Abstract
Zip code–binding protein 1 (ZBP-1) and its Xenopus laevis homologue, Vg1 RNA and endoplasmic reticulum–associated protein (VERA)/Vg1 RNA-binding protein (RBP), bind repeated motifs in the 3′ untranslated regions (UTRs) of localized mRNAs. Although these motifs are required for RNA localization, the necessity of ZBP-1/VERA remains unresolved. We address the role of ZBP-1/VERA through analysis of the Drosophila melanogaster homologue insulin growth factor II mRNA–binding protein (IMP). Using systematic evolution of ligands by exponential enrichment, we identified the IMP-binding element (IBE) UUUAY, a motif that occurs 13 times in the oskar 3′UTR. IMP colocalizes with oskar mRNA at the oocyte posterior, and this depends on the IBEs. Furthermore, mutation of all, or subsets of, the IBEs prevents oskar mRNA translation and anchoring at the posterior. However, oocytes lacking IMP localize and translate oskar mRNA normally, illustrating that one cannot necessarily infer the function of an RBP from mutations in its binding sites. Thus, the translational activation of oskar mRNA must depend on the binding of another factor to the IBEs, and IMP may serve a different purpose, such as masking IBEs in RNAs where they occur by chance. Our findings establish a parallel requirement for IBEs in the regulation of localized maternal mRNAs in D. melanogaster and X. laevis.
Introduction
mRNA localization is a commonly used intracellular trafficking mechanism that provides the means to restrict the translation of specific proteins to discrete cytoplasmic regions (for review see St Johnston, 2005). Localized mRNAs are directed to their destinations by cis-acting localization elements (LEs) that generally reside in the transcript's 3′ untranslated region (UTR). These elements must be recognized by specific RNA-binding proteins (RBPs) that link the mRNA to the localization machinery. However, this has only been clearly established in yeast, where four stem-loops in Ash1 mRNA are recognized by She2p, which links the mRNA to the myosin Myo4p through She3p (Gonzalez et al., 1999; Bohl et al., 2000; Long et al., 2000; Takizawa and Vale, 2000).
Apart from yeast, a definitive relationship has not been established between a localization signal, its cognate RBP, and the protein's function. Although genetic screens indicate that RBPs such as Staufen (Stau; St Johnston et al., 1991), HRP48 (Huynh et al., 2004), and Squid (Norvell et al., 1999) are required for localizing particular mRNAs, the elements that these proteins recognize are not well defined. On the other hand, RBPs such as hnRNPI (Cote et al., 1999), 40LoVe (Czaplinski et al., 2005), hnRNPA2 (Hoek et al., 1998), and VgRBP71 and Prrp (Zhao et al., 2001; Kroll et al., 2002) bind specific LEs, but in these cases, it remains to be conclusively proven that the protein is actually responsible for localizing the RNA.
One of the best candidates for an RBP that plays a direct role in mRNA transport is chicken and rat zip code–binding protein 1 (ZBP-1), as well as its Xenopus laevis homologue Vg1 RNA and endoplasmic reticulum–associated protein (VERA)/Vg1 RNA-binding protein (RBP), because it is highly conserved and binds to the localization signals of several different localized mRNAs (Ross et al., 1997; Deshler et al., 1998; Havin et al., 1998). ZBP-1 was first identified because it binds specifically to a 54-nt LE in chicken β-actin mRNA, called the zip code, and colocalizes with actin mRNA in the leading lamellae of motile fibroblasts (Kislauskis et al., 1994; Ross et al., 1997). Several lines of evidence support the hypothesis that this interaction is important for β-actin mRNA localization. The overexpression of a truncated version of ZBP-1 reduces the proportion of cells in which the RNA is localized, and the introduction of ZBP-1 into cells that do not express it can induce β-actin mRNA localization (Farina et al., 2003; Oleynikov and Singer, 2003). ZBP-1 colocalizes with β-actin mRNA in the growth cones and dendrites of cultured neurons, and both the localization of the mRNA and its colocalization with ZBP-1 are reduced by antisense oligonucleotides directed against either the zip code or ZBP-1 RNA (Zhang et al., 2001; Eom et al., 2003; Tiruchinapalli et al., 2003).
The X. laevis ZBP-1 homologue VERA/Vg1RBP was identified through its binding to the Vg1LE (Deshler et al., 1997, 1998; Havin et al., 1998). VERA/Vg1RBP recognizes a motif, UUCAC (called E2), which is repeated in the Vg1LE, where it is required for the RNA's localization to the vegetal pole of the oocyte. The same E2 motif occurs five times in the VegTLE, and these sites are likewise required for the accumulation of VegT mRNA at the vegetal pole (Bubunenko et al., 2002; Kwon et al., 2002). Consistent with a role for VERA binding, the injection of anti-VERA antibodies inhibits the localization of both Vg1 and VegT mRNAs by 50% (Kwon et al., 2002).
Although there is convincing evidence that mRNA localization requires the motifs recognized by VERA and ZBP-1, it is much harder in these experimental systems to demonstrate conclusively that the proteins themselves are required. Anti-sense treatments, antibody injections, and dominant-negative constructs against ZBP-1/VERA appear to inhibit RNA localization, but the effects are partial and variable (Kwon et al., 2002; Eom et al., 2003; Farina et al., 2003). Therefore, we have addressed whether the ZBP-1/VERA orthologue, insulin-like growth factor II mRNA–binding protein (IMP), is required for RNA localization in Drosphila melanogaster, where it is possible to evaluate mRNA localization in mutants that lack the protein completely.
One of the best systems to examine mRNA localization in D. melanogaster is in the oocyte, where the localizations of bicoid (bcd), oskar (osk), gurken (grk), and nanos (nos) mRNAs define the anterior–posterior and dorsal–ventral axes of the embryo (St Johnston et al., 1989; Ephrussi et al., 1991; Kim-Ha et al., 1991; Gavis and Lehmann, 1992; Neuman-Silberberg and Schupbach, 1993). The most relevant to our study is osk mRNA, which localizes to the oocyte posterior pole. Once there, Osk protein nucleates assembly of the polar granules, which contain the posterior determinant nos mRNA, as well as the germline determinants (Ephrussi et al., 1991; Kim-Ha et al., 1991; Ephrussi and Lehmann, 1992). osk RNA accumulates in the oocyte from early oogenesis onwards, localizes transiently to the anterior at stage 8, and then translocates to the posterior pole over a period of several hours during stages 8–9 (Ephrussi et al., 1991; Kim-Ha et al., 1993). Posterior localization involves two substeps, initial transport and long-term anchoring (Rongo and Lehmann, 1996). osk mRNA anchoring requires Osk protein, whose synthesis is triggered upon the RNA's arrival at the posterior pole (Markussen et al., 1995; Rongo and Lehmann, 1996; Gunkel et al., 1998; Vanzo and Ephrussi, 2002).
Premature translation of oskar mRNA produces a bicaudal phenotype in which an ectopic abdomen develops in place of the head and thorax, illustrating the importance of restricting translation to the posterior pole (Ephrussi et al., 1991; Smith et al., 1992). This is achieved by repressing the translation of unlocalized mRNA and relieving this repression once the mRNA reaches the posterior pole (Kim-Ha et al., 1995; Gunkel et al., 1998). Many gene products are required for repressing the translation of unlocalized osk RNA (Wilhelm and Smibert, 2005). In contrast to repression, very little is understood about the localization-dependent translational activation of osk, other than the potential involvement of the Aubergine, Orb, and Stau proteins and the requirement of sequences at the 5′ end of the mRNA (Wilson et al., 1996; Gunkel et al., 1998; Chang et al., 1999).
We have addressed whether the D. melanogaster ZBP/VERA homologue IMP is required for maternal mRNA localization in the oocyte. Upon finding that IMP localizes at the posterior with osk mRNA, we focused our analysis on the role of the protein and its binding sites in the regulation of osk mRNA localization and translation.
Results
IMP localization within the oocyte coincides with and depends on osk RNA
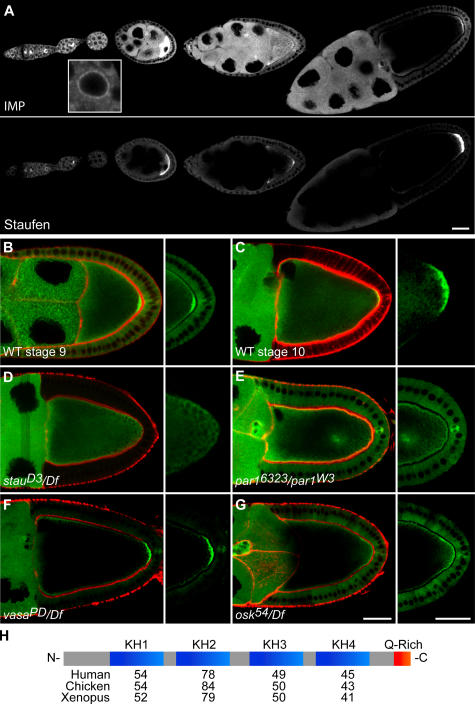
IMP contains the four signature KH-type RNA-binding domains and the glutamine-rich COOH terminus (Fig. 1 H) that are present in the vertebrate orthologues (Nielsen et al., 2000; Git and Standart, 2002). Affinity-purified antibodies against IMP reveal the protein in nurse cells and the oocyte early in oogenesis. However, the high concentration of IMP in the follicle cells blocks the penetration of the antibody into the oocyte after stage 4. Therefore, we evaluated IMP localization in a homozygous, viable, and fertile GFP–IMP protein trap line (Morin et al., 2001). GFP–IMP is enriched around the nurse cell nuclei (Fig. 1 A, inset) and accumulates in the oocyte as soon as it is specified in the germarium, where it shows a uniform distribution until stage 7 (Fig. 1 A). IMP accumulates transiently at the anterior of the oocyte during stages 8–9 and then localizes in a crescent at the posterior pole at stage 9, where it remains for the duration of oogenesis (Fig. 1, A–C). This pattern of localization is very similar to that observed for osk mRNA and Stau protein, which colocalize with IMP throughout oogenesis (Fig. 1 A; Ephrussi et al., 1991; Kim-Ha et al., 1991; St Johnston et al., 1991).
Figure 1.
IMP localization during oogenesis. (A–G) Localization of IMP during oogenesis visualized using G080, a GFP protein trap line (A, B, and E–G), or a GFP–IMP fusion construct specifically expressed in the germline (C and D). (A) IMP localizes to future oocyte in the germarium, accumulates in the oocyte through stage 7–8, appears enriched at both the anterior and posterior of the oocyte at stage 8, and is restricted to a crescent at the oocyte posterior pole by stage 9–10. These localizations are similar to those of Staufen protein, which marks the localization of osk mRNA. Within nurse cells, IMP is primarily cytoplasmic, but also rims the nucleus (inset). (B–G) IMP localization (green) and actin (red) in oocytes at stage 9 (B) or 10 (C) in WT and mutant backgrounds (D–G). (D) IMP is absent from the posterior crescent in a stau-null mutant that fails to localize osk RNA. (E) Like osk RNA (not depicted), IMP localizes ectopically in a transheterozygous par-1 allele combination that disrupts the polarity of the egg chamber. (F) IMP localizes normally in a vasa mutant that interferes with pole plasm formation. (G) IMP localizes to the posterior at stage 9 in an osk nonsense mutant (osk 54/Df(3R)pXT103) egg chamber, indicating its localization depends on osk RNA, not protein. (H) IMP contains four KH-type RNA-binding domains and a glutamine-rich COOH terminus. Numbers indicate the percentage of amino acid identity between IMP's KH domains and those of its homologues. Bars, 25 μm.
To ascertain whether IMP localization depends on osk, we examined whether it is perturbed in various mutants that affect the posterior accumulation of osk mRNA and protein. IMP does not localize to the posterior of the oocyte in staufen, barentsz, and hrp48 mutants, which block the transport of oskar mRNA to the posterior pole (Fig. 1 D and not depicted; Ephrussi et al., 1991; Kim-Ha et al., 1991; St Johnston et al., 1991). Furthermore, IMP colocalizes with osk RNA to an ectopic dot in the center of the oocyte in a par-1 mutant that disrupts microtubule polarity (Fig. 1 E; Shulman et al., 2000). Together, these results demonstrate that the localization of IMP to the oocyte posterior pole requires the localization of osk mRNA.
IMP could localize to the posterior through a direct interaction with osk mRNA or protein or could be recruited to the posterior by a downstream component of the pole plasm. To distinguish between these possibilities, we examined IMP localization in a strong vasa hypomorph (vasaPD/Df(2L) TW2), which prevents the posterior recruitment of Vasa by Osk and disrupts all subsequent steps in pole plasm assembly (Hay et al., 1990; Lasko and Ashburner, 1990; Breitwieser et al., 1996). IMP localizes normally to the posterior of these oocytes (Fig. 1 F), suggesting that its posterior accumulation depends on osk directly. Finally, we addressed whether IMP localization depends on Osk protein rather than osk mRNA by examining a nonsense mutation (osk54/Df) that disrupts the anchoring, but not the initial localization, of osk mRNA (Ephrussi et al., 1991; Kim-Ha et al., 1991; Markussen et al., 1995; Rongo et al., 1995). IMP still localizes to the posterior of these oocytes at stage 9, but the posterior crescent is weaker than in wild type (WT) and disappears at stage 10 (Fig. 1 G). Thus, IMP behaves like osk mRNA in every mutant combination examined, suggesting that it localizes to the posterior in association with the mRNA.
Identification of IMP's RNA targets using systematic evolution of ligands by exponential enrichment (SELEX)
KH domains recognize short, single-stranded RNA motifs (Lewis et al., 1999, 2000; Jensen et al., 2000) similar to the motifs that are required for localization of RNAs in chicken embryo fibroblasts (Kislauskis et al., 1993; Farina et al., 2003) and X. laevis oocytes (Deshler et al., 1997; Kwon et al., 2002; Lewis et al., 2004). To identify the motifs recognized by IMP, we performed in vitro selection experiments on a large pool of ∼7 × 1014 RNAs containing random 25-nt sequences. Because we were unable to obtain the first and second KH domains of IMP as soluble proteins, we selected RNAs that bind to the third and fourth KH domains. This seemed justified, as the vertebrate homologue ZBP-1 binds the β-actin zip code primarily through its third and fourth KH domains (Farina et al., 2003).
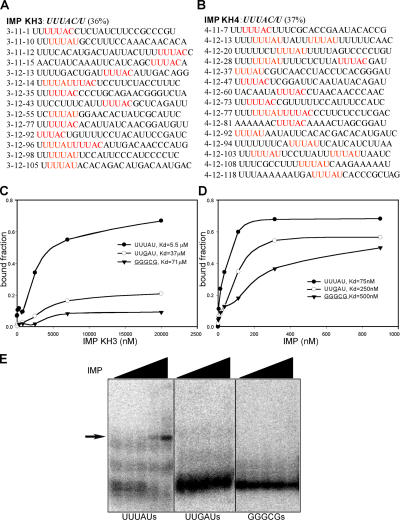
The structural basis of RNA recognition by KH domains was established through biochemical and x-ray diffraction studies of the KH domains from another protein, NOVA (Jensen et al., 2000; Lewis et al., 2000). Those studies used 11 rounds of in vitro selection against their isolated KH domain to identify its preferred recognition element, which is a particular sequence of four bases. On this basis, we chose to evaluate the 11th and 12th round “winner sequences” selected by the IMP KH domains in respect to the frequency of all tetramers. The most common tetramer retained by either KH3 or KH4 was UUUA, which occurred in 43% of the winning KH3 sequences and 46% of the KH4 winners. The base preferred by the IMP KH domains after the principal tetranucleotide was most frequently C (35%) or U (32%). Thus, SELEX indicates that the optimal binding sequence for both IMP KH3 and KH4 is UUUAY, which was present in 36% of clones bound by KH3 and 37% of clones bound by KH4 (Fig. 2 A).
Figure 2.
Characterization of the IBE UUUAY. (A and B) Representative RNA sequences selected after 12 rounds of SELEX against IMP's KH3 (A) and KH4 (B) domains. IBEs are in red. (C and D) Filter-binding assays between three tandem repeats of the winner sequence 4-12-13 (or the same RNA with IBEs mutated to UUgAU or gggcg) and either IMP KH3 (C) or the entire protein (D). (E) Electrophoretic mobility shift assay between IMP and three tandem copies of the winner sequence 4-12-13 containing either WT or mutant IBE motifs. Only RNAs with the WT (UUUAU) motifs induced a band shift.
To quantify the binding of KH3 and full-length IMP to UUUAY-containing RNA, we performed filter-binding assays using three tandem repeats of the 25-nt winner RNA, 4-12-13 (Fig. 2, C and D). When all five nucleotides of the motif are changed to GGGCG, the affinity of the RNA for the KH3 domain diminishes by an order of magnitude; and even a single nucleotide change, UUUAY to UUgAY, decreases the affinity significantly (Fig. 2 C). Full-length IMP binds to the UUUAY-containing RNA with an even higher affinity, and the mutations in the motif decrease binding to a similar extent to that observed for the single KH domains (Fig. 2 D). Electrophoretic mobility shift assays confirm the results of filter-binding assays; IMP shifts the mobility of RNAs with UUUAY, but not the mutant motifs (Fig. 2 E). These results indicate that IMP's KH domains 3 and 4 specifically recognize the UUUAY motif, which we refer to as an IMP-binding element (IBE).
IMP binds specifically to repeated IBEs in the osk 3′UTR
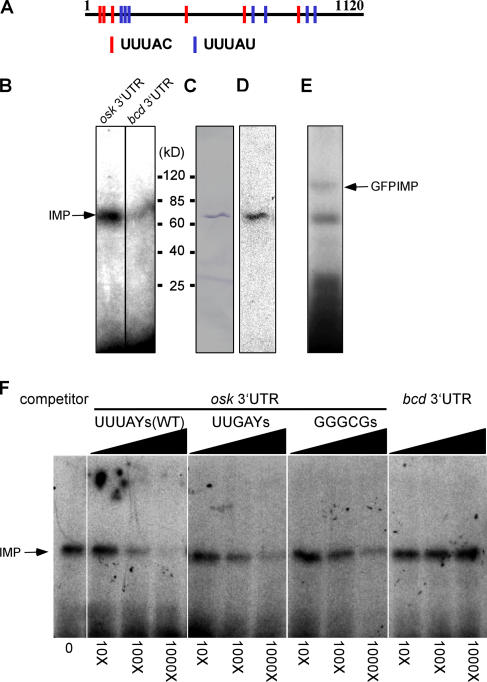
The IBE motif occurs 13 times in the 3′UTR of osk mRNA (Fig. 3 A), which is significantly more frequent than would be expected by chance. This contrasts with the 3′UTRs of other localized mRNAs, such as bicoid, which contains only two copies of the motif. Indeed, osk mRNA associates specifically with IMP in vivo because it coimmunopreciptitates with IMP from ovary extracts, whereas bicoid mRNA does not (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200510044/DC1).
Figure 3.
The osk 3′UTR contains 13 IBEs and UV cross-links to IMP. (A) Positions of the 13 IBEs in the osk 3′UTR. (B) A 65-kD protein (IMP) in D. melanogaster ovary extracts specifically cross-links the 1,120-nt osk 3′UTR, but not the 817-nt bcd 3′UTR, which contains only two UUUAY motifs. (C and D) Anti-IMP immunoblot of ovary extract (C) labels the same band that UV cross-links to the 32P-osk 3′UTR (D). (E) 32P-osk 3′UTR cross-links to an ∼95-kD polypeptide (GFP–IMP) in embryo extracts of the protein trap line G080. (F) Cross-linking reactions between the 32P-osk 3′UTR and oocyte extracts in the presence of increasing concentrations of cold, competitor RNAs, including WT and mutant osk 3′UTRs and the bicoid 3′UTR.
To characterize the interaction between IMP and the osk 3′UTR, we performed UV cross-linking assays with ovary extracts and a 32P-labeled RNA probe of the osk 3′UTR (Fig. 3, B–E). Using a procedure that optimized the binding of VERA/Vg1RBP to the LEs of Vg1 and VegT in X. laevis oocyte extracts (Deshler et al., 1997), we found that a single 65-kD polypeptide cross-links to the osk 3′UTR, but not to the bcd 3′UTR. This polypeptide co-migrates with the band detected by anti-IMP antibodies on immunoblots (Fig. 3, B–D). Similar cross-linking experiments, using extracts from embryos expressing the GFP–IMP fusion protein, labeled a second polypeptide, whose slower mobility corresponds to that expected of the GFP–IMP fusion (Fig. 3 E). This confirms that the protein cross-linked in the experiments is IMP.
To address whether the binding of IMP to the osk 3′UTR depends on the IBEs, we mutated all 13 copies of the motif to UUgAY or gggcg. Both mutant osk RNAs are significantly impaired in their ability to complete UV cross-linking of the WT osk 3′UTR to IMP in ovary extracts (Fig. 3 F). The predicted secondary structures (Mathews et al., 1999) of the WT and UUgAY osk 3′UTRs are virtually identical, suggesting that the single-base substitutions in osk's IBEs inhibit IMP binding not through a nonspecific effect on the RNA's folding, but instead through abrogation of sequence-selective binding of the IBEs by IMP's KH domains 3 and 4. The very specific effects of IBE base substitutions on osk RNA localization and translation provide additional, much stronger, evidence that the mutations do not affect RNA folding significantly.
Posterior IMP localization depends on the IBEs in the osk 3′UTR
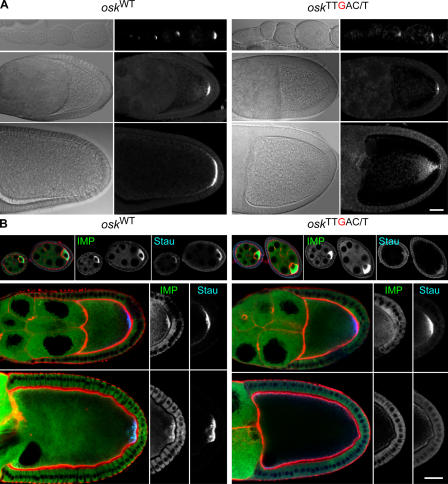
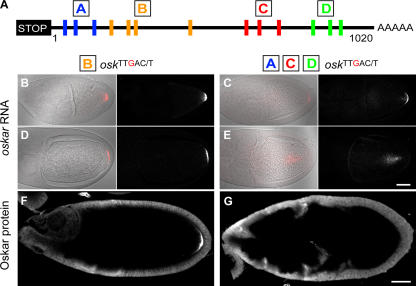
To address whether the IBEs in the osk 3′UTR are required for the posterior localization of IMP, we created transgenic lines in which all 13 copies of the IBE are mutated from UUUAC/U to UUUgC/U (osk 13 TTgAY) in an otherwise WT genomic osk fragment. Because we wanted to avoid the complication of mutant transgenic osk mRNAs localizing to the posterior by hitchhiking on the endogenous WT mRNA (Hachet and Ephrussi, 2004), we introduced this transgene, or a control unmutated transgene osk 13TTTAY, into an osk RNA-null background (oskA87/oskDf(3R)pXT103; unpublished data). Both the osk 13TTgAY and the control unmutated transgene (osk 13TTTAY) rescue the stage 6 oocyte-arrest phenotype of osk RNA-null flies completely (unpublished data). Through stage 9, the distribution of the mutant osk 13TTgAY mRNA is comparable to that of endogenous WT osk mRNA (Fig. 4 A). Thus, the IBEs are not necessary for osk mRNA's initial transport to the posterior pole.
Figure 4.
osk mRNA, IMP, and Stau distributions in osk13 TTgAY flies. (A) Fluorescent in situ hybridizations comparing WT and mutant transgenic osk RNAs in flies that otherwise lack endogenous osk RNA. Mutant osk RNA localizes normally through stage 9 (middle), but by stage 10 (bottom), is evident as diffuse fluorescence fanning out from the posterior pole. (B) IMP (green) and Stau (blue) proteins in WT and mutant osk oocytes before stage 9 (top), at stage 9 (middle), and at stage 10 (bottom). Actin is visualized with rhodamine-phalloidin (red). At stage 9, Stau is localized normally at the posterior pole in oocytes that express the mutant osk transgene, whereas IMP is diffuse and not concentrated at the posterior. Both Stau and IMP are missing from the posterior pole in stage 10 oocytes that express the mutant osk RNA. Bars, 25 μm.
To examine IMP localization, we introduced the GFP– IMP protein trap into oskA87/oskDf(3R)pXT103 flies carrying the osk 13 TTgAY or osk 13TTTAY transgenes. Whereas GFP–IMP colocalizes with endogenous Stau at the posterior of oocytes containing the WT osk 13TTTAY transgene, it never accumulates at the posterior of oocytes expressing osk 13 TTgAY mRNA, although Stau still localizes normally (Fig. 4 B). The IBEs in the osk 3′UTR are therefore essential for the posterior localization of IMP, confirming that these UUUAY motifs are bona fide IMP-binding sites in vivo.
Although the localization of osk 13 TTgAY mRNA is similar to that of the control osk 13TTTAY mRNA until the end of stage 9, the mutant mRNA disappears from the posterior at stage 10 b (Fig. 4 A). Furthermore, Stau protein displays an identical phenotype; it forms a WT posterior crescent at stage 9 and then disappears from the posterior at stage 10 b (Fig. 4 B). The IBEs in the osk 3′UTR are therefore necessary for the anchoring of osk mRNA at the posterior cortex.
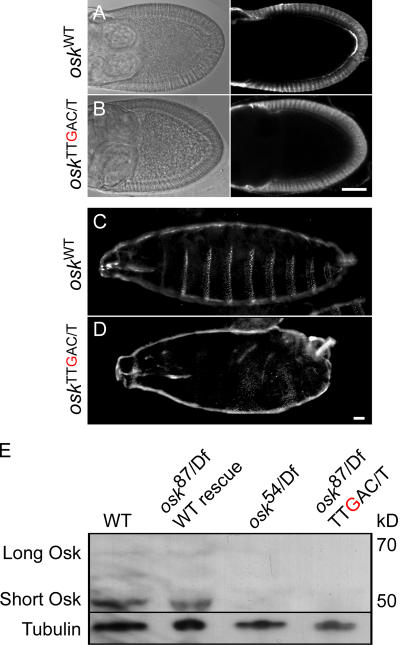
The osk IBEs are required for the translational activation of osk mRNA
The failure to maintain osk 13 TTgAY mRNA at the posterior at stage 10 could reflect a direct role for the IBEs in the anchoring of the mRNA. However, the maintenance of osk mRNA at the posterior requires Osk protein, which is only translated once the mRNA has been localized (Gunkel et al., 1998). Thus, an alternative possibility is that the IBEs are required for the activation of osk mRNA translation at the posterior, and that the anchoring defect is secondary to a lack of Osk protein. To address the effect of the IBE mutations on Osk protein synthesis, we stained oskA87/oskDf(3R)pXT103; osk 13 TTgAY or osk 13TTTAY ovaries with an anti-Osk antibody. The mRNA from a single copy of the WT osk transgene produces a robust posterior crescent of Osk protein from stage 9 onwards, whereas no Osk protein can be detected at any stage in the lines expressing osk 13 TTgAY mRNA (Fig. 5, A and B). Thus, the IBEs are essential for the derepression of osk mRNA translation at the posterior pole. The embryos from oskA87/oskDf(3R)pXT103; osk 13 TTgAY mothers display a fully penetrant osk maternal-effect phenotype in which the abdomen fails to form, consistent with the failure to translate Osk protein (Fig. 5, C and D). The absence of Osk protein was further confirmed by Western blot of ovarian extracts from oskA87/oskDf(3R)pXT103 flies that express either the WT or osk 13 TTgAY transgene (Fig. 5 E).
Figure 5.
IBE mutations abolish osk RNA translational activation. (A and B) Anti-Osk immunostaining of osk 87/Df(3R)pXT103 egg chambers expressing a WT osk transgene, showing a crescent of Osk protein (A). Osk protein is missing in egg chambers from osk TT g AY flies (B). (C and D) Cuticle preparations of larvae from osk 87/Df(3R)pXT103 that express the WT (C) or mutant osk TTgAY (D) transgene. (E) Western blot of ovarian protein extracts probed with anti-Osk antibody, followed by an anti-tubulin antibody. Extracts were from WT flies with no osk transgene (WT) or from osk 87/Df(3R)pXT103 flies expressing either a WT or the IBE mutant osk TTgAY osk transgene, or the nonsense mutant osk 54 transgene. Neither long nor short Osk is present in the osk TTgAY or osk 54 mutants. Bars, 25 μm.
Multiple copies of the IBE are necessary for osk mRNA translation
Although the phenotype of the osk 13 TTgAY suggests that the IBEs are important for osk mRNA translation and anchoring, an alternative possibility is that one of the IBE mutations prevents translation for some other reason; e.g., by chance one IBE might overlap the actual translational control element or all 13 IBE mutations might alter the folding of osk RNA. We therefore created four sets of transgenic lines in which nonoverlapping subsets (A–D) of three or four consecutive IBEs are mutant (Fig. 6 A). Three of these mutant lines (osk TTgAY A, C, and D) have phenotypes that are very similar to that of osk 13 TTgAY. They display a fully penetrant osk maternal-effect defect; Stau and the mutant osk RNAs localize to the oocyte posterior pole at stage 9, but appear dislodged from the posterior or disappear altogether during stage 10; and Osk protein is absent (Fig. 6, C, E, and G; and not depicted). In contrast, the fourth construct (osk TTgAY B) rescues the osk mRNA–null phenotype completely, and the localizations of osk mRNA and protein and Stau are normal (Fig. 6, B, D, and F; and not depicted). These findings support the hypothesis that multiple IBEs, and not some other control element that overlaps one IBE, are responsible for osk RNA translational activation and anchoring.
Figure 6.
Mutations to nonoverlapping subsets of osk's IBEs and their affects on osk RNA and protein distributions in the oocyte. (A) Four subsets of IBEs in the osk 3′UTR: A, B, C, and D. (B–E) osk in situ hybridizations at stages 9 (B and C) and 10 (D and E). (F and G) Osk protein detected by immunofluorescence at stage 10. Oocytes that express osk RNA with mutations to IBE subset B display normal localization of osk mRNA and protein. Mutations to subset D, or to subsets A and C (not depicted) cause osk RNA delocalization from the posterior pole at stage 10; Osk protein is absent in these oocytes. Bars, 25 μm.
Creation and analysis of imp mutants
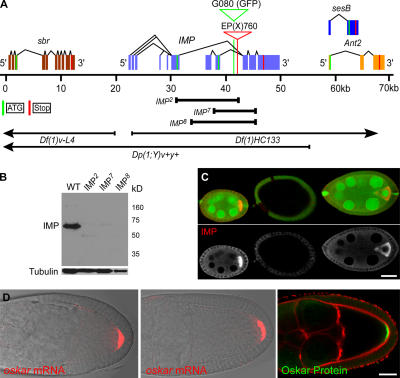
To test whether IMP is required for osk mRNA translation and anchoring, we generated null mutations in the protein through imprecise P excision. Screening by PCR revealed that three of these lines, imp2, imp7, and imp8, correspond to imprecise excisions that specifically removed parts of the IMP-coding region (Fig. 7 A). Both imp7 and imp8 remove a large portion of the IMP-coding region and are presumably null alleles, whereas imp2 removes both the alternate initiation codons, but may produce some protein from downstream in frame ATGs (Fig. 7, A and B). Furthermore, there is no detectable IMP staining in mutant germline clones, marked by the absence of GFP (Fig. 7 C).
Figure 7.
Analysis of oocytes that lack IMP protein. (A) The imp gene is flanked by sesB, Ant2, and sbr. Diagram shows alternatively spliced isoforms (exons in blue), the location of GFP in protein trap line G080, and P element insertion EP(X)760 used to create three mutant alleles of IMP. The positions of the two alternate ATGs are shown in green. (B) Immunoblots of preblastoderm embryos laid by mothers with IMP mutant germline clones. IMP2 may produce a truncated protein, whereas IMP7 and IMP8 are protein nulls. (C, top) A germline clone (center egg chamber) marked by the absence of GFP. (bottom) IMP is absent only from the mutant clone. (D) The distributions of osk mRNA and Osk protein in IMP-null egg chambers (middle and right) are indistinguishable from WT (left) in IMP mutant germline clones generated using the FLP/FRT OvoD1 DFS technique. Bars, 25 μm.
Although imp mutants are zygotic lethal, the complete removal of IMP from the germline has no obvious effect on oogenesis. Most importantly, osk mRNA localizes normally to the posterior of the oocyte at stage 9 in germline clones of all three alleles and remains anchored there throughout oogenesis (Fig. 7 D). Furthermore, the mRNA is translated at the posterior pole and produces a normal crescent of Osk protein (Fig. 7 D). Thus, despite being a bona fide component of the osk RNA localization complex and binding to the motifs required for osk translation and anchoring, IMP plays no essential role in the assembly or function of the pole plasm. However, maternal IMP is essential for embryogenesis, as 100% of the embryos from imp germline clones die in late embryogenesis and this phenotype is not rescued by a WT paternal copy of the gene.
Discussion
Our objective was to address whether D. melanogaster IMP is required for mRNA localization, as previous studies of its vertebrate homologues, ZBP-1 and VERA/Vg1RBP, had not resolved this question definitively (Zhang et al., 2001; Kwon et al., 2002; Eom et al., 2003; Farina et al., 2003; Tiruchinapalli et al., 2003). We have demonstrated that IMP binds directly to osk mRNA at well defined sites that are required for osk translation and anchoring. The best evidence that these sites are bona fide IBEs is that IMP is not recruited to the posterior by osk mRNA in which all 13 IBEs have been mutated with a single base change. Indeed, this is one of the only cases we are aware of where it has been possible to demonstrate that an RBP interacts in vivo with well defined elements identified biochemically in vitro. In vitro, mutant RNA still competes for binding of IMP, albeit less effectively than the WT osk RNA, suggesting that the 3′UTR may contain other lower affinity sites. However, these sites are not involved in the recruitment of IMP to the posterior in vivo, nor are they sufficient for translational activation. Although the IBEs are thus bona fide in vivo IMP-binding sites, their role in osk RNA translation and anchoring is independent of IMP, which is not required for these activities.
Two outcomes of this investigation seem particularly surprising. First, IBEs are required not for the initial localization of osk mRNA, but instead for its translational activation once it is localized and its subsequent anchoring at the posterior pole. Second, osk mRNA localization-dependent translation and anchoring require the IBEs in its 3′UTR, but not IMP itself.
Because Osk protein defines where the pole plasm forms, and hence where the pole cells and abdomen develop, it is essential that osk mRNA is only translated at the oocyte posterior. Indeed, translational control is arguably more important than localization in restricting Osk to the posterior, as normally only 18% of osk mRNA is actually localized (Bergsten and Gavis, 1999), and osk mRNA localization mutants such as barentsz (van Eeden et al., 2001) produce a normal abdomen. The translational repression of unlocalized osk mRNA occurs in different ways, depending on the stage of oogenesis. Mutants in RNA interference pathway components cause premature translation of osk mRNA during early oogenesis (Cook et al., 2004). Repression at later stages does not depend on these components, but instead requires the binding of Bruno and Hrp48 to three elements in the 3′UTR called Bruno response elements (Kim-Ha et al., 1995; Gunkel et al., 1998; Yano et al., 2004). This repression may occur at the level of translation initiation through the binding of Bruno to Cup protein and of Cup to the Cap-binding protein eIF4E, implying that the 5′ and 3′ ends of the mRNA are linked (Wilhelm et al., 2003; Nakamura et al., 2004).
Much less is known about how osk mRNA translation is derepressed at the posterior, apart from the findings that a 297-nt element at the 5′ end is required for the localization-dependent activation of a reporter RNA fused to the osk 3′UTR (Gunkel et al., 1998) and that the osk 3′UTR, although sufficient to repress the translation of heterologous coding sequences, is insufficient to activate their translation at the posterior (Rongo et al., 1995). Our data now provide direct evidence that the osk 3′UTR, through its IBEs, is required for translational derepression. Therefore, like activation, repression involves both the 5′ and 3′ ends. Moreover, three osk transgenes (osk TTgACYA, C, and D) with only 3 out of 13 sites mutated at a single base prevent osk translational derepression. These are much more subtle mutations than the deletions that have previously been used to define osk derepression elements (Gunkel et al., 1998) and will be useful for identifying the corresponding derepressor proteins.
Although the CPEB homologue, Orb, and the RISC component, Aubergine, have been proposed to play a role in osk translational activation (Wilson et al., 1996; Chang et al., 1999), mutants in these proteins also affect the initial localization of osk mRNA to the posterior, and this may account for the observed reduction in Osk protein levels (Castagnetti and Ephrussi, 2003; Martin et al., 2003). The only mutant combination that produces a similar phenotype to osk 13TTgAY is stau-null mutants that have been rescued by a transgene-expressing Stau protein that lacks the fifth double-stranded RNA–binding domain (Micklem et al., 2000). However, Stau is unlikely to be the putative factor that interacts with the IBEs in the osk 3′UTR to activate translation, both because it recognizes double-stranded RNA rather than short-sequence motifs (Ramos et al., 2000) and because the IBE mutations prevent osk mRNA translation without affecting Stau localization to the posterior pole at stage 9.
This brings us to the most significant outcome of our investigation: osk RNA translational activation and anchoring is disrupted by mutants in the IBEs, but not by the loss of IMP itself. The possibility that the IBE mutations prevent osk mRNA derepression and IMP localization indirectly by altering the structure of the RNA seems extremely unlikely, as single-base substitutions within three nonoverlapping sets of three IBEs in widely separated regions of the >1-kb osk 3′UTR produce an identical and very specific defect in translation, without affecting any of the earlier functions of the 3′UTR, such as the maintenance of oocyte fate, the transport of the mRNA from the nurse cells into the oocyte, the translational repression of unlocalized mRNA, or its localization to the posterior pole. Thus, none of these mutations disrupt the binding of any of the factors that mediate these earlier steps, including Staufen, which is thought to recognize the secondary structure of the RNA through the interaction of its double-stranded RNA-binding domains with multiple stem loops. This strongly argues against the possibility that the single base changes to the IBEs inhibit osk RNA translation through a nonspecific effect on RNA folding. This leads us to conclude that the IBEs play a direct role in the derepression of osk mRNA translation.
Because IMP itself is not necessary for derepression, this implies that the IBEs are also recognized by another factor, which we will call factor X. IMP and factor X could function redundantly to derepress osk translation, i.e., the two proteins might share osk's IBEs and compensate for each other's loss. However, factor X cannot be a ZBP-1/VERA family member because, unlike mammals, no such relatives are evident in the D. melanogaster genome.
Alternatively, IMP and factor X might function independently, i.e., osk derepression might occur exclusively through factor X binding. Rather than implementing osk's translational derepression, IMP's actual function might be to compete with factor X for IBE binding. In support of this, we have found that overexpression of IMP reduces Osk protein levels at the posterior (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200510044/DC1). Although the purpose of IMP competition is presently unclear, one possibility is that IMP serves to bind, and thereby mask, IBEs that occur by chance in RNAs for which factor X binding would be unnecessary or even detrimental. According to this view, competition with IMP would restrict factor X binding to those mRNAs, such as osk, that contain many copies of IBEs clustered within a restricted region. In the absence of IMP, factor X could bind to mRNAs with fewer IBEs and inappropriately regulate their translation. This may explain why embryos from imp-null oocytes always die, but from defects that appear unrelated to Osk function.
Our analysis of the interaction of IMP with osk mRNA closely parallels that of ZBP-1 and VERA/Vg1RBP with β-actin and Vg1 mRNA, respectively. (a) In each case, the protein has been shown to colocalize with the localized mRNA and can be UV cross-linked to it in extracts; (b) the precise binding sites of each protein have been determined and reveal that it recognizes a repeated motif in the target mRNA; (c) the function of these sites has then been analyzed by introducing specific point mutations that abrogate the binding of the protein, and these have been found to have a dramatic effect on translation or localization. In this study, we have gone one step further, and have compared the phenotype of the IBE mutants with that of mutations in IMP itself. The observation that the former gives a fully penetrant defect in osk mRNA translation, whereas the latter has no phenotype in the germline, conclusively demonstrates that IMP is not responsible for the function of the IBEs in the osk 3′UTR. This is important in light of the observation that many RBPs have been implicated in the posttranscriptional regulation of particular mRNAs by studying the effects of mutations in their binding sites. Our results highlight the potential limitations of this approach by demonstrating that one cannot necessarily infer the function of a protein from the phenotype of mutations in the cis-acting sequences that it recognizes.
The clear similarities between the localizations and functions of Vg1 and VegT mRNAs in X. laevis oocytes, and of osk mRNA in D. melanogaster oocytes, suggest that binding motifs for ZBP-1 proteins have a fundamental role in embryogenesis. Vg1, VegT, and osk localize as mRNAs to one pole of the oocyte, which is the site where the germ or pole plasm forms, and all three proteins play key roles in the formation of the primary body axis (Melton, 1987; Ephrussi et al., 1991; Kim-Ha et al., 1991; Zhang and King, 1996). Our findings extend this parallel by showing that the localized expression of all three proteins also depends on a repeated RNA motif, defined by its interaction with IMP or its homologues. Because our results rule out a function for IMP in the regulation of osk mRNA, this calls into question the role of VERA/Vg1RBP1 in the localization of Vg1 and Veg T mRNAs, and it may therefore be worth considering the possibility that there is also a factor X in X. laevis.
Materials and methods
SELEX
The cDNAs encoding IMP KH3 (residues Leu 301–Ala 396) and KH4 (residues Val 387–Gln 482) were subcloned into ProEX HTb (Life Technologies). These KH constructs included the canonical KH domain, as well as 20 additional residues at the COOH termini that, in a previous study of a different protein, were found essential for high affinity binding of the RNA recognition element (Jensen et al., 2000). The constructs were expressed in Escherichia coli and recovered by extraction of the bacteria with a solution of 8 M Urea, 100 mM NaH2PO4, and 10 mM Tris-Cl, pH 8.0. The fusion proteins were bound to Ni-NTA agarose (QIAGEN), eluted at pH 4.5, and dialyzed against 50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 5% glycerol, and 2 mM DTT.
To create a random 25mer RNA pool for SELEX, we used 1.2 nmol of the oligonucleotide 5′-GCGAATTCAGATAGTAAGTGCAATCT{25N}AATTGAATAAGCTGGTATCTCCC-3′ (Invitrogen), where N indicates the incorporation of nucleotides at random. EcoRI sites and sequences for RT-PCR amplication are included. This provided an oligonucleotide pool with an estimated complexity of 7.2 × 1014 sequences. To generate a double-stranded DNA library suitable for in vitro transcription, we PCR amplified the pool using 5′-GCGAAGCTTTAATACGACTCACTATAGGGAGATACCAGCTTATTCAATT-3′ and 5′-GCGAATTCAGATAGTAAGTGCAATCT-3′ as the forward primer containing the T7 promoter and HinDIII sites and the reverse primer containing an EcoRI site. We synthesized RNA from the double-stranded DNA using T7 RNA polymerase in the presence of α-32P-UTP and then purified the RNA pool on an acrylamide gel run under denaturing conditions.
To select for RNAs that bind the IMP KH domain, the gel-purified pool was split and each half applied to either KH3 or KH4 that was immobilized on separate Ni-NTA agarose. Bound and unbound RNAs were separated by centrifuging the beads. RNAs retained by the beads were extracted with phenol/chloroform and precipitated with ethanol. These eluted RNAs, which were enriched in sequences recognized by KH3 or KH4, were subjected to RT-PCR and in vitro transcription, thereby generating the RNAs for the next round of selection. After the 1st, 11th, and 12th rounds of selection, aliquots of the cDNAs corresponding to the RNAs selected by KH3 or KH4 were cloned and sequenced using standard techniques.
RNA-binding assays
32P-RNAs consisting of three tandem repeats of the winner sequence 4-12-13 (Fig. 2) were synthesized in vitro using the oligonucleotide 5′-GTTGAAAAAATAAAAATAATAAAAAGTTGAAAAAATAAAAATAATAAAAAGTTGAAAAAATAAAAATAATAAAAACTATAGTGAGTCGTATTA-3′ annealed to 5′-TAATACGACTCACTATAG-3′, which contains the T7 promoter. To create the corresponding motif mutants (UUgAY and gggcg) in the same context, the nucleotides encoding the IBEs (underlined) were altered (5′-ATcAA-3′ or 5′-CGCCC-3′) accordingly. RNA synthesis was performed with α-32P-UTP and an AmpliScribe T7 transcription kit (Epicentre Biotechnologies).
Filter-binding assays were performed in 100-μl reactions consisting of 1XB buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 0.5 mM DTT, and 20 μg/ml tRNA), 100 fmol of 32P-RNA, and varying concentrations of KH3 or IMP. Reactions were incubated at RT for 15 min. Protein-bound and -unbound RNA fractions were separated by filtration of the reactions through 0.22- (KH domain) or 0.45-μm (IMP) nitrocellulose filters.
For electrophoretic mobility shift assays, 60 fmol of 32P-RNA was incubated with Histidine-tagged IMP (30, 100, 300, and 900 nM) at RT for 30 min before electrophoresis under nondenaturing conditions using 8% acrylamide (37.5:1) gels. The gels were run at 100 V for 4 h at 4°C. Gels were dried and imaged on a PhosphorImager SI (Molecular Dynamics). UV cross-linking assays were performed as described previously (Kwon et al., 2002).
Transgenes
We engineered GFP fusions of IMP using imp cDNA that we obtained either from ESTs (provided by K. Korey and D. Van Vactor, Harvard Medical School, Boston, MA) or a D. melanogaster ovarian cDNA library (provided by N. Brown, The Gurdon Institute, Cambridge, UK). We cloned the imp ORF into pUMAT-GFP downstream of the maternal α-4-tubulin promoter, which drives expression in the germline (Micklem et al., 1997), or into pUAS-p, which allows for the tissue-specific expression of the transgene using the Gal4/UAS system (Brand and Perrimon, 1993; Rorth, 1998). The osk constructs were made from a 10-kb Xho1–Apa1 fragment of genomic DNA (a gift from U. Irion, The Gurdon Institute, Cambridge, UK). The osk 3′UTR TTTAY motifs were mutated using the Transformer site-directed mutagenesis kit (CLONTECH Laboratories, Inc.).
Fly stocks
imp excision mutants were recombined onto y,w,v,P{FRT(w{hs})}101. Germline clones were then generated by crossing the FRT recombinant lines to w,ovoD1,v,P{FRT(w[hs])}101/C(1)DX,y,f/Y; P{hsFLP}38 or y,w,P{Ubi-GFP}ID-1 P{FRT(w[hs])}101. Other lines used in this study include GFP-tagged IMP protein trap line G080 (Morin et al., 2001), stauD3, Df(2R)PC4 (St Johnston et al., 1991) and vasPD, Df(2L)A48 (Lasko and Ashburner, 1988), oskDf(3R)pXT103 (Lehmann and Nusslein-Volhard, 1986), par1W3, par16323 (Shulman et al., 2000), oskA87 (Vanzo and Ephrussi, 2002), and osk54 (Ephrussi et al., 1991).
Antibodies and histology
We generated rabbit antisera against full-length recombinant IMP or peptides. Antibodies were affinity purified against peptide immobilized to Sulfolink resin (Pierce Chemical Co.) or recombinant IMP immobilized to CnBr–Sepharose (Roche). Dilutions for immunoblots were as follows: 1:300 for anti-IMP antibodies, 1:3,000 for anti-Osk antibody (a gift from A. Ephrussi, European Molecular Biology Laboratory, Heidelberg, Germany), and 1:5,000 for anti-tubulin antibody (Sigma-Aldrich). Dilutions for immunostaining were 1:100 for anti-IMP, 1:500 for anti-Osk, and 1:500 for anti-Stau (St Johnston et al., 1991). Secondary antibodies were obtained from Jackson ImmunoResearch Laboratories. We performed osk RNA in situ hybridization as previously described, using dig-UTP–labeled RNA (Roche) and Cy3–anti-Dig (Jackson ImmunoResearch Laboratories; Huynh et al., 2001). Cuticle preparations were mounted in 1:1 Hoyers/lactic acid, and images were collected using a SPOT camera and software (Diagnostic Instruments) on an Axioplan microscope (Carl Zeiss MicroImaging, Inc.) using a 10× objective at RT. Fluorescent samples were mounted in Vectorshield (Vector Laboratories). Images were collected on a confocal system (models 1024 or Radiance 2100; BioRad Laboratories) with Lasersharp 2000 software (BioRad Laboratories), attached to a microscope (Eclipse E800; Nikon) using a 40×, 1.3 NA, objective at RT. Images were subsequently processed with Photoshop (Adobe).
P element excision mutants IMP2, IMP7, and IMP8
We used standard methods to generate and isolate P element excision lines that lack IMP gene segments. We obtained the EP(X) 760 P-insertion line (w1118, P{w+mC = EP}IMPEP760) generated by the Berkeley Gene Disruption Project from the Bloomington Stock Center. To characterize the excisions molecularly, we extracted DNA from homozygous mutant larvae and performed PCR with primers designed to identify lines that lack regions of the imp gene.
Online supplemental material
Fig. S1 depicts an experiment showing that oskar RNA is specifically immunoprecipitated with IMP. Fig. S2 shows that overexpression of IMP in the germline decreases the amount of Oskar protein at the posterior, as well as causing actin defects late in oogenesis. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200510044/DC1.
Supplementary Material
Acknowledgments
We thank Drs. David Van Vactor, Chris Korey, and Bill Theurkauf for guidance early in this project. We are grateful to Drs. Jan Christian and Marcel Wehrli for comments on the manuscript and to Dr. Wehrli for helping the Schnapp Laboratory with fly genetics and husbandry. We thank members of the Schnapp Laboratory, particularly Mr. Sean Wolfson, for their input to this project.
This work was supported by a National Institutes of Health grant and the Oregon Health and Science University Medical Research Foundation to B.J. Schnapp, by a Human Frontiers Network grant to B.J. Schnapp and D. St Johnston, and by a Wellcome Trust fellowship to D. St Johnston.
Abbreviations used in this paper: IBE, IMP-binding element; IMP, insulin-like growth factor II mRNA–binding protein; LE, localization element; RBP, RNA-binding protein; SELEX, systematic evolution of ligands by exponential enrichment; UTR, untranslated region; VERA, Vg1 RNA and endoplasmic reticulum–associated protein; WT, wild-type; ZBP-1, zip code–binding protein 1.
T.P. Munro and S. Kwon contributed equally to this paper.
B.J. Schnapp and D. St Johnston contributed equally to this paper.
References
- Bergsten, S.E., and E.R. Gavis. 1999. Role for mRNA localization in translational activation but not spatial restriction of nanos RNA. Development. 126:659–669. [DOI] [PubMed] [Google Scholar]
- Bohl, F., C. Kruse, A. Frank, D. Ferring, and R.P. Jansen. 2000. She2p, a novel RNA-binding protein tethers ASH1 mRNA to the Myo4p myosin motor via She3p. EMBO J. 19:5514–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, A.H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118:401–415. [DOI] [PubMed] [Google Scholar]
- Breitwieser, W., F.H. Markussen, H. Horstmann, and A. Ephrussi. 1996. Oskar protein interaction with Vasa represents an essential step in polar granule assembly. Genes Dev. 10:2179–2188. [DOI] [PubMed] [Google Scholar]
- Bubunenko, M., T.L. Kress, U.D. Vempati, K.L. Mowry, and M.L. King. 2002. A consensus RNA signal that directs germ layer determinants to the vegetal cortex of Xenopus oocytes. Dev. Biol. 248:82–92. [DOI] [PubMed] [Google Scholar]
- Castagnetti, S., and A. Ephrussi. 2003. Orb and a long poly(A) tail are required for efficient oskar translation at the posterior pole of the Drosophila oocyte. Development. 130:835–843. [DOI] [PubMed] [Google Scholar]
- Chang, J.S., L. Tan, and P. Schedl. 1999. The Drosophila CPEB homolog, orb, is required for oskar protein expression in oocytes. Dev. Biol. 215:91–106. [DOI] [PubMed] [Google Scholar]
- Cook, H.A., B.S. Koppetsch, J. Wu, and W.E. Theurkauf. 2004. The Drosophila SDE3 homolog armitage is required for oskar mRNA silencing and embryonic axis specification. Cell. 116:817–829. [DOI] [PubMed] [Google Scholar]
- Cote, C.A., D. Gautreau, J.M. Denegre, T. Kress, N.A. Terry, and K.L. Mowry. 1999. A Xenopus protein related to hnRNPI has a role in cytoplasmic RNA localization. Mol. Cell. 4:431–437. [DOI] [PubMed] [Google Scholar]
- Czaplinski, K., T. Kocher, M. Schelder, A. Segref, M. Wilm, and I.W. Mattaj. 2005. Identification of 40LoVe, a Xenopus hnRNP D family protein involved in localizing a TGF-beta-related mRNA during oogenesis. Dev. Cell. 8:505–515. [DOI] [PubMed] [Google Scholar]
- Deshler, J.O., M.I. Highett, and B.J. Schnapp. 1997. Localization of Xenopus Vg1 mRNA by vera protein and the endoplasmic reticulum. Science. 276:1128–1131. [DOI] [PubMed] [Google Scholar]
- Deshler, J.O., M.I. Highett, T. Abramson, and B.J. Schnapp. 1998. A highly conserved RNA-binding protein for cytoplasmic mRNA localization in vertebrates. Curr. Biol. 8:489–496. [DOI] [PubMed] [Google Scholar]
- Eom, T., L.N. Antar, R.H. Singer, and G.J. Bassell. 2003. Localization of a beta-actin messenger ribonucleoprotein complex with zipcode-binding protein modulates the density of dendritic filopodia and filopodial synapses. J. Neurosci. 23:10433–10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi, A., and R. Lehmann. 1992. Induction of germ cell formation by oskar. Nature. 358:387–392. [DOI] [PubMed] [Google Scholar]
- Ephrussi, A., L.K. Dickinson, and R. Lehmann. 1991. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 66:37–50. [DOI] [PubMed] [Google Scholar]
- Farina, K.L., S. Huttelmaier, K. Musunuru, R. Darnell, and R.H. Singer. 2003. Two ZBP1 KH domains facilitate β-actin mRNA localization, granule formation, and cytoskeletal attachment. J. Cell Biol. 160:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavis, E.R., and R. Lehmann. 1992. Localization of nanos RNA controls embryonic polarity. Cell. 71:301–313. [DOI] [PubMed] [Google Scholar]
- Git, A., and N. Standart. 2002. The KH domains of Xenopus Vg1RBP mediate RNA binding and self-association. RNA. 8:1319–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, I., S.B. Buonomo, K. Nasmyth, and U. von Ahsen. 1999. ASH1 mRNA localization in yeast involves multiple secondary structural elements and ASH1 protein translation. Curr. Biol. 9:337–340. [DOI] [PubMed] [Google Scholar]
- Gunkel, N., T. Yano, F.H. Markussen, L.C. Olsen, and A. Ephrussi. 1998. Localization-dependent translation requires a functional interaction between the 5′ and 3′ ends of oskar mRNA. Genes Dev. 12:1652–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachet, O., and A. Ephrussi. 2004. Splicing of oskar RNA in the nucleus is coupled to its cytoplasmic localization. Nature. 428:959–963. [DOI] [PubMed] [Google Scholar]
- Havin, L., A. Git, Z. Elisha, F. Oberman, K. Yaniv, S.P. Schwartz, N. Standart, and J.K. Yisraeli. 1998. RNA-binding protein conserved in both microtubule- and microfilament-based RNA localization. Genes Dev. 12:1593–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, B., L.Y. Jan, and Y.N. Jan. 1990. Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development. 109:425–433. [DOI] [PubMed] [Google Scholar]
- Hoek, K.S., G.J. Kidd, J.H. Carson, and R. Smith. 1998. hnRNP A2 selectively binds the cytoplasmic transport sequence of myelin basic protein mRNA. Biochemistry. 37:7021–7029. [DOI] [PubMed] [Google Scholar]
- Huynh, J.R., J.M. Shulman, R. Benton, and D. St Johnston. 2001. PAR-1 is required for the maintenance of oocyte fate in Drosophila. Development. 128:1201–1209. [DOI] [PubMed] [Google Scholar]
- Huynh, J.R., T.P. Munro, K. Smith-Litiere, J.A. Lepesant, and D.S. Johnston. 2004. The Drosophila hnRNPA/B homolog, Hrp48, Is specifically required for a distinct step in osk mRNA localization. Dev. Cell. 6:625–635. [DOI] [PubMed] [Google Scholar]
- Jensen, K.B., K. Musunuru, H.A. Lewis, S.K. Burley, and R.B. Darnell. 2000. The tetranucleotide UCAY directs the specific recognition of RNA by the nova K-homology 3 domain. Proc. Natl. Acad. Sci. USA. 97:5740–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Ha, J., J.L. Smith, and P.M. Macdonald. 1991. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 66:23–35. [DOI] [PubMed] [Google Scholar]
- Kim-Ha, J., P.J. Webster, J.L. Smith, and P.M. Macdonald. 1993. Multiple RNA regulatory elements mediate distinct steps in localization of oskar mRNA. Development. 119:169–178. [DOI] [PubMed] [Google Scholar]
- Kim-Ha, J., K. Kerr, and P.M. Macdonald. 1995. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell. 81:403–412. [DOI] [PubMed] [Google Scholar]
- Kislauskis, E.H., Z. Li, R.H. Singer, and K.L. Taneja. 1993. Isoform-specific 3′-untranslated sequences sort α-cardiac and β-cytoplasmic actin messenger RNAs to different cytoplasmic compartments. J. Cell Biol. 123:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislauskis, E.H., X. Zhu, and R.H. Singer. 1994. Sequences responsible for intracellular localization of β-actin messenger RNA also affect cell phenotype. J. Cell Biol. 127:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll, T.T., W.M. Zhao, C. Jiang, and P.W. Huber. 2002. A homolog of FBP2/KSRP binds to localized mRNAs in Xenopus oocytes. Development. 129:5609–5619. [DOI] [PubMed] [Google Scholar]
- Kwon, S., T. Abramson, T.P. Munro, C.M. John, M. Kohrmann, and B.J. Schnapp. 2002. UUCAC- and Vera-dependent localization of VegT RNA in Xenopus oocytes. Curr. Biol. 12:558–564. [DOI] [PubMed] [Google Scholar]
- Lasko, P.F., and M. Ashburner. 1988. The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature. 335:611–617. [DOI] [PubMed] [Google Scholar]
- Lasko, P.F., and M. Ashburner. 1990. Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes Dev. 4:905–921. [DOI] [PubMed] [Google Scholar]
- Lehmann, R., and C. Nusslein-Volhard. 1986. Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell. 47:141–152. [DOI] [PubMed] [Google Scholar]
- Lewis, H.A., H. Chen, C. Edo, R.J. Buckanovich, Y.Y. Yang, K. Musunuru, R. Zhong, R.B. Darnell, and S.K. Burley. 1999. Crystal structures of Nova-1 and Nova-2 K-homology RNA-binding domains. Structure. 7:191–203. [DOI] [PubMed] [Google Scholar]
- Lewis, H.A., K. Musunuru, K.B. Jensen, C. Edo, H. Chen, R.B. Darnell, and S.K. Burley. 2000. Sequence-specific RNA binding by a Nova KH domain: implications for paraneoplastic disease and the fragile X syndrome. Cell. 100:323–332. [DOI] [PubMed] [Google Scholar]
- Lewis, R.A., T.L. Kress, C.A. Cote, D. Gautreau, M.E. Rokop, and K.L. Mowry. 2004. Conserved and clustered RNA recognition sequences are a critical feature of signals directing RNA localization in Xenopus oocytes. Mech. Dev. 121:101–109. [DOI] [PubMed] [Google Scholar]
- Long, R.M., W. Gu, E. Lorimer, R.H. Singer, and P. Chartrand. 2000. She2p is a novel RNA-binding protein that recruits the Myo4p-She3p complex to ASH1 mRNA. EMBO J. 19:6592–6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markussen, F.H., A.M. Michon, W. Breitwieser, and A. Ephrussi. 1995. Translational control of oskar generates short OSK, the isoform that induces pole plasma assembly. Development. 121:3723–3732. [DOI] [PubMed] [Google Scholar]
- Martin, S.G., V. Leclerc, K. Smith-Litiere, and D. St Johnston. 2003. The identification of novel genes required for Drosophila anteroposterior axis formation in a germline clone screen using GFP-Staufen. Development. 130:4201–4215. [DOI] [PubMed] [Google Scholar]
- Mathews, D.H., J. Sabina, M. Zuker, and D.H. Turner. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911–940. [DOI] [PubMed] [Google Scholar]
- Melton, D.A. 1987. Translocation of a localized maternal mRNA to the vegetal pole of Xenopus oocytes. Nature. 328:80–82. [DOI] [PubMed] [Google Scholar]
- Micklem, D.R., R. Dasgupta, H. Elliott, F. Gergely, C. Davidson, A. Brand, A. Gonzalez-Reyes, and D. St Johnston. 1997. The mago nashi gene is required for the polarisation of the oocyte and the formation of perpendicular axes in Drosophila. Curr. Biol. 7:468–478. [DOI] [PubMed] [Google Scholar]
- Micklem, D.R., J. Adams, S. Grunert, and D. St Johnston. 2000. Distinct roles of two conserved Staufen domains in oskar mRNA localization and translation. EMBO J. 19:1366–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin, X., R. Daneman, M. Zavortink, and W. Chia. 2001. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA. 98:15050–15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, A., K. Sato, and K. Hanyu-Nakamura. 2004. Drosophila Cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev. Cell. 6:69–78. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg, F.S., and T. Schupbach. 1993. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 75:165–174. [PubMed] [Google Scholar]
- Nielsen, J., F. Cilius Nielsen, R. Kragh Jakobsen, and J. Christiansen. 2000. The biphasic expression of IMP/Vg1-RBP is conserved between vertebrates and Drosophila. Mech. Dev. 96:129–132. [DOI] [PubMed] [Google Scholar]
- Norvell, A., R.L. Kelley, K. Wehr, and T. Schupbach. 1999. Specific isoforms of Squid, a Drosophila hnRNP, perform distinct roles in Gurken localization during oogenesis. Genes Dev. 13:864–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleynikov, Y., and R.H. Singer. 2003. Real-time visualization of ZBP1 association with beta-actin mRNA during transcription and localization. Curr. Biol. 13:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, A., S. Grunert, J. Adams, D.R. Micklem, M.R. Proctor, S. Freund, M. Bycroft, D. St Johnston, and G. Varani. 2000. RNA recognition by a Staufen double-stranded RNA-binding domain. EMBO J. 19:997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongo, C., and R. Lehmann. 1996. Regulated synthesis, transport and assembly of the Drosophila germ plasm. Trends Genet. 12:102–109. [DOI] [PubMed] [Google Scholar]
- Rongo, C., E.R. Gavis, and R. Lehmann. 1995. Localization of oskar RNA regulates oskar translation and requires Oskar protein. Development. 121:2737–2746. [DOI] [PubMed] [Google Scholar]
- Rorth, P. 1998. Gal4 in the Drosophila female germline. Mech. Dev. 78:113–118. [DOI] [PubMed] [Google Scholar]
- Ross, A.F., Y. Oleynikov, E.H. Kislauskis, K.L. Taneja, and R.H. Singer. 1997. Characterization of a beta-actin mRNA zipcode-binding protein. Mol. Cell. Biol. 17:2158–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman, J.M., R. Benton, and D. St Johnston. 2000. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell. 101:377–388. [DOI] [PubMed] [Google Scholar]
- Smith, J.L., J.E. Wilson, and P.M. Macdonald. 1992. Overexpression of oskar directs ectopic activation of nanos and presumptive pole cell formation in Drosophila embryos. Cell. 70:849–859. [DOI] [PubMed] [Google Scholar]
- St Johnston, D. 2005. Moving messages: the intracellular localization of mRNAs. Nat. Rev. Mol. Cell Biol. 6:363–375. [DOI] [PubMed] [Google Scholar]
- St Johnston, D., W. Driever, T. Berleth, S. Richstein, and C. Nusslein-Volhard. 1989. Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development. 107:13–19. [DOI] [PubMed] [Google Scholar]
- St Johnston, D., D. Beuchle, and C. Nusslein-Volhard. 1991. staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 66:51–63. [DOI] [PubMed] [Google Scholar]
- Takizawa, P.A., and R.D. Vale. 2000. The myosin motor, Myo4p, binds Ash1 mRNA via the adapter protein, She3p. Proc. Natl. Acad. Sci. USA. 97:5273–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruchinapalli, D.M., Y. Oleynikov, S. Kelic, S.M. Shenoy, A. Hartley, P.K. Stanton, R.H. Singer, and G.J. Bassell. 2003. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J. Neurosci. 23:3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eeden, F.J., I.M. Palacios, M. Petronczki, M.J.D. Weston, and D. St Johnston. 2001. barentsz is essential for the posterior localization of oskar mRNA and colocalizes with it to the posterior pole. J. Cell Biol. 154:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzo, N.F., and A. Ephrussi. 2002. Oskar anchoring restricts pole plasm formation to the posterior of the Drosophila oocyte. Development. 129:3705–3714. [DOI] [PubMed] [Google Scholar]
- Wilhelm, J.E., and C.A. Smibert. 2005. Mechanisms of translational regulation in Drosophila. Biol. Cell. 97:235–252. [DOI] [PubMed] [Google Scholar]
- Wilhelm, J.E., M. Hilton, Q. Amos, and W.J. Henzel. 2003. Cup is an eIF4E binding protein required for both the translational repression of oskar and the recruitment of Barentsz. J. Cell Biol. 163:1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, J.E., J.E. Connell, and P.M. Macdonald. 1996. Aubergine enhances oskar translation in the Drosophila ovary. Development. 122:1631–1639. [DOI] [PubMed] [Google Scholar]
- Yano, T., S.L. De Quinto, Y. Matsui, A. Shevchenko, and A. Ephrussi. 2004. Hrp48, a Drosophila hnRNPA/B homolog, binds and regulates translation of oskar mRNA. Dev. Cell. 6:637–648. [DOI] [PubMed] [Google Scholar]
- Zhang, H.L., T. Eom, Y. Oleynikov, S.M. Shenoy, D.A. Liebelt, J.B. Dictenberg, R.H. Singer, and G.J. Bassell. 2001. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron. 31:261–275. [DOI] [PubMed] [Google Scholar]
- Zhang, J., and M.L. King. 1996. Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning. Development. 122:4119–4129. [DOI] [PubMed] [Google Scholar]
- Zhao, W.M., C. Jiang, T.T. Kroll, and P.W. Huber. 2001. A proline-rich protein binds to the localization element of Xenopus Vg1 mRNA and to ligands involved in actin polymerization. EMBO J. 20:2315–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.