Abstract
Multiple mitotic kinesins and microtubule-associated proteins (MAPs) act in concert to direct cytokinesis (Glotzer, M. 2005. Science. 307:1735–1739). In anaphase cells, many of these proteins associate with an antiparallel array of microtubules termed the central spindle. The MAP and microtubule-bundling protein PRC1 (protein-regulating cytokinesis 1) is one of the key molecules required for the integrity of this structure (Jiang, W., G. Jimenez, N.J. Wells, T.J. Hope, G.M. Wahl, T. Hunter, and R. Fukunaga. 1998. Mol. Cell. 2:877–885; Mollinari, C., J.P. Kleman, W. Jiang, G. Schoehn, T. Hunter, and R.L. Margolis. 2002. J. Cell Biol. 157:1175–1186). In this study, we identify an interaction between endogenous PRC1 and the previously uncharacterized kinesin KIF14 as well as other mitotic kinesins (MKlp1/CHO1, MKlp2, and KIF4) with known functions in cytokinesis (Hill, E., M. Clarke, and F.A. Barr. 2000. EMBO J. 19:5711–5719; Matuliene, J., and R. Kuriyama. 2002. Mol. Biol. Cell. 13:1832–1845; Kurasawa, Y., W.C. Earnshaw, Y. Mochizuki, N. Dohmae, and K. Todokoro. 2004. EMBO J. 23:3237–3248). We find that KIF14 targets to the central spindle via its interaction with PRC1 and has an essential function in cytokinesis. In KIF14-depleted cells, citron kinase but not other components of the central spindle and cleavage furrow fail to localize. Furthermore, the localization of KIF14 and citron kinase to the central spindle and midbody is codependent, and they form a complex depending on the activation state of citron kinase. Contrary to a previous study (Di Cunto, F., S. Imarisio, E. Hirsch, V. Broccoli, A. Bulfone, A. Migheli, C. Atzori, E. Turco, R. Triolo, G.P. Dotto, et al. 2000. Neuron. 28:115–127), we find a general requirement for citron kinase in human cell division. Together, these findings identify a novel pathway required for efficient cytokinesis.
Introduction
Cytokinesis is the physical cell division process that follows the duplication and spatial segregation of the genetic material (Glotzer, 2005). In animal cells, a complex microtubule structure termed the central spindle or spindle midzone forms between the retreating chromosomes in anaphase and organizes many proteins essential for the process of cytokinesis (Glotzer, 2005). One of the critical components of this structure is the microtubule-associated protein (MAP) PRC1 (protein-regulating cytokinesis 1; Jiang et al., 1998; Mollinari et al., 2002; Kurasawa et al., 2004). PRC1 not only promotes the formation of stable microtubule bundles (Mollinari et al., 2002) but also acts as a scaffold for the kinesin-4 family chromokinesin KIF4 (Kurasawa et al., 2004; Miki et al., 2005). In PRC1-depleted cells, KIF4 fails to localize to the central spindle, and, rather than forming a dense array, the central spindle microtubules spread out toward the cell cortex (Kurasawa et al., 2004). Like PRC1, KIF4 is required for cytokinesis (Jiang et al., 1998; Mollinari et al., 2002; Kurasawa et al., 2004) and for chromosome condensation and segregation (Mazumdar et al., 2004); however, the molecular details of these different functions are not understood.
More is known about the molecular functions of the kinesin-6 family motor proteins MKlp1 and MKlp2, which also have essential functions in cytokinesis (Adams et al., 1998; Powers et al., 1998; Raich et al., 1998; Hill et al., 2000). MKlp1 forms a heterotrimeric complex with the Rho GTPase–activating protein Cyk-4 and the Rho guanosine diphosphate–GTP exchange factor ECT2 (Tatsumoto et al., 1999; Mishima et al., 2002; Somers and Saint, 2003). This complex is needed to control the activation state of Rho at the cell cortex during the formation and ingression of the cleavage furrow (Oceguera-Yanez et al., 2005; Yuce et al., 2005). In vertebrates, MKlp2 is a docking partner of polo-like kinase 1 (Plk1) at the central spindle (Neef et al., 2003) and is also required for the transport of Aurora B kinase to the central spindle in anaphase cells (Gruneberg et al., 2004). In the absence of MKlp2, Aurora B is unable to phosphorylate its targets at the central spindle, including MKlp1, and cytokinesis fails. Whether or not PRC1 has a general function in regulating these mitotic kinesins in addition to KIF4 is not known.
Therefore, we have further investigated the function of the central spindle MAP PRC1 and uncovered links to multiple mitotic kinesins with reported functions in cytokinesis, including KIF4, MKlp1, and MKlp2. In addition, we identify a novel regulatory pathway in human cells involving citron kinase and the kinesin-3 family motor protein KIF14.
Results
KIF14 interacts with PRC1
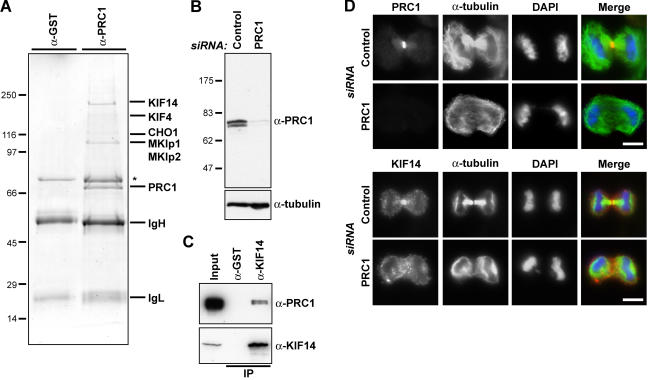
To investigate the function of PRC1 during cytokinesis in human cells, it was immunoaffinity purified from anaphase cell extracts, and the obtained complexes were analyzed by mass spectrometry (Fig. 1). This approach revealed that PRC1 complexes contain KIF4 and MKlp1 as expected as well as MKlp2 and KIF14 (Fig. 1 A). These proteins specifically precipitate together with PRC1 because the antibody used for the immune precipitation recognizes a doublet band at the size expected for the two PRC1 splice variants expressed in HeLa cells; this is depleted in cells treated with small interfering RNA (siRNA) duplexes targeting PRC1 (Fig. 1 B). The presence of KIF14 was unexpected because it was previously reported to function in chromosome segregation but not cytokinesis in a high through-put RNA interference screen (Zhu et al., 2005). Analysis of KIF14 complexes with specific antibodies confirmed that PRC1 was present (Fig. 1 C). Furthermore, KIF14 was found to localize to the central spindle and midbody (Fig. 1 D) and, like KIF4, to depend on the presence of PRC1 for this localization (Fig. 1 D; Kurasawa et al., 2004).
Figure 1.
The central spindle MAP PRC1 interacts with multiple kinesins. (A) Proteins immune precipitated from 10 mg of anaphase HeLa cell extract with affinity-purified rabbit antibodies to GST or PRC1 were separated on minigels and identified by mass spectrometry. The immunoglobulin G heavy (IgH) and light chains (IgL) are marked, and the asterisk denotes a nonspecific contaminant found with both control and KIF14 antibodies. (B) Lysates from HeLa cells treated with control or PRC1 siRNA duplexes for 36 h were Western blotted for PRC1 and α-tubulin as a loading control. (C) GST and KIF14 immune precipitates were Western blotted for KIF14 and PRC1. (D) HeLa cells treated with control or PRC1 siRNA duplexes for 30 h were fixed and stained with antibodies to PRC1 or KIF14 (red) and α-tubulin (green). DNA was stained with DAPI (blue). Bars, 10 μm.
KIF14 localizes to the central spindle and functions in cytokinesis
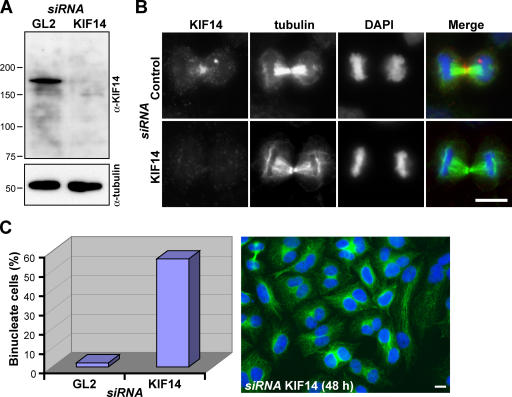
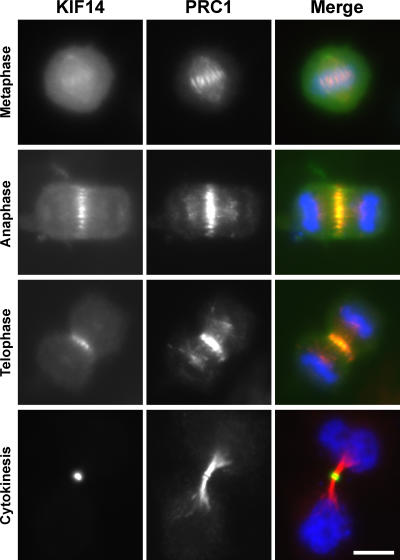
These observations suggest that KIF14 might function in cytokinesis. To test this idea, KIF14 was specifically depleted from cells using siRNA duplexes (Fig. 2). After 48 h of transfection, Western blotting for KIF14 showed that it was efficiently depleted (Fig. 2 A), central spindle staining was absent from anaphase cells (Fig. 2 B), and >60% of KIF14-depleted cells were binucleated (Fig. 2 C). No obvious defects in micro-tubule structures (Fig. 2, B and C) or chromosome segregation were observed (unpublished data), and, consistent with this, KIF14-depleted cells had two equally formed nuclei rather than unequal or micronuclei (Fig. 2 C). KIF4 is thought to be important for both chromosome structure and cytokinesis, and this dual function is reflected in its localization to both chromosomes and the central spindle (Kurasawa et al., 2004; Mazumdar et al., 2004). If KIF14 had a similar dual function, it, too, would be expected to display a similar pattern of localization. Like PRC1, KIF14 was present on the spindle midzone throughout mitosis but was highly enriched on the midbody during cytokinesis, whereas PRC1 remained spread along the microtubule bundles and apparently did not concentrate at the midbody (Fig. 3).
Figure 2.
Depletion of KIF14 results in a failure of cytokinesis. (A) Lysates from HeLa cells treated with control or KIF14 siRNA duplexes for 48 h were Western blotted for KIF14 and α-tubulin as a loading control. (B and C) HeLa cells treated with control or KIF14 siRNA duplexes for 48 h were fixed and stained with antibodies to KIF14 (red) and α-tubulin (green). (C) The number of binucleated cells was counted and is expressed as a percentage in control and KIF14-depleted cells. A typical field of KIF14-depleted cells from such an experiment is shown. DNA was stained with DAPI (blue). Bars, 10 μm.
Figure 3.
KIF14 localizes to the central spindle and midbody. HeLa cells expressing EGFP-KIF14 (green) were fixed and stained for KIF14 (green) and PRC1 (red) 24 h after transfection. DNA was stained with DAPI (blue). Bar, 10 μm.
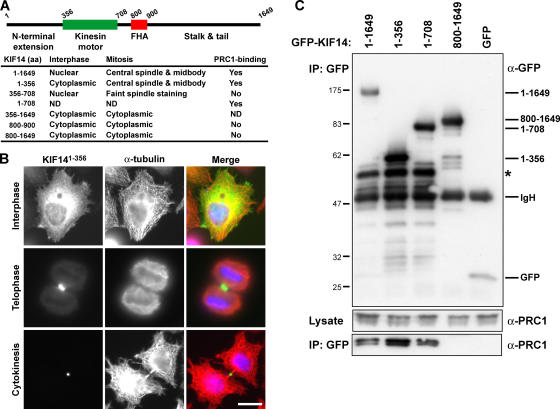
In metaphase cells, the bulk of KIF14 was cytoplasmic, and although a weak spindle microtubule staining was observed, it did not obviously localize to the chromosomes (Fig. 3). To further support the specific nature of this localization, mapping experiments were performed to identify the central spindle–targeting region of KIF14 (Fig. 4). The unique amino-terminal extension of KIF14 adjacent to the motor domain was found to localize to the central spindle and midbody (Fig. 4, A and B). None of the other regions of KIF14 displayed the same localization, although motor domain–containing fragments weakly associated with the spindle during metaphase (Fig. 4 A). At no time was any association with the chromosomes observed. Because KIF14 and PRC1 are present in a complex and have similar localizations, the role of PRC1 in KIF14 targeting was then investigated. A biochemical analysis of KIF14 using immune precipitation of tagged constructs revealed that amino acids 1–356, the extension amino terminal to the motor domain, also form the minimal region tested that was capable of binding to PRC1 (Fig. 4 C). In contrast, the forkhead-associated (FHA) domain-containing stalk and tail region of the motor was unable to bind PRC1 and also failed to target to the central spindle (Fig. 4, A and C). Therefore, the PRC1-binding site of KIF14 maps to the region containing the central spindle-targeting signal. This is different from the kinesin-6 family motors MKlp1 and MKlp2, which target to the central spindle via their carboxy-terminal stalk and tail domains (Gruneberg et al., 2004). Interestingly, in neither case does it appear that the kinesin motor domain is needed for specifying central spindle localization. Together, these findings indicate that KIF14, like PRC1, is required for cytokinesis but raise questions about the previously reported function in chromosome segregation (Zhu et al., 2005).
Figure 4.
Central spindle and midbody targeting of KIF14 involves the amino-terminal extension. (A and B) A schematic of human KIF14 indicating the unique extension amino terminal of the kinesin motor domain and the forkhead-associated (FHA) domain that defines the kinesin-3 family of motor proteins. HeLa cells were transfected with EGFP-tagged constructs encoding full-length KIF14 and the subdomains indicated in the table. Cells were fixed and stained for α-tubulin. The localizations of these proteins in interphase and mitotic HeLa cells is summarized in the table. (B) The localization of the KIF14 amino-terminal extension amino acids 1–356 (green) and α-tubulin (red) are shown in interphase, telophase, and in cells undergoing cytokinesis. DNA was stained with DAPI (blue). Bar, 10 μm. (C) Immune precipitations (IPs) were performed using GFP antibodies from HEK293T cells transfected for 40 h with GFP or GFP-tagged KIF14 constructs as indicated in the figure. Samples of the lysate and IP were analyzed by Western blotting for GFP and PRC1. The asterisk indicates a premature termination or possible breakdown product seen with all amino-terminal fragments of KIF14.
KIF14 is required for the localization of citron kinase
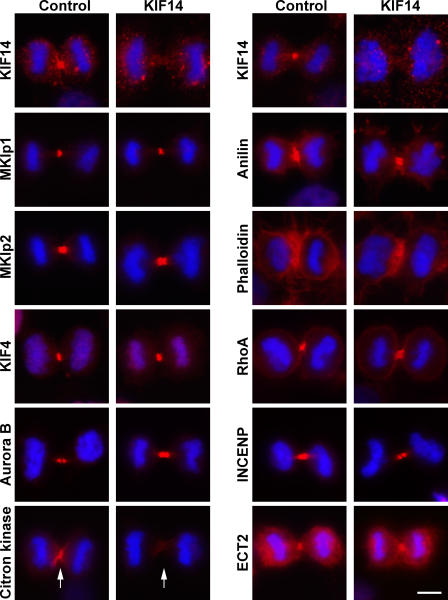
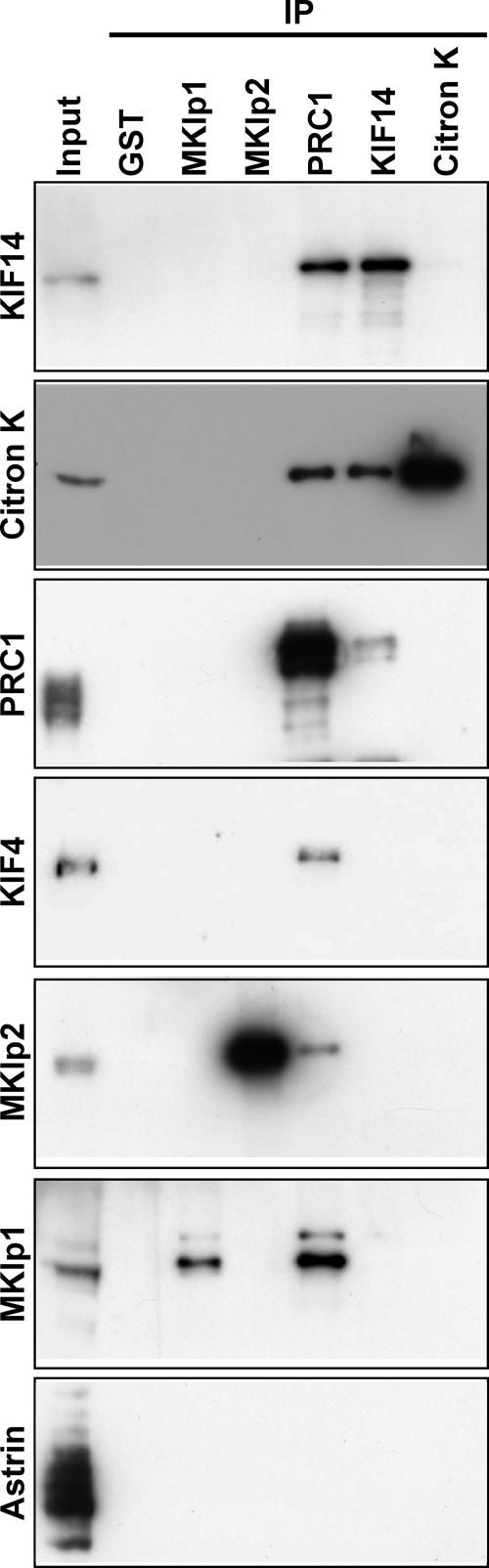
To establish why cytokinesis depends on KIF14, the localization of a panel of central spindle and cleavage furrow proteins was examined in KIF14-depleted cells (Fig. 5). In KIF14-depleted cells, citron kinase was lost from the central spindle and cleavage furrow region (Fig. 5). This was a specific effect because the central spindle proteins MKlp1, MKlp2, KIF4, ECT2, and the cleavage furrow proteins anillin, actin, and RhoA all displayed the expected normal localizations in both control and KIF14-depleted cells (Fig. 5). To test whether this was caused by a direct interaction between KIF14 and citron kinase, different central spindle proteins were immune precipitated from anaphase cell extracts using specific antibodies (Fig. 6). Consistent with the results presented in Fig. 1, KIF14, KIF4, MKlp1, and MKlp2 could be detected in PRC1 immune precipitates (Fig. 6). Furthermore, PRC1 could be detected in KIF14 immune precipitates (Fig. 6). Citron kinase was only found in PRC1 and KIF14 immune precipitates (Fig. 6), suggesting that it binds to KIF14 but not other mitotic kinesins. Finally, astrin, another mitotic spindle MAP, did not precipitate with PRC1, citron kinase, or any of the motor proteins tested (Fig. 6), supporting the idea that mitotic kinesins make specific interactions with PRC1.
Figure 5.
KIF14 is required for the localization of citron kinase but not other components of the central spindle or cleavage furrow. HeLa cells transfected with control or KIF14 siRNA duplexes for 48 h were fixed and stained with antibodies to the proteins (red) indicated in the figure. Actin was detected using 100 ng/ml rhodamine-phalloidin (red). DNA was stained with DAPI (blue). Bar, 10 μm. Arrows mark the positions of the cleavage furrow in control and KIF14-depleted cells stained for citron kinase.
Figure 6.
KIF14 interacts with PRC1 and citron kinase. Immune precipitations from anaphase HeLa cell extracts were performed using the antibodies indicated across the top of the figure and described in Materials and methods. The input lane represents 10% of material used for each IP. The immune precipitates were then separated by SDS-PAGE and analyzed by Western blotting with the antibodies indicated down the left side of the figure.
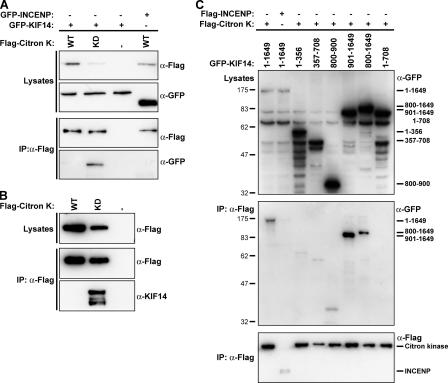
Fragments of KIF14 were then tested for their ability to interact with citron kinase, to provide more evidence for the direct interaction of these proteins, and to map the interaction site on KIF14 (Fig. 7). For this purpose, cells were transfected with GFP-tagged KIF14 and FLAG-tagged wild-type and kinase-dead forms of citron kinase. GFP-tagged KIF14 was only detected in immune precipitates with the kinase-dead form of citron kinase but not the wild-type kinase or the control protein INCENP (inner centromere protein; Fig. 7 A). This was despite the fact that kinase-dead citron kinase was typically expressed at a lower level than the wild-type protein (Fig. 7, A and B; compare the first two lanes), possibly because of its toxic effects on cell growth and division (Madaule et al., 1998). A similar result was seen for endogenous KIF14, which was also only found to efficiently coprecipitate with the kinase-dead form of citron kinase (Fig. 7 B). This interaction was not mediated by the amino-terminal extension involved in binding to PRC1 or the kinesin motor and FHA domains but required amino acids 901–1,649 forming the stalk and tail region (Fig. 7 C).
Figure 7.
Interaction of citron kinase with KIF14 is dependent on its kinase activity. (A and C) IPs were performed using FLAG antibodies from HEK293T cells cotransfected for 40 h with wild-type (WT; A) or kinase-dead (KD) FLAG-tagged citron kinase and GFP-tagged KIF14 or INCENP constructs as indicated in the figure. Samples of the lysates and FLAG IP were analyzed by Western blotting for GFP and the FLAG epitope. (B) IPs were performed using FLAG antibodies from HEK293T cells transfected for 40 h with wild-type or kinase-dead FLAG-tagged citron kinase. Samples of the lysates and FLAG IP were analyzed by Western blotting for KIF14 and the FLAG epitope. (C) The running positions of the various KIF14 constructs are indicated to the right of the top panel. KIF14 constructs that efficiently coprecipitated with citron kinase are indicated to the right of the middle panel.
Therefore, KIF14 makes interactions with at least two other components of the central spindle components. First, amino acids 1–356 interact with PRC1, and this is needed for central spindle targeting of the motor protein. Second, the stalk and tail region encompassing amino acids 901–1,649, although unable to target to the central spindle alone, form the binding site for citron kinase. Interestingly, this interaction appears to depend on the activation state of citron kinase, although the significance of this is unclear at present. The inactive mutant of citron kinase has a dominant-negative effect on cytokinesis (Madaule et al., 1998), and the results presented here suggest this may, in part, be caused by sequestration or improper regulation of KIF14.
Citron kinase is required for KIF14 localization and cytokinesis
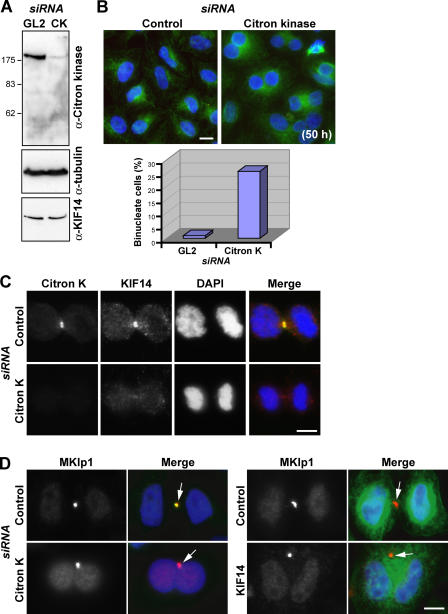
Citron kinase has been reported to be required downstream of Rho for the final steps of cytokinesis in Drosophila melanogaster (Naim et al., 2004; Shandala et al., 2004). However, in mammalian cells, the situation is a little more confusing because it is unclear if citron kinase is an essential component of the cytokinesis machinery (Di Cunto et al., 2000; Matsumura, 2005). Thus, this issue was reinvestigated in HeLa cells using siRNA duplexes to deplete citron kinase (Fig. 8). Western blotting showed that citron kinase was efficiently depleted from HeLa cells using siRNA without altering the levels of KIF14 or α-tubulin (Fig. 8 A). After 50 h of citron kinase depletion, a 15-fold increase in binucleated cells was found compared with the control (Fig. 8 B), but no obvious defects on chromosome segregation or cleavage furrow ingression were observed (Fig. 8 C, telophase figures; and not depicted). KIF14 targeting to the central spindle and midbody was significantly reduced in these cells (Fig. 8 C). Formation of the MKlp1-containing midbody matrix structure is one of the latest stages of cytokinesis before abscission (Matuliene and Kuriyama, 2002, 2004). Interestingly, the binucleated cells formed after the depletion of citron kinase or KIF14 contain MKlp1-positive midbody remnant structures (Fig. 8 D, arrows). This is different from cells depleted for MKlp2 or PRC1, which fail to form a midbody (Mollinari et al., 2002; Neef et al., 2003; Gruneberg et al., 2004). These observations suggest that citron kinase is required for the localization of KIF14 and that citron kinase and KIF14-depleted human cells fail to complete cytokinesis at a late stage after or in parallel with formation of the midbody.
Figure 8.
Citron kinase is required for cytokinesis and for the localization of KIF14 to the midbody. (A) Cell lysates from HeLa cells treated with control or citron kinase (CK) siRNA duplexes for 50 h were Western blotted for citron kinase, KIF14, and α-tubulin as a loading control. (B and C) HeLa cells treated with control or citron kinase siRNA duplexes for 50 h were fixed and stained with antibodies to citron kinase (green) and KIF14 (red). (B) A typical field of KIF14-depleted cells from such an experiment is shown. The number of binucleated cells was counted and is expressed as a percentage in control and citron kinase–depleted cells. (C) Localization of KIF14 in control and citron kinase–depleted cells. DAPI, blue. (D) HeLa cells treated with control, citron kinase, or KIF14 siRNA duplexes for 50 h were fixed and stained with antibodies to MKlp1 (red) and citron kinase or tubulin (green). Arrows on the merged images indicate midbody remnants. Bars, 10 μm.
Discussion
In this study, we have identified a network of interactions between KIF14, citron kinase, the central spindle MAP, and the microtubule-bundling protein PRC1. Although it has recently been reported that KIF14 is needed for chromosome segregation (Zhu et al., 2005), our data indicate that it is, in fact, required for the process of cytokinesis. This is consistent with observations made on the D. melanogaster KIF14 homologue KLP38B (Ohkura et al., 1997). Mutations in the Klp38B gene result in the formation of polyploid cells without causing defects in other aspects of mitosis such as chromosome segregation (Ohkura et al., 1997). Although it is unknown whether KLP38B interacts with sticky, the D. melanogaster homologue of citron kinase, sticky mutants and insect S2 cells in culture depleted of sticky using RNA interference have severe defects in cell division similar to those we reported in human cells (D'Avino et al., 2004; Echard et al., 2004; Naim et al., 2004; Shandala et al., 2004). The presence of an FHA domain is one of the defining features of the kinesin-3 family motor proteins (Lawrence et al., 2004; Miki et al., 2005). FHA domains are phosphopeptide-binding motifs (Li et al., 2000), implying that KIF14 either makes phospho-dependent interactions with other proteins or itself. Neither the interaction of KIF14 with PRC1 nor citron kinase appears to depend on the FHA domain, and, to date, we have been unable to identify any other interaction partners of KIF14. Observations on KIF1A, another kinesin-3 family motor protein in which the FHA domain negatively regulates the kinesin motor activity (Lee et al., 2004), support the idea that this could also be a regulatory feature in KIF14.
The observations that KIF14 interacts more strongly with the kinase-dead form of citron kinase perhaps indicate that it is a substrate for this kinase. However, preliminary observations indicate that this may not be the case (unpublished data), and this issue requires further investigation. An alternative possibility is that citron kinase interacts with KIF14, depending on its activation state. It has been reported that citron kinase acts downstream of Rho and depends on Rho for its activity (Di Cunto et al., 1998; Madaule et al., 1998, 2000; Yamashiro et al., 2003; Shandala et al., 2004), so this could provide a regulatory pathway modulating the interaction of citron kinase with KIF14. Citron kinase is reported to phosphorylate myosin light chain and, thus, exert a regulatory effect on actomyosin-mediated contractility of the cleavage furrow (Yamashiro et al., 2003; Matsumura, 2005). How this relates to KIF14 function is unclear because KIF14-depleted cells do not appear to have defects in the localization of central spindle and cleavage furrow components other than citron kinase (Fig. 6). One explanation for this could be that human citron kinase is only required very late in cytokinesis, perhaps to control the final stages of abscission in cooperation with anillin as in insect cells (Naim et al., 2004; Shandala et al., 2004).
These findings may also have a wider relevance because KIF14 is encoded on chromosome 1 within the minimal region at 1q31-1q32 amplified in a wide variety of different tumors (Corson et al., 2005). Of the 14 genes encoded in this region, only KIF14 was overexpressed in tumors and tumor cell lines, indicating it may be an important factor in oncogenesis (Corson et al., 2005). Defective regulation of cytokinesis has been proposed to be a possible route to the generation of aneuploid cells and, hence, tumorigenesis (Nigg, 2001). Our observations that KIF14 is needed for efficient cytokinesis, together with the finding that it is overexpressed in some human tumors, support this idea and indicate that KIF14 and other components of this pathway such as citron kinase may be potentially interesting targets for therapeutic intervention.
We have previously shown that MKlp2 is necessary for the relocation of the Aurora B kinase from the centromeres to the central spindle at the metaphase to anaphase transition (Gruneberg et al., 2004) and is also an important docking partner for Plk1 at the central spindle (Neef et al., 2003). Our observations on KIF14 and citron kinase suggest that spatial control of protein kinases is a general principle of mitotic regulation (Pines, 1999). Based on the data presented here and published observations on the insect homologue of citron kinase (D'Avino et al., 2004; Naim et al., 2004), we propose a model for KIF14 and citron kinase function (Fig. 9). In this model, the heterotrimeric centralspindlin complex of MKlp1, Cyk-4, and ECT2 locally regulates the activation state of Rho family GTP-binding proteins at the cleavage furrow during furrow ingression (Somers and Saint, 2003; Glotzer, 2005; Yuce et al., 2005). KIF14 is required for the localization of citron kinase, whereas Rho controls the activation state of citron kinase and, hence, the phosphorylation of myosin light chain (Yamashiro et al., 2003; Shandala et al., 2004; Matsumura, 2005). PRC1 can be seen as a master regulator or central spindle matrix protein that is required not only to organize antiparallel microtubule bundles (Mollinari et al., 2002) but also to localize the many motor proteins associated with this structure. This is supported by our observations that endogenous PRC1 interacts with MKlp1, MKlp2, and KIF14 (Figs. 1 A and 6) as well as KIF4 (Kurasawa et al., 2004). Together, these pathways, each defined by a different kinesin motor protein, contribute to the temporal regulation of cleavage furrow ingression and the final stages of cytokinesis. Whether citron kinase has substrates in addition to myosin light chain and the role these might play in controlling cytokinesis are the subjects of further investigation. These findings add another layer to the complex regulatory network of MAPs, kinesin motor proteins, and mitotic kinases necessary for the process of animal cell division.
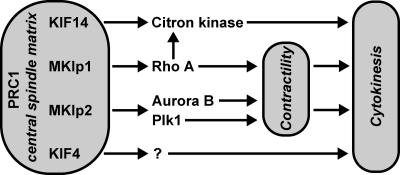
Figure 9.
PRC1 forms a central spindle matrix directing kinesin function during cytokinesis. PRC1 is a master regulator of the central spindle interacting with multiple motor proteins that are involved in cytokinesis. Centralspindlin, shown as MKlp1 for simplicity, controls Rho activation and inactivation, whereas KIF14 is needed for citron kinase localization to the central spindle, midbody, and, thus, its site of activity. One function of Rho is to control the activation state of citron kinase. MKlp2 is needed for both the targeting of Plk1 and the chromosomal passenger complex and, thus, for spatial control of both Plk1 and Aurora B. These kinases phosphorylate proteins required for cytokinesis, including components of the centralspindlin complex. The molecular function of KIF4 is less clear, but it too appears to be required for cytokinesis. In the absence of MKlp1 and MKlp2, early events in central spindle and cleavage furrow formation are abnormal. This leads to defects in furrow positioning and contractility. KIF14 and citron kinase are not required for these early events or late events such as midbody formation but are needed for efficient cytokinesis.
Materials and methods
Antibody reagents
Antibodies were obtained as follows: mouse monoclonals to α-tubulin (clone DM1a; Sigma-Aldrich); Aurora B (clone AIM-1; Becton Dickinson); mouse monoclonal to citron kinase (Becton Dickinson); mouse monoclonal to Rho A (clone 55; Becton Dickinson); rabbit polyclonals to MKlp1 SC-867 (0.2 mg/ml; Santa Cruz Biotechnology, Inc.); the MKlp1 motor domain (Gruneberg et al., 2004); the KIF4 amino acids 738–1,232; KIF14 (BL358; Bethyl Laboratories Inc.); ECT2 amino acids 1–388; INCENP (Honda et al., 2003); astrin amino acids 1,014–1,193; anillin amino acids 417–656; and affinity-purified sheep polyclonal to MKlp2 (Hill et al., 2000; Gruneberg et al., 2004). PRC1 antibodies were raised in rabbits against the full-length hexahistidine-tagged protein purified from insect cells and were affinity purified against maltose-binding protein–tagged full-length PRC1 expressed in bacteria. Antibodies are directed to human proteins unless indicated otherwise. Secondary antibodies conjugated to HRP, CY2, and CY3 were obtained from Jackson ImmunoResearch Laboratories.
Molecular biology
KIF14 was amplified from a human testis cDNA library (CLONTECH Laboratories, Inc.) using pfu polymerase (Stratagene). PRC1 constructs have been described previously (Neef et al., 2003). Mouse citron kinase constructs were obtained from F. Di Cunto (University of Torino, Torino, Italy) and have been described previously (Di Cunto et al., 1998). All constructs were confirmed by DNA sequencing (Medigenomix). Mammalian expression constructs were made in pEGFP-C2 (CLONTECH Laboratories, Inc.) or pcDNA3.1+ (Invitrogen) modified to encode either myc- or triple FLAG-epitope tags.
Immune precipitations
HeLa S3 cells were grown and arrested with 1.6 μg/ml aphidicolin for 19 h, released for 6 h in fresh growth medium, and arrested for 14 h with 100 ng/ml nocodazole. Mitotic cells obtained by shake-off were plated in fresh growth medium and released for 70 min. Cell pellets were lysed in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 40 mM β-glycerophosphate, 10 mM NaF, 1% [vol/vol] IGEPAL, 0.1% [wt/vol] deoxycholate, 100 μM ATP, 100 μM MgCl2, 2 mM Pefabloc, and complete protease inhibitor cocktail [Roche Diagnostics]). For immune precipitations, 2 μg of affinity-purified antibody, 20 μl protein G– or A–Sepharose beads, and 8 mg of extract in a total volume of 500 μl were incubated for 2 h at 4°C. Beads were washed twice with 1 ml lysis buffer and twice with 1 ml wash buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 40 mM β-glycerophosphate, 10 mM NaF, 0.1% [vol/vol] IGEPAL, and 1 mM Pefabloc). HEK293T cells plated on 15-cm–diameter dishes were transfected using 8 μg of the required plasmid DNA and 24 μl Fugene-6 (Roche Diagnostics) according to the manufacturer's instructions. After 40 h, cells were washed three times in ice-cold PBS and were lysed in immunoprecipitation (IP) buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% [vol/vol] IGEPAL, 2 mM Pefabloc, and complete protease inhibitor cocktail). Beads were washed twice with 1 ml IP buffer and twice with 1 ml of wash buffer. Bound proteins were eluted in 50 μl of sample buffer and were analyzed by SDS-PAGE followed by mass spectrometry (Perkins et al., 1999) or Western blotting.
Cell culture and RNA interference
HeLa S3 and U2OS cells were cultured at 37°C and 5% CO2 in DME containing 10% FCS. Plasmid transfection and RNA interference were performed as described previously (Neef et al., 2003). Proteins were targeted with the following sequences: MKlp2 with 5′-AACCACCTATGTAATCTCATG-3′; MKlp1 with 5′-AAGCAGTCTTCCAGGTCATCT-3′; PRC1 with 5′-AAGGCTTCTAGGCGTGAGGAG-3′; KIF14 with 5′-TTCCCGATCTCATTCAGTTTT-3′; and citron kinase with 5′-ATGGAAGGCACTATTTCTCAA-3′. The GL2 and lamin A controls were described previously (Elbashir et al., 2001). For Western blotting, cells from three wells of six-well plates were washed in 2 ml PBS and lysed in 70–80 μl of 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 0.1% (wt/vol) Triton X-100. For each lane of a minigel, 10 μg of the protein lysate was loaded.
Image acquisition
Cells to be imaged were fixed for 12 min in PTEMF (20 Pipes-KOH, pH 6.8, 0.2% [vol/vol] Triton X-100, 10 mM EGTA, 2 mM MgCl2, and 4% [wt/vol] formaldehyde) and blocked with 2% (wt/vol) BSA in PBS. Cells to be stained with phalloidin to detect actin were fixed for 20 min with 3% (wt/vol) PFA, quenched for 10 min with 50 mM ammonium chloride, and permeabilized with 0.1% (vol/vol) Triton X-100 for 5 min. For RhoA staining, cells were fixed and permeabilized by incubation in 10% (wt/vol) tricholoracetic acid for 15 min. All solutions were made in PBS, and antibody staining was performed for 60 min using a 1,000-fold dilution of antiserum or purified antibody at a final concentration of 1 μg/ml. Coverslips were mounted in 10% (wt/vol) Moviol 4–88, 1 μg/ml DAPI, and 25% (wt/vol) glycerol in PBS. Images were collected using a microscope (Axioskop-2; Carl Zeiss MicroImaging, Inc.) equipped with a 63× NA 1.4 plan Apochromat oil immersion objective and standard filter sets (Carl Zeiss MicroImaging, Inc.), a 1,300 × 1,030 pixel cooled CCD camera (CCD-1300-Y; Princeton Instruments), and Metavue software (Visitron Systems). Images were cropped in Adobe Photoshop 7.0 and were sized and placed using Adobe Illustrator 10.0 (Adobe Systems).
Acknowledgments
We thank Dr. Herman Silljé for discussion of citron kinase function and comments on the manuscript.
The Max-Planck Society supports research in the department of E.A. Nigg and in the group of F.A. Barr.
Abbreviations used in this paper: FHA, forkhead associated; INCENP, inner centromere protein; MAP, microtubule-associated protein; PRC1, protein-regulating cytokinesis 1; siRNA, small interfering RNA.
References
- Adams, R.R., A.A. Tavares, A. Salzberg, H.J. Bellen, and D.M. Glover. 1998. pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 12:1483–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson, T.W., A. Huang, M.S. Tsao, and B.L. Gallie. 2005. KIF14 is a candidate oncogene in the 1q minimal region of genomic gain in multiple cancers. Oncogene. 24:4741–4753. [DOI] [PubMed] [Google Scholar]
- D'Avino, P.P., M.S. Savoian, and D.M. Glover. 2004. Mutations in sticky lead to defective organization of the contractile ring during cytokinesis and are enhanced by Rho and suppressed by Rac. J. Cell Biol. 166:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cunto, F., E. Calautti, J. Hsiao, L. Ong, G. Topley, E. Turco, and G.P. Dotto. 1998. Citron rho-interacting kinase, a novel tissue-specific ser/thr kinase encompassing the Rho-Rac-binding protein Citron. J. Biol. Chem. 273:29706–29711. [DOI] [PubMed] [Google Scholar]
- Di Cunto, F., S. Imarisio, E. Hirsch, V. Broccoli, A. Bulfone, A. Migheli,C. Atzori, E. Turco, R. Triolo, G.P. Dotto, et al. 2000. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron. 28:115–127. [DOI] [PubMed] [Google Scholar]
- Echard, A., G.R. Hickson, E. Foley, and P.H. O'Farrell. 2004. Terminal cytokinesis events uncovered after an RNAi screen. Curr. Biol. 14:1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir, S.M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 411:494–498. [DOI] [PubMed] [Google Scholar]
- Glotzer, M. 2005. The molecular requirements for cytokinesis. Science. 307:1735–1739. [DOI] [PubMed] [Google Scholar]
- Gruneberg, U., R. Neef, R. Honda, E.A. Nigg, and F.A. Barr. 2004. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 166:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, E., M. Clarke, and F.A. Barr. 2000. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 19:5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, R., R. Körner, and E.A. Nigg. 2003. Exploring the functional interactions between Aurora B, INCENP and surviving in mitosis. Mol. Biol. Cell. 14:3325–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, W., G. Jimenez, N.J. Wells, T.J. Hope, G.M. Wahl, T. Hunter, and R. Fukunaga. 1998. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol. Cell. 2:877–885. [DOI] [PubMed] [Google Scholar]
- Kurasawa, Y., W.C. Earnshaw, Y. Mochizuki, N. Dohmae, and K. Todokoro. 2004. Essential roles of KIF4 and its binding partner PRC1 in organized central spindle midzone formation. EMBO J. 23:3237–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, C.J., R.K. Dawe, K.R. Christie, D.W. Cleveland, S.C. Dawson, S.A. Endow, L.S. Goldstein, H.V. Goodson, N. Hirokawa, J. Howard, et al. 2004. A standardized kinesin nomenclature. J. Cell Biol. 167:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.R., H. Shin, J. Choi, J. Ko, S. Kim, H.W. Lee, K. Kim, S.H. Rho, J.H. Lee, H.E. Song, et al. 2004. An intramolecular interaction between the FHA domain and a coiled coil negatively regulates the kinesin motor KIF1A. EMBO J. 23:1506–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., G.I. Lee, S.R. Van Doren, and J.C. Walker. 2000. The FHA domain mediates phosphoprotein interactions. J. Cell Sci. 113:4143–4149. [DOI] [PubMed] [Google Scholar]
- Madaule, P., M. Eda, N. Watanabe, K. Fujisawa, T. Matsuoka, H. Bito, T. Ishizaki, and S. Narumiya. 1998. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 394:491–494. [DOI] [PubMed] [Google Scholar]
- Madaule, P., T. Furuyashiki, M. Eda, H. Bito, T. Ishizaki, and S. Narumiya. 2000. Citron, a Rho target that affects contractility during cytokinesis. Microsc. Res. Tech. 49:123–126. [DOI] [PubMed] [Google Scholar]
- Matsumura, F. 2005. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 15:371–377. [DOI] [PubMed] [Google Scholar]
- Matuliene, J., and R. Kuriyama. 2002. Kinesin-like protein CHO1 is required for the formation of midbody matrix and the completion of cytokinesis in mammalian cells. Mol. Biol. Cell. 13:1832–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuliene, J., and R. Kuriyama. 2004. Role of the midbody matrix in cytokinesis: RNAi and genetic rescue analysis of the mammalian motor protein CHO1. Mol. Biol. Cell. 15:3083–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar, M., S. Sundareshan, and T. Misteli. 2004. Human chromokinesin KIF4A functions in chromosome condensation and segregation. J. Cell Biol. 166:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki, H., Y. Okada, and N. Hirokawa. 2005. Analysis of the kinesin superfamily: insights into structure and function. Trends Cell Biol. 15:467–476. [DOI] [PubMed] [Google Scholar]
- Mishima, M., S. Kaitna, and M. Glotzer. 2002. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell. 2:41–54. [DOI] [PubMed] [Google Scholar]
- Mollinari, C., J.P. Kleman, W. Jiang, G. Schoehn, T. Hunter, and R.L. Margolis. 2002. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J. Cell Biol. 157:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim, V., S. Imarisio, F. Di Cunto, M. Gatti, and S. Bonaccorsi. 2004. Drosophila citron kinase is required for the final steps of cytokinesis. Mol. Biol. Cell. 15:5053–5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef, R., C. Preisinger, J. Sutcliffe, R. Kopajtich, E.A. Nigg, T.U. Mayer, and F.A. Barr. 2003. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell Biol. 162:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg, E.A. 2001. Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2:21–32. [DOI] [PubMed] [Google Scholar]
- Oceguera-Yanez, F., K. Kimura, S. Yasuda, C. Higashida, T. Kitamura, Y. Hiraoka, T. Haraguchi, and S. Narumiya. 2005. Ect2 and MgcRacGAP regulate the activation and function of Cdc42 in mitosis. J. Cell Biol. 168:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura, H., T. Torok, G. Tick, J. Hoheisel, I. Kiss, and D.M. Glover. 1997. Mutation of a gene for a Drosophila kinesin-like protein, Klp38B, leads to failure of cytokinesis. J. Cell Sci. 110:945–954. [DOI] [PubMed] [Google Scholar]
- Perkins, D.N., D.J. Pappin, D.M. Creasy, and J.S. Cottrell. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 20:3551–3567. [DOI] [PubMed] [Google Scholar]
- Pines, J. 1999. Four-dimensional control of the cell cycle. Nat. Cell Biol. 1:E73–E79. [DOI] [PubMed] [Google Scholar]
- Powers, J., O. Bossinger, D. Rose, S. Strome, and W. Saxton. 1998. A nematode kinesin required for cleavage furrow advancement. Curr. Biol. 8:1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich, W.B., A.N. Moran, J.H. Rothman, and J. Hardin. 1998. Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol. Biol. Cell. 9:2037–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandala, T., S.L. Gregory, H.E. Dalton, M. Smallhorn, and R. Saint. 2004. Citron kinase is an essential effector of the Pbl-activated Rho signalling pathway in Drosophila melanogaster. Development. 131:5053–5063. [DOI] [PubMed] [Google Scholar]
- Somers, W.G., and R. Saint. 2003. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell. 4:29–39. [DOI] [PubMed] [Google Scholar]
- Tatsumoto, T., X. Xie, R. Blumenthal, I. Okamoto, and T. Miki. 1999. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J. Cell Biol. 147:921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro, S., G. Totsukawa, Y. Yamakita, Y. Sasaki, P. Madaule, T. Ishizaki, S. Narumiya, and F. Matsumura. 2003. Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Mol. Biol. Cell. 14:1745–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuce, O., A. Piekny, and M. Glotzer. 2005. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 170:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, C., J. Zhao, M. Bibikova, J.D. Leverson, E. Bossy-Wetzel, J.B. Fan, R.T. Abraham, and W. Jiang. 2005. Functional analysis of human microtubule-based motor proteins, the kinesins and dyneins, in mitosis/cytokinesis using RNA interference. Mol. Biol. Cell. 16:3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]