Abstract
The centrosome is the major microtubule organizing structure in somatic cells. Centrosomal microtubule nucleation depends on the protein γ-tubulin. In mammals, γ-tubulin associates with additional proteins into a large complex, the γ-tubulin ring complex (γTuRC). We characterize NEDD1, a centrosomal protein that associates with γTuRCs. We show that the majority of γTuRCs assemble even after NEDD1 depletion but require NEDD1 for centrosomal targeting. In contrast, NEDD1 can target to the centrosome in the absence of γ-tubulin. NEDD1-depleted cells show defects in centrosomal microtubule nucleation and form aberrant mitotic spindles with poorly separated poles. Similar spindle defects are obtained by overexpression of a fusion protein of GFP tagged to the carboxy-terminal half of NEDD1, which mediates binding to γTuRCs. Further, we show that depletion of NEDD1 inhibits centriole duplication, as does depletion of γ-tubulin. Our data suggest that centriole duplication requires NEDD1-dependent recruitment of γ-tubulin to the centrosome.
Introduction
The centrosome is a center of microtubule organization in animal cells. It consists of a pair of cylindrical centrioles surrounded by fibrous pericentriolar material. During S phase, the centriole pair splits and each centriole duplicates, resulting in two new centrosomes. Before mitosis, these centrosomes increase their microtubule nucleation capacity and form two microtubule asters that are pushed apart from each other by the forces of motor proteins associated at the microtubule surface. Upon nuclear envelope breakdown, centrosomal microtubules attach to the kinetochores of chromosomes and a functional spindle apparatus is formed. The two centrosomes thus become the focal points of the spindle in mitosis. One of the best characterized proteins at the centrosome is γ-tubulin, a member of the tubulin family that catalyzes the nucleation of microtubule polymers from α/β-tubulin dimers. γ-Tubulin itself associates with additional proteins into two differently sized complexes (Oegema et al., 1999): a small complex (γ-tubulin small complex [γTuSC]) and a larger complex that acquires a ring-shaped morphology (γ-tubulin ring complex [γTuRC]). γTuSCs contain two copies of γ-tubulin, associated with the γ-complex proteins (GCPs) 2 and 3 (Knop et al., 1997; Murphy et al., 1998; Tassin et al., 1998; Gunawardane et al., 2000). In contrast, γTuRCs are assembled from multiple γTuSCs associated with the additional proteins GCP4, -5, and -6 (Fava et al., 1999; Gunawardane et al., 2000; Murphy et al., 2001). Although budding yeast exclusively contains proteins of the γTuSC, both γTuSC and γTuRC complexes are found in vertebrate and Drosophila melanogaster cells. The respective roles of these two complexes are not well understood. In particular, it is unclear in which form γ-tubulin is targeted to the centrosomes and which proteins mediate the sudden increase in γ-tubulin recruitment immediately before mitosis (Khodjakov and Rieder, 1999). An additional protein associated with the γTuRC has recently been identified in D. melanogaster: Dgp71WD, a 71-kD protein that contains an amino-terminal region with multiple WD repeats but lacks the signature motifs of GCP/grip proteins (Gunawardane et al., 2003). Pair-wise expression in insect SF9 cells has shown that Dgp71WD interacts directly with Dgrip 84, 91, 128, and 163 (homologous to GCP2, -3, -5, and -6). Based on these interactions, Dgp71WD has been proposed to have a role in the scaffolding of the γTuRC. Sequence comparison revealed the existence of a related protein in mammals termed NEDD1, first described as a developmentally regulated protein in neuronal cells of mice (Neural precursor cell Expressed, Developmentally Down-regulated protein 1; Kumar et al., 1992). NEDD1 appears to be expressed in most animal tissues, and overexpression of NEDD1 in various mammalian cell lines suppresses cell growth (Kumar et al., 1994). NEDD1 has also been identified in a proteomic screen for centrosome proteins (Andersen et al., 2003), but it is currently unknown whether NEDD1 is a functional homologue of Dgp71WD and what role it plays at the centrosome or at the spindle apparatus. In the present study, we show that γTuRCs require NEDD1 for targeting to the centrosome. We demonstrate that the interaction between NEDD1 and γ-tubulin complexes is essential for centriole duplication and spindle assembly.
Results
NEDD1 is a new centrosomal protein homologous to Dgp71WD
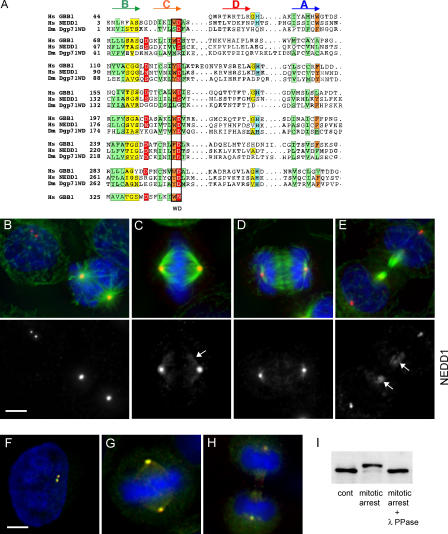
Both Dgp71WD and NEDD1 are suspected to contain WD repeats in their amino-terminal half. To further investigate their structural similarity, we performed sequence alignment and structure prediction using bidimensional hydrophobic cluster analysis (Callebaut et al., 1997). This method allowed the identification of two WD repeats in addition to the five repeats described in Dgp71WD by Gunawardane et al. (2003). We used as a template the sequence of human transducin β chain 1, of which the three-dimensional structure has been solved (pdb identifier 1got). Fig. 1 A shows that the three proteins are structurally similar within a region of seven repetitions of four β strands—A, B, C, and D. These strands are predicted to form seven blades of a β propeller structure, with strand C carrying the WD signature (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200510028/DC1). Within their WD domain, NEDD1 and Dgp71WD are more similar to each other than to any other WD protein. The two proteins show an overall identity of 20.8% (Gunawardane et al., 2003), have a similar length, and possess a coiled-coil domain of ∼100 amino acids at their carboxy-terminal ends (unpublished data). These similarities suggest that they perform similar functions. To test this, we raised antibodies against the carboxy-terminal domain of the protein and performed localization studies in HeLa cells. Small amounts of NEDD1 are present at the centrosome during interphase (Fig. 1 B). However, NEDD1 recruitment to the centrosome increases significantly at the onset of mitosis (Fig. 1 B). Immunoblotting revealed that NEDD1 in arrested mitotic cells has a reduced mobility (Fig. 1 I). Phosphatase treatment suggests that this shift in mobility is attributable to the mitotic phosphorylation of NEDD1. In metaphase, most of NEDD1 is concentrated at the centrosome, but small quantities are also seen along the spindle fibers (Fig. 1 C). During anaphase, the centrosomal localization of NEDD1 is gradually reduced (Fig. 1 D), and in telophase, small amounts are also seen along the midbody microtubules (Fig. 1 E). This dynamic distribution resembles γ-tubulin localization during the cell cycle (Zheng et al., 1991; Julian et al., 1993; Lajoie-Mazenc et al., 1994; Khodjakov and Rieder, 1999). Indeed, we observed a perfect colocalization of NEDD1 with γ-tubulin (Fig. 1, F–H). We performed quantitative immunoblotting of NEDD1 in HeLa extracts using the carboxy-terminal fusion protein of NEDD1 for calibration and determined that the abundance of NEDD1 is ∼0.002% of total cellular protein (unpublished data). In comparison, we found γ-tubulin to be significantly more abundant, with an estimated 13–20 molecules of γ-tubulin per molecule of NEDD1.
Figure 1.
NEDD1 is recruited to the mitotic centrosome and to spindle microtubules and colocalizes with γ-tubulin. (A) Alignment of the WD repeats of human NEDD1 (available from GenBank/EMBL/DDBJ under accession no. 74762597) and Dgp71WD (accession no. 28628541), together with those of human transducin β chain 1 (GBB; accession no. 51317302). The positions of predicted β strands (A, B, C, and D) are indicated above the alignment. Green and orange boxes indicate conservation of hydrophobic and aromatic residues, respectively. Additional sequence hallmarks found in WD repeats are indicated in yellow, red, and blue. (B–E) HeLa cells immunostained for NEDD1 (red), α-tubulin (green), and DNA (blue). NEDD1 is enriched at the mitotic centrosome and is found along spindle and midbody microtubules (arrows). (B) Interphase and prometaphase cells; (C) metaphase cell; (D) anaphase cells; (E) telophase cell. Bar, 5 μm. (F–H) Cells stained for NEDD1 (red), γ-tubulin (green), and DNA (blue). NEDD1 colocalizes with γ-tubulin throughout cell cycle, at interphase (F), metaphase (G), and telophase (H). Bar,5 μm. (I) HeLa cell lysates immunoblotted for NEDD1: untreated cells (cont), after overnight incubation in 500 nM taxol (mitotic arrest), or lysate of cells in mitotic arrest treated with λ-phosphatase (400 U for 30 min at 30°C).
NEDD1 is required for mitotic spindle organization
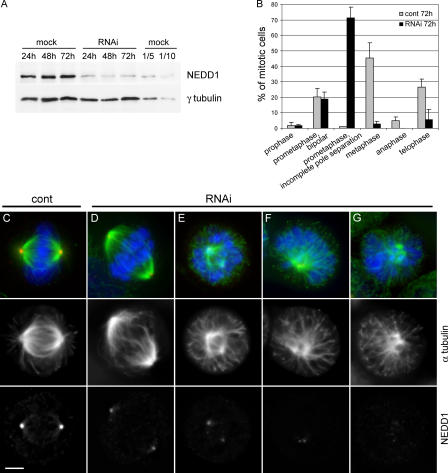
To study the function of NEDD1, we reduced the levels of the protein using small interfering RNA (siRNA). We show by immunoblotting that after 24 h of siRNA treatment, NEDD1 levels are reduced to 20% (Fig. 2 A). Considering a transfection efficiency of ∼85%, as determined by cytoplasmic incorporation of rhodamine-labeled RNA oligomers (unpublished data), we reached a nearly quantitative depletion in individual cells. These low levels were maintained also after prolonged siRNA treatment of up to 72 h. We show as a control that the levels of γ-tubulin remain unaffected after NEDD1 depletion (Fig. 2 A). After 72 h of treatment, cell cultures show a significantly increased mitotic index of 47% (n = 200), compared with 5% (n = 550) of mitotic cells in control cultures. NEDD1-depleted cells accumulate mitotic aberrations and are arrested in a prometaphaselike state of mitosis (Fig. 2 B). In the majority of these cells, an aberrant mitotic apparatus is formed, with unseparated or poorly separated poles and with microtubules arranged in a monoastral pattern (Fig. 2, E–G). The chromosomes are distributed randomly in the cytoplasm, and MAD2 staining reveals the absence of microtubule attachment to kinetochores (unpublished data). The overall density of microtubules in these cells seemed to be reduced. Few mitotic cells were observed where NEDD1 was reduced to a lesser extent. These cells still form bipolar spindles but lack astral microtubules and show an enlarged pole-to-pole distance (Fig. 2 D). Identical results were obtained with two different siRNA oligomers (unpublished data), confirming the specificity of this phenotype.
Figure 2.
Silencing of NEDD1 induces mitotic defects. HeLa cells were treated without siRNA (mock), with control siRNA (cont), or with NEDD1 siRNA (RNAi) for various time points. (A) Immunoblot of crude cell lysates (40 μg) showing reduction of NEDD1 levels but no γ-tubulin reduction. NEDD1 levels in mock-treated cells diluted 1/5 and 1/10 are presented to compare with depleted cells. (B) Histogram indicating percentage of mitotic cells at different stages of mitosis (mean of three experiments ± SEM; 350–400 total cells scored per condition). Most of the cells are arrested in a prometaphase-like state with poorly separated spindle poles. (C–G) Mitotic cells stained for NEDD1 (red), α-tubulin (green), and DNA (blue). Bar, 5 μm.
NEDD1 targets γTuRCs to the centrosome
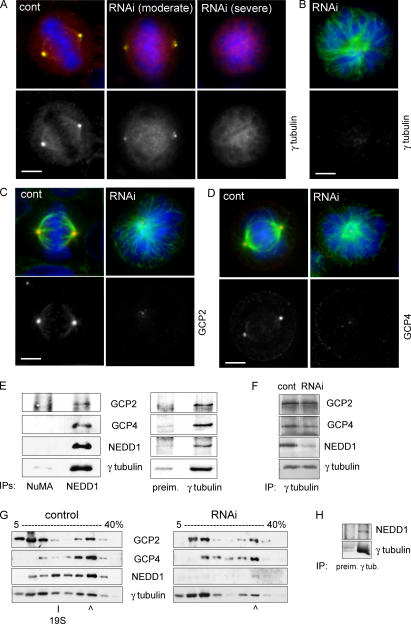
We then investigated whether NEDD1 depletion affects the recruitment of γ-tubulin to the centrosome. Fig. 3 A shows that partial reduction of NEDD1 in mitosis results in partial loss of γ-tubulin staining. More efficient removal of NEDD1 results in almost complete loss of γ-tubulin at the centrosome, correlated with the formation of monoastral microtubule organization in mitosis (Fig. 3 B). Consistently, the localization of the γTuRC proteins GCP2 and -4 to the centrosome is suppressed in these monoastral structures (Fig. 3, C and D). Our data leave open the question of whether NEDD1 depletion prevents γTuRC assembly or whether γTuRCs are still assembled but fail to be recruited to the centrosome in the absence of NEDD1. To address this, we examined the association between NEDD1 and the γTuRC by coimmunoprecipitation. Purified antibody against NEDD1 coprecipitated components of the γTuRC, including γ-tubulin, GCP2, and GCP4 (Fig. 3 E). In the reverse experiment, antibody against γ-tubulin also coprecipitated NEDD1, in addition to the γ-complex proteins GCP2 and -4 (Fig. 3 E). Further, NEDD1 sediments at ∼32S together with γTuRC proteins in sucrose-gradient centrifugation experiments (Fig. 3 G, control). In addition, we coimmunoprecipitated NEDD1 and γ-tubulin from the peak fraction of the sucrose gradient containing the γTuRC (Fig. 3 H). This indicates that NEDD1 associates with the γTuRC. We performed additional control experiments using gel filtration of HeLa cell lysates, demonstrating that all γTuRC proteins elute in a peak together with NEDD1 and that NEDD1 coimmunoprecipitates with γ-tubulin from this peak (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200510028/DC1). Sucrose-gradient centrifugation showed a significant amount of γTuRC components sedimenting at sizes smaller than 19S (Fig. 3 G). These might represent breakdown products of γTuRCs preferentially formed under the conditions of sucrose-gradient experiments, as compared with gel filtration. We then examined the composition of the γTuRC in cells depleted of NEDD1. RNA silencing in three different experiments reduced the levels of NEDD1 coimmunoprecipitating with γ-tubulin; in contrast, GCP4 was only slightly affected and GCP2 coprecipitated at nearly regular levels (Fig. 3 F; coprecipitating protein amounts compared with controls: NEDD1, 28% ± 6; GCP2, 96% ± 4; GCP4, 88% ± 21, after normalization over amounts of immunoprecipitated γ-tubulin). In addition, immunoblots of cell extracts separated on sucrose gradients reveal that the sedimentation of the majority of γTuRC proteins is unaffected after depletion of NEDD1, with small levels of GCP4 and γ-tubulin sedimenting more slowly (Fig. 3 G, RNAi [RNA interference]). We conclude that the majority of γTuRCs can still form in the absence of NEDD1, although we cannot exclude the possibility that residual levels of NEDD1 after depletion support the assembly of reduced amounts of γTuRCs.
Figure 3.
Silencing of NEDD1 inhibits recruitment of the γTuRC to the mitotic centrosome, but the majority of γTuRCs can still form. (A) NEDD1-depleted HeLa cells (RNAi) or cells treated with control siRNA (cont) that were stained for NEDD1 (green), γ-tubulin (red), and DNA (blue). (B–D) Cells stained for α-tubulin (green) and γ-tubulin, GCP2 or -4 (red), and DNA (blue). Bars, 5 μm. (E) Coimmunoprecipitation of NEDD1 and γTuRC proteins. (left) Immunoprecipitation from HeLa cells using antibodies against NuMA or NEDD1. (right) Immunoprecipitation with polyclonal antibody R75 against γ-tubulin or with preimmune serum from the same animal (preim.). Precipitates w ere separated by gel electrophoresis, blotted, and probed with antibodies against GCP2, GCP4, NEDD1, and γ-tubulin. (F) Coimmunoprecipitation of γTuRC proteins from cells treated with control siRNA or with siRNA against NEDD1 (RNAi). Conditions were as in the right column of E. (G) Comparison of extracts from HeLa cells treated with control and NEDD1 siRNA (RNAi) after fractionation in gradients of 5–40% sucrose. The fractions were precipitated using methanol and immunoblotted with antibodies against GCP2, GCP4, NEDD1, and γ-tubulin. Arrowheads indicate the position of the 32S γTuRC, and 19S indicates the sedimentation of thyroglobulin. (H) Coimmunoprecipitation of NEDD1 and γ-tubulin from the purified 32S fraction of undepleted extracts, using the polyclonal antibody R75 against γ-tubulin and control precipitation with preimmune serum from the same animal.
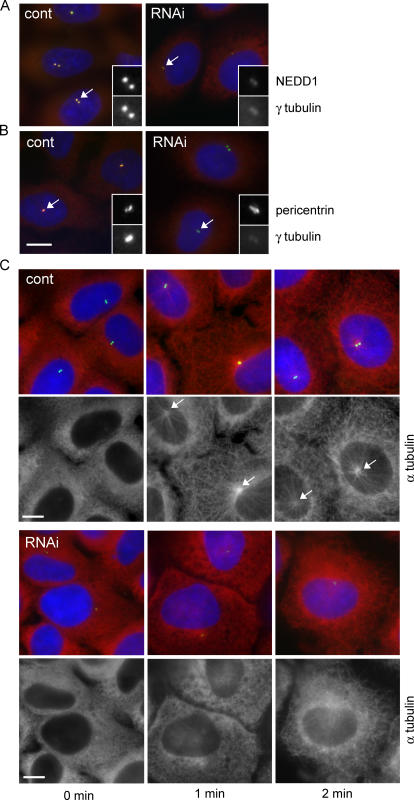
To determine whether the association between NEDD1 and γ-tubulin complexes is cell cycle dependent, we also investigated NEDD1 silencing effects in interphase cells. As seen before in mitosis, γTuRC localization to the centrosome was also dependent on NEDD1 in interphase cells. Reduced levels of NEDD1 resulted in reduced amounts of γ-tubulin at the centrosome (Fig. 4 A). Localization of other centrosomal components, such as pericentrin, was not affected (Fig. 4 B). The reduction ofγ-tubulin correlated with a delay in microtubule regrowth after cold treatment (Fig. 4 C). Moreover, in U2OS cells that usually contain a well-focused microtubule network, microtubules appeared disorganized and regrowth did not originate from a single centrosomal focus, as seen in control cells (Fig. 4 C, 2 min).
Figure 4.
Silencing of NEDD1 in interphase cells affects centrosomal localization of γ-tubulin and microtubule nucleation. (A and B) HeLa cells treated with control siRNA (cont) or NEDD1 siRNA (RNAi) and stained for NEDD1 or pericentrin (green), γ-tubulin (red), and DNA (blue). Centrosomes appear in yellow in control cells because NEDD1 or pericentrin colocalize with γ-tubulin. Insets show enlarged views of the centrosomes indicated by arrows. (C) Microtubule regrowth assay in U2OS cells treated with control siRNA or in cells treated with siRNA against NEDD1. Cells were cold treated and reheated for 0, 1, or 2 min at 37°C before staining for NEDD1 (green), α-tubulin (red), and DNA (blue). Arrows point to microtubule asters nucleated in control cells; in RNAi-treated cells, asters are barely visible. Bars, 10 μm.
The carboxy-terminal domain of NEDD1 mediates binding to γTuRC proteins
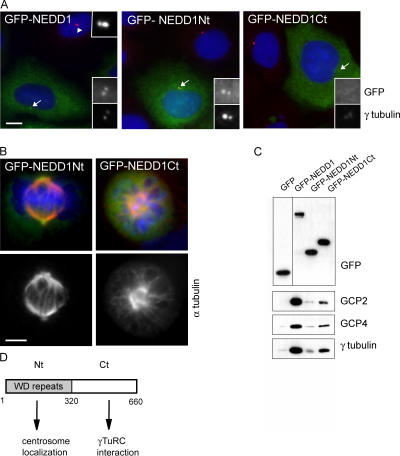
Next, we wanted to determine which region of the NEDD1 protein mediates an interaction with the γTuRC. We separately expressed the amino- and carboxy-terminal halves of the protein (amino acids 1–319 and 321–660, respectively), fused to GFP, in U2OS cells. The amino-terminal half corresponds to the WD-repeat domain. 24 h after transfection, overexpressed full-length NEDD1 localizes to the centrosome, in addition to diffuse localization in the cytoplasm (Fig. 5 A). Interestingly, the soluble pool seemed to increase with the expression level of the protein, indicating that the fraction associated with the centrosome is limited, probably via an endogenous anchoring factor. The amino-terminal domain also localizes to the centrosome. In contrast, the carboxy-terminal domain does not exhibit any centrosomal localization. In mitosis, the amino-terminal domain localizes to the centrosome and to the spindle poles, whereas the carboxy-terminal domain remains cytoplasmic (Fig. 5 B). We further noticed that overexpressing the carboxyterminal domain caused a dominant-negative phenotype in mitosis, inhibiting spindle pole separation, similar to NEDD1-silencing experiments (Fig. 5 B). Colocalization of γ-tubulin shows that overexpression of the carboxy-terminal domain, but not the amino-terminal domain, displaces γ-tubulin from the centrosome (Fig. 5 A). Full-length NEDD1, when highly expressed, also partly displaces γ-tubulin. This suggests that excess NEDD1 associates with the centrosome and blocks the attachment of NEDD1–γTuRC complexes or, alternatively, that excess NEDD1 is able to sequester γ-tubulin in the cytoplasm via its carboxy-terminal domain. We further performed biochemical experiments to determine whether γTuRC components could be coprecipitated with the amino- or carboxy-terminal half of NEDD1. Coimmunoprecipitations of the GFP-tagged, overexpressed NEDD1 fragments were performed with an antibody against GFP. These experiments revealed that complexes containing γ-tubulin, GCP2, and GCP4 are able to bind to the carboxy-terminal fragment of NEDD1, although less efficiently than to full-length NEDD1 (Fig. 5 C). Our mapping experiments thus indicate that the amino-terminal domain of NEDD1 is responsible for centrosome targeting, whereas the carboxy-terminal domain mediates the interaction with the γTuRC (Fig. 5 D).
Figure 5.
The carboxy-terminal domain of NEDD1 binds to γTuRCs, and the amino-terminal domain mediates centrosome attachment. (A) Transient overexpression of GFP fusion proteins with full-length NEDD1 (GFP-NEDD1) and amino acids 1–319 (GFP-NEDD1Nt) and 321–660 (GFP-NEDD1Ct) in U2OS cells. Cells are stained for γ-tubulin (red) and DNA (blue). Arrows indicate the positions of centrosomes that are shown enlarged in insets, displaying the fluorescence of the various GFP fusion proteins or γ-tubulin. For comparison, an untransfected cell is indicated by an arrowhead in the left panel (inset in top right corner shows an enlarged view). Bar, 10 μm. (B) Mitotic U2OS cells overexpressing GFP-NEDD1Nt or GFP-NEDD1Ct (green) that were stained for α-tubulin (red) and DNA (blue). GFP-NEDD1Ct induces spindle defects. Bar, 5 μm. (C) Immunoprecipitation of the overexpressed proteins by anti-GFP antibody in Cos-7 cells. (top) Detection of precipitated proteins by anti-GFP antibody (note that GFP is resolved in a 10% polyacrylamide gel, whereas the other proteins are separated in a 7.5% gel). (bottom) γTuRC proteins (γ-tubulin, GCP2, and GCP4) coprecipitate with GFP-NEDD1 and GFP-NEDD1Ct. (D) Schematic drawing depicting functional domains of NEDD1.
NEDD1 depletion induces dispersion of the microtubule organizing centers
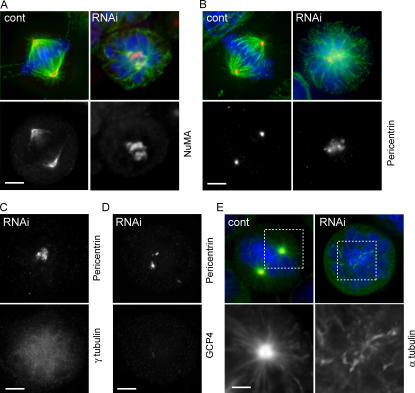
The loss of γTuRC recruitment to the centrosome after NEDD1 silencing led us to examine in more detail the resulting spindle defects, particularly the structure of the spindle poles. We noticed that, in mitotic cells lacking detectable amounts of NEDD1, microtubules were often organized from broad irregularly shaped areas rather than from focused organizing centers. This was also clear in cells overexpressing the carboxy-terminal domain of NEDD1 (Fig. 5 B). Staining of nuclear mitotic apparatus protein (NuMA) to indicate the localization of microtubule minus ends confirmed the presence of dispersed microtubule organizing centers (Fig. 6 A). Consistently, the pericentriolar material was found scattered, as shown by pericentrin staining, which distributed either more diffusely or dispersed in multiple foci (Fig. 6, B–D). In contrast, components of the γTuRC were almost completely absent from the microtubule organizing centers and instead found diffuse throughout the cytoplasm (Fig. 6, C and D). This suggested that although γTuRC proteins failed to localize to the centrosome both in interphase and mitosis, pericentrin was able to target to microtubule organizing centers, although less focused in mitosis (Fig. 3, A–D; Fig. 4, A and B; and Fig. 6, B–D). Dispersal of microtubule organizing proteins correlated with defects of centrosome-dependent microtubule regrowth after cold treatment. After short times (30 s), microtubule nucleation occurred from multiple sites in the cytoplasm, in contrast to centrosomal nucleation in controls (Fig. 6 E).
Figure 6.
Dispersion of the microtubule organization centers in NEDD1-depleted mitotic cells. (A and B) HeLa cells stained for α-tubulin (green) and DNA (blue), NuMA (A, red) as a marker of microtubule minus ends, and pericentrin (B, red) as a marker of the pericentriolar material. NEDD1-depleted cells (RNAi) show poorly separated and disorganized spindle poles. (C and D) NEDD1-depleted cells stained for pericentrin and the γTuRC proteins γ-tubulin (C) and GCP4 (D). (E) Microtubule regrowth assay in mitotic cells. Cells were cold treated and reheated for 30 s at 37°C before staining for NEDD1 (green), α-tubulin (red), and DNA (blue). Magnified areas show microtubules nucleated from the centrosome in control cells and from dispersed sites in silenced cells. Bars, 5 μm.
NEDD1 depletion interferes with centriole assembly
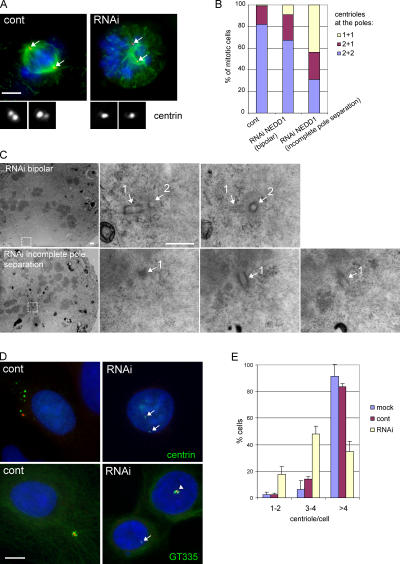
We then investigated whether NEDD1 depletion also affected the formation of the centrosomal core structures, i.e., the centrioles. We used centrin staining to visualize the centrioles in mitotic cells (Fig. 7 A). In control cells, a pair of centrioles can be clearly distinguished at both spindle poles (<10% of the poles present a single centrin dot, and <1% of the spindles show only one dot at both poles). In NEDD1-depleted cells, we selected spindles showing incomplete pole separation. In those spindles, we counted only one centrin dot at each pole, at a frequency of >40% (Fig. 7 B). This suggests that NEDD1 is necessary for daughter centriole assembly during duplication or for centriole maturation. Electron microscopy analysis of serial sections of multiple NEDD1-depleted cells confirmed that only one centriole was present at each pole (Fig. 7 C). To determine whether centriole duplication was dependent on NEDD1, we performed an assay previously published by Balczon et al. (1995): U2OS cells were arrested in S phase in the presence of aphidicolin, allowing the centrioles to undergo multiple rounds of duplication. In the vast majority of control cells (84% after control RNA and 92% after mock treatment; Fig. 7 E), this led to the formation of more than four structures per cell containing centriolar markers, as detected by immunostaining of centrin and polyglutamylated tubulin (Fig. 7 D). Because NEDD1 was not present on all of these structures, these could either represent imperfectly assembled centrioles or immature centrioles that might accumulate visible amounts of NEDD1 later in the cycle. In contrast, cells depleted of NEDD1 mainly contained four centriolar structures or less (only 35% contained more than four; Fig. 7 E).
Figure 7.
Silencing of NEDD1 inhibits centriole duplication. (A) HeLa cells stained for α-tubulin (green), DNA (blue), and centrin as a marker of the centrioles (red and insets). Cells that were efficiently depleted of NEDD1 (RNAi) show poorly separated poles, containing only one centriole at each pole. Arrows indicate positions of the centrosomes. Bar, 5 μm. (B) Quantification of the number of centrin-stained centrioles per pole (mean of three experiments; 228 control RNA-treated cells, 138 NEDD1-depleted cells with fully separated poles, and 322 depleted cells with incompletely separated poles were scored). (C) Electron microscopy of a cell with a bipolar mitotic spindle (RNAi bipolar) and a depleted cell with a monopolar phenotype (RNAi incomplete pole separation). The pictures on the left show low magnifications, and selected areas at high magnifications are at the right. Serial sections of spindle poles reveal a pair of closely associated centrioles (1 and 2) oriented at a 90° angle in the bipolar spindle, whereas only a single centriole (1) could be found at the pole of the depleted cell. Bar, 500 nm. (D) U2OS cells treated with aphidicolin and stained for NEDD1 (red), DNA (blue), and centrin or polyglutamylated tubulin (GT335; green). Control cells or poorly depleted cells (arrowhead in bottom right image) show clouds of numerous centriole dots, whereas efficiently depleted cells have mainly 1–4 centrioles (arrows). Bar, 10 μm. (E) Quantification of centriole numbers per cell was based on centrin staining. Numbers were obtained from selected cells that displayed a strong reduction of NEDD1 levels (mean of three experiments ± SEM; 350–400 cells scored per condition).
NEDD1-dependent centrosome and spindle defects are reproduced by γ-tubulin depletion
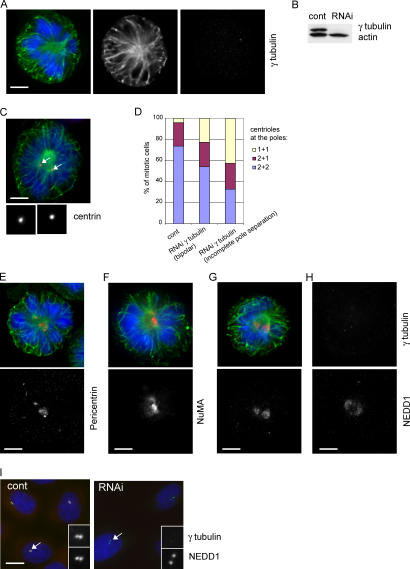
Because our experiments on NEDD1 depletion or overexpression of the carboxy-terminal half of NEDD1 both resulted in displacement of γ-tubulin from the centrosome, we suspected that the NEDD1-dependent defects on centriole assembly and spindle formation might have been indirectly caused by the absence of γTuRCs at the centrosome. To test this idea, we performed γ-tubulin depletion by siRNA treatment and investigated the effects on spindle formation and on centriole duplication (Fig. 8). Our RNA oligomers were directed against a sequence present in both human forms of γ-tubulin and resulted in a nearly quantitative reduction of γ-tubulin levels (Fig. 8, A and B). As seen previously in NEDD1-depleted cells, γ-tubulin depletion also led to defects in spindle pole separation and to a high number of cells with unreplicated centrioles in mitosis (Fig. 8 C). Only 33% of γ-tubulin–depleted cells displayed centriole pairs at each pole, whereas the remaining cells contained at least one pole with an unreplicated centriole (Fig. 8 D). When investigating the morphology of the spindle poles, we observed scattering of pericentrin and NuMA staining as seen in NEDD1-depleted cells (Fig. 6, A–D; and Fig. 8, E and F). NEDD1 is still found localized to the area of microtubule organization in mitosis, although more dispersed and at variable amounts (Fig. 8, G and H). In interphase, regular levels of NEDD1 are recruited to the centrosome, even in the absence of γ-tubulin (Fig. 8 I).
Figure 8.
Silencing of γ-tubulin inhibits centriole duplication and causes spindle defects similar to those of NEDD1 depletion. (A) Mitotic HeLa cell depleted of γ-tubulin and stained for α-tubulin (green), γ-tubulin (red), and DNA (blue). Bar, 5 μm. (B) Immunoblot of lysates (40 μg) of cells treated with control siRNA (cont) or γ-tubulin siRNA (RNAi) and probed with antibodies against γ-tubulin and actin. (C) A depleted cell stained for centrin (red), α-tubulin (green), and DNA (blue). Insets show magnified views of centrin staining of left and right spindle poles, respectively. Arrows indicate positions of the centrosomes. Bar, 5 μm. (D) Quantification of centrin-stained centrioles per pole (mean of two experiments; 232 control RNA-treated cells, 35 γ-tubulin–depleted cells with fully separated poles, and 180 depleted cells with incompletely separated poles were scored). (E–G) Cells depleted of γ-tubulin and stained in green for α-tubulin and in red for pericentrin (E), NuMA (F), and NEDD1 (G). (H) Cell depleted of γ-tubulin and stained for γ-tubulin and NEDD1. Mitotic cells depleted of γ-tubulin show disorganized spindle poles and diffuse staining of NEDD1. (I) Control and γ-tubulin–depleted interphase cells (RNAi) stained for γ-tubulin (red), NEDD1 (green), and DNA (blue). NEDD1 is concentrated at the centrosome (arrows and bottom insets), even in the absence of γ-tubulin (top insets).
Discussion
We have shown that NEDD1 is an essential protein for the recruitment of γ-tubulin complexes to the centrosome. NEDD1 depletion prevents the localization of γ-tubulin and GCPs to the centrosome both in interphase and in mitosis. In contrast, the absence of γ-tubulin does not prevent centrosomal targeting of NEDD1 itself. Our biochemical studies as well as previous work on the D. melanogaster homologue Dgp71WD show that NEDD1 associates directly with the γTuRC (Gunawardane et al., 2003). It has previously been suggested that the D. melanogaster homologue is a scaffold protein of the γTuRC.Although we see the majority of the γTuRC still forming after NEDD1 depletion, our experiments are consistent with the idea that NEDD1 could be required for efficient γTuRC assembly because cosedimentation and coimmunoprecipitation of GCP4 with other γTuRC components seem slightly reduced at low levels of NEDD1. However, NEDD1 is different both structurally and functionally from proteins of the GCP/grip family, of which several were shown to have a strong effect on γTuRC assembly (Martin et al., 1998; Zhang et al., 2000). Because the majority of γTuRCs still sedimented at ∼32S after reducing NEDD1 levels to ∼20%, we conclude that NEDD1 associates with γTuRCs at a low stoichiometry; otherwise, its absence would have resulted in a significant shift. Consistently, comparison of γ-tubulin and NEDD1 amounts indicates a large excess of γ-tubulin over NEDD1 in the cytoplasm. If γ-tubulin assembled into γTuRCs in excess over NEDD1, multiple γ-tubulin molecules and associated GCPs could build a ring-shaped template for microtubule nucleation, whereby a single molecule of NEDD1 bound to the periphery of the γTuRC could increase the binding competence of the complex to the centrosome. Our biochemical data suggest that the carboxy-terminal half of NEDD1 binds to the γTuRC, whereas the amino-terminal half corresponding to the WD-repeat domain mediates its attachment to the centrosome. Additional interactions between γTuRC proteins and the centrosome are thought to be mediated by the binding of GCP2 and -3 to pericentrin and AKAP450/CG-NAP (Takahashi et al., 2002; Kawaguchi and Zheng, 2004; Zimmerman et al., 2004) and by ninein or ninein-like protein (Mogensen et al., 2000; Casenghi et al., 2003; Delgehyr et al., 2005). A different group of proteins known to mediate γ-tubulin interaction includes centrosomin in D. melanogaster and the fission yeast proteins Mto1 and Pcp1, all sharing a conserved 60–amino acid motif that putatively binds to the γ-tubulin complex (Terada et al., 2003; Sawin et al., 2004; Samejima et al., 2005).
The significant increase of NEDD1 on mitotic centrosomes led us to wonder whether NEDD1 is responsible for the increased recruitment of γ-tubulin complexes at the onset of mitosis (Khodjakov and Rieder, 1999). Consistently, the most striking defects after depletion of NEDD1 are monopolar spindles or spindles with poorly separated poles, which are reminiscent of the spindle defects found in cells depleted of γ-tubulin (Sunkel et al., 1995; Strome et al., 2001; Hannak et al., 2002; Raynaud-Messina et al., 2004; Yuba-Kubo et al., 2005; this study). Moreover, similar phenotypes are found in cells depleted of CeGrip-1 and -2 and pericentrin and in mutants of Dgrip91 (Barbosa et al., 2000, 2003; Hannak et al., 2002; Zimmerman et al., 2004). In all these examples, cells lack proper targeting of γ-tubulin to the centrosome, which is thought to affect microtubule nucleation and microtubule minus end dynamics. Altered microtubule-end dynamics have been reported to cause the formation of monopolar spindles (Ganem and Compton, 2004). Consistently, defects at microtubule ends are a possible explanation for the for-mation of monopolar spindles in NEDD1- or γ-tubulin–depleted cells. However, loss of bipolar spindle organization is a complex phenotype, and additional factors might contribute to this defect: loss of astral microtubules in NEDD1-depleted mitotic cells might eliminate astral pulling forces and therefore favor spindle collapse. In addition, NEDD1-depleted cells show defects in centriole duplication, which in turn might favor monopolarity (Habedanck et al., 2005), although acentriolar pathways have been reported to support bipolar spindle formation, even in somatic vertebrate cells (Khodjakov et al., 2000). Besides NEDD1, a large number of kinases and structural proteins have been implicated in centriole duplication. In particular, proteins such as SPD-2; SAS-4, -5, and -6; and centrin are not only present in the pericentriolar material but also form part of the centriolar cylinder and could therefore be structural components directly responsible for the formation of new centrioles (Salisbury et al., 2002; Kirkham et al., 2003; Leidel and Gonczy, 2003; Kemp et al., 2004; Dammermann et al., 2004; Delattre et al., 2004; Pelletier et al., 2004; Leidel et al., 2005). Other proteins, such as SPD-5 or ɛ-tubulin, are located at the pericentriolar material or at subdistal appendages of the centriole, and it was suggested that their role in the duplication process could be to recruit additional protein for daughter centriole assembly (Chang et al., 2003; Dammermann et al., 2004). Likely, one of the key components that need to be recruited for centriole duplication is γ-tubulin itself: several kinases and structural proteins that are involved in centrosome duplication are also involved in attracting γ-tubulin, such as aurora A, SPD-2, and SPD-5 (Hannak et al., 2001; Hamill et al., 2002; Pelletier et al., 2004). Moreover, γ-tubulin has been shown to be present in the centriolar cylinder, besides localizing to the pericentriolar material (Fuller et al., 1995; Moudjou et al., 1996; Klotz et al., 2003). It is quite possible that γ-tubulin plays a role in nucleating the microtubule triplets of the centriolar cylinder during centriole duplication, similar to the way it nucleates regular microtubules. Thus, the failure of centriole duplication after NEDD1 depletion can be explained by an indirect effect that is attributable to the failure of γ-tubulin recruitment. Our data on centriole duplication defects after depletion of γ-tubulin support this idea. In agreement with this concept are further reports on defective centriole formation in D. melanogaster Dgrip91 mutants that fail to recruit γ-tubulin to the centrosome, in Caenorhabditis elegans embryos depleted of γ-tubulin by RNAi, as well as observations in the ciliates Paramecium tetraurelia and Tetrahymena thermophila, where the absence of γ-tubulin leads to defects in basal body formation (Ruiz et al., 1999; Barbosa et al., 2000; Shang et al., 2002; Dammermann et al., 2004). Further, it appears that duplication of centrioles and basal bodies is regulated by posttranslational modifications of γ-tubulin: in the flagellate Naegleria gruberi, basal body duplication is believed to require phosphorylation of γ-tubulin, and in mammalian cells ubiquitination of γ-tubulin is thought to prevent excess rounds of centriole duplication (Starita et al., 2004; Kim et al., 2005). We have shown that NEDD1 is another key component in controlling γ-tubulin levels at the mammalian centrosome. Considering the putative relationship between aberrant centrosome duplication and cancer, the regulation of γ-tubulin recruitment to the centrosome will be of continued prime interest to cell biologists.
Materials and methods
Cloning of human NEDD1 cDNA and generation of expression vectors
EST clones DKFZp313N211Q2 and IRATp970F0222D6 containing the full-length cDNA of NEDD1 were obtained from Deutsches Ressourcenzentrum für Genomforschung GmbH. The full-length NEDD1 construct was amplified by PCR and cloned into the pCR BluntII-TOPO vector (Invitrogen), and the internal NdeI site was removed without change of amino acid sequence. Sequencing of the first EST revealed two mutations that induced the amino acid changes P97T and S379N. To correct the sequence, a SwaI–AclI fragment encoding amino acids 97–379 was swapped between the two ESTs. NEDD1 was then subcloned into pEGFPC2 (CLONTECH Laboratories, Inc.) using the SalI and KpnI restriction sites flanking the cDNA in pCR BluntII-TOPO. GFP-NEDD1Nt (corresponding to amino acids 1–319) and Ct (corresponding to amino acids 321–660) were generated by introducing the 5′ SalI–AleI and the 3′ AleI–KpnI fragments, respectively, into pEGFPC2. Transfection of the GFP constructs into cells was performed with Fugene reagent (Roche) according to the manufacturer.
Cell culture
HeLa, U2OS, and Cos-7 cell lines were grown at 37°C in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. HeLa-GFP tubulin-expressing cells were generated by stable transfection with pEGFP-Tub (CLONTECH Laboratories, Inc.). Mitotic indexes were ascertained by determining the percentage of live cells containing a fluorescent spindle, to prevent underestimation because of loss of mitotic cells during staining procedures.
RNA silencing
Double-stranded siRNA oligomers were transfected using oligofectamine (Invitrogen) according to the manufacturer. In brief, cells were seeded in 6-well dishes at a density of 170,000 cells/well and transfected after 24 h of growth with 2.5 μl of 100 μM siRNA. Two siRNAs targeting NEDD1 mRNA were used (Ambion). Results presented here correspond to the targeting of nucleotides 229–247. Targeting of nucleotides 286–303 induced similar depletion levels and cellular phenotypes. Note that a dTdT overhang was added to the 3′ of the RNA oligomers. For depletion of γ-tubulin, we used two siRNAs, both targeting the two human isoforms, at nucleotide positions 259–279 and 1257–1281. Maximal depletion was reached by transfecting the second siRNA twice with a 96-h interval and stopping the cells 48 h after the second transfection. However, both induced similar cellular phenotypes. Control (nonsilencing) siRNA was provided by Xeragon (QIAGEN). Mock depletion was performed with oligofectamine but no siRNA. In general, except for the centriole duplication assay where the control siRNA showed a weak effect, we did not observe any phenotypic difference between transfection in the presence of control siRNA or without siRNA.
Antibodies
The NEDD1-His fusion protein corresponding to amino acids 279–660 was expressed in Escherichia coli and affinity purified on Ni-NTA agarose (Invitrogen) under denaturing conditions. The eluted protein was injected into a rabbit for antibody production. The resulting antibody was affinity purified over the same antigen. A rabbit antibody recognizing GCP2 was raised against a synthetic peptide, LRGPPAPAPRVA, corresponding to amino acids 887–898 at the carboxy terminus of the protein. Rabbit anti-GCP2 and anti-GCP4 antibodies (Fava et al., 1999) were affinity purified over the respective full-sized proteins produced in E. coli. Other primary antibodies used in this study were anti–α-tubulin (mouse B-5-1-2 [Sigma-Aldrich] or rabbit [Abcam]), anti–γ-tubulin (mouse GTU-88 [Sigma-Aldrich] or rabbit serum R75 [Julian et al., 1993]), anti-pericentrin (mouse [Dammermann and Merdes, 2002] or rabbit [Covance]), anti-NuMA (mouse Ab-2 [Calbiochem] or rabbit serum [a gift from D. Compton, Dartmouth Medical School, Hanover, NH]), anti-centrin (mouse 20H5; a gift from J. Salisbury, Mayo Clinic, Rochester, MN), and anti–polyglutamylated tubulin (mouse GT335; a gift from B. Eddé, Centre de Recherches de Biochimie Macromoléculaire, Montpellier, France). Different mouse anti-GFP antibodies have been used for immunoprecipitation (mAb 3E6; Invitrogen) and immunodetection (Roche) of the GFP-NEDD1 derivatives.
Microscopical techniques
Cells grown on coverslips were fixed in methanol at −20°C and processed for immunofluorescence following conventional protocols. For detection, secondary antibodies conjugated to Alexa 488 or 568 (Invitrogen) were used. Fluorescence microscopy was performed on a microscope (Axiovert; Carl Zeiss MicroImaging, Inc.) equipped with a Z motor, using 63× or 100× 1.4 NA objectives. Z series images were acquired with a camera (AxioCam MRm; Carl Zeiss MicroImaging, Inc.) and AxioVision software (Carl Zeiss MicroImaging, Inc.). Images were subsequently deconvolved using AxioVision, and z planes were projected onto a single view. Image processing and quantification of fluorescence were done using Photoshop (Adobe). Note that Figs. 4, 5 A, 7 D, and 8 I were not deconvolved and single representative planes are shown. In Figs. 1 (B–E), 2 (C–G), and 5 B, gray-scale images show single representative planes.
For electron microscopy analysis, NEDD1-depleted and control cells were fixed in 0.5% glutaraldehyde in 60 mM Pipes, 25 mM Hepes, 1 mM EGTA, and 2 mM MgCl2, pH 6.9; scraped and pelleted; and subsequently postfixed in 2% osmium tetroxide. After dehydration, cells were embedded in araldite, serially sectioned, and contrasted with uranyl acetate and lead citrate. Pictures were taken with an electron microscope (CM120 Biotwin; Philips).
Coimmunoprecipitation and sucrose gradients
Cells were scraped in the culture medium and rinsed twice in PBS. They were resuspended in IP buffer (50 mM Hepes, pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM MgCl2, and 0.25 mM GTP) with 0.2–0.5% Triton X-100 and a protease inhibitor mix (Sigma-Aldrich). Lysates were prepared after 5 min of incubation on ice by repeated pipetting and centrifugation in a microfuge for 10 min at 12,000 g, at 4°C. For coimmunoprecipitations, appropriate antibodies (2 μg anti-GFP, 25 μg anti-NEDD1, 22 μg anti-NuMA Ab-2, or 10 μl anti–γ-tubulin R75 serum) were immobilized on 50 μl Dynabeads/protein A (Dynal) and incubated with 700 μg of cell extract supernatant for 1 h at 4°C. The immunoprecipitates were washed three times in IP buffer and eluted in 30 μl of gel sample buffer containing SDS. For detection of protein in total extracts, 40 μg of extract was loaded per gel lane. For sedimentation analysis, 750 μg of total cell extracts was fractionated on gradients of 5–40% sucrose in IP buffer, in a SW55Ti rotor (Beckman Coulter) at 55,000 rpm for 3 h. Proteins were detected by immunoblotting using an ECL kit (GE Healthcare). NEDD1 protein levels were determined with a Li-Cor imager (Odyssey).
Microtubule regrowth assay
Cells grown on coverslips were transferred into precooled medium on ice for 1 h and then into prewarmed medium at 37°C. Regrowth was stopped at different time points by methanol fixation.
Centriole duplication assay
Cells grown on coverslips were treated with 1.6 μg/ml aphidicolin (Coger) 12 h after transfection of siRNAs. Cells were fixed 72 h after transfection (i.e., after 60 h of incubation in the presence of the drug) and processed for immunofluorescence.
Online supplemental material
Fig. S1 shows the predicted three-dimensional structure of the NEDD1 WD-repeat domain. Fig. S2 shows that NEDD1 copurifies with the γTuRC. Online supplemental material is available at www.jcb.org/cgi/content/full/jcb.200510028/DC1.
Supplementary Material
Acknowledgments
We would like to thank Drs. Duane Compton, Bernard Eddé, and Jeff Salisbury for the gift of antibodies; Dr. Laurent Mazzolini (Institut de Sciences et Technologies du Médicament de Toulouse, Toulouse, France) for help with NEDD1 cloning; Anne-Marie Cirinesi for help with immunological techniques; and all our colleagues for helpful discussions.
This work was supported by a grant from the Association pour la Recherche sur le Cancer (4X20XP 0230F) and funds from Fonds Européen de Développement Economique Régional, Action Concertée Incitative (045566), Centre National de la Recherche Scientifique, and Laboratories Pierre Fabre.
Note added in proof. During revision of this article, characterization of NEDD1 was independently described by another group (Luders, J., U.K. Patel, and T. Stearns. 2005. Nat. Cell. Biol. 10.1038/ncb1349).
L. Haren and M.-H. Remy contributed equally to this paper.
Abbreviations used in this paper: γTuRC, γ-tubulin ring complex; γTuSC, γ-tubulin small complex; GCP, γ-complex protein; NuMA, nuclear mitotic apparatus protein; RNAi, RNA interference; siRNA, small interfering RNA.
References
- Andersen, J.S., C.J. Wilkinson, T. Mayor, P. Mortensen, E.A. Nigg, and M. Mann. 2003. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 426:570–574. [DOI] [PubMed] [Google Scholar]
- Balczon, R., L. Bao, W.E. Zimmer, K. Brown, R.P. Zinkowski, and B.R. Brinkley. 1995. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J. Cell Biol. 130:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa, V., R.R. Yamamoto, D.S. Henderson, and D.M. Glover. 2000. Mutation of a Drosophila gamma tubulin ring complex subunit encoded by discs degenerate-4 differentially disrupts centrosomal protein localization. Genes Dev. 14:3126–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa, V., M. Gatt, E. Rebollo, C. Gonzalez, and D.M. Glover. 2003. Drosophila dd4 mutants reveal that gammaTuRC is required to maintain juxtaposed half spindles in spermatocytes. J. Cell Sci. 116:929–941. [DOI] [PubMed] [Google Scholar]
- Callebaut, I., G. Labesse, P. Durand, A. Poupon, L. Canard, J. Chomilier, B. Henrissat, and J.P. Mornon. 1997. Deciphering protein sequence information through hydrophobic cluster analysis (HCA): current status and perspectives. Cell. Mol. Life Sci. 53:621–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casenghi, M., P. Meraldi, U. Weinhart, P.I. Duncan, R. Korner, and E.A. Nigg. 2003. Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev. Cell. 5:113–125. [DOI] [PubMed] [Google Scholar]
- Chang, P., T.H. Jr. Giddings, M. Winey, and T. Stearns. 2003. Epsilon-tubulin is required for centriole duplication and microtubule organization. Nat. Cell Biol. 5:71–76. [DOI] [PubMed] [Google Scholar]
- Dammermann, A., and A. Merdes. 2002. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 159:255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann, A., T. Muller-Reichert, L. Pelletier, B. Habermann, A. Desai, and K. Oegema. 2004. Centriole assembly requires both centriolar and pericentriolar material proteins. Dev. Cell. 7:815–829. [DOI] [PubMed] [Google Scholar]
- Delattre, M., S. Leidel, K. Wani, K. Baumer, J. Bamat, H. Schnabel, R. Feichtinger, R. Schnabel, and P. Gonczy. 2004. Centriolar SAS-5 is required for centrosome duplication in C. elegans. Nat. Cell Biol. 6:656–664. [DOI] [PubMed] [Google Scholar]
- Delgehyr, N., J. Sillibourne, and M. Bornens. 2005. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 118:1565–1575. [DOI] [PubMed] [Google Scholar]
- Fava, F., B. Raynaud-Messina, J. Leung-Tack, L. Mazzolini, M. Li, J.C. Guillemot, D. Cachot, Y. Tollon, P. Ferrara, and M. Wright. 1999. Human 76p: a new member of the γ-tubulin–associated protein family. J. Cell Biol. 147:857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, S.D., B.E. Gowen, S. Reinsch, A. Sawyer, B. Buendia, R. Wepf, and E. Karsenti. 1995. The core of the mammalian centriole contains gamma-tubulin. Curr. Biol. 5:1384–1393. [DOI] [PubMed] [Google Scholar]
- Ganem, N.J., and D.A. Compton. 2004. The KinI kinesin Kif2a is required for bipolar spindle assembly through a functional relationship with MCAK. J. Cell Biol. 166:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane, R.N., O.C. Martin, K. Cao, L. Zhang, K. Dej, A. Iwamatsu, and Y. Zheng. 2000. Characterization and reconstitution of Drosophila γ-tubulin ring complex subunits. J. Cell Biol. 151:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane, R.N., O.C. Martin, and Y. Zheng. 2003. Characterization of a new gammaTuRC subunit with WD repeats. Mol. Biol. Cell. 14:1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habedanck, R., Y.D. Stierhof, C.J. Wilkinson, and E.A. Nigg. 2005. The polo kinase Plk4 functions in centriole duplication. Nat. Cell Biol. 7:1140–1146. [DOI] [PubMed] [Google Scholar]
- Hamill, D.R., A.F. Severson, J.C. Carter, and B. Bowerman. 2002. Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev. Cell. 3:673–684. [DOI] [PubMed] [Google Scholar]
- Hannak, E., M. Kirkham, A.A. Hyman, and K. Oegema. 2001. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 155:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak, E., K. Oegema, M. Kirkham, P. Gonczy, B. Habermann, and A.A. Hyman. 2002. The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is γ-tubulin dependent. J. Cell Biol. 157:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian, M., Y. Tollon, I. Lajoie-Mazenc, A. Moisand, H. Mazarguil, A. Puget, and M. Wright. 1993. Gamma-tubulin participates in the formation of the midbody during cytokinesis in mammalian cells. J. Cell Sci. 105:145–156. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, S., and Y. Zheng. 2004. Characterization of a Drosophila centrosome protein CP309 that shares homology with kendrin and CG-NAP. Mol. Biol. Cell. 15:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, C.A., K.R. Kopish, P. Zipperlen, J. Ahringer, and K.F. O'Connell. 2004. Centrosome maturation and duplication in C. elegans require the coiled-coil protein SPD-2. Dev. Cell. 6:511–523. [DOI] [PubMed] [Google Scholar]
- Khodjakov, A., and C.L. Rieder. 1999. The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov, A., R.W. Cole, B.R. Oakley, and C.L. Rieder. 2000. Centrosome-independent mitotic spindle formation in vertebrates. Curr. Biol. 10:59–67. [DOI] [PubMed] [Google Scholar]
- Kim, H.K., J.G. Kang, S. Yumura, C.J. Walsh, J.W. Cho, and J. Lee. 2005. De novo formation of basal bodies in Naegleria gruberi: regulation by phosphorylation. J. Cell Biol. 169:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham, M., T. Muller-Reichert, K. Oegema, S. Grill, and A.A. Hyman. 2003. SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell. 112:575–587. [DOI] [PubMed] [Google Scholar]
- Klotz, C., F. Ruiz, N. Garreau de Loubresse, M. Wright, P. Dupuis-Williams, and J. Beisson. 2003. Gamma-tubulin and MTOCs in Paramecium. Protist. 154:193–209. [DOI] [PubMed] [Google Scholar]
- Knop, M., G. Pereira, S. Geissler, K. Grein, and E. Schiebel. 1997. The spindle pole body component Spc97p interacts with the gamma-tubulin of Saccharomyces cerevisiae and functions in microtubule organization and spindle pole body duplication. EMBO J. 16:1550–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S., Y. Tomooka, and M. Noda. 1992. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem. Biophys. Res. Commun. 185:1155–1161. [DOI] [PubMed] [Google Scholar]
- Kumar, S., T. Matsuzaki, Y. Yoshida, and M. Noda. 1994. Molecular cloning and biological activity of a novel developmentally regulated gene encoding a protein with beta-transducin-like structure. J. Biol. Chem. 269:11318–11326. [PubMed] [Google Scholar]
- Lajoie-Mazenc, I., Y. Tollon, C. Detraves, M. Julian, A. Moisand, C. Gueth-Hallonet, A. Debec, I. Salles-Passador, A. Puget, H. Mazarguil, et al. 1994. Recruitment of antigenic gamma-tubulin during mitosis in animal cells: presence of gamma-tubulin in the mitotic spindle. J. Cell Sci. 107:2825–2837. [DOI] [PubMed] [Google Scholar]
- Leidel, S., and P. Gonczy. 2003. SAS-4 is essential for centrosome duplication in C. elegans and is recruited to daughter centrioles once per cell cycle. Dev. Cell. 4:431–439. [DOI] [PubMed] [Google Scholar]
- Leidel, S., M. Delattre, L. Cerutti, K. Baumer, and P. Gonczy. 2005. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat. Cell Biol. 7:115–125. [DOI] [PubMed] [Google Scholar]
- Martin, O.C., R.N. Gunawardane, A. Iwamatsu, and Y. Zheng. 1998. Xgrip109: a γ tubulin–associated protein with an essential role in γ tubulin ring complex (γTuRC) assembly and centrosome function. J. Cell Biol. 141:675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen, M.M., A. Malik, M. Piel, V. Bouckson-Castaing, and M. Bornens. 2000. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J. Cell Sci. 113:3013–3023. [DOI] [PubMed] [Google Scholar]
- Moudjou, M., N. Bordes, M. Paintrand, and M. Bornens. 1996. Gamma-tubulin in mammalian cells: the centrosomal and the cytosolic forms. J. Cell Sci. 109:875–887. [DOI] [PubMed] [Google Scholar]
- Murphy, S.M., L. Urbani, and T. Stearns. 1998. The mammalian γ-tubulin complex contains homologues of the yeast spindle pole body components spc97p and spc98p. J. Cell Biol. 141:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S.M., A.M. Preble, U.K. Patel, K.L. O'Connell, D.P. Dias, M. Moritz, D. Agard, J.T. Stults, and T. Stearns. 2001. GCP5 and GCP6: two new members of the human gamma-tubulin complex. Mol. Biol. Cell. 12:3340–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema, K., C. Wiese, O.C. Martin, R.A. Milligan, A. Iwamatsu, T.J. Mitchison, and Y. Zheng. 1999. Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 144:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier, L., N. Ozlu, E. Hannak, C. Cowan, B. Habermann, M. Ruer, T. Muller-Reichert, and A.A. Hyman. 2004. The Caenorhabditis elegans centrosomal protein SPD-2 is required for both pericentriolar material recruitment and centriole duplication. Curr. Biol. 14:863–873. [DOI] [PubMed] [Google Scholar]
- Raynaud-Messina, B., L. Mazzolini, A. Moisand, A.M. Cirinesi, and M. Wright. 2004. Elongation of centriolar microtubule triplets contributes to the formation of the mitotic spindle in gamma-tubulin-depleted cells. J. Cell Sci. 117:5497–5507. [DOI] [PubMed] [Google Scholar]
- Ruiz, F., J. Beisson, J. Rossier, and P. Dupuis-Williams. 1999. Basal body duplication in Paramecium requires gamma-tubulin. Curr. Biol. 9:43–46. [DOI] [PubMed] [Google Scholar]
- Salisbury, J.L., K.M. Suino, R. Busby, and M. Springett. 2002. Centrin-2 is required for centriole duplication in mammalian cells. Curr. Biol. 12:1287–1292. [DOI] [PubMed] [Google Scholar]
- Samejima, I., P.C. Lourenco, H.A. Snaith, and K.E. Sawin. 2005. Fission yeast mto2p regulates microtubule nucleation by the centrosomin-related protein mto1p. Mol. Biol. Cell. 16:3040–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin, K.E., P.C. Lourenco, and H.A. Snaith. 2004. Microtubule nucleation at non-spindle pole body microtubule-organizing centers requires fission yeast centrosomin-related protein mod20p. Curr. Biol. 14:763–775. [DOI] [PubMed] [Google Scholar]
- Shang, Y., B. Li, and M.A. Gorovsky. 2002. Tetrahymena thermophila contains a conventional γ-tubulin that is differentially required for the maintenance of different microtubule-organizing centers. J. Cell Biol. 158:1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starita, L.M., Y. Machida, S. Sankaran, J.E. Elias, K. Griffin, B.P. Schlegel, S.P. Gygi, and J.D. Parvin. 2004. BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number. Mol. Cell. Biol. 24:8457–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S., J. Powers, M. Dunn, K. Reese, C.J. Malone, J. White, G. Seydoux, and W. Saxton. 2001. Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos. Mol. Biol. Cell. 12:1751–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkel, C.E., R. Gomes, P. Sampaio, J. Perdigao, and C. Gonzalez. 1995. Gamma-tubulin is required for the structure and function of the microtubule organizing centre in Drosophila neuroblasts. EMBO J. 14:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M., A. Yamagiwa, T. Nishimura, H. Mukai, and Y. Ono. 2002. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol. Biol. Cell. 13:3235–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin, A.M., C. Celati, M. Moudjou, and M. Bornens. 1998. Characterization of the human homologue of the yeast spc98p and its association with γ-tubulin. J. Cell Biol. 141:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada, Y., Y. Uetake, and R. Kuriyama. 2003. Interaction of Aurora-A and centrosomin at the microtubule-nucleating site in Drosophila and mammalian cells. J. Cell Biol. 162:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuba-Kubo, A., A. Kubo, M. Hata, and S. Tsukita. 2005. Gene knockout analysis of two gamma-tubulin isoforms in mice. Dev. Biol. 282:361–373. [DOI] [PubMed] [Google Scholar]
- Zhang, L., T.J. Keating, A. Wilde, G.G. Borisy, and Y. Zheng. 2000. The role of Xgrip210 in γ-tubulin ring complex assembly and centrosome recruitment. J. Cell Biol. 151:1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y., M.K. Jung, and B.R. Oakley. 1991. Gamma-tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell. 65:817–823. [DOI] [PubMed] [Google Scholar]
- Zimmerman, W.C., J. Sillibourne, J. Rosa, and S.J. Doxsey. 2004. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell. 15:3642–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.