Abstract
Central synapses exhibit spontaneous neurotransmitter release that is selectively regulated by cAMP-dependent protein kinase A (PKA). We now show that synaptic vesicles contain synaptotagmin-12, a synaptotagmin isoform that differs from classical synaptotagmins in that it does not bind Ca2+. In synaptic vesicles, synaptotagmin-12 forms a complex with synaptotagmin-1 that prevents synaptotagmin-1 from interacting with SNARE complexes. We demonstrate that synaptotagmin-12 is phosphorylated by cAMP-dependent PKA on serine97, and show that expression of synaptotagmin-12 in neurons increases spontaneous neurotransmitter release by approximately threefold, but has no effect on evoked release. Replacing serine97 by alanine abolishes synaptotagmin-12 phosphorylation and blocks its effect on spontaneous release. Our data suggest that spontaneous synaptic-vesicle exocytosis is selectively modulated by a Ca2+-independent synaptotagmin isoform, synaptotagmin-12, which is controlled by cAMP-dependent phosphorylation.
Introduction
Presynaptic terminals release neurotransmitters in two modes: evoked release, which is induced by Ca2+ flowing into the nerve terminal when stimulated by an action potential, and spontaneous release, which occurs in the absence of massive Ca2+ influx (Katz, 1969). Evoked release is clearly the more important form of release in terms of how the brain processes information, but many observations indicate that spontaneous release may also be physiologically important, and not just an “accident” of turbocharged presynaptic release machinery (for reviews see Otsu and Murphy, 2003; Zucker, 2005). This evidence suggests that “spontaneous” synaptic vesicle exocytosis causing miniature postsynaptic currents (minis) may be mechanistically distinct from evoked exocytosis and independently regulated, and that minis may have a biological function.
Mechanistically, minis appear to derive from a vesicle pool that differs from that which feeds evoked release (Sara et al., 2005). Although evoked and spontaneous release both require SNARE proteins, deletions of the vesicular SNARE protein synaptobrevin/VAMP2 differentially alter evoked and spontaneous release (Deitcher et al., 1998; Schoch et al., 2001), and structure/function studies suggest that the sequences of synaptobrevin required for evoked and spontaneous release differ (Deak et al., 2006). Moreover, spontaneous and evoked release appear to be differentially regulated by Ca2+ (Llano et al., 2000; Angleson and Betz, 2001).
Physiologically, minis may have a substantial function in regulating neural networks. In cultured hippocampal slices, blocking all release by botulinum toxin had a dramatic effect on spine morphology, whereas blocking only evoked release by tetrodotoxin did not (McKinney et al., 1999). Similarly, in cultured hippocampal neurons, spontaneous release was shown to stabilize synaptic function through tonic suppression of dendritic protein synthesis (Sutton et al., 2006). In addition, in small cerebellar interneurons, a single excitatory or inhibitory quantum, which is what is released by a mini event, can trigger or inhibit, respectively, the generation of an action potential (Carter and Regehr, 2002).
In synapses of mouse cortical and hippocampal neurons, and in Drosophila melanogaster neuromuscular junctions, evoked synchronous neurotransmitter release is triggered by Ca2+ binding to synaptotagmin-1 (Geppert et al., 1994; Fernández-Chacón et al., 2001; Yoshihara and Littleton, 2002; Nishiki and Augustine, 2004; Maximov and Südhof, 2005), whereas synaptotagmin-2 performs a similar role in brainstem synapses (Pang et al., 2006). In addition to mediating synchronous Ca2+-triggered release, synaptotagmin-1 and -2 both normally restrict spontaneous release (Pang et al., 2006), suggesting that they are intrinsic components of the release machinery. We now find that another member of the synaptotagmin family, synaptotagmin-12, is colocalized with synaptotagmin-1 on synaptic vesicles, but is expressed much later in development. Synaptotagmin-12 was originally described as a thyroid hormone–inducible protein (Thompson, 1996) that is homologous to synaptotagmin-1, but lacks its Ca2+-binding sequences, suggesting that it does not participate in Ca2+ triggering of release. We demonstrate that expression of synaptotagmin-12 in cultured neurons at a time when no endogenous synaptotagmin-12 can be detected causes a dramatic and selective increase in spontaneous release. Moreover, we show that synaptotagmin-12 is phosphorylated by cAMP-dependent protein kinase A (PKA) at a single site, and that mutation of this site blocks the effect of synaptotagmin-12 on spontaneous release, suggesting that this phosphorylation activates its up-regulation of spontaneous release. Finally, we demonstrate that synaptotagmin-12 forms a tight constitutive complex with synaptotagmin-1 on synaptic vesicles, but regulates spontaneous release independently from synaptotagmin-1. Our data suggest a function for a Ca2+-independent synaptotagmin isoform in modulating spontaneous release.
Results
Expression of synaptotagmin-12
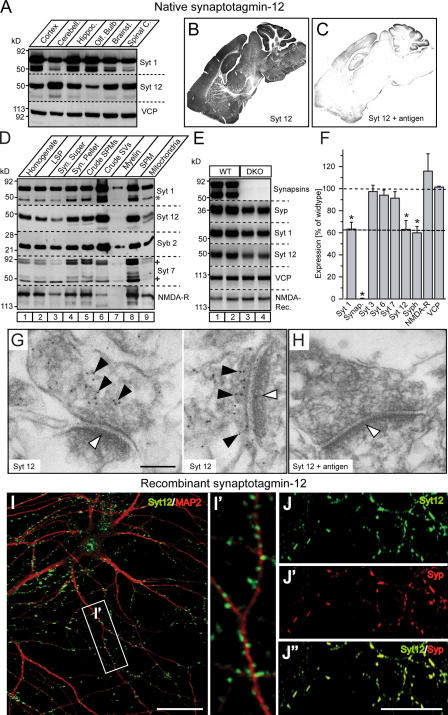
We generated an antibody against the linker sequence between the transmembrane region and C2 domains of synaptotagmin-12. As expected from the lack of sequence homology between synaptotagmin isoforms in the linker region, the synaptotagmin-12 antibody recognized only this isoform, but no other synaptotagmin (Fig. 1 A). Immunoblotting revealed that synaptotagmin-12 is expressed in the brain and adrenal medulla (Fig. 1 B), but not in other nonneuronal tissues tested (Fig. 1 D), and is also absent from various cell lines, including PC12 cells (Fig. 1 C).
Figure 1.
Expression of native synaptotagmin-12 in various tissues and neuronal cultures. (A) Lysates of COS7 cells transfected with cDNAs encoding full-length synaptotagmins 1–13 (Syt 1–13) were analyzed with indicated polyclonal antibodies to various synaptotagmins and valosin-containing protein (VCP; used as a loading control). (B–F) Various tissues, cultured cell lines, and primary neuronal cultures were analyzed by immunoblotting with antibodies to synaptotagmin-12 (Syt 12), -1 (Syt 1), and VCP as a loading control. Equal amounts of total protein were loaded into each lane. (B) Synaptotagmin-12 is expressed in adult mouse brain and adrenal medulla. (C) Expression of synaptotagmin-12 could not be detected in cultured cell lines. (D) Analysis of indicated rat tissues isolated at different stages of development ranging from embryonic day 18 to postnatal day 40 (as shown on the right side of each image), shows that expression of synaptotagmin-12 is restricted to postnatal brain and displays progressive increase during development. (E and F) Relative expression levels of synaptotagmin-1 and -12 were determined by quantitative immunoblotting of total brain homogenates isolated from mouse at different stages of postnatal development and cortical neurons isolated from newborn mouse pups and cultured in vitro for 5–15 d. (E) Raw immunoblotting data. (F) Quantification of expression with I125-labeled secondary antibodies. The relative expression levels of each protein were plotted as the percentage of expression in adult brains (n ≥ 4). Similar results were obtained with cultured hippocampal neurons (not depicted). Data are shown as the mean ± the SEM.
Analysis of different developmental time points showed that synaptotagmin-12 is undetectable during embryonic development (embryonic day 18) when synaptotagmin-1 is already robustly expressed, is present at very low levels postnatally, and becomes abundant after the first postnatal week (Fig. 1 D). Quantitation of the developmental time course of expression revealed that synaptotagmin-12 levels increase by ∼10-fold during the second postnatal week (Figs. 1, E and F). This expression profile is very different from that of synaptotagmin-1, the levels of which increase less than twofold over the same time period, but agrees well with the expression of the D. melanogaster synaptotagmin-12 homologue that also exhibits low levels throughout embryonic development (Adolfsen et al., 2004).
Localization of synaptotagmin-12 to synaptic vesicles
Immunoblotting of tissue homogenates isolated from various regions of adult brain (Fig. 2 A) and immunolabeling of brain sections (Fig. 2 B) revealed that synaptotagmin-12 is abundantly expressed throughout the brain, with the highest expression levels in cerebellum. This labeling was specific because preincubation with the antigen greatly reduced synaptotagmin-12 immunoreactivity in brain sections (Fig. 2 C). To establish the localization of native synaptotagmin-12, we fractionated the brain homogenates and analyzed the distribution of synaptotagmin-12 in different subcellular fractions. Synaptotagmin-12 was highly enriched in the fraction that contains synaptic vesicles (Fig. 2 D).
Figure 2.
Expression and localization of synaptotagmin-12. (A) Total homogenates of cortex, cerebellum, hippocampus, olfactory bulb, brain stem, and spinal cord isolated from the brains of adult rats were analyzed with antibodies to synaptotagmin-12, -1, and VCP (loading control). 20 μg of total protein was loaded into each lane. Note that synaptotagmin-12 is expressed at different levels in various brain regions, with the highest expression level in cerebellum. (B and C) Sections of brains isolated from adult mice were labeled with antibody to synaptotagmin-12. (B) Labeling of brain sections indicates that synaptotagmin-12 is abundantly expressed in various brain regions. (C) Brain sections were labeled with antibody to synaptotagmin-12 mixed with ∼1 mg/ml recombinant GST-linker fusion protein that was used for immunization. Note that synaptotagmin-12 immunoreactivity is greatly reduced in the presence of the antigen. (D) Distribution of native synaptotagmin-12 in subcellular fractions of adult brain separated by centrifugation. Similar to synaptotagmin-1 (Syt 1) and synaptobrevin/VAMP2 (Syb 2), synaptotagmin-12 is enriched in synaptic vesicle fraction (crude SVs). Asterisks indicate proteolitically cleaved fragment of synaptotagmin-1 and various splice variants of synaptotagmin-7. (E) Expression of indicated proteins in brains of adult wild-type and synapsin double-knockout mice (DKO) was analyzed by quantitative immunoblotting with I125-labeled secondary antibodies. (F) The relative expression levels of indicated proteins in DKO brains were normalized to VCP and presented as the percentage of wild type. Note that expression of synaptotagmin-12 and other synaptic vesicle proteins is reduced by ∼40%. Data are shown as the mean ± the SEM. (G and H) Electron micrographs of adult mouse hippocampal sections labeled with synaptotagmin-12 antibody alone (G) or in the presence of ∼1 mg/ml of the antigen (synaptotagmin-12 linker region) used for immunization (H). The postsynaptic densities are marked by white arrowheads. Note that synaptotagmin-12 immunoreactivity is restricted to presynaptic sites (G; black arrowheads) and inhibited in the presence of the antigen (H). (I and J) Localization of recombinant synaptotagmin-12 in cultured hippocampal neurons. The neurons were infected at 5 d after plating (5 DIV) with lentivirus expressing full-length synaptotagmin-12 cDNA, and double labeled at 14 DIV with antibodies to synaptotagmin-12 and the dendritic marker MAP2 (I; Syt 12, green; MAP2, red) or Syt 12 and synaptic marker synaptophysin (J; Syt 12, green; synaptophysin, red). Inset (I′) illustrates enlarged fragment of dendrite marked by white box. Note that recombinant synaptotagmin-12 is concentrated in discrete clusters apposed to dendrites and colocalized with synaptophysin. Bars: (G) 100 nM; (I and J") 20 μM.
Because subcellular fractionation is imprecise, we next tested the localization of synaptotagmin-12 by measuring its levels in the brains of synapsin 1/2 double knockout mice. In these brains, synaptic vesicles are selectively decreased in numbers, and thus the levels of all synaptic vesicle proteins are decreased, whereas the levels of other proteins, e.g., active zone proteins, plasma membrane proteins, or cytosolic proteins, are unchanged (Rosahl et al., 1995). Indeed, we found that synaptotagmin-12 was reduced by ∼40% in the synapsin-deficient brains, which exactly corresponds to the decrease observed for other synaptic vesicle proteins (Fig. 2, E and F). The levels of synaptic proteins that are not localized on synaptic vesicles, such as the synaptotagmin isoforms 3, 6, and 7 or NMDA-receptors, were not decreased in synapsin double knockout mice (Fig. 2, E and F).
To obtain a higher resolution localization, we cultured cortical neurons from newborn mice or rats. We first attempted to localize endogenous synaptotagmin-12 in the cultured neurons, but surprisingly, could not detect any synaptotagmin-12 in the neurons even after prolonged culture (Fig. 1 E). Quantitations of the levels of synaptotagmin-1 and -12 revealed that whereas the concentration of synaptotagmin-1 continuously increased in the neurons as a function of culture time, synaptotagmin-12 remained at levels below the sensitivity of our assay, even after 15 d in vitro (DIV; Fig. 1 E). Thus, interestingly, synaptotagmin-12 is present in cultured neurons below the levels observed in the brains from which the neurons were obtained, suggesting that additional factors in brain that are absent under culture conditions must contribute to the regulation of synaptotagmin-12 expression.
To be able to localize synaptotagmin-12 in cultured neurons by immunofluorescence labeling, we therefore expressed recombinant synaptotagmin-12 with a lentivirus. We found that recombinant synaptotagmin-12 was targeted to synapses where it precisely colocalized with synaptophysin (Fig. 2, I and J). Synaptic vesicle localization of native synaptotagmin-12 was supported by immunohistochemical analysis of brain sections from adult mice that showed that synaptotagmin-12 is distributed in a typically synaptic pattern (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200607021/DC1), and by immunoelectron microscopy that revealed synaptotagmin-12 immunoreactivity over the vesicle cluster in presynaptic terminals (Fig. 2, G and H). Together, these data show that synaptotagmin-12 is a synaptic vesicle protein.
Biochemical properties of synaptotagmin-12
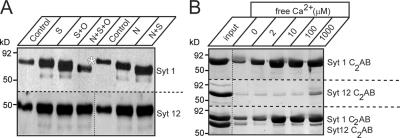
Vesicular targeting of synaptotagmin-1 is regulated by N-glycosylation (Han et al., 2004). To determine whether synaptotagmin-12 is also glycosylated, we tested the effects of various deglycosylating enzymes on the mobility of native synaptotagmin-12 during SDS-PAGE. In agreement with a previously published report (Han et al., 2004), removal of neuraminic acid and O- and N-linked sugars with sialidase, O-glycanase, PNGase, or different combinations of these enzymes resulted in a dramatic change in the mobility of synaptotagmin-1, but had no effect on the mobility of synaptotagmin-12, suggesting that synaptotagmin-12 is not glycosylated (Fig. 3 A).
Figure 3.
Synaptotagmin-12 is not glycosylated and lacks phospholipid binding. (A) Deglycosylation analysis of synaptotagmin-12. Proteins from rat brain homogenate were treated with sialidase (S), o-glycanase (O), PNGase (N), or different combinations of these enzymes to remove neuraminic acid and O- and N-linked sugars. The samples were then analyzed by immunoblotting with the antibodies to synaptotagmin-1 and -12. Treatment with deglycosylation enzymes results in a substantial change in mobility of synaptotagmin-1 in SDS-PAGE, but has no effect on mobility of synaptotagmin-12, indicating that synaptotagmin-12 is not glycosylated. (B) C2 domains of synaptotagmin-12 do not bind phospholipids in a Ca2+-dependent manner. Recombinant C2AB domains of synaptotagmins-1 and -12 were expressed as GST-fusion proteins and tested in phospholipid-binding assay at different concentrations of free Ca2+ (as indicated on the top of the gel). Unlike C2 domains of synaptotagmin-1 (Syt 1 C2AB; top), C2 domains of synaptotagmin-12 (Syt 12 C2AB) do not bind phospholipids when tested alone or in the presence of Syt 1 C2AB.
To test whether synaptotagmin-12 interacts with phospholipids, we performed phospholipid-binding assays with the recombinant C2AB domain of synaptotagmin-12, using the C2AB domain of synaptotagmin-1 as a positive control. Consistent with the primary sequence analysis that indicated that synaptotagmin-12 lacks Ca2+-binding sequences, no Ca2+-dependent phospholipid binding was observed when the C2AB domain of synaptotagmin-12 was tested alone or in the presence of C2AB domains of synaptotagmin-1 (Fig. 3 B).
PKA-dependent phosphorylation of synaptotagmin-12
A screen of the phosphorylation of different synaptotagmins in synaptosomes that were incubated with [32P]orthophosphate revealed that the strongest labeling with 32P under the conditions used was obtained for synaptotagmin-12 (Fig. 4 B and not depicted). To identify which protein kinase may mediate synaptotagmin-12 phosphorylation, we immunoprecipitated synaptotagmin-12 from nonlabeled brain extracts and incubated the immunoprecipitated synaptotagmin-12 with brain lysate in the presence of 1 mM ATP and 10 μCi γ-[32P]ATP alone or with addition of 0.1 mM cAMP, 0.1 mM cGMP, or 1 mM Ca2+. Without additions, no synaptotagmin-12 phosphorylation was detected, but cAMP caused strong phosphorylation (Fig. 4 C). cGMP induced marginal phosphorylation, possibly because cGMP at the high dose used (0.1 mM) can stimulate PKA, whereas Ca2+ did not stimulate any synaptotagmin-12 phosphorylation (Fig. 4 C).
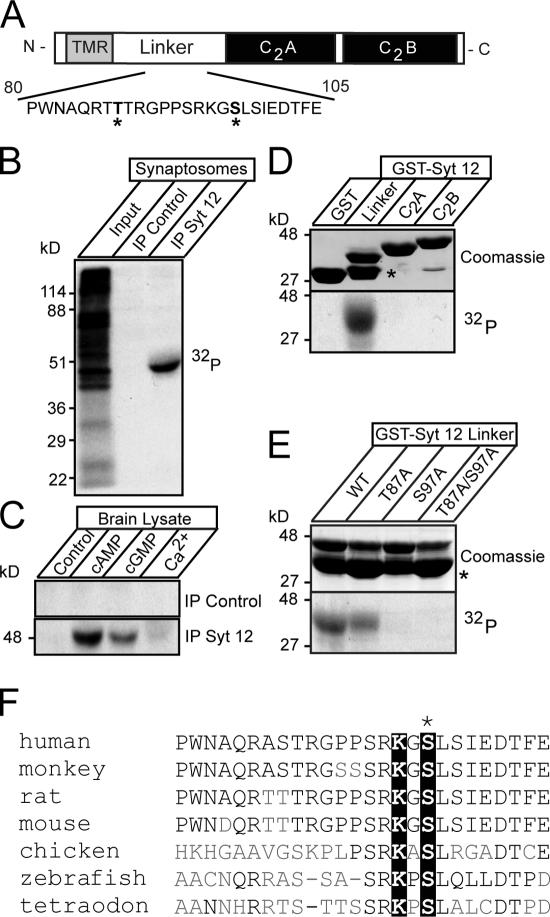
Figure 4.
Synaptotagmin-12 is phosphorylated in a cAMP-dependent manner at serine97. (A) Schematic diagram of synaptotagmin-12 domain structure. A fragment of linker sequence containing two putative sites for PKA-dependent phosphorylation (T87 and S97, marked by asterisks) is illustrated below. (B) In vivo phosphorylation of native synaptotagmin-12. The proteins from rat brain synaptosomes preincubated with [32P]orthophosphate were extracted in detergent and immunoprecipitated with antibody to synaptotagmin-12 or preimmune serum. Incorporation of 32P was measured with x-ray film. Note that a single ∼50-kD band is observed in synaptotagmin-12, but not in control immunoprecipitation. (C) In vitro phosphorylation of native synaptotagmin-12. Proteins extracted from brain were immunoprecipitated with synaptotagmin-12 antibody or preimmune serum attached to Sepharose beads. The beads were incubated with brain cytosol mixed with 1 mM ATP and 10 μCi γ-[32P]ATP alone or with addition of 0.1 mM cAMP, 0.1 mM cGMP, or 1 mM Ca2+. Note that synaptotagmin-12 is strongly phosphorylated in a cAMP-dependent manner. (D and E) Identification of a functional PKA phosphorylation site in synaptotagmin-12 sequence. (D) Indicated fragments of synaptotagmin-12 were expressed as recombinant GST fusion proteins (top shows Coomassie staining of all fusion proteins; the asterisk corresponds to GST) and phosphorylated in vitro in the presence of catalytic subunit of PKA and γ-[32P]ATP. Note that only linker region of synaptotagmin-12 is phosphorylated. (E) Phosphorylation of wild-type synaptotagmin-12 linker GST fusion protein and linkers containing single alanine substitutions in residues T87, S97, or a combination of these mutations. Note that phosphorylation is completely abolished in S97A mutant. The asterisk corresponds to GST. (F) Multiple sequence alignment of synaptotagmin-12 homologues from different species shows the fragments of the linker sequences containing conserved PKA phosphorylation site (marked by black boxes).
We searched the synaptotagmin-12 sequence for potential PKA phosphorylation sites and identified two such sites in the synaptotagmin-12 linker region (T87 and S97; Fig. 4 A). To determine whether one or both of these are being used, we first tested whether recombinant fragments of synaptotagmin-12, produced as GST-fusion proteins, were phosphorylated by PKA. Only the linker region, but not the C2 domains, were phosphorylated (Fig. 4 D). We next tested whether mutation of either T87 or S97 alters phosphorylation of the linker by PKA. We found that phosphorylation was completely abolished by substituting serine97 with alanine, whereas substituting threonine87 with alanine had no effect (Fig. 4 E), suggesting that synaptotagmin-12 is phosphorylated by PKA on serine97. To determine whether the PKA phosphorylation site in synaptotagmin-12 is evolutionarily conserved, we compared the linker sequences from different species. Although no significant homology was found between fly and vertebrate synaptotagmin-12 linker sequences (unpublished data), serine97 with a canonical preceding lysine residue was found to be conserved in all vertebrate synaptotagmin-12 homologues from zebrafish to humans (Fig. 4 F).
Synaptotagmin-12 modulates spontaneous, but not evoked, release
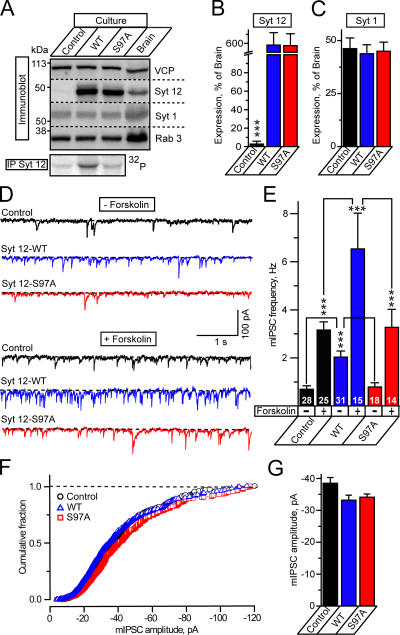
We next explored whether synaptotagmin-12 regulates synaptic transmission using cultured cortical neurons isolated from newborn mice or rats. Because the cultured neurons do not contain any detectable synaptagmin-12, even after prolonged culture (Fig. 1, E and F), the cultured neurons resemble a synaptotagmin-12 loss-of-function model. We infected the neurons with lentiviruses encoding full-length wild-type or S97A mutant synaptotagmin-12 and compared the expression levels of recombinant proteins after 15 DIV (and 10 d after lentivirus infection) with the levels of native synaptotagmin-12 in noninfected cultures and in brain (Fig. 5 A). We found that recombinant wild-type and S97A mutant synaptotagmin-12 were expressed in cultured neurons at equal levels, i.e., were properly translated, with an approximately sixfold higher concentration than that of synaptotagmin-12 in P15 brain (Fig. 5 B).
Figure 5.
Synaptotagmin-12 regulates spontaneous transmitter release. (A) Analysis of expression and phosphorylation of native and recombinant synaptotagmin-12 in cultured cortical neurons. Noninfected neurons or neurons infected with wild-type or S97A synaptotagmin-12 lentiviruses were analyzed by immunoblotting with antibodies to synaptotagmin-12, -1, Rab3, and VCP as a loading control. Radioimmunoprecipitation analysis (bottom) shows that recombinant wild-type synaptotagmin-12 is phosphorylated in cultured neurons and phosphorylation is abolished in S97A mutant. (B and C) Expression levels of synaptotagmin-12 and -1 in noninfected neurons and neurons infected with wild-type and S97A synaptotagmin-12 lentiviruses were determined by quantitative immunoblotting with I125-labeled secondary antibodies, normalized to VCP, and plotted as the percentage of expression in P15 brains. (D–G) Overexpression of wild-type synaptotagmin-12 increases the rate of spontaneous release in inhibitory synapses. Spontaneous mIPSCs were monitored in the absence or presence of 50 μM forskolin from noninfected neurons (control) or neurons infected with the lentiviruses expressing wild-type synaptotagmin-12 or S97A mutant. mIPSCs were recorded in the presence of CNXQ and APV to block excitatory responses and Tetrodotoxin (TTX) to block spontaneous firing. (D) Typical mIPSCs recorded from control neurons (shown in black) or neurons infected with wild-type (shown in blue) or S97A (shown in red) synaptotagmin-12 lentiviruses. mIPSCs were recorded in control conditions (traces 1–3) or in the presence of forskolin (traces 4–6). Scale bars apply to all traces. (E) Mean frequencies of mIPSCs monitored in various conditions (indicated on the bottom of the graph) from three different cortical cultures. The total numbers of analyzed neurons are shown on each column. (F and G) Overexpression of synaptotagmin-12 does not affect the amplitudes of spontaneous mIPSCs. F shows a cumulative amplitude histogram constructed from 550 individual mIPSCs collected from control noninfected neurons and neurons infected with wild-type and S97A synaptotagmin-12 lentiviruses, and G shows the plot of average mIPSC amplitudes. For each group, n ≥ 12 neurons (data from three independent cultures). Data are shown as the mean ± the SEM.
Both wild-type and S97A mutant synaptotagmin-12 were targeted to synapses (Fig. 2 J and not depicted). When compared with noninfected neurons, the neurons infected with synaptotagmin-12 lentiviruses did not display significant changes in general morphology, and the levels of endogenous synaptic proteins (Fig. 5, A and C, and not depicted). To test whether expressed synaptotagmin-12 is phosphorylated under physiological conditions, we analyzed noninfected neurons and neurons infected with wild-type and S97A mutant synaptotagmin-12 lentiviruses by immunoprecipitation. We labeled the neurons with [32P]orthophosphate, lysed them, and measured 32P incorporation into immunoprecipitated synaptotagmin-12. Significant synaptotagmin-12 phosphorylation was only detected in neurons expressing wild-type synaptotagmin-12, whereas noninfected neurons and neurons expressing mutant synaptotagmin-12 exhibited no significant 32P labeling (Fig. 5 A, bottom). These data suggest that under the conditions used, synaptotagmin-12 is phosphorylated only on serine97 either because another constitutively active protein kinase phosphorylates this amino acid or because, in these cultured neurons, PKA is activated.
To determine whether synaptotagmin-12 regulates evoked or spontaneous neurotransmitter release, we monitored inhibitory postsynaptic currents (IPSCs) in noninfected neurons or neurons expressing recombinant wild-type or S97A mutant synaptotagmin-12. We found that expression of wild-type synaptotagmin-12 increased the frequency of spontaneous release events threefold, but had no effect on their amplitude (Fig. 5, D–G). In contrast to spontaneous minis, synaptotagmin-12 had no effect on the sizes or kinetics of evoked responses, either when these were elicited by isolated single-action potentials or by high-frequency stimulus trains, indicating that both synchronous and asynchronous components or evoked release were unaffected (Fig. 6). Importantly, expression of S97A mutant synaptotagmin-12 had no effect on spontaneous release (Fig. 5, D and E), even though the mutant protein was expressed at the same level as wild-type synaptotagmin-12 (Fig. 5, A and B).
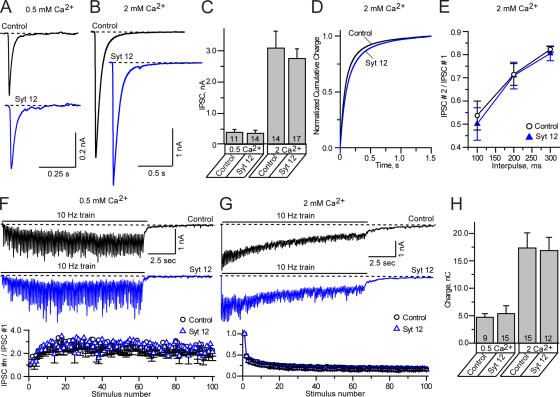
Figure 6.
Analysis of evoked IPSCs in neurons expressing synaptotagmin-12. Primary cortical neurons isolated from newborn pups were infected with lentivirus expressing wild-type synaptotagmin-12 and analyzed at 14–17. DIV Noninfected neurons or neurons infected with GFP-lentivirus were used as a control. Inhibitory synaptic responses were evoked by local extracellular stimulation and recorded in a whole-cell mode. AMPA and NMDA currents were suppressed by the addition of CNQX and APV to the extracellular bath solution. Recordings were performed in 0.5 or 2 mM extracellular Ca2+. The holding potential was –70 mV. The data are shown as the mean ± the SEM. (A–D) Amplitudes and the time course of individual IPSCs recorded form control neurons (shown in black) or neurons infected with synaptotagmin-12 lentivirus (shown in blue). IPSCs were triggered by single-action potentials applied at low frequency (0.1 Hz). (A and B) IPSCs recorded in 0.5 (A) or 2 mM (B) extracellular calcium from the neurons cultured for 14–16 DIV. The scale bars apply to all traces. (C) Mean amplitudes of IPSCs monitored in indicated concentrations of extracellular calcium from three different cortical cultures. The total numbers of analyzed neurons are shown on each column. (D) Cumulative histograms illustrate the time course of total charge transferred over 1.5 s after single-action potential stimulation. (E) Paired pulse IPSC ratios measured in 2 mM extracellular calcium in control neurons and neurons infected with synaptotagmin-12 lentivirus. IPSCs were triggered by two action potentials spaced by various interpulse periods, as indicated on the plot. Control, n = 10; Syt 12, n = 11. (F and G) Typical inhibitory responses recorded in 0.5 (F) or 2 mM (G) extracellular calcium from control (current traces on the top, shown in black) or synaptotagmin-12–infected neurons (current traces on the bottom, shown in blue) triggered by 100 action potentials applied at 10 Hz. The scale bars apply to all traces. The plots on the bottom show the average amplitudes of synchronous IPSCs during stimulus trains. (H) Average total charges transferred during trains of 100 action potentials applied at 10 Hz. The total numbers of analyzed neurons are shown on each column. Data are shown as the mean ± the SEM.
Several previous studies indicated that spontaneous release is up-regulated by cAMP-dependent pathways (Chavez-Noriega and Stevens, 1994; Capogna et al., 1995; Yoshihara et al., 2000; Doi et al., 2002). To test the possibility that the effect of synaptotagmin-12 on spontaneous release—which depends on the PKA-substrate site at serine97—intersects with the PKA-dependent regulation of spontaneous release, we examined the effect of forskolin (an activator of adenylate cyclase) on spontaneous release. We found that in control cultures, forskolin caused an ∼4.5-fold increase in the rate of spontaneous release (Fig. 5, D and E), but had no effect on evoked release (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200607021/DC1). In cultures expressing wild-type synaptotagmin-12, the increase in mini frequency induced by forskolin was much more potent than in control cultures, whereas in cultures expressing S97A mutant synaptotagmin-12, the effect of forskolin was identical to that observed in control cultures (Fig. 5, D and E).
Synaptotagmin-12 binding to synaptotagmin-1
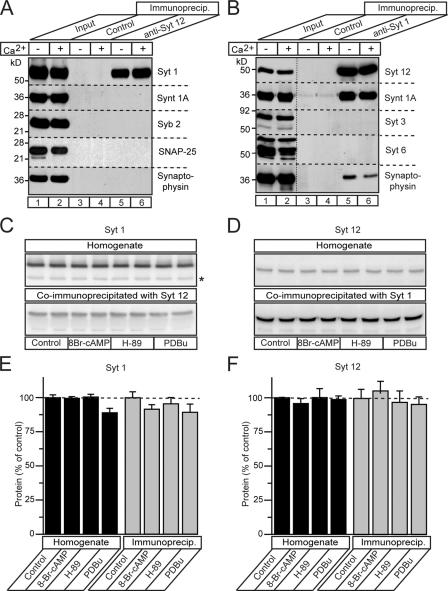
To obtain clues to the mechanism of action of synaptotagmin-12 in its selective effect on spontaneous release, we investigated whether synaptotagmin-12 might interact with SNARE proteins because binding to SNARE proteins is a salient property of other synaptotagmins (Bennett et al., 1992; Yoshida et al., 1992; Li et al., 1995; Chapman et al., 1995; Kee and Scheller, 1996; Banerjee et al., 1996; Rickman and Davletov, 2003; Rickman et al., 2004; Bowen et al., 2005; Tang et al., 2005). We performed immunoprecipitations of detergent-solubilized brain proteins with antibodies to synaptotagmin-1 and -12, and analyzed the immunoprecipitates for the presence of SNARE proteins and synaptotagmins. These experiments failed to reveal binding of synaptotagmin-12 to SNARE proteins, but showed that synaptotagmin-1 was coimmunoprecipitated with synaptotagmin-12 (Fig. 7 A), and that synaptotagmin-12 was coimmunoprecipitated with synaptotagmin-1 (Fig. 7 B). SNARE proteins were selectively absent from the synaptotagmin-12 immunoprecipitates, but were present in the synaptotagmin-1 immunoprecipitates. Equal amounts of synaptotagmin-1 and -12 were coimmunoprecipitated in the presence of either 1 mM free Ca2+ or EGTA, suggesting that the interaction between these proteins is Ca2+-independent (Fig. 7, A and B). Quantitations revealed that the equal amounts of synaptotagmin-1 and -12 were coimmunoprecipitated from nontreated brain homogenates and homogenates preincubated with PKA activator 8-Br-cAMP (1 mM), PKA inhibitor H-89 (5 μM), or PKC activator PDBu (1 μM), suggesting that interaction between synaptotagmin-1 and -12 is not regulated by phosphorylation (Fig. 7, C–F).
Figure 7.
Synaptotagmin-12 forms a Ca2+-independent complex with synaptotagmin-1. Brain proteins were extracted in 1% of Triton X-100 and immunoprecipitated with polyclonal antibody to synaptotagmin-12 (A), monoclonal antibody to synaptotagmin-1 (B), corresponding preimmune serum or irrelevant control antibody in the presence of 1 mM Ca2+ or EGTA. Immunoprecipitates were extensively washed and analyzed by immunoblotting with various antibodies, as indicated on the right side of each image. Input lines were loaded with 1% of total protein extract used for immunoprecipitation. (A) Synaptotagmin-1, but not SNARE, proteins are coimmunoprecipitated with synaptotagmin-12. (B) Synaptotagmin-12 and syntaxin-1 are coimmunoprecipitated with synaptotagmin-1 in a Ca2+-independent manner. (C–F) Synaptotagmin-1 and -12 were coimmunoprecipitated form nontreated brain homogenates or homogenates incubated with PKA activator 8-Br-cAMP (1 mM), PKA inhibitor H-89 (5 μM), or PKC activator PDBu (1 μM). Immunoblots (C and D) and protein quantifications with I125-labeled secondary antibodies (E and F) show the relative amounts of synaptotagmin-1 and -12 in total protein extracts and immunoprecipitations under indicated conditions. Data are shown as the mean ± the SEM.
Synaptotagmin-12 regulates spontaneous release independent of synaptotagmin-1
To determine whether synaptotagmin-12 regulates spontaneous release through interaction with synaptotagmin-1, we infected cortical neurons isolated from newborn synaptotagmin-1–deficient mice (Geppert et al., 1994) with lentiviruses expressing wild-type and S97A mutant synaptotagmin-12. We then monitored spontaneous release and release triggered by action potentials (Fig. 8).
Figure 8.
Synaptotagmin-12 regulates spontaneous transmitter release independently from synaptotagmin-1. Primary cortical neurons isolated from newborn pups of synaptotagmin-1–deficient mice were infected with lentiviruses expressing GFP (control), wild-type synaptotagmin-12, or S97A mutant and analyzed at 15–17 DIV. (A–D) Evoked IPSCs triggered in 2 mM of extracellular Ca2+ by single-action potentials or trains of 100 action potentials applied at 10 Hz. (A) Typical asynchronous IPSCs triggered by single-action potentials in control neurons (black) or neurons expressing wild-type synaptotagmin-12 (blue). Scale bars apply to both traces. (B) Average charge transferred over 1.5 s by asynchronous responses triggered by single action potentials. The total numbers of analyzed neurons are shown on each column. (C) Asynchronous responses triggered by high frequency trains of 100 action potentials applied at 10 Hz. Scale bars apply to both traces. (D) Average total synaptic charge transfer during trains or 100 potentials in control neurons or neurons expressing wild-type synaptotagmin-12. The total numbers of analyzed neurons are shown on each column. (E and F) Synaptotagmin-12 increases the rate or spontaneous release in synaptotagmin-1–deficient neurons. (E) Typical mIPSCs recorded from control neurons (black) or neurons infected with wild-type (blue) or S97A (red) synaptotagmin-12 lentiviruses. mIPSCs were recorded in 0.2 mM of extracellular Ca2+ in the presence of tetrodotoxin, APV, and CNQX. Scale bars apply to all traces. (F) Average frequencies of mIPSCs monitored from two different cortical cultures. The total numbers of analyzed neurons are shown on each column. Data are shown as the mean ± the SEM.
Because deletion of synaptotagmin-1 results in an increase in the rate of spontaneous release (Maximov and Südhof, 2005; Pang et al., 2006,), we measured spontaneous release in a lower concentration of extracellular Ca2+ that allows us to resolve individual miniature IPSC events. We found that similar to wild-type neurons, expression of synaptotagmin-12 in synaptotagmin-1–deficient neurons enhanced the rate of spontaneous release (Fig. 8, E and F), but did not alter evoked release triggered by action potentials (Fig. 8, A–D). Because evoked release is completely asynchronous in synaptotagmin-1– deficient neurons (Maximov and Südhof, 2005), synaptotagmin- 12, thus, does not significantly alter asynchronous release. Although the increase in spontaneous release in synaptotagmin-1– deficient neurons expressing synaptotagmin-12 was not as dramatic as in wild-type cultures, the increase was still dependent on the phosphorylation of synaptotagmin-12 because the S97A mutant synaptotagmin-12 was unable to potentiate the mini IPSC (mIPSC) rate (Fig. 8, E and F). In these experiments, we could not determine the effects of forskolin on mIPSC frequency because the rate of spontaneous release was already too high.
Discussion
In this study, we demonstrate that synaptotagmin-12 is a synaptic vesicle protein that is widely expressed in the brain with a developmentally delayed onset (Fig. 1). Although synaptotagmin-12 is highly homologous to synaptotagmin-1 (which functions as the Ca2+ sensor for fast synaptic vesicle exocytosis), and is colocalized with synaptotagmin-1 on synaptic vesicles (Fig. 2 and Fig. S1), synaptotagmin-12 differs from synaptotagmin-1 in that it does not bind Ca2+ and phospholipids (Fig. 3 and not depicted). We also demonstrate that it is phosphorylated in a cAMP-dependent manner at a single residue, serine97 (Fig. 4). Because it is expressed in a developmentally delayed pattern, cultured neurons contain very low levels of endogenous synaptotagmin-12. Overexpression of recombinant synaptotagmin-12 in these neurons dramatically increased the rate of spontaneous release (Fig. 5), but had no effect on synchronous and asynchronous components of release triggered by action potentials (Fig. 6).
At least two alternative hypotheses could potentially explain how synaptotagmin-12 controls spontaneous neurotransmitter release: (1) synaptotagmin-12 regulates spontaneous release via its interaction with synaptotagmin-1, and (2) synaptotagmin-12 acts as an independent modulator of spontaneous release.
The first hypothesis is supported by the observation that synaptotagmin-12 forms a constitutive Ca2+-independent heterooligomeric complex with synaptotagmin-1 that is incompatible with the interaction of synaptotagmin-1 with SNARE complexes (Fig. 7). Previous studies indicate that synaptotagmin-1 is associated with SNARE complexes in a Ca2+-independent manner (Kee and Scheller, 1996; Rickman and Davletov, 2003), and that deletion of synaptotagmin-1 increases the rate of excitatory and inhibitory minis (Maximov and Südhof, 2005; Pang et al., 2006), suggesting that freeing SNARE complexes from synaptotagmin-1 may disinhibit spontaneous release. Our finding that synaptotagmin-12 interacts with synaptotagmin-1 suggests that synaptotagmin-12 increases spontaneous neurotransmitter release by pulling synaptotagmin-1 off SNARE complexes, and thereby disinhibiting spontaneous exocytosis. The alternative hypothesis would be that that synaptotagmin-12 regulates spontaneous release by a synaptotagmin-1–independent (but phosphorylation-dependent) mechanism, and that the interaction of synaptotagmin-12 with synaptotagmin-1 performs an additional, as yet undetermined, role.
To test the two hypotheses, we determined the effect of overexpression of synaptotagmin-12 in synaptotagmin-1–deficient neurons (Fig. 8). We found that in the absence of synaptotagmin-1, synaptotagmin-12 still produced an increase, albeit a moderate one, in the rate of spontaneous exocytosis. This result favors the second hypothesis, suggesting that the phosphorylation site–dependent modulation of mini release by synaptotagmin-12 does not require synaptotagmin-1. It is also unlikely that the effect of expression of synaptotagmin-12 on spontaneous release is caused by a massive change in synapse density or in the size of the readily releasable pool of synaptic vesicles because we observed no differences between neurons expressing or lacking wild-type or mutant synaptotagmin-12 in evoked synaptic responses; this applies both for responses triggered by single-action potentials or by trains of action potentials (Figs. 6 and 8). A recent study has shown that spontaneous release is driven by an isolated pool of synaptic vesicles containing synaptotagmin-1 and controlled by synaptobrevin/VAMP2 (Sara et al., 2005). It is conceivable that synaptotagmin-12 selectively regulates spontaneous release because it is only localized on this subpopulation of vesicles, but further biochemical analysis with an antibody against the luminal domain of synaptotagmin-12 will be required to test this possibility.
Our findings have several implications for the understanding of synaptic transmission. First, the possible biological role of spontaneous release events is widely debated, with opinions ranging from considering such events as mere accidental byproducts of turbo-charged fusion machinery to biologically meaningful processes of synaptic communication (Otsu and Murphy, 2003; Zucker, 2005). Our data, by identifying a synaptic vesicle synaptotagmin isoform that selectively regulates spontaneous release in cultured neurons, lend credence to the notion that spontaneous release is a highly specific and regulated event. Notably, the rate of spontaneous release increases with age (Hsia et al., 1998), as do the expression levels of synaptotagmin-12. This finding suggests a connection, but several other factors such as developmental changes in connectivity and synapse maturation must also play a major role in this process (Hsia et al., 1998).
Although the effect of synaptotagmin-12 in cultured neurons is specific for spontaneous as opposed to evoked release, this does not exclude the possibility that in an intact brain, synaptotagmin-12 could participate in PKA-dependent forms of plasticity. It is noticeable that synaptotagmin-12 was discovered as a thyroid hormone–inducible gene (Thompson, 1996; Dong et al., 2005) and comes on relatively late in development, suggesting that its role is under further regulation beyond the phosphorylation by PKA. Moreover, extensive studies indicate that cAMP-dependent phosphorylation regulates exocytosis in nonneuronal cells (Koh et al., 2000) and neurons (Chavez-Noriega and Stevens, 1994; Capogna et al., 1995; Yoshihara et al., 2000; Doi et al., 2002). Multiple pathways have been implicated in the cAMP-dependent modulation of synaptic strength (Chavez-Noriega and Stevens, 1994; Capogna et al., 1995; Carroll et al., 1998; Sakaba and Neher, 2001; Kaneko and Takahashi, 2004). The requirement of serine97 for the effect of synaptotagmin-12 on spontaneous release indicates that synaptotagmin-12 may be generally involved in the regulation of synaptic vesicle exocytosis by PKA-dependent phosphorylation. Finally, expression of synaptotagmin-12 was detected in adrenal glands (Fig. 1 B), suggesting that synaptotagmin-12 may also be localized on dense core vesicles and play a role in regulating calcium-independent secretion in nonneuronal cells.
Materials and methods
Plasmid construction
The following vectors were constructed for expression of various regions of rat synaptotagmin-12 as GST-fusion proteins: pGEX-KG-synaptotagmin-12-linker (aa 47–150); pGEX-KG-synaptotagmin-12-C2A (aa 151–281); and pGEX-KG-synaptotagmin-12-C2B (aa 282–421). For construction of synaptotagmin-12 lentivirus, full-length cDNA (aa 1–421) was subcloned into pFUGW shuttle vector. The point mutations were generated by PCR and verified by sequencing.
Antibody production
To generate a polyclonal antibody against synaptotagmin-12, the linker region of rat synaptotagmin-12 (aa 47–150) was expressed in BL-21 Escherichia coli strain as GST fusion protein and purified by affinity chromatography on glutathione–Agarose beads (GE Healthcare). The protein was eluted from the beads by 20 mM glutathione, dialyzed twice against PBS, and used for immunization at concentration of ∼1 mg/ml. The specificity of the serum was confirmed by immunoblotting of full-length recombinant synaptotagmins 1–13 expressed in COS7 cells. The antibody was then purified by sequential affinity chromatography on the columns containing GST and GST-synaptotagmin-12-linker covalently attached to NHS–Sepharose beads (Pierce Chemical Co.)
Subcellular fractionation
Whole rat brains, including cerebellum and olfactory bulb, were homogenized in 0.32 M sucrose, 25 mM Hepes, pH 7.2, and centrifuged at 800 g to remove the nuclear fraction. The postnuclear supernatant (LSP) was centrifuged at 12,000 g to separate the soluble (synaptosomal supernatant) and membrane (synaptosomal pellet) fractions. The synaptosomal pellet was hypotonically lysed by incubation for 30 min in 5 mM Tris, pH 7.2, and recentrifuged at 27,000 g to separate the crude synaptic plasma membranes and crude synaptic vesicles. The crude synaptic plasma membrane fraction was adjusted to 1.1 M sucrose, loaded to the bottom of the centrifuge tube, and layered by solutions of 0.86 and 0.32 M sucrose. The samples were then centrifuged at 19,000 RPM (SW41 Ti rotor; Beckman Coulter) to separate synaptic plasma membranes, myelin, and mitochondria. All fractions were adjusted to the total protein concentration of 4 mg/ml and analyzed by immunoblotting. Approximately 40 μg of total protein was loaded into each lane (Fig. 3 C).
Immunoprecipitations
Rat brains were homogenized in 50 mM Hepes, 100 mM NaCl, 2 mM MgCl2, 4 mM EGTA, pH 6.8, and solubilized for 2 h at 4°C in the same buffer containing 1% Triton X-100. The samples were then centrifuged for 1 h at 50,000 RPM (70 Ti rotor; Beckman Coulter) to remove insoluble material and incubated for 2 h at 4°C with protein A–or protein G–Sepharose beads (GE Healthcare) covered with polyclonal antibody to synaptotagmin-12, monoclonal antibody to synaptotagmin-1, corresponding preimmune serum, or control antibody. The protein complexes attached to the beads were washed five times with the extraction buffer, eluted with SDS sample buffer, and analyzed by SDS-page and immunoblotting. The input lanes were loaded with 1% of total protein extract used for immunoprecipitation. To determine the effects of activators or inhibitors of PKA-dependent phosphorylation, the brain homogenates were preincubated for 30 min with 1 mM 8-Br-cAMP, 5 μM H-89, or 1 μM PDBu (used as a negative control) before protein extraction, and the immunoprecipitations were carried in the presence of EGTA, as described above.
Cortical and hippocampal primary neuronal cultures
The cortexes or hippocampi were dissected from the brains of embryonic day 18 embryos or newborn pups, dissociated by trypsin digestion, and plated on circle glass coverslips coated with Matrigel. The cultures were maintained in MEM medium (Invitrogen) supplemented with B-27 (Invitrogen), l-glutamine, 0.5% glucose, 5% fetal bovine serum, and 2 mM Ara-C (Sigma-Aldrich). The cultures were used for experiments at 14–17 DIV.
Immunocytochemistry
Neurons attached to the glass coverslips were rinsed once in PBS, fixed for 15 min on ice in 4% formaldehyde, 4% sucrose in PBS and permeabilized for 5 min at room temperature in 0.2% Triton X-100 (Roche) in PBS. After permeabilization, the neurons were incubated for 30 min in blocking solution containing 5% BSA (Sigma-Aldrich; fraction V) in PBS, followed by 1-h incubation with primary and rhodamine- and FITC-conjugated secondary antibodies diluted in blocking solution. The coverslips were then mounted on glass slides with Aqua-Poly/Mount medium (Polysciences, Inc.) and analyzed at room temperature using a confocal microscope (DMIRE2; Leica) and 63×/1.32–0.6 oil immersion objective. The images were collected using confocal software (Leica) and processed using Photoshop software (Adobe). The background fluorescence was digitally reduced by 25% using the “color balance” function in Photoshop. All digital manipulations were equally applied to the entire image. Brain sections from perfusion-fixed rats were permeabilized for 10 min in 0.5% Triton X-100 in PBS and incubated in blocking buffer containing 2% goat serum and 0.1% Triton X-100 in PBS. The sections were then incubated sequentially for 1 h in primary and secondary antibodies diluted in blocking buffer and developed in DAB substrate. For dehydration, the sections were incubated for 10 min in 70% ethanol, followed by 10-min incubation in 90% ethanol and 10-min incubation in 100% ethanol. The sections were then mounted on glass slides and analyzed by light microscopy.
Immunoelectron microscopy
Adult mouse brains were perfusion fixed in 4% paraformaldehyde and 0.1% glutaraldehyde in PBS, pH 7.4, followed by overnight immersion fixation in 0.1% glutaraldehyde and 2% paraformaldehyde. The brain sections (120 μm) were permeabilized in 0.2% Triton X-100, blocked in 4% normal goat serum, and incubated overnight with primary antibody alone or primary antibody mixed with the antigen (∼1 mg/ml). The sections were then incubated with the secondary antibody conjugated with 1.4-nm gold particles (1:100 dilution; Nanoprobes) for 24 h, and immunogold signal was enhanced with the HQ silver enhancement kit (Nanoprobes). Sections were further fixed with 0.5% osmium tetroxide, dehydrated through a graded series of ethanol, and embedded in Poly/Bed 812 epoxy resin (Polysciences, Inc.). Ultrathin sections (65 nm) were stained with 5% uranyl acetate solution and examined under a transmission electron microscope (FEI Tecnai; FEI) at 120 kV accelerating voltage.
Phosphorylation
Synaptosomes (∼1 mg of total protein) were prelabeled for 30 min at 37°C in phosphate-free aerated Krebs-Henseleit-Hepes buffer (118 mM NaCl, 3.5 mM KCl, 1.25 mM CaCl2, 1.2 mM MgSO4, 25 mM, NaHCO3, 5 mM Hepes-NaOH, pH 7.4, and 115 mM glucose) containing 0.3 mCi of [32P]orthophosphate and incubated for an additional 10 min with 1 μM of okadaic acid. 32P-labeled synaptosomes were precipitated by centrifugation at 14,000 rpm on a microcentrifuge (Eppendorf) and solubilized for 1 h at 4°C in Krebs-Henseleit-Hepes buffer containing 2 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 50 mM NaF, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, 0.2% SDS, 1 mM PMSF, 5 μg/ml leupeptin, 2 μg/ml aprotinin, and 1 mM DTT. Solubilized proteins were diluted with equal volume of lysis buffer without detergents and immunoprecipitated with the antibody to synaptotagmin-12 or preimmune serum, as described in Immunoprecipitations. For in vitro phosphorylation of native synaptotagmin-12, brain proteins were extracted in 1% of Triton X-100 and immunoprecipitated with synaptotagmin-12 antibody or preimmune serum. Immunoprecipitates were extensively washed and incubated for 10 min at 37°C with 50 μl of rat brain cytosol (10 mg/ml in Tris buffer without protease inhibitors) mixed with 2.5 mM ATP and 20 μCi γ-[32P]ATP alone with addition of 0.1 mM cAMP, 0.1 mM cGMP, or 1 mM Ca2+. For in vitro phosphorylation of recombinant proteins, 30 μg of each GST-fusion protein immobilized on glutathione–Sepharose beads were mixed with 500 μl of reaction mixture containing 50 mM Hepes, pH 7.2, 100 mM NaCl, 4 mM EGTA, 2 mM MgCl2, 50 units of catalytic subunit of PKA (Sigma-Aldrich), 1 mM ATP, and 10 μCi γ-[32P]ATP and incubated for 30 min at 30°C. The proteins attached to the beads were then washed three times with Hepes buffer (50 mM Hepes, pH 7.2, 100 mM NaCl, 4 mM EGTA, 2 mM MgCl2, 2 mM sodium orthovanadate, 10 mM sodium pyrophosphate, and 50 mM NaF) and analyzed by SDS- PAGE. For detection, gels were dried and exposed to x-ray film for 24–48 h at −80°C.
Electrophysiology
Cortical neurons were infected with lentiviruses encoding wild-type synaptotagmin-12, -12-S97A, or GFP and analyzed at 14–17 DIV (9–12 d after infection). Because efficiency of lentivaral infections exceeded 95% (as determined by GFP fluorescence and immunocytochemistry with antibody to synaptotagmin-12), we randomly selected the neurons for whole-cell recording assuming that most presynaptic inputs in infected cultures are formed by neurons expressing recombinant protein of interest. Inhibitory synaptic responses were triggered by 1-ms current injection (900 μA) through a local extracellular electrode (FHC, Inc.) and recorded in a whole-cell mode using Multiclamp 700A amplifier (Axon Instruments, Inc.). All experiments were performed at room temperature. The frequency, duration, and magnitude of extracellular stimulus were controlled with Model 2100 Isolated Pulse Stimulator (A-M Systems, Inc.). The whole-cell pipette solution contained 135 mM CsCl2, 10 mM Hepes, 1 mM EGTA, 1 mM Na-GTP, 4 mM Mg-ATP, and 1 mM QX-314, pH 7.4. The bath solution contained 140 mM NaCl, 5 mM KCl, 0.5 mM or 2 mM CaCl2, 0.8 mM MgCl2, 10 mM Hepes, and 10 mM glucose, pH 7.4. Excitatory AMPA and NMDA currents were suppressed by the addition of 50 μM APV and 20 μM CNQX to the bath solution. Spontaneous mIPSCs were monitored in the presence of 1 μM tetrodotoxin to block action potentials. Forskolin (Sigma-Aldrich) was used at 50 μM. The currents were sampled at 10 kHz and analyzed off-line using pClamp9 (Axon Instruments, Inc.) and Origin7 (Microcal, Inc.) software. mIPSCs event detection was performed with pClamp template search function using a template constructed by averaging >100 manually collected individual mIPSCs. Statistical analysis was performed with t test; ***, P ≤ 0.001. All data are shown as the mean ± the SEM.
Miscellaneous
Production of recombinant lentiviruses, phospholipid-binding assays, deglycosylation, and immunoblotting were performed as previously described (Shin et al., 2003; Han et al., 2004; Maximov and Südhof, 2005).
Online supplemental material
Fig. S1 shows localization of synaptotagmin-12 in brain slices. Fig. S2 shows the effect of Forskolin on evoked release in inhibitory synapses of cultured cortical neurons. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200607021/DC1.
Acknowledgments
We would like to thank I. Kornblum and A. Roth for their expert technical assistance.
Abbreviations used in this paper: DIV, days in vitro; IPSC, inhibitory postsynaptic current; mIPSC, mini IPSC; PKA, protein kinase A; VCP, valosin-containing protein.
References
- Adolfsen, B., S. Saraswati, M. Yoshihara, and J.T. Littleton. 2004. Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment. J. Cell Biol. 166:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angleson, J.K., and W.J. Betz. 2001. Intraterminal Ca(2+) and spontaneous transmitter release at the frog neuromuscular junction. J. Neurophysiol. 85:287–294. [DOI] [PubMed] [Google Scholar]
- Banerjee, A., J.A. Kowalchyk, B.R. DasGupta, and T.F. Martin. 1996. SNAP-25 is required for a late postdocking step in Ca2+-dependent exocytosis. J. Biol. Chem. 271:20227–20230. [DOI] [PubMed] [Google Scholar]
- Bennett, M.K., N. Calakos, and R.H. Scheller. 1992. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 257:255–259. [DOI] [PubMed] [Google Scholar]
- Bowen, M.E., K. Weninger, J. Ernst, S. Chu, and A.T. Brunger. 2005. Single-molecule studies of synaptotagmin-1 and complexin binding to the SNARE complex. Biophys. J. 89:690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogna, M., B.H. Gahwiler, and S.M. Thompson. 1995. Presynaptic enhancement of inhibitory synaptic transmission by protein kinases A and C in the rat hippocampus in vitro. J. Neurosci. 15:1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, R.C., R.A. Nicoll, and R.C. Malenka. 1998. Effects of PKA and PKC on miniature excitatory postsynaptic currents in CA1 pyramidal cells. J. Neurophysiol. 80:2797–2800. [DOI] [PubMed] [Google Scholar]
- Carter, A.G., and W.G. Regehr. 2002. Quantal events shape cerebellar interneuron firing. Nat. Neurosci. 5:1309–1318. [DOI] [PubMed] [Google Scholar]
- Chapman, E.R., P.I. Hanson, S. An, and R. Jahn. 1995. Ca2+ regulates the interaction between synaptotagmin-1 and syntaxin-1. J. Biol. Chem. 270:23667–23671. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega, L.E., and C.F. Stevens. 1994. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J. Neurosci. 14:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak, F., O.H. Shin, E.T. Kavalali, and T.C. Sudhof. 2006. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J. Neurosci. 26:6668–6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitcher, D.L., A. Ueda, B.A. Stewart, R.W. Burgess, Y. Kidokoro, and T.L. Schwarz. 1998. Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J. Neurosci. 18:2028–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi, A., H. Ishibashi, S. Jinno, T. Kosaka, and N. Akaike. 2002. Presynaptic inhibition of GABAergic miniature currents by metabotropic glutamate receptor in the rat CNS. Neuroscience. 109:299–311. [DOI] [PubMed] [Google Scholar]
- Dong, H., M. Wade, A. Williams, A. Lee, G.R. Douglas, and C. Yauk. 2005. Molecular insight into the effects of hypothyroidism on the developing cerebellum. Biochem. Biophys. Res. Comm. 330:1182–1193. [DOI] [PubMed] [Google Scholar]
- Fernández-Chacón, R., A. Königstorfer, S.H. Gerber, J. García, M.F. Matos, C.F. Stevens, N. Brose, J. Rizo, C. Rosenmund, and T.C. Südhof. 2001. Synaptotagmin I functions as a Ca2+ regulator of release probability. Nature. 410:41–49. [DOI] [PubMed] [Google Scholar]
- Geppert, M., Y. Goda, R.E. Hammer, C. Li, T.W. Rosahl, C.F. Stevens, and T.C. Südhof. 1994. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 79:717–727. [DOI] [PubMed] [Google Scholar]
- Han, W., J.S. Rhee, A. Maximov, Y. Lao, T. Mashimo, C. Rosenmund, and T.C. Südhof. 2004. N-glycosylation is essential for vesicular targeting of synaptotagmin 1. Neuron. 41:85–99. [DOI] [PubMed] [Google Scholar]
- Hsia, A.Y., R.C. Malenka, and R.A. Nicoll. 1998. Development of excitatory circuitry in the hippocampus. J. Neurophysiol. 79:2013–2024. [DOI] [PubMed] [Google Scholar]
- Kaneko, M., and T. Takahashi. 2004. Presynaptic mechanism underlying cAMP-dependent synaptic potentiation. J. Neurosci. 24:5202–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz, B. 1969. The Release of Neural Transmitter Substances. Liverpool University Press. Liverpool, UK. 60 pp.
- Kee, Y., and R.H. Scheller. 1996. Localization of synaptotagmin-binding domains on syntaxin. J. Neurosci. 16:1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, D.S., M.W. Moody, T.D. Nguyen, and B. Hille. 2000. Regulation of exocytosis by protein kinases and Ca2+ in pancreatic duct epithelial cells. J. Gen. Physiol. 116:507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., B. Ullrich, Z.Z. Zhang, R.G.W. Anderson, N. Brose, and T.C. Südhof. 1995. Ca2+-dependent and Ca2+-independent activities of neural and nonneural synaptotagmins. Nature. 375:594–599. [DOI] [PubMed] [Google Scholar]
- Llano, I., J. Gonzalez, C. Caputo, F.A. Lai, L.M. Blayney, Y.P. Tan, and A. Marty. 2000. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat. Neurosci. 3:1256–1265. [DOI] [PubMed] [Google Scholar]
- Maximov, A., and T.C. Südhof. 2005. Autonomous function of synaptotagmin 1 in triggering synchronous release independent of asynchronous release. Neuron. 48:547–554. [DOI] [PubMed] [Google Scholar]
- McKinney, R.A., M. Capogna, R. Durr, B.H. Gahwiler, and S.M. Thompson. 1999. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat. Neurosci. 2:44–49. [DOI] [PubMed] [Google Scholar]
- Nishiki, T., and G.J. Augustine. 2004. Synaptotagmin I synchronizes transmitter release in mouse hippocampal neurons. J. Neurosci. 24:6127–6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu, Y., and T.H. Murphy. 2003. Miniature transmitter release: accident of nature or careful design? Sci. STKE. 211:pe54. [DOI] [PubMed]
- Pang, Z.P., J. Sun, J. Rizo, A. Maximov, and T.C. Sudhof. 2006. Genetic analysis of synaptotagmin-2 in spontaneous and Ca(2+)-triggered neurotransmitter release. EMBO J. 25:2039–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman, C., and B. Davletov. 2003. Mechanism of calcium-independent synaptotagmin-1 binding to target SNAREs. J. Biol. Chem. 278:5501–5504. [DOI] [PubMed] [Google Scholar]
- Rickman, C., D.A. Archer, F.A. Meunier, M. Craxton, M. Fukuda, R.D. Burgoyne, and B. Davletov. 2004. Synaptotagmin-1 interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 279:12574–12579. [DOI] [PubMed] [Google Scholar]
- Rosahl, T.W., D. Spillane, M. Missler, J. Herz, D.K. Selig, J.R. Wolff, R.E. Hammer, R.C. Malenka, and T.C. Südhof. 1995. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 375:488–493. [DOI] [PubMed] [Google Scholar]
- Sakaba, T., and E. Neher. 2001. Preferential potentiation of fast-releasing synaptic vesicles by cAMP at the calyx of Held. Proc. Natl. Acad. Sci. USA. 98:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara, Y., T. Virmani, F. Deak, X. Liu, and E.T. Kavalali. 2005. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 45:563–573. [DOI] [PubMed] [Google Scholar]
- Schoch, S., F. Deak, A. Konigstorfer, M. Mozhayeva, Y. Sara, T.C. Sudhof, and E.T. Kavalali. 2001. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 294:1117–1122. [DOI] [PubMed] [Google Scholar]
- Shin, O.H., J.S. Rhee, J. Tang, S. Sugita, C. Rosenmund, and T.C. Sudhof. 2003. 2003. Sr2+ binding to the Ca2+ binding site of the synaptotagmin 1 C2B domain triggers fast exocytosis without stimulating SNARE interactions. Neuron. 37:99–108. [DOI] [PubMed] [Google Scholar]
- Sutton, M.A., H. Ito, P. Cressy, C. Kempf, J. Woo, and E.M. Schuman. 2006. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 125:785–789. [DOI] [PubMed] [Google Scholar]
- Tang, J., A. Maximov, O.H. Shin, H. Dai., J. Rizo, and T.C. Südhof. 2006. A complexin/synaptotagmin-1 switch controls fast synaptic vesicle exocytosis. Cell. 126:1175–1187. [DOI] [PubMed] [Google Scholar]
- Thompson, C.C. 1996. Thyroid hormone-responsive genes in developing cerebellum include a novel synaptotagmin and a hairless homolog. J. Neurosci. 16:7832–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara, M., and J.T. Littleton. 2002. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 36:897–908. [DOI] [PubMed] [Google Scholar]
- Yoshida, A., C. Oho, A. Omori, R. Kuwahara, T. Ito, and M. Takahashi. 1992. HPC-1 is associated with synaptotagmin-1 and omega-conotoxin receptor. J. Biol. Chem. 267:24925–24928. [PubMed] [Google Scholar]
- Yoshihara, M., K. Suzuki, and Y. Kidokoro. 2000. Two independent pathways mediated by cAMP and protein kinase A enhance spontaneous transmitter release at Drosophila neuromuscular junctions. J. Neurosci. 20:8315–8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker, R.S. 2005. Minis: whence and wherefore? Neuron. 45:482–484. [DOI] [PubMed] [Google Scholar]