Abstract
Objectives. Groove pancreatitis (GP) describes a form of segmental pancreatitis, which affects the pancreatic head at the interface with the duodenum, and is frequently associated with ectopic pancreatic tissue in the duodenal wall. We present a series of symptomatic patients with complicated GP who underwent pancreaticoduodenectomy, and review the diagnostic challenges, imaging modalities, pathological features and clinical outcome of this rare condition. Patients and methods. This was a prospective case base study of clinical, radiological and pathological data collected between the years 2000 and 2005 on patients diagnosed with severe GP – confirmed by histopathological examination following pancreaticoduodenectomy. Results. In total 11 patients were included, presenting with chronic abdominal pain (n=11), gastric outlet obstruction (n=5) and jaundice (n=1). Exocrine dysfunction with associated weight loss (median > 9 kg) was present in 10 patients, and type 2 diabetes in 2 patients. Radiological imaging (CT/MRCP/EUS) provided complementary investigations and correlated well with classic histopathological findings (duodenal wall thickening, mucosal irregularity and Brunner's gland hyperplasia, duodenal wall cysts and pancreatic heterotropia). Following pancreaticoduodenectomy (median follow-up period 52 weeks) all patients experienced significant pain alleviation and weight gain (average 3 kg at 2 months). Conclusion. Pancreaticoduodenectomy is associated with significant improvements in weight gain and alleviates the chronic pain associated with severe GP.
Introduction
A distinct form of chronic pancreatitis occurring predominantly in and around the duodenal wall has been reported under various names, including cystic dystrophy of heterotopic pancreas 1, pancreatic hamartoma of duodenum 2, para-duodenal wall cyst, myoadenomatosis 3 and groove pancreatitis (GP) 4. The underlying pathology involves inflammatory changes of the duodenal wall resulting in thickening and cyst formation, as first described by Potet and Duclert 1. The term groove pancreatitis was coined by Becker 4 to describe a form of segmental pancreatitis, which affects the pancreatic head at the interface with the duodenum, and is frequently associated with ectopic pancreatic tissue in the duodenal wall. This rare but distinct entity is often confused clinically with ampullary neoplasms, duodenal tumours, cystic tumours of the head of pancreas and acute relapsing pancreatitis. Patients with GP have duodenal stenosis with a clinical pattern similar to chronic pancreatitis (CP), although CP is often associated with the disease itself 2,5,6,7. Clinical complications associated with GP are related to inflammatory changes affecting the duodenal wall, these include major gastrointestinal haemorrhage, perforation, chronic debilitating abdominal pain, recurrent pancreatitis, duodenal stenosis, and albeit a small risk of malignant transformation of ectopic pancreas 5,6,7,8,9.
We present a series of symptomatic patients with GP who underwent pancreaticoduodenectomy, and review the diagnostic challenges, imaging modalities, pathological features and clinical outcome of this rare condition.
Patients and methods
Patients undergoing pancreaticoduodenectomy (PD) for symptomatic GP following a clinical diagnosis of GP supported by radiological imaging that included computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP) and trans-duodenal endoscopic ultrasonography (EUS) were prospectively included in the study at the Leeds Teaching Hospitals NHS Trust during a 6-year period (2000–2005). GP was confirmed histologically in all cases following pancreaticoduodenectomy by a specialist pancreatic histopathologist (C.V.). Collated data included demography, clinical presentation, disease complications, diagnostic work-up (tumour markers, liver function tests and endoscopic/radiological investigations) and clinical outcome (treatment, morbidity and mortality).
Results
Eleven patients were included in the study (10 men and 1 woman), with a median age at diagnosis of 48 years (range 35–61 years) (Table I). All patients had chronic abdominal pain requiring a minimum of regular non-opiate analgesia. Pain was often described as a dull epigastric pain radiating to the back. Nine of 11 patients were on regular opiate analgesia, 2 of whom were also taking tricyclic antidepressants. Five patients were under regular review by the chronic pain team. Intermittent nausea and vomiting occurring for more than 2 months was featured in eight patients (73%). An acute presentation of gastric outflow obstruction occurred in five patients, four of whom could not tolerate solids or liquids. One patient presented with jaundice.
Table I. Baseline patient demographics, pancreatic functional status and blood biochemistry.
| Case | Age Dx | Abdominal pain | Weight loss | Pancreatitis | Steatorrhoea | Diabetes | Alcohol | Smoking | GOO | Analgesia |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | Y | Y | N | N | N | Y | Y | Y | NSAIDS, opiates |
| 2 | 42 | Y | Y | N | N | N | Y | Y | Y | NSAIDS, opiates |
| 3 | 35 | Y | Y | Y; 5× | Y | Y | Y | Y | Y | NSAIDS, opiates |
| 4 | 46 | Y | Y | Y; 4× | Y | N | Y | Y | N | NSAIDS, opiates |
| 5 | 52 | Y | Y | Y; 4× | Y | N | Y | Y | N | NSAIDS, opiates |
| 6 | 49 | Y | N | N | N | N | Y | Y | N | NSAIDS |
| 7 | 48 | Y | Y | Y; 2× | N | N | Y | Y | Y | NSAIDS, opiates |
| 8 | 57 | Y | Y | Y; 2× | N | Y | Y | N | Y | NSAIDS |
| 9 | 47 | Y | Y | Y; 3× | N | N | Y | Y | N | NSAIDS, opiates |
| 10 | 39 | Y | Y | Y; 3× | N | N | Y | Y | N | NSAIDS, opiates |
| 11 | 61 | Y | Y | Y; 1× | Y | N | Y | Y | N | NSAIDS, opiates |
GOO, gastric outlet obstruction; NSAIDs, non-steroidal anti-inflammatory drugs; Y, yes; N, no.
The majority of patients were heavy alcohol drinkers with an average weekly intake > 30 units per week (range 20–50 units) (Table I). All but one patient were heavy smokers (> 25 pack years). Eight patients had a history of recurrent attacks of pancreatitis, with a median number of three attacks (range 1–5) over the last 3 years (Table I).
Evidence of exocrine insufficiency was present in 10 of 11 patients, manifest as steatorrhoea in 4 patients (36%), and significant weight loss in 10 patients (mean weight loss of 9 kg in 3 months, range 0–64 kg). Only two patients (18%) were diabetics, reporting increased insulin requirements prior to diagnosis.
Derangements in liver function tests were detected in three patients (one had an isolated elevation in alanine-L-transpeptidase (ALT), one had an isolated elevation in alkaline phosphatase (AlkPhos) and one had elevated ALT, AlkPhos and bilirubin (TBil 76 IU/L) and was clinically jaundiced at presentation.
Mild hyperamylasaemia was detected in two patients (serum amylase of 408 and 421 IU/L), and both these patients had normal liver function tests (Table I). Serum CEA and CA 19.9 levels were within normal range in all patients where measured.
Diagnostic imaging
Nine of 11 patients underwent a contrast-enhanced multi-slice CT scan of the abdomen with particular emphasis on the pancreas and duodenum (Table II). Features diagnostic of GP on CT were present in six patients (67%). Cystic lesions in the head of pancreas at the interface with the duodenum were observed in four cases, although a mass, either cystic or solid, was observed in five patients. Duodenal wall thickening with or without cystic changes was reported in five patients, and calcification of the pancreatic parenchyma was present in only two cases (18%). Following CT scan, four patients went on to have MRI/MRCP as their next investigation (of which three required EUS). A further four patients underwent EUS alone.
Table II. Radiological and histopathologically determined characteristics of groove pancreatitis.
| Histopathology |
Diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | CT | MRCP | EUS | Suspected diagnosis | Microscopy | No. of cysts | Special features | Distance into HOP (cm) | |
| 1 | 4 cm MHOP, BDD, PDD | Not done | CP | Pancreatic cancer | GF, BGH, EP, antral gastritis | Multiple Max. size 0.2 cm | Necrosis <1 mm to PD | 1.5 | Pure GP |
| 2 | 4 cm CMHOP | CCDW, BDD | DS, CCDW | GP | CCDW (×3), EP, BGH, GF | 3 Max. size 1.5 cm | – | 1.5 | Pure GP |
| 3 | 2 cm CMHOP | Not done | Not done | Cystic tumour | CCDW, GF, EP, CPHOP | Multiple Max. size 1.8 cm | – | NA | Segmental GP |
| 4 | DWT, CCDW | Not done | CP, SHOP, DWT, BDD, PDD | GP | BDD, PDD, CCDW, GF, EP, BGH | 15 Max. size 0.3 cm | Rupture of Santorini duct | 0.8 | Pure GP |
| 5 | DWT, CP, CCDW | Not done | CP, DS, DWT, BDD, PDD | GP | CCDW, GF, EP, BGH, CPHOP | 2 Max. size 1.5 cm | – | 1.2 | Segmental GP |
| 6 | Not done | MHOP, BDD | DPDD, CMHOP | Cystic tumour | CCDW, EP, GF | Multiple Max. size 1.1 cm | Ruptured Santorini duct | 2.5 | Pure GP |
| 7 | DWT, DS, CDDW, CP | DWT, CCDW | Not done | GP | CCDW, GF, BGH, CPHOP, antral gastritis | Multiple Max. size 1.0 cm | – | 1 | Segmental GP |
| 8 | Not done | 5 cm CMHOPDWT | CCDW, DS | GP | CCDW, EP, BGH, DS, antral gastritis | 3 Max. size 2.0 cm | – | 0.7 | Pure GP |
| 9 | DWT, BDD | BDD, PDD | CP, BDD, PDD | Ampullary tumour | CCDW, GF, BGH, EP | Multiple Max. size 1.0 cm | – | 1.3 | Pure GP |
| 10 | CMHOPDWT | Not done | DWT, CCDWCP | GP | CCDW, GF, BGH, EP | Multiple Max. size 0.2 cm | – | 0.8 | Pure GP |
| 11 | 3 cm CMHOP | 1.5 cm MHOP, BDD, PDD | 1.3 cm CMHOP, BDD, CP | Pancreatic cancer | GF, BGH, EP | 1 Max. size 0.5 cm | – | 0.5 | Pure GP |
CT, computed tomography; MRCP, magnetic resonance cholangiopancreatogram; EUS, endoscopic ultrasound; HOP, head of pancreas; MHOP, mass head of pancreas; BDD, biliary duct dilatation; CMHOP, cystic mass head of pancreas; SHOP, swollen head of pancreas; DWT, duodenal wall thickening; CCDW, cystic changes duodenal wall; CP, chronic pancreatitis; CPHOP, chronic pancreatitis head of pancreas; DS, duodenal stenosis; PDD, pancreatic duct dilatation; DPDD, distal pancreatic duct dilatation; GP, groove pancreatitis; GF, groove fibrosis and scarring; BGH, Brunners’ gland hyperplasia (duodenum); EP, ectopic pancreatic tissue.
MRI/MRCP was carried out in six patients (following CT in four patients, and as a primary modality following ultrasound scan in two patients). Cystic changes in the duodenal wall were confirmed in three patients as reported on CT imaging. One patient was reported to have a complex mass, and two patients had inflammatory changes at the pancreaticoduodenal groove (Table II). Isolated biliary dilatation was observed in two patients (one with cystic changes in the duodenal wall and the other with a mass in the head of pancreas), and dilatation of both common bile duct (CBD) and pancreatic duct (PD) was seen in two further cases (one with a cystic mass of the head of pancreas and the other with an ill-defined lesion at the distal end of the CBD). Isolated PD dilatation was not observed in any of the cases. MRI observations correlated well with CT findings (all four patients), and provided additional information with respect to PD dilatation in a patient reported to have only biliary dilatation on CT. In the two patients who had an MRI as first line imaging, one was shown to have a complex mass in the pancreaticoduodenal groove and the second had a mass in the head of pancreas with a dilated CBD.
Overall groove pancreatitis was suggested in three of six patients (50%), and of the three remaining patients, two had indeterminate masses in the head of pancreas and one had a cystic lesion in the head of pancreas.
Nine patients underwent trans-duodenal endoscopic ultrasound – four had prior CT only, three had CT and MRI, and two had MRI alone. EUS demonstrated a combination of cystic changes in the duodenal wall and duodenal wall thickening in four patients and duodenal wall thickening alone in two patients. Of the remaining three patients, one had features of CP only, while the other two had cystic masses in the head of pancreas. On EUS features suggestive of CP were present in two-thirds of the patients (67%).
Correlation of EUS findings with CT and MRI
Correlation of CT and EUS findings showed agreement in five of seven cases (71%). A cystic lesion observed within the duodenal wall on CT (in one patient) was shown to have duodenal wall thickening alone on EUS. Duodenal wall thickening observed on CT was in agreement with EUS findings (one patient); however, cystic masses seen in the head of pancreas on CT (two patients) were thought to be in the duodenal wall in one patient and head of pancreas in the other. EUS provided additional information with respect to the presence of CP (five additional cases to the one observed on CT). EUS observations differed from those on CT in two patients. In the first case, CT revealed a 4 cm mass in the uncinate process associated with mild CBD and PD dilatation, while EUS suggested CP confined to the body and tail of pancreas and no masses. In the second patient, CT correctly identified inflammatory changes confined to the pancreaticoduodenal groove, whereas EUS suggested a more diffuse CP of the head of pancreas.
MRCP agreed with EUS findings in all five cases (100%), although CP was more frequently detected or suspected on EUS. Of the two patients in which GP could not be diagnosed on MRI, EUS was able to define a solid lesion on MR to be cystic lesion with CP, and an ill-defined lesion in another patient to be changes associated with CP.
All patients underwent a pancreaticoduodenectomy. One patient, with underlying restrictive airways disease (COPD), developed postoperative adult respiratory distress syndrome (ARDS) and multi-organ failure (MOF). After a median follow-up period of 52 weeks (range 4–156), all patients experienced significant pain alleviation. Nine of the 10 patients no longer require any analgesia, and one patient is currently on non-opiate analgesia (Table II). At 2 months follow-up, significant weight gain (average 3 kg) was reported in all patients.
Histopathology
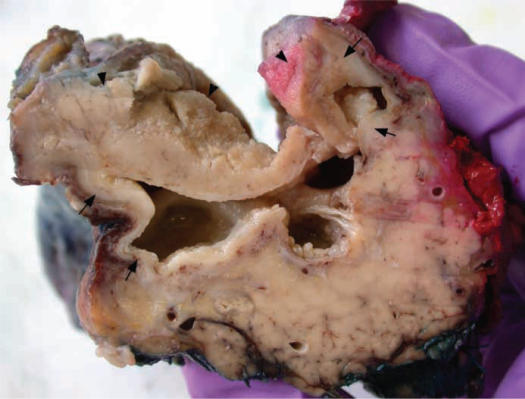
All cases showed active chronic inflammation of the pancreaticoduodenal interface and adjacent duodenal wall and pancreatic head, with extensive scarring and widening of the pancreaticoduodenal groove (Table II). In all cases cystic spaces were identified, which varied in number (1–15) and size (0.2–2.0 cm). Most of the cysts were located within the duodenal muscularis propria and submucosa and/or pancreaticoduodenal groove, and some cavities extended into the adjacent pancreatic parenchyma (Figure 1). The cyst walls consisted of either granulation tissue, often with palisaded histiocytes, or pancreatic duct-type columnar epithelium. Some cysts contained foci of squamous or mucinous epithelial lining.
Figure 1. .
Four irregular cystic spaces measuring up to 1.5 cm in diameter are present within the duodenal submucosa and muscularis propria (arrows). The overlying duodenal (sub)mucosa (arrowheads) is thickened.
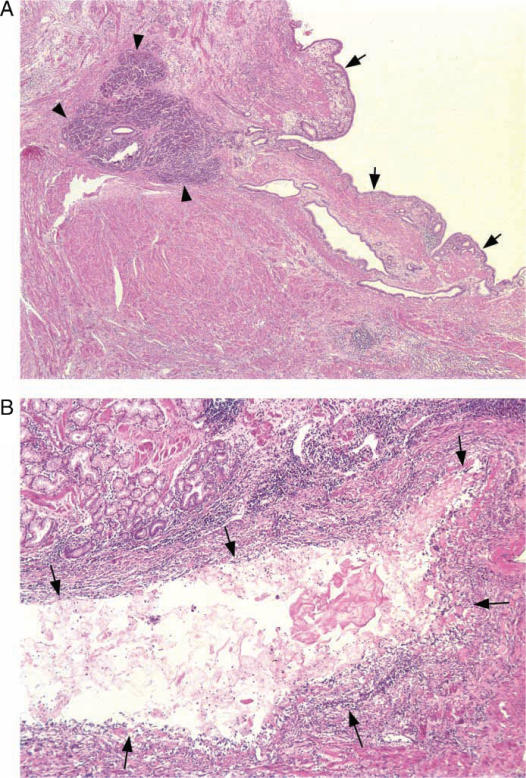
Ectopic pancreatic tissue, including acini, islets and ducts, was identified in the duodenal wall of all cases (Figure 2). In one of the cases, ectopic pancreatic tissue was also found in the wall of the gastric antrum. The volume of the ectopic tissue varied from microscopic foci to grossly visible nodular lesions in the major and minor papilla of two cases. Intramural ectopic pancreatic tissue was often associated with disarray of the surrounding muscularis propria. Many of the ectopic pancreatic ducts were dilated, and some showed evidence of rupture and/or inflammation.
Figure 2. .
(A) Ectopic pancreatic tissue, composed of acini (arrow heads) and ductules is present within the duodenal muscularis propria. One of the ectopic ducts is cystically dilated (arrows) (×50). (B) Some of the cystic lesions (arrows) within the duodenal wall are inflamed and have lost their epithelial lining (×200).
None of the above features were found below the level of the ampulla of Vater, and in most cases, inflammatory changes appeared more pronounced in the superior portion of the pancreatic head, at the level of the minor papilla or above.
Brunner's gland hyperplasia was seen in all cases, and in combination with the above-described inflammatory changes; in seven cases this resulted in marked thickening and irregularity of the duodenal wall, swelling of the mucosal folds and luminal narrowing of the duodenum over a median distance of 6 cm (5.5–8 cm).
The severity of inflammation of the adjacent pancreatic parenchyma varied markedly. Active chronic inflammation with or without cystic alteration extended over a median distance of 1.5 cm (0.7–2.5 cm) into the pancreatic head. In two cases, there was inflammatory rupture of the Santorini duct with severe inflammation and necrosis of adjacent pancreatic parenchyma, and in one case extension of necrotizing inflammation into the anterior peripancreatic fat. In both cases, ectopic pancreatic tissue was present in and compressed the minor papilla. In two further cases, necrotizing inflammation extended to within 1 mm from the main pancreatic duct.
Evidence of chronic pancreatitis, affecting the pancreas at a distance from the pancreaticoduodenal groove, was identified in two cases. In all other cases, there was a varying degree of pancreatic duct dilatation, with prominent intraductal protein plugs. Two cases contained a peri-ampullary (duodenal) diverticulum.
Discussion
In the original report by Becker and Mischke, groove pancreatitis was described as an inflammatory process occurring between the C-loop of the duodenum and head of pancreas and scarring of the pancreaticoduodenal groove 4. In a topographical analysis of over 600 surgical resected cases of CP, of which 19.5% had groove involvement, Becker and Mischke described 3 types of GP 4: that which oc o ocurred in the groove alone – termed ‘pure GP’ (2%), that which in in in addition had segmental pancreatitis of head of pancreas – termed segmental GP (6.5%), and a chronic homogenous pancreatitis with groove involvement (non-segmental GP) – (11%).
Common symptoms in all our patients were intractable chronic abdominal pain, often requiring opiate-based analgesia, along with nausea and vomiting, although only 5 of 11 patients presented with overt symptoms of gastric outlet obstruction. Duodenal stenosis occurs as a consequence of compression from GP and cystic degenerative changes in the duodenal wall 4,6,10. It has been reported in 50–70% of cases of GP 4,6, and is less frequently observed in CP (31%) and pancreatic cancer (16.7%). Duodenal wall cysts are often multiple and appear to be related to pancreatic heterotopia 4,10,11. Duodenal wall cysts were observed in 49% of all cases of groove scarring in Becker and Mischke's series 4, although only in 9% and 16% of cases of pure GP and segmental GP, respectively, in the series of Stolte et al. 6. However, the incidence of solitary cysts in the groove was much higher, 25% in non-segmental GP, 36% in GP and 26% in segmental GP. Duodenal wall thickening and duodenal wall cysts were observed on CT in 90% and 70% of our cases, respectively 10,11,12. In a retrospective series of 20 patients with GP, Vullierme et al. described these duodenal changes in all cases, whereby the cysts were often multiple and large 12. Marked Brunner's gland hyperplasia was a constant contributing factor to the thickening of the duodenal wall, and a complex pathophysiological relationship between chronic alcohol consumption, GP and Brunner's gland hyperplasia has been suggested previously 6.
Histopathological examination of our specimens demonstrated cystic lesions in the duodenal wall of all cases. These were mostly multiple and varied in size from 0.2 cm to 2 cm. Some of the cysts had retained their epithelial lining, which was of pancreatic duct type, while others showed evidence of rupture, and were demarcated by granulation tissue (so-called pseudocysts). Ectopic pancreatic tissue was present in all our specimens, and there seemed to be a gradual transition from small or dilated ectopic pancreatic ducts to the above-described cysts and pseudocysts. The ectopic pancreatic tissue and inflammatory changes were localized along to the pancreaticoduodenal interface superior to the ampulla of Vater, while the pancreas below the ampulla remained mainly unaffected. This distribution pattern, which has been described previously 5, is intriguing and raises the possibility of a link with the complex embryogenesis of the pancreatic head. Interestingly, the ventral anlage, from which most of the inferior (infra-papillary) part of the pancreatic head is derived, fuses with the duodenum at a later stage than the dorsal anlage, and it can be speculated that this is associated with a lower incidence of pancreatic heterotopia.
Inflammation was often not limited to the pancreaticoduodenal groove and in some cases this extended deep into the pancreatic head over a distance of up to 2.5 cm. In two cases extensive and severe inflammation of pancreatic parenchyma and peripancreatic fat was associated with rupture of the Santorini duct. In a further two cases, necrotizing inflammation extended to within 1 mm of the main pancreatic duct.
Scarring of the CBD is reportedly the mechanism which leads to stenosis of the distal CBD, with dilatation of the proximal biliary tree 4,5,6. Pancreatic ductal dilatation is less frequent and is related to segmental GP, and often described to be absent in pure GP 4,6. In segmental GP fibrosis of the pancreatic parenchyma leads to stenosis of the PD of Santorini, sparing the duct of Wirsung. Inflammation, atrophy and fibrosis associated small ductal rupture in pancreatic parenchyma adjacent to the groove was commonly observed, although pancreatic ductal dilatation with protein plugging and intraductal calculi was observed in only three cases – deemed segmental GP.
GP is likely a consequence of inflammation of ectopic pancreatic tissue, the extent of which may explain the varying clinical presentation. The presence of intractable pain and/or pancreatic insufficiency (weight loss, steatorrhoea or diabetes), can be attributed to the severe inflammatory and fibrotic changes observed in the head of pancreas – often associated with duodenal obstruction secondary to scarring or cystic dystrophy. In such patients, a pancreaticoduodenectomy affords symptomatic relief and can lead to adequate weight gain.
Acknowledgements
We would like to thank Drs Maria Sheridan FRCR, Keith Harris FRCS FRCR and Alan Chalmers FRCR, for their expert review of the radiology.
References
- 1.Potet F, Duclert N. Dystrophie kystique sur pancreas aberrant de la paroi duodenale. Arch F Mal App Dig. 1970;59:223–38. [PubMed] [Google Scholar]
- 2.McFaul CD, Vitone LJ, Campbell F, Azadeh B, Hughes ML, Garvey CJ, et al. Pancreatic hamartoma. Pancreatology. 2004;4:533–8. doi: 10.1159/000080528. [DOI] [PubMed] [Google Scholar]
- 3.Ryan A, Lafnitzegger JR, Lin DH, Jakate S, Staran ED. Myoepithelial hamartoma of the duodenal wall. Virchows Arch. 1998;432:191–4. doi: 10.1007/s004280050155. [DOI] [PubMed] [Google Scholar]
- 4.Becker V, Mischke U. Groove pancreatitis. Int J Pancreatol. 1991;10:173–82. doi: 10.1007/BF02924155. [DOI] [PubMed] [Google Scholar]
- 5.Shudo R, Obara T, Tanno S, Fujii T, Nishino N, Sagawa M, et al. Segmental groove pancreatitis accompanied by protein plugs in Santorini's ducts. J Gastroenterol. 1998;33:289–94. doi: 10.1007/s005350050086. [DOI] [PubMed] [Google Scholar]
- 6.Stolte M, Weiss W, Volkholz H, Roesch W. A special form of segmental pancreatitis: “groove pancreatitis”. Hepatogastroenterology. 1982;29:198–208. [PubMed] [Google Scholar]
- 7.Holstege A, Barner S, Brambs HJ, Wenz W, Gerok W, Farthmann EH. Relapsing pancreatitis associated with duodenal wall cysts. Gastroenterology. 1985;88:814–19. doi: 10.1016/0016-5085(85)90157-x. [DOI] [PubMed] [Google Scholar]
- 8.Jeng KS, Yang KC, Kuo SH. Malignant degeneration of heterotropic pancreas. Gastrointest Endosc. 1991;37:196–8. doi: 10.1016/s0016-5107(91)70687-1. [DOI] [PubMed] [Google Scholar]
- 9.Lai EC, Tompkins RC. Heterotropic pancreas: review of a 26-year experience. Am J Surg. 1986;51:697–700. doi: 10.1016/0002-9610(86)90045-0. [DOI] [PubMed] [Google Scholar]
- 10.Itoh S, Yamakawa K, Shimamoto K, Endo T, Ishigaki T. CT findings in groove pancreatitis: correlation with histopathological findings. J Comput Assist Tomogr. 1994;18:911–15. doi: 10.1097/00004728-199411000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Flejou J-F, Potet F, Molas G, Bernades P, Amouyal P, Fekete F. Cystic dystrophy of the gastric and duodenal wall developing in heterotropic pancreas: an unrecognised entity. Gut. 1993;34:343–7. doi: 10.1136/gut.34.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vullierme M-P, Vilgrain V, Flejou JF, Zins M, O'Toole D, Ruszniewski P, et al. Cystic dystrophy of the duodenal wall in the heterotropic pancreas: radiopathological correlations. J Comput Assist Tomogr. 2000;24:635–43. doi: 10.1097/00004728-200007000-00023. [DOI] [PubMed] [Google Scholar]