behavioral lateralization in the chick. In: Andrew RJ, editor. Neural and behavioral plasticity. The use of the chick as a model. Oxford: Oxford University Press; 1991. pp. 536–54. [Google Scholar]
- Annett M. Left, right, hand, and brain: The right-shift theory. London: Erlbaum; 1985. [Google Scholar]
- Annett M. Handedness and brain asymmetry: The right shift theory. Hove, East Sussex, UK: Psychology Press; 2002. [Google Scholar]
- Annett M, Annett J. Handedness for eating in gorillas. Cortex. 1991;27:269–285. doi: 10.1016/s0010-9452(13)80131-1. [DOI] [PubMed] [Google Scholar]
- Aruguete MA, Ely EA, King JE. Laterality in spontaneous motor activity of chimpanzees and squirrel monkeys. American Journal of Primatology. 1992;27:13–21. doi: 10.1002/ajp.1350270303. [DOI] [PubMed] [Google Scholar]
- Biddle FG, Coffaro CM, Ziehr JE, Eales BA. Genetic variation in paw preference (handedness) in the mouse. Genome. 1993;36:935–943. doi: 10.1139/g93-123. [DOI] [PubMed] [Google Scholar]
- Bisazza A, Cantalupo C, Capocchiano M, Vallortigara G. Population lateralization and social behavior: A study with sixteen species of fish. Laterality. 2000;5:269–284. doi: 10.1080/713754381. [DOI] [PubMed] [Google Scholar]
- Bisazza A, Cantalupo C, Robins A, Rogers LJ, Vallortigara G. Right pawedness in toads. Nature. 1996;379:408. [Google Scholar]
- Bisazza A, Facchin L, Vallortigara G. Heritability of lateralization in fish: Concordance of right-left asymmetry between parents and offspring. Neuropsychologia. 2000;38:907–912. doi: 10.1016/s0028-3932(00)00018-x. [DOI] [PubMed] [Google Scholar]
- Bisazza A, Sovrano VA, Vallortigara G. Consistency among different tasks of left-right asymmetries in lines of fish originally selected for opposite direction of lateralization in a detour task. Neuropsychologia. 2001;39:1077–1085. doi: 10.1016/s0028-3932(01)00034-3. [DOI] [PubMed] [Google Scholar]
- Boesch C. Handedness in wild chimpanzees. International Journal of Primatology. 1991;6:541–558. [Google Scholar]
- Bradshaw B, Rogers L. The evolution of lateral asymmetries, language, tool-use and intellect. San Diego: Academic Press; 1993. [Google Scholar]
- Brenot PH. Liberalized handedness, bipedalism and cortical specialization. In: Preuschoft H, Chivers DJ, editors. Hands of primates. New York: Springer-Verlag; 1992. pp. 45–53. [Google Scholar]
- Bresard B, Bresson F. Handedness in Pongo pygmaeus and Pan troglodytes. Journal of Human Evolution. 1983;12:659–666. [Google Scholar]
- Butterworth G, Itakura S. Development of precision grips in chimpanzees. Developmental Science. 1998;1:39–43. [Google Scholar]
- Byrne RW, Byrne JM. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla gorilla berengei) Cortex. 1991;27:521–536. doi: 10.1016/s0010-9452(13)80003-2. [DOI] [PubMed] [Google Scholar]
- Calvin WH. The throwing Madonna: Essays on the brain. New York: MacGraw-Hill; 1983. [Google Scholar]
- Cant JGH. Positional behavior and body size of arboreal primates: A theoretical framework for field studies and an illustration of its application. American Journal of Physical Anthropology. 1992;88:273–283. doi: 10.1002/ajpa.1330880302. [DOI] [PubMed] [Google Scholar]
- Cantalupo C, Freeman H, Hopkins WD. Cerebellar asymmetries in great apes. Brain, Behavior and Evolution in press. [Google Scholar]
- Cantalupo C, Hopkins WD. Asymmetric Broca's area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo C, Pilcher D, Hopkins WD. Are asymmetries in sylvian fissure length associated with the planum temporale? Neuropsychologia. 2003;41:1975–1981. doi: 10.1016/s0028-3932(02)00288-9. [DOI] [PubMed] [Google Scholar]
- Carpenter CR. An observational study of two captive mountain gorillas (Gorilla berengei) Human Biology. 1937;9:175–196. [Google Scholar]
- Carter-Saltzman L. Biological and sociocultural effects on handedness: Comparison between biological and adoptive parents. Science. 1980 September;209:1263–1265. doi: 10.1126/science.7403887. [DOI] [PubMed] [Google Scholar]
- Chorazyna H. Shifts in laterality in a baby chimpanzee. Neuropsychologia. 1976;14:381–384. doi: 10.1016/0028-3932(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Christel MI. Catarrhine primates grasping small objects: Techniques and hand preferences. In: Anderson JR, Roeder JJ, Thierry B, Herrenschmidt N, editors. Current primatology Vol III: Behavioral neuroscience, physiology and reproduction. Strasbourg: Universite Louis Pasteur; 1994. pp. 37–49. [Google Scholar]
- Christel MI, Kitzel S, Niemitz C. How precisely do bonobos (Pan paniscus) grasp small objects? International Journal of Primatology. 1998;19:165–194. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Colell M, Segarra MD, Sabater-Pi J. Manual laterality in chimpanzees (Pan troglodytes) in complex tasks. Journal of Comparative Psychology. 1995a;109:298–307. doi: 10.1037/0735-7036.109.3.298. [DOI] [PubMed] [Google Scholar]
- Colell M, Segarra MD, Sabater-Pi J. Hand preferences in chimpanzees (Pan troglodytes), bonobos (Pan paniuscus) and orangutans (Pongo pygmaeus) in food-reaching and other daily activities. International Journal of Primatology. 1995b;16:413–434. [Google Scholar]
- Collins RL. When left-handed mice live in a right handed world. Science. 1975;187:181–184. doi: 10.1126/science.1111097. [DOI] [PubMed] [Google Scholar]
- Collins RL. On the inheritance of direction and degree of asymmetry. In: Glick S, editor. Cerebral lateralization in non-human species. Orlando, FL: Academic Press; 1985. pp. 150–164. [Google Scholar]
- Collins RL. Observational learning of a left-right asymmetry by mice (Mus musculus) Journal of Comparative Psychology. 1988;102:222–224. doi: 10.1037/0735-7036.102.3.222. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The lopsided brain: Evolution of the generative mind. New York: Oxford University Press; 1992. [Google Scholar]
- Corballis MC. The genetics and evolution of handedness. Psychological Review. 1997;104:714–727. doi: 10.1037/0033-295x.104.4.714. [DOI] [PubMed] [Google Scholar]
- Corballis MC. From hand to mouth: The origins of language. Princeton: Princeton University Press; 2002. [Google Scholar]
- Corp N, Byrne RW. Sex difference in chimpanzee handedness. American Journal of Physical Anthropology. 2004;123:62–68. doi: 10.1002/ajpa.10218. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Sexual selection, timing and the descent of man: A theory of the genetic origins of language. Current Psychology of Cognition. 1998;17:1079–1114. [Google Scholar]
- Cunningham D, Forsythe C, Ward JP. A report of behavioral lateralization in an infant orangutan. Primates. 1989;30:249–253. [Google Scholar]
- Curt F, De Agostini M, Maccario M, Dellatolas G. Parental hand preference and manual functional asymmetry in preschool children. Behavior Genetics. 1995;25:525–536. doi: 10.1007/BF02327576. [DOI] [PubMed] [Google Scholar]
- Damerose E, Hopkins WD. A comparison of scan and focal sampling procedures in the assessment of laterality for maternal cradling and infant nipple preferences in olive baboons (Papio anubis) Animal Behaviour. 2002;63:511–518. [Google Scholar]
- Damerose E, Vauclair J. Posture and laterality in human and non-human primates: Asymmetries in maternal handling and infant's early motor asymmetries. In: Rogers L, Andrews R, editors. Comparative vertebrate lateralization. Oxford: Oxford University Press; 2002. pp. 306–362. [Google Scholar]
- Dienske H, Hopkins B, Reid AK. Lateralisation of infant holding in chimpanzees: New data do not confirm previous findings. Behaviour. 1995;132:801–809. [Google Scholar]
- Dimond S, Harries R. Face touching monkeys, apes and man. Evolutionary origins and cerebral asymmetry. Neuropsychologia. 1984;22:227–233. doi: 10.1016/0028-3932(84)90065-4. [DOI] [PubMed] [Google Scholar]
- Doran DM. Sex differences in adult chimpanzee positional behavior. The influence of body size on locomotion and posture. American Journal of Physical Anthropology. 1993;91:99–115. doi: 10.1002/ajpa.1330910107. [DOI] [PubMed] [Google Scholar]
- Ettlinger GF. Hand preference, ability and hemispheric specialization. How far are these factors related in the monkey? Cortex. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Fagot J, Bard KA. Asymmetric grasping response in neonate chimpanzees (Pan troglodytes) Infant Behavior and Development. 1995;18:253–255. [Google Scholar]
- Fagot J, Vauclair J. Handedness and bimanual coordination in the lowland gorilla. Brain, Behavior and Evolution. 1988;32:89–95. doi: 10.1159/000116536. [DOI] [PubMed] [Google Scholar]
- Fernandez-Carriba S, Gomez R, Loeches A, Moricello A, Perez I. V Congreso de la Asociación Primatológica Española (APE“01) Madrid, Spain: 2001. Differences in the level of handedness in chimpanzees (Pan troglodytes) according to the complexity of the task. [Google Scholar]
- Fernandez-Carriba S, Loeches A. Fruit smearing by captive chimpanzees: A newly observed food processing behavior. Current Anthropology. 2001;42:143–147. [Google Scholar]
- Ferster CB. Concurrent schedules of reinforcement in the chimpanzee. Science. 1957;126:509. doi: 10.1126/science.125.3257.1090. [DOI] [PubMed] [Google Scholar]
- Finch G. Chimpanzee handedness. Science. 1941;94:117–118. doi: 10.1126/science.94.2431.117. [DOI] [PubMed] [Google Scholar]
- Fischer RB, Meunier GF, White PJ. Evidence of laterality in the lowland gorilla. Perceptual and Motor Skills. 1982;54:1093–1094. [Google Scholar]
- Fletcher AW, Weghorst JA. Laterality of hand function in naturalistically housed chimpanzees (Pan troglodytes) Laterality. 2005;10:219–242. doi: 10.1080/13576500442000049. [DOI] [PubMed] [Google Scholar]
- Foucart J, Bril B, Hirata S, Morimura M, Houki C, Ueno Y, et al. A preliminary analysis of nut-cracking movements in a captive chimpanzee: Adaptation to the properties of tools and nuts. In: Rouz V, Bril B, editors. Stone knapping: The necessary conditions for a uniquely hominoid behavior. Cambridge, MA: McDonald Institute Series Monograph; in press. [Google Scholar]
- Francks C, Fisher SE, MacPhie L, Richardson AJ, Marlow AJ, Stein JF, et al. A genomewide linkage screen for relative hand skill in sibling pairs. American Journal of Human Genetics. 2002;70:800–805. doi: 10.1086/339249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: Humanlike pattern of Wernicke's brain language area homologue. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Gazzaniga M. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Miller BL. Molecular approaches to cerebral laterality: Development and neurogeneration. American Journal of Medical Genetics. 2001;101:370–381. [PubMed] [Google Scholar]
- Ghirlanda S, Vallortigara G. The evolution of brain lateralization: A game-theoretical analysis of population structure. Proceedings of the Royal Society of London B. 2004;271:853–857. doi: 10.1098/rspb.2003.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijzen A. Bonnes manieres a table chez les anthropoids en captivite. Societe et e Royale de Zoologie d'Anvers. 1972;38:15–20. [Google Scholar]
- Glaser HSR. Differentiation of scribbling in a chimpanzee. In: Kummer H, editor. Proceeding of the Third International Congress of Primatology. Basil, Switzerland: Karger; 1971. pp. 142–149. [Google Scholar]
- Goodall J. Feeding behaviour of wild chimpanzees: A preliminary report. In: Napier J, Barnicot NA, editors. Symposia of the zoological society of London, Number 10. London: Zoological Society of London; 1963. pp. 39–47. [Google Scholar]
- Grzimek D. Rechtsund linkshndigkeit bei Pferden, Papageien, and affen. Zeitschrift Tierpsychologie. 1949;6:405–432. [Google Scholar]
- Güntürkün O. Avian visual lateralization: A review. Neuroreport. 1997;8:3–11. [PubMed] [Google Scholar]
- Haas G. Handigkeitsbeobachtungen bei Gorillas. Saugetierlaundliche Mitteilungen. 1958;6:59–62. [Google Scholar]
- Harrison R, Nystrom P. Limb preferences in Pan paniscus. American Journal of Physical Anthropology. 2001;112:149. Abstract. [Google Scholar]
- Heestand J. Behavioral lateralization in four species of ape. University of Washington; Seattle: 1986. Unpublished doctoral dissertation. [Google Scholar]
- Hicks R, Kinsbourne M. Genetic basis for human handedness: Evidence from a partial cross-fostering study. Science. 1976;192:908–910. doi: 10.1126/science.1273577. [DOI] [PubMed] [Google Scholar]
- Holder MK. Influences and constraints on manual asymmetry in wild African primates: Reassessing implications for the evolution of human handedness and brain lateralization. Rutgers University; Newark, NJ: 2000. Unpublished doctoral dissertation. [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Hand preferences in New World primates. International Journal of Comparative Psychology. 1997;9:173–207. [Google Scholar]
- Hopkins B, Ronnqvist L. Human handedness: Developmental and evolutionary perspectives. In: Simion F, Butterworth G, editors. The development of sensory, motor and cognitive capacities of early infancy: From perception to cognition. East Sussex, UK: Psychology Press; 1998. pp. 191–236. [Google Scholar]
- Hopkins WD. Laterality in monkeys and apes. In: Ehara A, Kimura T, Takenaka O, Iwamoto M, editors. Primatology today. Amsterdam: Elsevier; 1991. pp. 271–274. [Google Scholar]
- Hopkins WD. Posture and reaching in chimpanzees (Pan) and orangutans (Pongo) Journal of Comparative Psychology. 1993;17:162–168. doi: 10.1037/0735-7036.107.2.162. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for bimanual feeding in 140 captive chimpanzees (Pan troglodytes): Rearing and ontogenetic factors. Developmental Psychobiology. 1994;27:395–407. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences in juvenile chimpanzees: Continuity in development. Developmental Psychology. 1995a;31:619–625. [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. Journal of Comparative Psychology. 1995b;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Chimpanzee handedness revisited: 54 years since Finch (1941) Psychonomic Bulletin and Review. 1996;3:449–457. doi: 10.3758/BF03214548. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Heritability of hand preference in chimpanzees: Evidence from a partial interspecies cross-fostering study. Journal of Comparative Psychology. 1999a;113:307–313. doi: 10.1037/0735-7036.113.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in non-human primates. International Journal of Primatology. 1999b;20:851–866. [Google Scholar]
- Hopkins WD. Laterality in maternal cradling and infant positional biases: Implications for the evolution and development of hand preferences in nonhuman primates. International Journal of Primatology. 2004;25:1243–1265. doi: 10.1023/B:IJOP.0000043961.89133.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. Hemispheric specialization in infant chimpanzees (Pan troglodytes): Evidence for a relation with gender and arousal. Developmental Psychobiology. 1993;26:219–235. doi: 10.1002/dev.420260405. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. Evidence of asymmetries in spontaneous head turning in infant chimpanzees (Pan troglodytes) Behavioral Neuroscience. 1995;109:808–812. doi: 10.1037//0735-7044.109.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. A longitudinal study of laterality in chimpanzees (Pan troglodytes) Developmental Psychobiology. 2000;36:292–300. [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA, Griner KM. Locomotor adaptation and leading limb asymmetries in neonatal chimpanzees (Pan troglodytes) International Journal of Primatology. 1997;18:104–114. [Google Scholar]
- Hopkins WD, Bard KA, Jones A, Bales S. Chimpanzee hand preference for throwing and infant cradling: Implications for the origin of human handedness. Current Anthropology. 1993;34:786–790. [Google Scholar]
- Hopkins WD, Bennett A, Bales S, Lee J, Ward JP. Behavioral laterality in captive bonobos (Pan paniscus) Journal of Comparative Psychology. 1993;107:403–410. doi: 10.1037/0735-7036.107.4.403. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Does variation in sample size explain individual differences in hand preferences of chimpanzees: A reply to Palmer (2002) American Journal of Physical Anthropology. 2003;121:878–881. doi: 10.1002/ajpa.10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries of the primary motor cortex but not with homologous language areas. Behavioral Neuroscience. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Individual and setting differences in the hand preferences of chimpanzees (Pan troglodytes): A critical analysis and some alternative explanations. Laterality. 2005;10:65–80. doi: 10.1080/13576500342000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C, Freeman H, Russell J, Kachin M, Nelson E. Chimpanzees are right-handed when recording bouts of hand use. Laterality. 2005;10:149–159. doi: 10.1080/13576500342000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C, Wesley MJ, Hostetter A, Pilcher D. Grip morphology and hand use in chimpanzees (Pan troglodytes): Evidence of a left hemisphere specialization in motor skill. Journal of Experimental Psychology: General. 2002;131:412–423. doi: 10.1037//0096-3445.131.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Dahl JF. Birth order and hand preference in chimpanzees (Pan troglodytes): Implications for pathological models of human handedness. Journal of Comparative Psychology. 2000;114:302–306. doi: 10.1037/0735-7036.114.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Dahl JF, Pilcher D. Genetic influence on the expression of hand preferences in chimpanzees (Pan troglodytes): Evidence in support of the right shift theory and developmental instability. Psychological Science. 2001;12:299–303. doi: 10.1111/1467-9280.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, de Waal FD. Behavioral laterality in captive bonobos (Pan paniscus): Replication and extension. International Journal of Primatology. 1995;16:261–276. [Google Scholar]
- Hopkins WD, Fernanadez-Carriba S, Wesley MJ, Hostetter A, Pilcher D, Poss S. The use of bouts and frequencies in the evaluation of hand preferences for a coordinated bimanual task in chimpanzees (Pan troglodytes): An empirical study comparing two different indices of laterality. Journal of Comparative Psychology. 2001;115:294–299. doi: 10.1037//0735-7036.115.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Hook M, Braccini S, Schapiro S. Population-level right handedness for a coordinated bimanual task in chimpanzees (Pan troglodytes): Replication and extension in a second colony of apes. International Journal of Primatology. 2003;24:677–689. doi: 10.1023/A:1023752816951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Pilcher D, Cantalupo C. Neural basis of cognition, communication and handedness in non-human primates. In: Maestripieri D, editor. Primate psychology. Cambridge, MA: Harvard University Press; 2003. [Google Scholar]
- Hopkins WD, Rabinowitz DM. Manual specialization and tool-use in captive chimpanzees (Pan troglodytes): The effect of unimanual and bimanual strategies on hand preference. Laterality. 1997;2:267–277. doi: 10.1080/713754273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL. Further evidence of a right hand advantage in motor skill by chimpanzees (Pan troglodytes) Neuropsychologia. 2004;42:990–996. doi: 10.1016/j.neuropsychologia.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Cantalupo C, Freeman H, Schapiro S. Factors influencing the prevalence and handedness for throwing in captive chimpanzees (Pan troglodytes) Journal of Comparative Psychology. doi: 10.1037/0735-7036.119.4.363. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Freeman H, Buehler N, Reynolds E, Schapiro SJ. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes) Psychological Science. 2005;16:456–462. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Hook M, Braccini S, Schapiro S. Simple reaching is not so simple: Association between hand use and grip preferences in captive chimpanzees. International Journal of Primatology. 2005;26:259–277. doi: 10.1007/s10764-005-2924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Stoinski T, Lukas K, Ross S, Wesley MJ. Comparative assessment of handedness for a coordinated bimanual task in chimpanzees (Pan), gorillas (Gorilla), and orangutans (Pongo) Journal of Comparative Psychology. 2003;117:302–308. doi: 10.1037/0735-7036.117.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Wesley MJ, Izard MK, Hook M, Schapiro SJ. Chimpanzees are predominantly right-handed: Replication in three colonies of apes. Behavioral Neuroscience. 2004;118:659–663. doi: 10.1037/0735-7044.118.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Wesley MJ, Izard MK, Hook M, Schapiro SJ. Determinants of chimpanzees: Genetics or social learning? 2005 Manuscript submitted for publication. [Google Scholar]
- Hopkins WD, Wesley MJ, Russell J, Schapiro SJ. Parental and perinatal factors influencing the development of handedness in chimpanzees. Developmental Psychobiology. doi: 10.1002/dev.20165. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingmanson EJ. Hand use preference among Pan paniscus at Wamba, Zaire; Paper presented at the annual meeting of the American Association of Physical Anthropologist; Raleigh-Durham, NC. 1996. Apr, [Google Scholar]
- Ingmanson EJ. Right and left hand use preference in a power grip task among Pan paniscus at Wamba; Paper presented at the annual meeting of the American Association of Physical Anthropologist; Salt Lake City, UT. 1998. Apr, [Google Scholar]
- Itakura S. Manual action in infant chimpanzees. A preliminary study. Perceptual and Motor Skills. 1996;83:611–614. doi: 10.2466/pms.1996.83.2.611. [DOI] [PubMed] [Google Scholar]
- Jones-Engel LE, Bard KA. Precision grips in young chimpanzees. American Journal of Primatology. 1996;39:1–15. doi: 10.1002/(SICI)1098-2345(1996)39:1<1::AID-AJP1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kellogg WN, Kellogg LN. The ape and the child: A study of environmental influence on early behavior. New York: Whittlesey House; 1933. [Google Scholar]
- Kim HS, Wadekar RV, Takenala O, Winstaney C, Mitsunaga F, Kageyama, et al. SINE-R. C2 (a Homo sapiens specific retroposon) is homologous to CDNA in a post-mortem brain in schizophrenia and to two loci in the Xq21.3Yp block linked to handedness and psychosis. American Journal of Medical Genetics. 1999;88:560–566. [PubMed] [Google Scholar]
- Klar AJ. Genetic models of handedness, brain lateralization, schizophrenia, and manic-depression. Schizophrenia Research. 1999;39:207–218. doi: 10.1016/s0920-9964(99)00075-4. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Draeger B, Bobe L, Lohman H, Ringelstein EB, et al. Language lateralization in healthy right-handers. Brain. 2000;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- Krause MA, Fouts RS. Chimpanzee (Pan troglodytes) pointing: Hand shapes, accuracy, and the role of eye gaze. Journal of Comparative Psychology. 1997;111:330–336. doi: 10.1037/0735-7036.111.4.330. [DOI] [PubMed] [Google Scholar]
- Laland KN, Kumm J, Van Horn JD, Feldman MW. A gene-culture model of human handedness. Behavior Genetics. 1995;25:433–445. doi: 10.1007/BF02253372. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Aureli F, Hopkins WD, Hyatt CW. Effect of cognitive challenge on self-directed behaviors by chimpanzees (Pan troglodytes) American Journal of Primatology. 2001;55:1–14. doi: 10.1002/ajp.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman RAW. Manual preference in prosimians, monkeys, and apes. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. pp. 107–124. [Google Scholar]
- Lockard J. Handedness in a captive group of lowland gorillas. International Journal of Primatology. 1984;5:356. Abstract. [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population-level handedness for tool use. Proceedings of the National Academy of Sciences, USA. 2005;102:12634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz-Maki SG, MacNeilage PF. Right side preference for force application. American Journal of Primatology. 1991;24:117. Abstract. [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–303. [Google Scholar]
- Manning JT, Heaton R, Chamberlain AT. Left-side cradling: Similarities and differences between apes and humans. Journal of Human Evolution. 1994;26:77–83. [Google Scholar]
- Marchant LF. Hand preferences among captive island groups of chimpanzees. The State University of New Jersey; New Brunswick: 1983. Unpublished doctoral dissertation, Rutgers. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: Comprehensive study of spontaneous activities. Journal of Human Evolution. 1996;30:427–443. [Google Scholar]
- Marchant LF, Steklis HD. Hand preference in a captive island group of chimpanzees. American Journal of Primatology. 1986;10:301–313. doi: 10.1002/ajp.1350100403. [DOI] [PubMed] [Google Scholar]
- Matsuzawa T, Biro D, Humle T, Inoue-Nakamura N, Tonooka R, Yamakoshi G. Emergence of culture in wild chimpanzees: Education by master-apprenticeship. In: Matsuzawa T, editor. Primate origins of human cognition and behavior. Tokyo: Springer; 2001. pp. 557–574. [Google Scholar]
- McGee MG, Cozad T. Population genetic analysis of human hand preference: Evidence for generation differences, familial resemblance and maternal effects. Behavior Genetics. 1980;10:263–275. doi: 10.1007/BF01067772. [DOI] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF. Chimpanzees, tools, and termites: Hand preference or handedness? Current Anthropology. 1992;33:114–119. [Google Scholar]
- McGrew WC, Marchant LF. Are gorillas right-handed or not? Human Evolution. 1993;8:17–23. [Google Scholar]
- McGrew WC, Marchant LF. On which side of the apes? In: McGrew WC, Marchant LF, Nishida T, editors. Great ape societies. Cambridge: Cambridge University Press; 1996. pp. 255–272. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- McGrew WC, Marchant LF. Laterality in hand use pays off in foraging for wild chimpanzees. Primates. 1999;40:509–513. [Google Scholar]
- McGrew WC, Marchant LF. Ethological study of manual laterality in the chimpanzees of the Mahale mountains, Tanzania. Behaviour. 2001;138:329–358. [Google Scholar]
- McGrew WC, Marchant LF, Wrangham RW, Klein H. Manual laterality in anvil use: Wild chimpanzees cracking Strychnos fruits. Laterality. 1999;4:79–87. doi: 10.1080/03069887600760101. [DOI] [PubMed] [Google Scholar]
- McManus C. Handedness, language dominance, and aphasia. A genetic model. Psychological Medicine. 1985;18:347–355. [PubMed] [Google Scholar]
- McManus IC, Bryden MP. The genetics of handedness, cerebral dominance and lateralization. In: Rapin I, Segalowitz SJ, editors. Handbook of neuropsychology. Vol 6: Developmental neuropsychology. Pt 1. Amsterdam: Elsevier; 1992. pp. 115–144. [Google Scholar]
- Michel GF. Right-handedness: A consequence of infant supine head-orientation preference? Science. 1981;212:685–687. doi: 10.1126/science.7221558. [DOI] [PubMed] [Google Scholar]
- Miles L. Hand preference in a signing ape; Paper presented at the Laterality: Evolution and Mechanism Conference; Memphis, TN: Memphis State University; 1990. [Google Scholar]
- Milliken GW, Stafford DK, Ward JP. Laterality in face, body and conspecific touching in two family groups of orangutan (Pongo pygmaeus) American Journal of Primatology. 1994;33:229. Abstract. [Google Scholar]
- Morange F. Handedness in two chimpanzees in captivity. In: Anderson JR, Roeder JJ, Thierry B, Herrenschmidt N, editors. Current primatology. Vol III: Behavioral neuroscience, physiology and reproduction. Strasbourg: Universite Louis Pasteur; 1994. pp. 61–67. [Google Scholar]
- Moricello A, Fernandez-Carriba D, Loeches A. Motor asymmetries in locomotion and postural control in chimpanzees (Pan troglodytes) American Journal of Primatology. doi: 10.1002/ajp.20280. in press. [DOI] [PubMed] [Google Scholar]
- Morris RD, Hopkins WD, Bolser-Gilmore L. Assessment of hand preference in two language-trained chimpanzees (Pan troglodytes): A multi-method analysis. Journal of Clinical and Experimental Neuropsychology. 1993;15:487–502. doi: 10.1080/01688639308402573. [DOI] [PubMed] [Google Scholar]
- Nishida T. Left nipple suckling preference in wild chimpanzees. Ethology and Sociobiology. 1993;14:45–52. [Google Scholar]
- Nishida T, Hiraiwa M. Natural history of tool making behavior by wild chimpanzees in feeding upon wood-bearing ants. Journal of Human Evolution. 1982;14:73–99. [Google Scholar]
- Olson DA, Ellis JE, Nadler RD. Hand preferences in captive gorillas, orangutans, and gibbons. American Journal of Primatology. 1990;20:83–94. doi: 10.1002/ajp.1350200203. [DOI] [PubMed] [Google Scholar]
- O'Neil CR, Stratton HTR, Ingersoll RH, Fouts R. Conjugate lateral eye movements in Pan troglodytes. Neuropsychologia. 1978;20:759–762. doi: 10.1016/0028-3932(78)90013-1. 1978. [DOI] [PubMed] [Google Scholar]
- Palmer AR. Chimpanzee right-handedness reconsidered: Evaluating the evidence with funnel plots. American Journal of Physical Anthropology. 2002;118:191–199. doi: 10.1002/ajpa.10063. [DOI] [PubMed] [Google Scholar]
- Palmer AR. Reply to Hopkins and Cantalupo: Chimpanzee right-handedness reconsidered—sampling issues and data presentation. American Journal of Physical Anthropology. 2003;121:382–384. [Google Scholar]
- Papademetriou E, Sheu CF, Michel GF. A meta-analysis of primate hand preferences for reaching and other hand-use measures. Journal of Comparative Psychology. 2005;119:33–48. doi: 10.1037/0735-7036.119.1.33. [DOI] [PubMed] [Google Scholar]
- Parnell RJ. Hand preference for food processing in wild western lowland gorillas (Gorilla gorilla gorilla) Journal of Comparative Psychology. 2001;115:365–375. [PubMed] [Google Scholar]
- Perelle IB, Ehrman L. An international study of human handedness: The data. Behavior Genetics. 1994;24:217–227. doi: 10.1007/BF01067189. [DOI] [PubMed] [Google Scholar]
- Peters H. Behavioral lateralization in the orangutan (Pongo pygmaeus pygmaeus) University of New England; Australia: 2005. Unpublished doctoral dissertation. [Google Scholar]
- Porac C, Coren S. Lateral preferences and human behavior. New York: Springer; 1981. [Google Scholar]
- Previc FH. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychological Review. 1991;98:299–334. doi: 10.1037/0033-295x.98.3.299. [DOI] [PubMed] [Google Scholar]
- Provins KA. Handedness and speech: A critical reappraisal of the role of genetic and environmental factors in the cerebral lateralization of function. Psychological Review. 1997;104:554–571. doi: 10.1037/0033-295x.104.3.554. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech function. Annals of the New York Academy of Sciences. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Raymond M, Pontier D. Is there geographical variation in human handedness? Laterality. 2004;9:35–51. doi: 10.1080/13576500244000274. [DOI] [PubMed] [Google Scholar]
- Redshaw ME. The development of precision in human and gorilla infants. In: Preuschoft H, Chivers DJ, editors. Hands of primates. New York: Springer-Verlag; 1993. pp. 45–53. [Google Scholar]
- Reiss BF, Ross S, Lyerly SB, Birch HG. The behavior of two captive specimens of lowland gorillas (Gorilla gorilla) Zoologica. 1949;34:111–118. [PubMed] [Google Scholar]
- Remis MJ. Tree structure and sex differences in arboreality among western lowland gorillas (Gorilla gorilla gorilla) at Bai Hokou, Central African Republic. Primates. 1999;40:383–396. [Google Scholar]
- Rensch BV, Ducker G. Manipulierfahigheit eines jungen orangutans and eines jungen gorilla mit Ammerkungen uder das Speilverhalten. Zeitschrift Tierpsychologie. 1966;23:874–896. [PubMed] [Google Scholar]
- Robinson C. The development of hand preference in children and young chimpanzees. University of Nevada; Reno: 1979. Unpublished doctoral dissertation. [Google Scholar]
- Rogers LJ, Andrew JR. Comparative vertebrate lateralization. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Rogers LJ, Kaplan G. Hand preferences and other lateral biases in rehabilitated orangutans, Pongo pygmaeus pygmaeus. Animal Behaviour. 1995;51:13–25. [Google Scholar]
- Rogers LJ, Kaplan G. Teat preference for suckling in common marmosets: Relationship to side of being carried and hand preference. Laterality. 1998;3:269–281. doi: 10.1080/713754301. [DOI] [PubMed] [Google Scholar]
- Schaller GB. The mountain gorilla: Ecology and behavior. Chicago: University of Chicago Press; 1963. [Google Scholar]
- Schiller PF. Figural preferences in the drawing of a chimpanzee. Journal of Comparative and Physiological Psychology. 1951;44:101–111. doi: 10.1037/h0053604. [DOI] [PubMed] [Google Scholar]
- Searleman A, Porac C, Coren S. Relationship between birth order, birth stress, and lateral preferences: A critical review. Psychological Bulletin. 1989;105:397–408. doi: 10.1037/0033-2909.105.3.397. [DOI] [PubMed] [Google Scholar]
- Shafer DD. Handedness in gorillas. Gorilla Foundation. 1988;8:2–5. [Google Scholar]
- Shafer DD. Patterns of hand preference in gorillas and children. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. pp. 267–283. [Google Scholar]
- Shafer DD. Hand preference behaviors shared by two groups of captive bonobos. Primates. 1997;38:303–313. [Google Scholar]
- Sicotte NL, Woods RP, Mazziotta JC. Handedness in twins: A meta-analysis. Laterality. 1999;4:265–286. doi: 10.1080/713754339. [DOI] [PubMed] [Google Scholar]
- Signore P, Chaoui M, Nosten-Bertrand M, Perez-Diaz F, Marchaland C. Handedness in mice: Comparison across 11 different strains. Behavior Genetics. 1991;21:421–429. doi: 10.1007/BF01065977. [DOI] [PubMed] [Google Scholar]
- Steiner SM. Handedness in chimpanzees. Friends of Washoe. 1990;9:9–19. [Google Scholar]
- Sugiyama Y. Tool-use for catching ants by chimpanzees at Bossou and Monts Nimba, West Africa. Primates. 1995;36:193–205. [Google Scholar]
- Sugiyama Y, Fushimi T, Sakura O, Matsuzawa T. Hand preference and tool use in wild chimpanzees. Primates. 1993;34:151–159. [Google Scholar]
- Toback EL. Laterality of manual and pedal activity in captive chimpanzees (Pan troglodytes) University of Sterling; Sterling, Scotland, UK: 1999. Unpublished doctoral dissertation. [Google Scholar]
- Tomasello M, Call J. Primate cognition. London: Oxford University Press; 1997. [Google Scholar]
- Tonooka R, Matsuzawa T. Hand preferences in captive chimpanzees (Pan troglodytes) in simple reaching for food. International Journal of Primatology. 1995;16:17–34. [Google Scholar]
- Turtle R. Apes of the world. Their social behavior, communication, mentality, and ecology. Park Ridge, NJ: Noyes; 1986. [DOI] [PubMed] [Google Scholar]
- Vallortigara G. Right hemisphere advantage for social recognition in the chick. Neuropsychologia. 1992;30:761–768. doi: 10.1016/0028-3932(92)90080-6. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behavioral and Brain Sciences. 2005;28:575–589. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- Vleeschouwer KD, van Elsacker L, Verheyen RF. Reaching, individual digit control and handedness in great apes; Paper presented at the International Primatological Society meeting in Strasbourg; France. 1994. [Google Scholar]
- Vleeschouwer KD, van Elsacker L, Verheyen RF. Effects of posture on hand preferences during experimental food reaching in bonobos (Pan paniscus) Journal of Comparative Psychology. 1995;109:203–207. doi: 10.1037/0735-7036.109.2.203. [DOI] [PubMed] [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Waters NS, Denenberg VH. Analysis of two measures of paw preference in a large population of inbred mice. Behavioural Brain Research. 1994;63:195–204. doi: 10.1016/0166-4328(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Weidauer G. Neue Handigkeitsbeobachtungen bei Menschenaffen. Biologische Rundshau. 1972;10:63–64. [Google Scholar]
- Welles J. A comparative study of manual prehension in Anthropoids. Saugetierlaundliche Mitteilungen. 1976;24:26–37. [Google Scholar]
- Westergaard GC, Kuhn HE, Suomi SJ. Bipedal posture and hand preference in humans and other primates. Journal of Comparative Psychology. 1998;112:56–63. doi: 10.1037/0735-7036.112.1.55. [DOI] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGew WC, Nishida T, Reynolds V, Sugiyama Y, et al. Charting cultural variation in chimpanzees. Behaviour. 2001;138:1489–1525. [Google Scholar]
- Yanez-Gonzalez, Leavens DA. Handedness for social behaviors in captive chimpanzees. 2004 Unpublished findings. [Google Scholar]
- Yeager C. Orangutan (Pongo pygmaeus) hand preference in the Tanjung Putting National Park. American Journal of Primatology. 1991;24:142. Abstract. [Google Scholar]
- Yeo R, Gangestad SW. Developmental origins of variation in human hand preference. Genetica. 1993;89:281–296. [Google Scholar]
- Yeo R, Thoma RJ, Gangestad SW. Human handedness: A biological perspective. In: Segalowitz SJ, Rapin I, editors. Handbook of neuropsychology. London: Elsevier; 2002. pp. 329–364. [Google Scholar]
- Yerkes R. The mind of the gorilla. Genetic Psychological Monographs. 1927;2:1–193. [Google Scholar]


