Abstract
Rho guanosine triphosphatases (GTPases) are critical regulators of cytoskeletal dynamics and control complex functions such as cell adhesion, spreading, migration, and cell division. It is generally accepted that localized GTPase activation is required for the proper initiation of downstream signaling events, although the molecular mechanisms that control targeting of Rho GTPases are unknown. In this study, we show that the Rho GTPase Rac1, via a proline stretch in its COOH terminus, binds directly to the SH3 domain of the Cdc42/Rac activator β-Pix (p21-activated kinase [Pak]–interacting exchange factor). The interaction with β-Pix is nucleotide independent and is necessary and sufficient for Rac1 recruitment to membrane ruffles and to focal adhesions. In addition, the Rac1–β-Pix interaction is required for Rac1 activation by β-Pix as well as for Rac1-mediated spreading. Finally, using cells deficient for the β-Pix–binding kinase Pak1, we show that Pak1 regulates the Rac1–β-Pix interaction and controls cell spreading and adhesion-induced Rac1 activation. These data provide a model for the intracellular targeting and localized activation of Rac1 through its exchange factor β-Pix.
Introduction
Directional cell migration is essential for development, chemotaxis, and wound healing and requires cell polarization in combination with a motility stimulus. Key to the cells' capacity to migrate is the dynamics of the microtubular and actin cytoskeleton. Whereas microtubules primarily play a role in dictating and sustaining cell polarity (Fukata et al., 2002; Etienne-Manneville and Hall, 2003; Schmoranzer et al., 2003; Watanabe et al., 2005), forward movement results from actin polymerization at the front and actomyosin-based contraction at the rear of the cell (Ridley et al., 2003). In parallel, the actin and microtubule cytoskeleton also orchestrates cell substrate adhesion to further promote directional migration (Waterman-Storer and Salmon, 1999; Geiger et al., 2001; Ezratty et al., 2005).
Cytoskeletal dynamics and cell adhesion are controlled by small GTPases of the Rho family, in particular RhoA, Rac1, and Cdc42 (Ridley et al., 2003; Xu et al., 2003). These GTPases act as master switches, cycling between an inactive, guanosine diphosphate (GDP)–bound state and an active, GTP-bound state. This cycling is regulated through guanine nucleotide exchange factors (GEFs) that exchange bound GDP for GTP (Rossman et al., 2005), GTPase-activating proteins (GAPs) that promote the intrinsic GTP hydrolysis, and guanine nucleotide dissociating inhibitors (GDIs) that bind Rho-like GTPases through their COOH-terminal lipid anchor (del Pozo et al., 2002) and retain the GTPases in the cytosol (Olofsson, 1999; del Poz et al., 2002). Activation of Rho GTPases is associated with membrane translocation (Kranenburg et al., 1997; del Pozo et al., 2000) and release from the cytosolic GDI protein. At the plasma membrane, Rho GTPases activate a wide range of effector proteins that regulate adhesion and migration as well as gene transcription (Bishop and Hall, 2000).
It has been suggested that integrins indirectly regulate the recruitment of Rac1 to the membrane and promote the dissociation of RhoGDI, which would allow localized Rac1 activation and signaling (del Pozo et al., 2002). In line with this, Rac1 association with the membrane was found to be independent of interactions with effector proteins (del Pozo et al., 2002), suggesting that other regulatory proteins mediate membrane targeting. Membrane localization of Rho GTPases is mediated by the COOH-terminal lipid moiety. However, it is unlikely that membrane association through this lipid anchor mediates spatially restricted GTPase targeting and signaling. More likely, GTPases are specifically targeted to membrane-associated signaling complexes via protein–protein interactions (Michaelson et al., 2001; van Hennik et al., 2003). Supporting this notion, the Rho-like GTPase Rac1 is activated at the leading edge of migrating cells, which is in line with its role in cell motility (Kraynov et al., 2000; Itoh et al., 2002). The related GTPase Cdc42 is also activated in cellular protrusions, albeit in a narrower, peripheral area compared with Rac1 (Itoh et al., 2002; Nalbant et al., 2004). This differentially localized GTPase activation will result in coordinated spatially confined signaling, which is required for directional cell migration.
Our laboratory has previously shown that the hypervariable COOH-terminal domain of Rac1 mediates its association with the DAG kinase–phosphatidylinositol-4-phosphate 5-kinase complex as well as with adaptor proteins such as CrkII and Nck (Tolias et al., 1998; van Hennik et al., 2003). These protein interactions suggest that Rac1 and, most likely, Rho GTPases in general use their hypervariable COOH terminus to target to specific protein complexes. This is in agreement with studies showing that the COOH termini of small GTPases mediate both subcellular targeting (Michaelson et al., 2001) and signaling specificity (Filippi et al., 2004).
In this study, we show that β-Pix (p21-activated kinase [Pak]–interacting exchange factor) can interact with the hypervariable domain of Rac1. β-Pix is a Rac/Cdc42GEF that also acts as a signaling organizer by binding to the ArfGAP paxillin kinase linker (PKL)/GIT as well as to the Rac effector Pak1 (Bagrodia et al., 1998, 1999; Manser et al., 1998). We demonstrate that Rac1, through the proline stretch in its COOH terminus, binds directly to the SH3 domain of β-Pix. This interaction mediates Rac1 targeting to membrane ruffles and to focal adhesions (FAs) as well as Rac1 activation and Rac1-mediated cell spreading. Finally, we show that Rac1 and Pak1 compete for binding to β-Pix and that Pak1 controls the Rac1–β-Pix interaction and adhesion-induced Rac1 activation. These data provide a model for Rac1 targeting through its activator β-Pix, which drives integrin-mediated localized Rac1 activation in polarized migrating cells.
Results
β-Pix interacts with the COOH terminus of Rac1
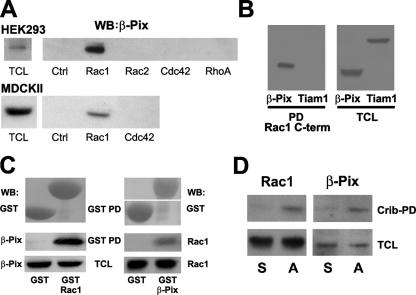
Recently, we showed that cell-permeable versions of the COOH termini of Rac1, RhoA, and Cdc42 act as selective inhibitors of the respective GTPases in a variety of cell types, presumably by interfering with the proper targeting of these GTPases (van Hennik et al., 2003). We noticed that the COOH terminus of Rac1, which binds the SH3 domain of Crk (van Hennik et al., 2003), shows homology to a SH3 domain–binding motif in Pak1 and SPIN90. Interestingly, both Pak1 and SPIN90 interact with the SH3 domain of Rac1/Cdc42 GEF β-Pix (Manser et al., 1998; Lim et al., 2003), suggesting that Rac1 might also interact with β-Pix. Streptavidin-based pull-down assays with a series of biotinylated RhoGTPase COOH-terminal peptides using lysates from human embryonal kidney 293 (HEK293) or MDCKII cells confirmed that endogenous β-Pix specifically binds the COOH terminus of Rac1 but not of Rac2, Cdc42, or RhoA (Fig. 1 A). In contrast to β-Pix, the Rac GEFs Tiam1 (Fig. 1 B) or Vav2 (not depicted) did not bind the COOH-terminal domain of Rac1. These data show that the Rac1 COOH terminus binds specifically to β-Pix.
Figure 1.
Analysis of the Rac1–β-Pix interaction. (A) Endogenous β-Pix interacts specifically with the Rac1 COOH terminus. Pull-down experiments were performed using cell lysates from HEK293 and MDCKII cells with control peptide (Ctrl) or the indicated GTPase COOH-terminal peptides. β-Pix was detected by Western blotting (WB). TCL, total cell lysate. (B) The COOH terminus of Rac1 binds β-Pix but not Tiam1. Pull-down assays (PD) were performed with the Rac1 COOH-terminal peptide in lysates from COS-7 cells transfected with β-Pix or Tiam1. (C) Full-length Rac1 binds to β-Pix. Reciprocal GST pull-down experiments were performed with lysates from COS-7 cells transfected, as indicated, with β-Pix or Rac1. (D) Interaction between endogenous Rac1 and β-Pix. Cells were incubated in suspension or on collagen-coated dishes for 1 h before lysis. Active Rac1 was precipitated with a biotinylated Pak1-Crib peptide, and the precipitate was washed and analyzed by Western blotting for Rac1 (left) and β-Pix (right). S, cells in suspension; A, cells that are adherent.
To demonstrate that β-Pix also binds full-length Rac1, we expressed either β-Pix or Rac1 in COS-7 cells and performed reciprocal pull-down assays with bacterially purified GST, GST-Rac1, or GST–β-Pix (Fig. 1 C). These experiments showed that β-Pix interacts with the full-length Rac1 protein and, conversely, that bacterially purified full-length Rac1 binds β-Pix.
To test whether endogenous Rac1 binds to endogenous β-Pix, we performed pull-down assays with the biotinylated Crib domain of Pak1 (Price et al., 2003). This domain binds activated Rac from stimulated cells and was used instead of specific anti-Rac1 antibodies because these are directed against the Rac1 COOH terminus. These experiments were performed with cell lysates derived from cells in suspension or adherent to collagen to induce Rac1 activation (Price et al., 1998). We found that endogenous β-Pix coprecipitated with the Crib domain of Pak1 in lysates from the adherent cells (i.e., where Rac1 was activated) but not in lysates from the cells in suspension (Fig. 1 D). The latter finding also excludes the possibility that β-Pix interacts with the Pak-Crib domain directly. In addition, pull-down assays with GST-Rhotekin or GST–Wiskott-Aldrich syndrome protein, which bind active Rho or Cdc42, respectively, did not show any interaction with β-Pix (not depicted).
The interaction of β-Pix with Rac1 is mediated by their respective SH3 and COOH-terminal domains
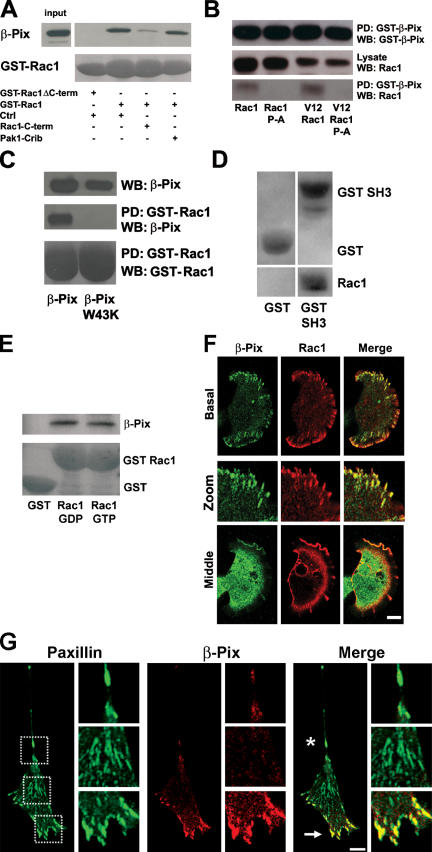
To further characterize the Rac1–β-Pix interaction, we performed pull-down assays with purified GST-Rac1, GST-Rac1ΔC (a Rac1 COOH-terminal deletion mutant; Haeusler et al., 2003), and purified β-Pix in the absence or presence of the Rac1 COOH-terminal peptide or, as a control, the Pak-Crib peptide. Indeed, GST-Rac1 but not GST-Rac1ΔC binds to β-Pix (Fig. 2 A). Moreover, the binding between Rac1 and β-Pix was blocked by the Rac1 COOH-terminal peptide but not by a control peptide or the Pak1-Crib peptide (Fig. 2 A). These data show that the interaction between full-length Rac1 and β-Pix is direct and is mediated via the COOH terminus of Rac1 but not via its effector loop.
Figure 2.
The Rac1–β-Pix interaction is mediated via the β-Pix SH3 domain and the Rac1 COOH terminus. (A) The Rac1–β-Pix interaction is direct and is mediated via the COOH terminus of Rac1. Bacterially purified β-Pix, GST-Rac1, and GST-RacΔC were used in GST pull-down experiments in the presence of control (Ctrl), Rac1 COOH-terminal, or Pak1-Crib peptides. GST-RacΔC does not bind to β-Pix, and the interaction between GST-Rac1 and purified β-Pix is competed only by the COOH-terminal peptide. (B) The Rac1 COOH-terminal proline residues mediate β-Pix binding. Binding of transfected Rac1wt, Rac1 P-A, V12Rac1, or V12Rac1 P-A to GST–β-Pix was analyzed by pull-down (PD) assays followed by Western blotting (WB). (C) The β-Pix SH3 domain mediates binding to Rac1. Bacterially purified GST-Rac1, β-Pix, and β-PixW43K were used in pull-down assays as indicated. (D) The β-Pix SH3 domain is sufficient for Rac1 binding. Pull-down assays were performed with either GST or GST-SH3 in the presence of purified Rac1. (E) Binding of β-Pix to Rac1 is independent of the nucleotide, which is bound to Rac1. COS-7 cells were transfected with a β-PixΔDH mutant, and pull-down assays were performed with GST, GST Rac1-GDP, or GST Rac1-GTPγS. Western blotting was used to detect an association with β-Pix and to detect GST. (F) β-Pix and Rac1 colocalize in FAs and membrane ruffles. HA-Rac1 was expressed in HEK293 cells and detected by immunostaining. Endogenous β-Pix is in green, Rac1 is in red, and colocalization appears in yellow. (G) β-Pix is differentially localized at FAs at the leading edge of polarized cells. NIH3T3 cells were seeded on fibronectin for 18 h, after which they were fixed and stained for endogenous paxillin and β-Pix. Paxillin is in green, β-Pix is in red, and colocalization in the merged images appears in yellow. Arrow indicates the leading edge, and the asterisk indicates the contractile back of the cell. Magnified images of the front, middle, and back of the cell are indicated by the dashed boxes. Bars, 10 μm.
The SH3 domain of β-Pix and a proline stretch in Pak1 mediate the Pak–β-Pix association (Bagrodia et al., 1998; Manser et al., 1998). To determine whether the homologous proline stretch in the COOH terminus of Rac1 is required for the interaction with β-Pix, we generated wild-type (WT) and activated Rac1 mutants in which the proline residues at positions 179, 180, and 181 were mutated to alanines (Rac1 P-A). Pull-down experiments with GST–β-Pix in lysates from COS-7 cells expressing either Rac1, Rac1 P-A, V12Rac1, or V12Rac1 P-A show that the P-A mutants no longer interact with β-Pix. This confirms that the proline stretch in the COOH terminus of Rac1 is required for the interaction with β-Pix and indicates that the interaction between Rac1 and β-Pix is nucleotide independent (Fig. 2 B).
Subsequent pull-down experiments with WT GST-Rac1 in the presence of purified β-Pix or of a β-Pix SH3 domain mutant, β-PixW43K (Bagrodia et al., 1998), showed that β-Pix can only bind Rac1 when the SH3 domain of β-Pix is intact (Fig. 2 C). Moreover, we observed that the isolated β-Pix SH3 domain is sufficient for binding to purified Rac1 (Fig. 2 D). Together, these findings indicate that the direct interaction of Rac1 with β-Pix is mediated via the proline stretch in the COOH terminus of Rac1 and the SH3 domain of β-Pix. Moreover, the Rac1 sequence is specific for the β-Pix SH3 domain because the Rac2 or Cdc42 COOH-terminal peptides, which harbor potential SH3 domain–binding motifs, do not bind β-Pix (Fig. 1 A).
To further show that the interaction between β-Pix and Rac1 is independent of the bound nucleotide, we performed pull-down assays with GST or with GST-Rac1 bound to GDP or GTP. We used COS-7 cell lysates expressing an exchange-deficient β-Pix mutant (Manser et al., 1998) to exclude a potential effect of β-Pix activity on the nucleotide-bound state of Rac1. Fig. 2 E shows that this β-Pix mutant binds both the GDP- and the GTP-bound forms of Rac1 to a similar extent, which shows that the Rac1–β-Pix interaction is independent of the Dbl homology (DH) domain of β-Pix and occurs with both inactive and activated Rac1.
To explore the intracellular localization of the Rac1–β-Pix complex, we transfected HEK293 cells with HA-tagged Rac1 and seeded these cells on fibronectin for 1 h. Immunostainings for endogenous β-Pix and HA-tagged Rac1 showed that in the basal section of the cell, Rac1 and β-Pix colocalize to FAs (Manser et al., 1998; Turner et al., 1999) particularly at the periphery of the cell (Fig. 2 F, basal and zoom). In the middle section of the cell, the two proteins colocalize markedly at the periphery but not in the center and colocalize most prominently in membrane ruffles (Fig. 2 F, middle).
Rac1 is usually localized primarily in the cytosol as a result of its association to RhoGDI. However, activated Rac1 translocates to the plasma membrane in an integrin-dependent fashion (del Pozo et al., 2000). In polarized cells, Rac1 activity has been found in the leading edge, which is in line with its function in actin polymerization, cell protrusion, and motility (Kraynov et al., 2000; Ridley et al., 2003). Given the fact that β-Pix binds Rac1 and, thus, may drive local Rac1 activation, we analyzed β-Pix localization in polarized cells. For these experiments, we cultured NIH3T3 cells on fibronectin-coated coverslips for 18 h. Cells were fixed and immunostained for endogenous β-Pix and for paxillin, a marker for FAs. We found that although paxillin is found in FAs both in the leading edge as well as in the contracting rear of polarized cells, β-Pix is concentrated in FAs in the leading edge and is virtually absent from the paxillin-positive FAs in the cell body or in the rear of the cell (Fig. 2 G). These data suggest that the differential localization of β-Pix in polarized cells drives localized activation of Rac1.
β-Pix–mediated Rac1 activation is dependent on the hypervariable COOH terminus of Rac1
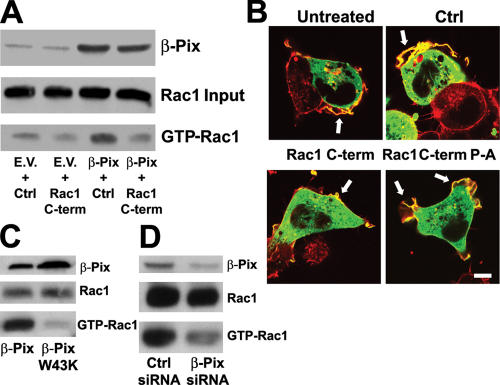
To establish the functional relevance of the Rac1–β-Pix interaction, we generated HEK293 and MDCKII cells that express β-Pix at levels approximately twofold more than endogenous and incubated these cells briefly with the cell-permeable version of the Rac1 COOH-terminal peptide that blocks the Rac1–β-Pix interaction. Rac1 activity was subsequently determined using the Pak-Crib pull-down assay (Price et al., 2003). We found that the exogenous expression of β-Pix resulted in higher levels of active Rac1 in these cells, which was reduced in cells that were incubated with the Rac1 COOH-terminal peptide (Fig. 3 A). Importantly, the Rac1 COOH-terminal peptide does not interfere with the isolation of activated Rac1 by the Pak1-Crib peptide (van Hennik et al., 2003). Furthermore, GFP–β-Pix–induced membrane ruffling in HEK293 cells was almost completely inhibited by the Rac1 COOH-terminal peptide but not by the control peptide or by a Rac1 P-A COOH-terminal peptide (Fig. 3 B), which cannot bind β-Pix (not depicted). Moreover, expression of the β-PixW43K mutant inhibited Rac1 activation compared with the expression of WT β-Pix, again indicating that the SH3 domain of β-Pix is indispensable for β-Pix–mediated Rac1 activation. In addition, when β-Pix–expressing cells were kept in suspension, Rac1 was not activated, suggesting that adhesion to an extracellular matrix is required for β-Pix–mediated Rac1 activation (unpublished data). To further test this, we reduced the levels of β-Pix in mouse embryonal fibroblasts (MEFs) using short inhibitory RNA (siRNA) expression and seeded the cells on fibronectin for 15 min, after which the Rac1 activity was assayed. Indeed, the cells with reduced β-Pix expression also showed reduced Rac1 activity after adhesion (Fig. 3 D), confirming that β-Pix mediates adhesion-induced Rac1 activation.
Figure 3.
Binding of the COOH terminus of Rac1 to β-Pix is important for β-Pix–mediated Rac activation and membrane ruffling. (A) β-Pix–mediated Rac1 activation is dependent on the Rac1 COOH terminus. HEK293 cells transfected with empty vector (EV) or β-Pix were seeded on fibronectin for 1 h and incubated with the control (Ctrl) or Rac1 COOH-terminal peptide (Rac1 C-term) 15 min before lysis. A Pak1-Crib peptide was used to isolate GTP-loaded Rac1, and total Rac1 and β-Pix were detected by Western blotting. (B) β-Pix–induced membrane ruffling is inhibited by the Rac1 COOH-terminal peptide. HEK293 cells were transfected with GFP–β-Pix, seeded on fibronectin, and treated with a control peptide, the Rac1 COOH-terminal peptide, or a Rac1 COOH-terminal peptide in which the prolines were replaced by alanines (Rac1 C-term P-A). Cells were subsequently fixed and stained for β-Pix (green) and F-actin (red). Arrows indicate membrane ruffles. Bar, 10 μm. (C) The SH3 domain of β-Pix is necessary for β-Pix–mediated Rac1 activation. HEK293 cells expressing β-Pix or β-PixW43K were seeded on fibronectin for 15 min before lysis. A biotinylated Pak1-Crib peptide was used to isolate GTP-loaded Rac1. Total Rac1 and β-Pix were detected by Western blotting. (D) β-Pix mediates adhesion-induced Rac1 activation. MEFs were transfected with control siRNA or β-Pix siRNA, and cells were seeded on fibronectin for 15 min before lysis. Active Rac1 was pulled down with a biotinylated Pak1-Crib peptide. Rac1 and β-Pix were detected by Western blotting.
β-Pix can target Rac1 to the plasma membrane
Cell adhesion induces the activation of integrins and recruitment of paxillin (Brown and Turner, 2004), which can associate with the GIT–β-Pix–Pak complex (Turner et al., 1999), possibly driving localized Rac1 activation by β-Pix (Zhao et al., 2000a,b). Our observation that β-Pix binds specifically to Rac1 via its COOH terminus (Fig. 1) suggests that β-Pix mediates the targeting of Rac1 to the membrane. To test this, we used a nonprenylated Rac1 mutant (V12Rac1C189S; del Pozo et al., 2000) that no longer localizes to the plasma membrane but accumulates in the nucleus (Fig. 4 A). However, when this Rac1 mutant is cotransfected with β-Pix, membrane localization of Rac1 is restored (Fig. 4 A). Furthermore, the membrane recruitment of V12Rac1C189S by β-Pix is blocked by the cell-permeable Rac1 COOH-terminal peptide (Fig. 4 A). This shows that the Rac1 COOH terminus by itself is not sufficient for the targeting of full-length nonprenylated Rac1 to the plasma membrane but that the interaction with β-Pix is both necessary and sufficient for the membrane targeting of Rac1, even in the absence of a lipid anchor.
Figure 4.
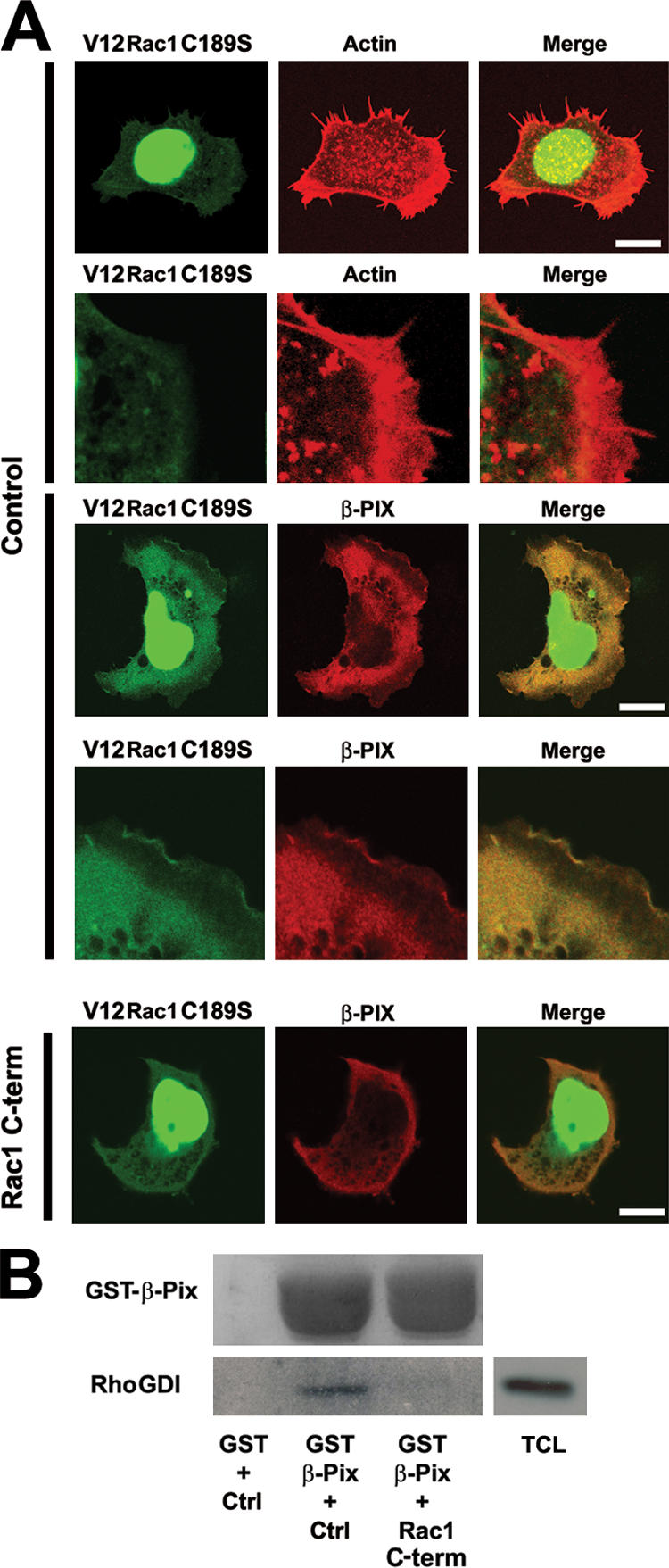
The Rac1–β-Pix interaction mediates Rac1 targeting to the plasma membrane. (A) The nongeranylgeranylated V12Rac1C189S mutant is targeted to the plasma membrane in β-Pix–expressing cells. HEK293 cells were transfected with GFPV12Rac1C189S (green) with or without myc–β-Pix (red) and were seeded on fibronectin for 1 h. Cotransfection of β-Pix results in the detection of GFPV12Rac1C189S in membrane ruffles, which is blocked by the Rac1 COOH-terminal peptide (bottom three panels). Bars, 10 μm. (B) Rac1, β-Pix, and RhoGDI can form a trimeric complex. Pull-down experiments were performed with GST or GST–β-Pix in COS-7 cell lysates in the presence of control (Ctrl) or the COOH-terminal Rac1 peptide (Rac1 C-term). RhoGDI and GST–β-Pix were detected by immunoblotting. TCL, total cell lysate.
In the cytosol, Rac1 is tightly bound to RhoGDI, and, therefore, it is likely that RhoGDI is either released from Rac1 before Rac1 targeting to the membrane or that Rac1 and RhoGDI are targeted as a complex. Based on structural analysis, it was suggested that the COOH terminus of Rac1 is not required for the interaction of Rac1 with RhoGDI (Di Poi et al., 2001; Grizot et al., 2001). This, in turn, suggests that Rac1 can bind β-Pix through its COOH terminus even when complexed to RhoGDI. In line with this, GST–β-Pix pull-down experiments showed that RhoGDI indeed complexes with β-Pix. This interaction is blocked by the COOH-terminal Rac1 peptide (Fig. 2 B), indicating that the β-Pix–RhoGDI interaction is mediated via Rac1. Of note, the COOH-terminal Rac1 peptide does not bind RhoGDI and does not interfere with the Rac1–RhoGDI interaction (van Hennik et al., 2003; unpublished data). These data suggest that Rac1 can be targeted to the membrane by β-Pix while in complex with RhoGDI.
The COOH-terminal prolines in Rac1 mediate localization to FAs and are involved in Rac1-mediated spreading
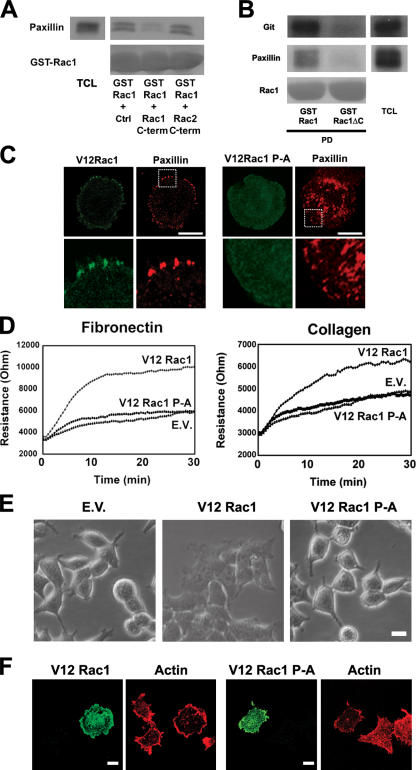
To further establish the Rac1 targeting to a β-Pix–GIT–paxillin complex, we performed GST-Rac1 pull-down experiments with lysates of COS-7 cells and found that endogenous paxillin associates with Rac1 (Fig. 5 A). This interaction is inhibited by the Rac1 COOH-terminal peptide but not by the Rac2 COOH-terminal peptide or by a control peptide (Fig. 5 A). Furthermore, we observed that GST-Rac1ΔC no longer associates with endogenous paxillin or PKL/GIT (Fig. 5 B). These data indicate that FA targeting of Rac1 is mediated by the β-Pix–GIT–paxillin complex and is dependent on the Rac1 COOH terminus. In line with this result, we found that V12Rac1 localizes to paxillin-containing FAs in spreading HEK293 cells, which is in contrast to the V12Rac1 P-A mutant (Fig. 5 C). Interestingly, the distribution of FAs in spreading cells that express V12Rac1 P-A appeared disorganized compared with the cells expressing V12Rac1. Moreover, the V12Rac1 P-A mutant showed no discrete localization in these cells, suggesting that the targeting of Rac1 to FAs requires their proper organization as well as an intact Rac1 COOH terminus.
Figure 5.
Rac1 targeting to FAs is important for Rac1-mediated cell spreading. (A) The Rac1–paxillin interaction can be inhibited by the Rac1 COOH-terminal peptide. Pull-down experiments with GST-Rac1 in COS-7 cell lysates was performed in the presence of 100 μg of the indicated peptides. Paxillin was detected by immunoblotting. TCL, total cell lysate. (B) The association of Rac1 with paxillin and GIT is mediated by the Rac1 COOH terminus. Pull-down (PD) experiments were performed with COS-7 cell lysates using GST-Rac1 or GST-Rac1ΔC. Paxillin and GIT were detected by immunoblotting. (C) Rac1 colocalization with paxillin in spreading cells is dependent on the Rac1 COOH-terminal proline residues. HEK293 cells were transfected with HA-tagged V12Rac1 or V12Rac1 P-A and stained with antibodies to the HA immunotag (green) and to paxillin (red). The boxed areas are magnified in the corresponding bottom panels. (D) Rac1-mediated cell spreading is dependent on the proline stretch in the COOH terminus of Rac1. HEK293 cells stably expressing empty vector (EV), V12Rac1, or V12Rac1 P-A were seeded on fibronectin- or collagen-coated gold electrodes, and resistance was measured for 30 min. n = 75 (per line). (E) HEK293 cells stably expressing empty vector, V12Rac1, or V12Rac1 P-A were seeded on collagen dishes and fixed after 30 min. Images were obtained with phase-contrast microscopy. (F) HEK293 transiently expressing V12Rac1 or V12 Rac1 P-A were seeded on collagen for 1 h. Cells were fixed and immunostained for Rac1 (green) and actin (red). Bars, 10 μm.
To assess whether the spreading of V12Rac1- or V12Rac1 P-A–expressing cells is quantitatively different, we seeded HEK293 cells expressing either empty vector, V12Rac1, or V12Rac1 P-A on fibronectin or collagen and monitored their spreading by means of electrical cell substrate impedance sensing (ECIS; Schmidt et al., 2003). This technique allows real-time analysis of cell spreading on gold electrodes coated with extracellular matrix proteins. V12Rac1-transfected cells spread two- to threefold more efficiently compared with the V12Rac1 P-A mutant or empty vector–transfected cells (Fig. 5 D). This was confirmed by phase-contrast imaging (Fig. 5 E) as well as by staining for F-actin (Fig. 5 F). Together, these data show that Rac1 binding to β-Pix is important for Rac1 targeting to FAs and for efficient Rac1-mediated cell spreading on extracellular matrix proteins.
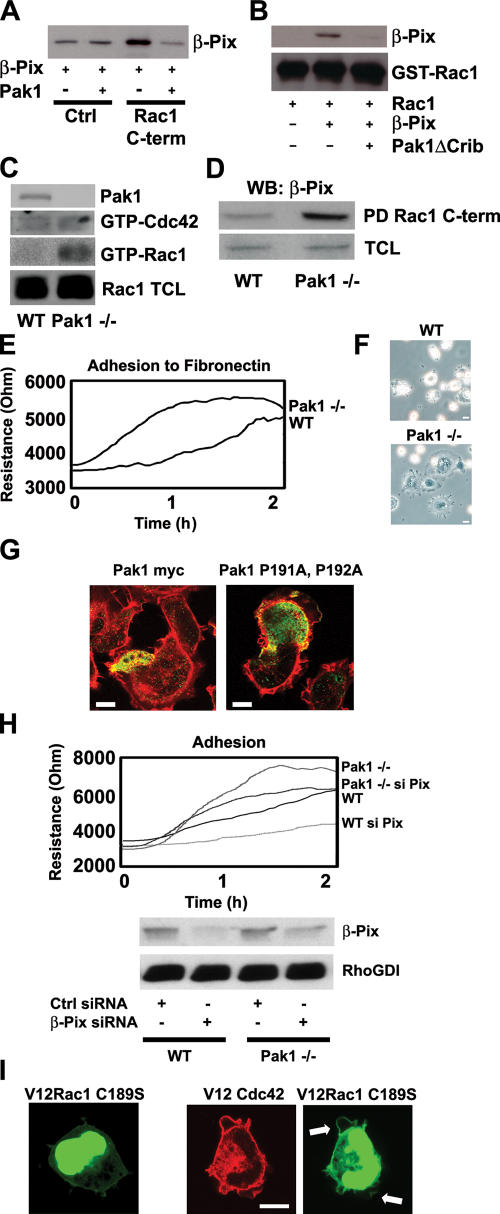
Rac1 and Pak1 are competitive interactors for β-Pix
β-Pix interacts via its SH3 domain not only with Rac1 (Fig. 2) but also with Pak1 (Bagrodia et al., 1998; Manser et al., 1998). This suggests that Rac1 and Pak1 may compete for β-Pix binding, which could represent a means of regulating Rac1 binding to β-Pix. Therefore, association of the Rac1 COOH-terminal peptide with purified GST–β-Pix was analyzed in the presence or absence of purified GST-Pak1. The data show that GST–β-Pix directly binds to the Rac1 COOH-terminal peptide (Fig. 6 A, third lane) and that GST-Pak1 inhibits this interaction (Fig. 6 A, fourth lane). It is noteworthy that the Rac1 peptide does not interact with Pak1 (not depicted). Furthermore, we found that Pak1ΔCrib, a Pak1 mutant that cannot bind active Rac1 but can associate to the SH3 domain of β-Pix, blocks the interaction between full-length Rac1 and β-Pix (Fig. 6 B). These data show that Rac1 and Pak1 compete for binding to β-Pix.
Figure 6.
Pak1 regulates the interaction of Rac1 or Cdc42 with β-Pix. (A) Direct binding of the Rac1 COOH terminus to β-Pix is inhibited by Pak1. GST–β-Pix and GST-Pak1 were incubated with control (Ctrl) or Rac1 COOH-terminal peptides (Rac1 C-term). After peptide pull-down, β-Pix was detected by immunoblotting. The bands in the first and second lanes represent a specific binding of GST–β-Pix. (B) Pak1 inhibits the interaction of full-length Rac1 with β-Pix. GST-Rac1, GST–β-Pix, and GST-Pak1ΔCrib were purified from bacteria. GST-Rac1 was loaded with GTPγS, and pull-down experiments were performed with a biotinylated Pak1-Crib peptide in the presence or absence of GST–β-Pix and GST-Pak1ΔCrib. β-Pix binding and GST-Rac1 loading were detected by immunoblotting. (C) Pak1 specifically regulates Rac1 activity. WT and Pak1−/− cells were seeded on fibronectin-coated dishes for 30 min before lysis. A Pak1-Crib peptide was used to isolate GTP-loaded Rac1 as well as to isolate GTP-Cdc42. Pak1, Cdc42, and Rac1 were detected by Western blotting. TCL, total cell lysate. (D) Pak1 and Rac1 are competitive binders for β-Pix. WT and Pak1−/− cells were seeded on fibronectin-coated dishes for 30 min before lysis. Pull-down (PD) assays were performed with the COOH-terminal Rac1 peptide, and β-Pix binding was detected by Western blotting (WB). (E) Loss of Pak1 results in enhanced cell spreading. WT and Pak1−/− cells were seeded on fibronectin-coated ECIS electrodes, and resistance was measured for 2 h. (F) Loss of Pak1 expression induces cell spreading. WT and Pak1−/− cells were seeded on fibronectin-coated dishes and fixed after 15 min. Images were obtained by phase-contrast microscopy. (G) Pak1-dependent cell spreading is mediated by β-Pix. Pak1−/− cells were transfected with myc-tagged Pak1 or Pak1 P191A and P192A and seeded on fibronectin for 15 min before fixation. Pak1 is indicated in green, and F-actin is indicated in red. (H) β-Pix regulates cell spreading. WT and Pak1−/− MEFs were transfected with control (Ctrl) or β-Pix siRNA and seeded on fibronectin-coated ECIS electrodes. β-Pix and RhoGDI expression were determined by Western blotting. (E and H) n = 300 (per line). (I) V12Cdc42 induces translocation of the nongeranylgeranylated V12Rac1C189S mutant to the plasma membrane. HEK293 cells were transfected with GFPV12Rac1C189S (green) and cotransfected or left untransfected with V12Cdc42 (red). Arrows indicate the membrane-targeted V12Rac1C189S. Bars, 10 μm.
The competitive binding of Rac1 and Pak1 to β-Pix suggests that in the absence of Pak1, more β-Pix–Rac1 complexes could be formed, resulting in higher levels of activated Rac1. To test this, we used immortalized MEFs that were derived from Pak1-null (Pak1−/−) and WT (Pak+/+) mice and tested the Rac1 activity in these cells after seeding on fibronectin. We found that there is clearly more activated Rac1 present in the Pak1−/− cells compared with the WT cells (Fig. 6 C). The low but detectable levels of GTP-Cdc42 are similar in the Pak1−/− cells versus the WT cells (Fig. 6 C). Subsequent pull-down assays with the COOH-terminal Rac1 peptide showed that more β-Pix associates to the Rac1 COOH terminus in the Pak1−/− cells compared with the WT cells (Fig. 6 D). This indicates that in the absence of Pak1, there is more β-Pix available for Rac1 binding, resulting in increased Rac1 activation.
The increased levels of activated Rac1 in the Pak1−/− cells suggest that these cells will spread more efficiently than the WT cells (Bishop and Hall, 2000). We tested this by seeding the WT and Pak1−/− cells on fibronectin-coated ECIS electrodes. In agreement with the aforementioned hypothesis, we found that the Pak1−/− cells spread more efficiently than the WT cells (Fig. 6 E). To confirm this, we seeded cells on fibronectin (Fig. 6 F) or collagen (not depicted) and visualized the cells' morphology by phase-contrast microscopy. The Pak1−/− cells did indeed spread more efficiently, forming clearly detectable lamellipodia, whereas most WT cells were round with small membrane extensions (Fig. 6 F). Furthermore, reconstitution experiments with Pak1 WT or Pak1 P191A and P192A, Pak1 mutants that do not bind β-Pix (Manser et al., 1998), showed that the enhanced spreading of Pak1−/− cells can be restored with WT Pak1 but not by the Pix-binding mutant (Fig. 6 G). These data suggest that Pak1 controls cell spreading by regulating the β-Pix–Rac1 interaction and, as a result, β-Pix–mediated Rac1 activation. To further confirm this, we reduced the expression of β-Pix by means of siRNA in WT and Pak1−/− cells and analyzed their spreading on fibronectin-coated ECIS electrodes. The enhanced spreading of Pak1−/− cells was reduced when β-Pix expression is knocked down (Fig. 6 H). Similarly, reducing the expression of β-Pix in WT cells also impaired their spreading on fibronectin (Fig. 6 H). This indicates that β-Pix plays a pivotal role in the enhanced spreading of the Pak1−/− and WT cells.
The interaction between Pak1 and β-Pix is negatively regulated by the autophosphorylation of Pak1 (Zhao et al., 2000a; Mott et al., 2005). Because activated Cdc42 promotes Pak1 autophosphorylation and activation (Manser et al., 1994), Cdc42 could induce the dissociation of Pak1 from β-Pix to allow Rac1–β-Pix binding and targeting of Rac1 to the membrane. In line with this, we observed that V12Cdc42 induced the translocation of V12Rac1C189S to the plasma membrane. This confirms that Cdc42 activation promotes Rac1 membrane targeting (Fig. 6 I), which is consistent with the observation that the expression of V12Cdc42 results in the activation of Rac1 (Nobes and Hall, 1995).
Discussion
In this study, we describe a novel mode of interaction between a Rho-like GTPase and its GEF (i.e., Rac1 and β-Pix). We show that Rac1 interacts specifically and directly through its hypervariable COOH-terminal domain with the SH3 domain of β-Pix (Figs. 1 and 2). The data further show that Rac1 and its effector Pak1 act as competitors for β-Pix binding. Importantly, this Rac1–β-Pix interaction is required for Rac1 targeting to the membrane and to FAs, which supports the notion that β-Pix plays an important role in the integrin-mediated recruitment and localized activation of Rac1.
Rho-like GTPases signal at membranes, where the relevant GEFs as well as their downstream targets are localized. A recent in vivo study has demonstrated that the COOH termini of Rac1 and Rac2 dictate both subcellular localization and differential signaling (Filippi et al., 2004). Earlier studies had already shown that the COOH termini of a large number of small GTPases mediate differential intracellular localization, which is suggestive of targeting through specific protein interactions (Michaelson et al., 2001; van Hennik et al., 2003). Our finding that the COOH terminus of Rac1 mediates specific protein–protein interactions that regulate its intracellular targeting and signaling may therefore apply to many, if not all, small GTPases. Indeed, recent studies in our laboratory show that the COOH termini of the different Rho-GTPases have their own, only partially overlapping repertoire of specific interactors (unpublished data).
As for R-Ras and Nck (Wang et al., 2000) or Cdc42/Rac1 and CAPRI (Zhang et al., 2005), the interaction between Rac1 and β-Pix is nucleotide independent. In line with this finding, we found no competition of the Rac1–β-Pix interaction by the Crib domain of Pak1, which binds activated Rac1. Moreover, we found that Rac1 also associates with a GEF-deficient form of β-Pix. In addition, the Rac P-A mutant, which does not bind β-Pix, was not targeted to paxillin-containing FAs and was no longer able to promote adhesion-induced cell spreading. Finally, Rac1 activation by β-Pix was abrogated when their interaction was blocked by the Rac1 COOH-terminal peptide or when the β-Pix W43K mutant was used. Together, these results indicate that binding of the Rac1 COOH terminus to the SH3 domain of β-Pix is essential for Rac1 targeting and for subsequent localized activation.
In resting cells, Rho GTPases are associated with RhoGDI, which retains the GTPases in their inactive state in the cytosol (Bishop and Hall, 2000). The fact that we could isolate a GDI–Rac–β-Pix complex suggests that GDI may be recruited along with Rac1 to the plasma membrane and to FAs, where it will dissociate from Rac1 to allow β-Pix–mediated nucleotide exchange. RhoGDI is likely to sterically hinder nucleotide exchange because it binds the switch I and II regions of Rac1, which are also required for interaction with the DH–pleckstrin homology domain of the Rac1 GEF Tiam1 and likely for other GEFs as well (Di Poi et al., 2001; Karnoub et al., 2001). In addition, association to GDI blocks Rac1 effector binding (del Pozo et al., 2002), preventing signaling before GDI dissociation and activation. The Rac1 effector Pak1 has been implicated in Rac1 activation, as it can phosphorylate RhoGDI, leading to its dissociation from Rac1 (DerMardirossian et al., 2004). However, our finding that Rac1 activity is increased in Pak1−/− cells suggests that alternative means or kinases exist to dissociate Rac1 from RhoGDI.
The notion that β-Pix interacts with Pak1 through its SH3 domain (Bagrodia et al., 1998; Manser et al., 1998) suggests that Rac1 and Pak1 act as competitive binding partners for β-Pix. Indeed, we observed that purified WT Pak1 as well as a Pak1 mutant that does not bind activated Rac1 interferes with the β-Pix–Rac1 interaction in vitro. In line with this, we found more β-Pix binding to the COOH terminus of Rac1 and, consequently, more Rac1 activation in Pak1−/− cells. Interestingly, the Pak1−/− cells show an increase in cell spreading, suggesting that Pak1 is not essential for Rac1-induced cell spreading. It is unknown how the competition of Rac1 and Pak1 for binding to the SH3 domain of β-Pix is regulated. However, activation and autophosphorylation of Pak1 blocks its binding to β-Pix (Zhao et al., 2000a; Mott et al., 2005) and leads to its dissociation from FAs (Zhao et al., 2000a). This indicates that Pak activation would allow the association of Rac1 to β-Pix. Pak autophosphorylation can be induced by activated Cdc42 (Manser et al., 1994), suggesting that the Pak1–β-Pix complex mediates Cdc42-induced Rac1 activation (Nobes and Hall, 1995). Activated Cdc42, which localizes to the leading edge of motile cells (Nalbant et al., 2004), has also been shown to recruit β-Pix to peripheral focal contacts (Manser et al., 1998). In agreement with these findings, we found that V12RacC189S is recruited from the nucleus to the membrane both by β-Pix as well as by activated Cdc42.
β-Pix is found at FAs and associates through PKL with the FA protein paxillin. We found β-Pix in particular at FAs in the leading edge of polarized cells, which corresponds with earlier findings on the localized activation of Rac1 in the front of migrating cells (Kraynov et al., 2000). Intriguingly, we observed that paxillin-positive FAs in the center and at the rear of the cell were virtually devoid of β-Pix. This suggests that leading edge FAs have a preference for recruiting β-Pix. These findings are in agreement with earlier results showing the preferential localization of activated Pak1 in lamellipodia and FAs in the leading edge of motile fibroblasts (Sells et al., 2000). Furthermore, we show that reducing the levels of β-Pix results in less adhesion-induced Rac1 activation and cell spreading, indicating that β-Pix is pivotal for integrin-mediated Rac1 activation (del Pozo et al., 2004), specifically at the leading edge of cells. Nishiya et al. (2005) recently showed that phosphorylation of α4 integrins in the leading edge of polarized cells modulates their association with paxillin. As a result, paxillin and the ArfGAP PKL/GIT become preferentially associated with α4 integrins in the back of the cell. The ArfGAP was shown to be essential for inhibiting Arf6 and Rac activation in the lateral side and the rear of the cell. These findings are in agreement with our data on the preferential localization of β-Pix in FAs at the leading edge, albeit that other paxillin-binding integrins may be important in these cells because the α4 integrin is expressed at low levels in fibroblasts. Together, these differential integrin-dependent signaling cascades may explain the polarized activation of Rac1 in the leading edge of migrating cells.
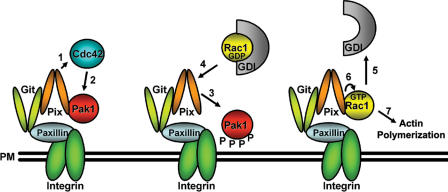
In conclusion, our data support a model (Fig. 7) in which integrin engagement leads to the activation of Cdc42 in the leading edge of motile cells. Activated Cdc42 will subsequently activate FA-associated Pak1, which is bound to β-Pix. Activated Pak dissociates from β-Pix, allowing Rac1 recruitment to FAs through the β-Pix SH3 domain. This is then followed by Rac1 activation, which will trigger actin polymerization, membrane protrusion, and directional cell migration.
Figure 7.
Model for β-Pix–mediated membrane/FA targeting and activation of Rac1. Activation of Cdc42 at the plasma membrane (PM), e.g., by β-Pix (1), will lead to the activation of Pak1 (2), resulting in its autophosphorylation. Activated Pak1 dissociates from the β-Pix–GIT complex (3), enabling the Rac1–RhoGDI complex to associate with the β-Pix–GIT complex (4). Subsequently, the release of RhoGDI from Rac1 (5) enables the activation of Rac1 by β-Pix (6) and allows Rac1-mediated actin polymerization (7). See Discussion for details. (middle) P, protein phosphorylation event.
Materials and methods
Antibodies and constructs
The following antibodies were used: HA tag (12CA5) was obtained from Boehringer; myc tag and β-Pix were purchased from Santa Cruz Biotechnology, Inc.; and Rac1 (610651), paxillin (610051), and GIT (611388) were obtained from Transduction Laboratories. F-actin was stained with rhodamine-labeled phalloidin (Invitrogen).
Myc–β-Pix WT and W43K (Bagrodia et al., 1998) were cloned in pEGFP-C1 (CLONTECH Laboratories, Inc.) using BamHI and EcoRI restriction sites and in GST-6P-1 (27–4597-01; GE Healthcare) using BamHI and NotI. The exchange-deficient β-Pix mutant was a gift from E. Manser (Institute of Molecular and Cell Biology, Singapore). Rac1-HA (Guthrie Healthcare System), V12Rac1C189S (gift from M.A. Schwartz, University of Virginia, Charlottesville, VA; del Pozo et al., 2000), V12Cdc42-myc (Nobes and Hall, 1995), GST-Rac1 and GST-Rac1ΔC (gift from R. Ahmadian, European Molecular Biology Laboratory, Heidelberg, Germany; Haeusler et al., 2003), GST-Pak1 (Zhao et al., 1998), and GST-Pak1ΔCrib (Reeder et al., 2001) and Rac1 P-A mutants were generated with the QuikChange Site-Directed Mutagenesis Kit (200518; Stratagene). The sequences for β-Pix siRNA was GUCCCAGGAUGAGUGGUUU (Eurogentec) and for control siRNA was AUCAUUAGCAUCAAACGUC (Eurogentec).
Peptide synthesis
Synthetic peptides were synthesized on a peptide synthesizer (Syro II; MultiSynTech) using Fmoc solid phase chemistry and encoded a protein transduction domain (Biotin-YARAAARQARAG; Soderling et al., 2002) followed by the 10 amino acids preceding the CAAX motif of Rac1, Rac2, Rac3, RhoA, RhoB, and Cdc42. The sequence of the Rac1 P-A peptide is CAAAVKKRKRK.
Cell culture and transfections
The HEK293, MDCKII, and COS-7 cell lines were maintained in Iscove's Modified Dulbecco's Medium (IMDM; BioWhittaker) containing 10% heat-inactivated FCS (Invitrogen) at 37°C and 5% CO2. Cells were passaged by trypsinization. HEK293 and COS-7 cells were transiently transfected with FuGENE (Roche). For this, we mixed 2 μg DNA and 6 μl FuGENE in 100 μl IMDM that was incubated for 15 min at RT. Subsequently, 2 ml IMDM with FCS containing 500,000 cells were added to the DNA–FuGENE mixture, which was then incubated in a six-well plate for 6 h. Transfections of siRNAs were performed with LipofectAMINE 2000 (Invitrogen). In short, 25 μl siRNA (100 μM) and 25 μl LipofectAMINE 2000 were separately diluted in 1,250 μl IMDM, after which the two solutions were mixed and incubated for 30 min at RT. Subsequently, 10 ml of cell suspension (500,000 cells/ml in IMDM/10% FCS) was added, and cells were plated in a 10-cm dish. After 6 h, the medium was replaced with fresh IMDM/10% FCS. HEK293 and MDCKII cells with stable expression of β-Pix, V12Rac1, and V12Rac1 P-A were generated by retroviral transduction. Transfection and production of amphotropic retroviruses were described previously (Michiels et al., 1995). MEFs were isolated from day 13.5 WT or Pak1−/− embryos. WT and Pak1−/− MEFs were immortalized with SV40 T antigen and were maintained in DME supplemented with 10% FBS.
Pull-down assays
To assay the binding of β-Pix, 10 × 106 HEK293 or COS-7 cells were seeded 1 d before the experiment. Cells were lysed in lysis buffer A (50 mM Tris-HCl, pH 7.5, 400 mM NaCl, 10 mM MgCl2, 10% glycerol, 1% NP-40, and 0.1% SDS) and centrifuged for 10 min at 14,000 rpm and 4°C. The supernatant was then incubated with the indicated COOH-terminal peptides (5 μg) in the presence of streptavidin-coated beads (Sigma-Aldrich) at 4°C for 1 h while rotating. Beads were washed five times in lysis buffer B (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10 mM MgCl2, 10% glycerol, and 1% NP-40) and resuspended in 25 μl SDS sample buffer. β-Pix association was determined by Western blot analysis.
To determine Rac activity, 5 × 106 HEK293 cells were seeded on fibronectin-coated dishes for 1 h. After a 15-min incubation with the different COOH-terminal peptides (200 μg/ml), GTP-bound Rac1 was isolated with biotinylated Pak1-Crib peptide (Price et al., 2003). Rac1 binding was detected by Western blotting.
GST fusion proteins were purified from BL21 bacteria. After overnight culture, protein expression was induced with 0.1 mM IPTG for 4 h at 37°C. Bacteria were centrifuged and resuspended in PBS, 1% Triton X-100, and 10% glycerol and lysed by sonication (2 × 30 s; duty cycle of 50% and output of 6; Sonifier 250; Branson). Lysates were cleared by centrifugation for 15 min at 14,000 rpm and 4°C. GST fusion proteins were isolated by glutathione-coated beads while rotating head over head at 4°C for 30 min. Samples were then washed five times with lysis buffer B and used as indicated (25 μg per pull-down). GST tag was cleaved from GST–β-Pix and GST–β-PixW43K when indicated with PreScission Protease (27–0843-01; GE Healthcare). For competition experiments, we used 100 μg of peptide and 25 μg Pak protein.
Confocal and phase-contrast microscopy
24 h after transfection, cells were seeded for the indicated time on either fibronectin- or collagen-coated glass coverslips and fixed by 3.7% formaldehyde (Merck) in PBS for 5 min and permeabilized with 0.5% Triton X-100 in PBS. Immunostainings were performed at 37°C for 1 h with the indicated antibodies. Fluorescent imaging was performed with a confocal laser scanning microscope (Axiovert 100 M; Carl Zeiss MicroImaging, Inc.) using a 63×/NA 1.40 oil lens (Carl Zeiss MicroImaging, Inc.). Image acquisition was performed with LSM 510 software (Carl Zeiss MicroImaging, Inc.). For phase-contrast microscopy, cells were seeded on fibronectin- or collagen-coated dishes and fixed at the indicated time points. Images were obtained with a microscope (model DMIL; Leica), L20/0.30 PH1 lens, a camera (DC 300; Leica), and IM500 software (Leica).
Electrical resistance measurements
For ECIS-based adhesion experiments, gold ECIS electrodes were coated with either fibronectin or collagen in 0.9% NaCl for 1 h at 37°C. Next, HEK293 or MEF cells were seeded at a concentration of 400,000 cells per well in 400 μl IMDM with 10% FCS. ECIS was subsequently monitored for up to 2 h with the ECIS equipment (Applied Biophysics).
Acknowledgments
We would like to thank M.A. Schwartz and M.R. Ahmadian for Rac1 cDNAs and E. Manser for the inactive mutant of β-Pix.
J.P. ten Klooster is supported by the Landsteiner Foundation of Blood Transfusion Research (grant 203). Z.M. Jaffer and J. Chernoff are supported by the National Institutes of Health (grant GM54168). P.L. Hordijk is a fellow of the Landsteiner Foundation of Bloodtransfusion Research (grant 112).
Abbreviations used in this paper: DH, Dbl homology; ECIS, electrical cell substrate impedance sensing; FA, focal adhesion; GAP, GTPase-activating protein; GDI, guanine nucleotide dissociating inhibitor; GDP, guanosine diphosphate; GEF, guanine nucleotide exchange factor; HEK293, human embryonal kidney 293; MEF, mouse embryonal fibroblast; Pak, p21-activated kinase; Pix, Pak-interacting exchange factor; PKL, paxillin kinase linker; siRNA, short inhibitory RNA; WT, wild type.
References
- Bagrodia, S., S.J. Taylor, K.A. Jordon, L. Van Aelst, and R.A. Cerione. 1998. A novel regulator of p21-activated kinases. J. Biol. Chem. 273:23633–23636. [DOI] [PubMed] [Google Scholar]
- Bagrodia, S., D. Bailey, Z. Lenard, M. Hart, J.L. Guan, R.T. Premont, S.J. Taylor, and R.A. Cerione. 1999. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem. 274:22393–22400. [DOI] [PubMed] [Google Scholar]
- Bishop, A.L., and A. Hall. 2000. Rho GTPases and their effector proteins. Biochem. J. 348:241–255. [PMC free article] [PubMed] [Google Scholar]
- Brown, M.C., and C.E. Turner. 2004. Paxillin: adapting to change. Physiol. Rev. 84:1315–1339. [DOI] [PubMed] [Google Scholar]
- del Pozo, M.A., L.S. Price, N.B. Alderson, X.D. Ren, and M.A. Schwartz. 2000. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19:2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo, M.A., W.B. Kiosses, N.B. Alderson, N. Meller, K.M. Hahn, and M.A. Schwartz. 2002. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 4:232–239. [DOI] [PubMed] [Google Scholar]
- del Pozo, M.A., N.B. Alderson, W.B. Kiosses, H.H. Chiang, R.G. Anderson, and M.A. Schwartz. 2004. Integrins regulate Rac targeting by internalization of membrane domains. Science. 303:839–842. [DOI] [PubMed] [Google Scholar]
- DerMardirossian, C., A. Schnelzer, and G.M. Bokoch. 2004. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol. Cell. 15:117–127. [DOI] [PubMed] [Google Scholar]
- Di Poi, N., J. Faure, S. Grizot, G. Molnar, E. Pick, and M.C. Dagher. 2001. Mechanism of NADPH oxidase activation by the Rac/Rho-GDI complex. Biochemistry. 40:10014–10022. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and A. Hall. 2003. Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr. Opin. Cell Biol. 15:67–72. [DOI] [PubMed] [Google Scholar]
- Ezratty, E.J., M.A. Partridge, and G.G. Gundersen. 2005. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7:581–590. [DOI] [PubMed] [Google Scholar]
- Filippi, M.D., C.E. Harris, J. Meller, Y. Gu, Y. Zheng, and D.A. Williams. 2004. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat. Immunol. 5:744–751. [DOI] [PubMed] [Google Scholar]
- Fukata, M., T. Watanabe, J. Noritake, M. Nakagawa, M. Yamaga, S. Kuroda, Y. Matsuura, A. Iwamatsu, F. Perez, and K. Kaibuchi. 2002. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 109:873–885. [DOI] [PubMed] [Google Scholar]
- Geiger, B., A. Bershadsky, R. Pankov, and K.M. Yamada. 2001. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2:793–805. [DOI] [PubMed] [Google Scholar]
- Grizot, S., J. Faure, F. Fieschi, P.V. Vignais, M.C. Dagher, and E. Pebay-Peyroula. 2001. Crystal structure of the Rac1-RhoGDI complex involved in nadph oxidase activation. Biochemistry. 40:10007–10013. [DOI] [PubMed] [Google Scholar]
- Haeusler, L.C., L. Blumenstein, P. Stege, R. Dvorsky, and M.R. Ahmadian. 2003. Comparative functional analysis of the Rac GTPases. FEBS Lett. 555:556–560. [DOI] [PubMed] [Google Scholar]
- Itoh, R.E., K. Kurokawa, Y. Ohba, H. Yoshizaki, N. Mochizuki, and M. Matsuda. 2002. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 22:6582–6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnoub, A.E., D.K. Worthylake, K.L. Rossman, W.M. Pruitt, S.L. Campbell, J. Sondek, and C.J. Der. 2001. Molecular basis for Rac1 recognition by guanine nucleotide exchange factors. Nat. Struct. Biol. 8:1037–1041. [DOI] [PubMed] [Google Scholar]
- Kranenburg, O., M. Poland, M. Gebbink, L. Oomen, and W.H. Moolenaar. 1997. Dissociation of LPA-induced cytoskeletal contraction from stress fiber formation by differential localization of RhoA. J. Cell Sci. 110:2417–2427. [DOI] [PubMed] [Google Scholar]
- Kraynov, V.S., C. Chamberlain, G.M. Bokoch, M.A. Schwartz, S. Slabaugh, and K.M. Hahn. 2000. Localized Rac activation dynamics visualized in living cells. Science. 290:333–337. [DOI] [PubMed] [Google Scholar]
- Lim, C.S., S.H. Kim, J.G. Jung, J.K. Kim, and W.K. Song. 2003. Regulation of SPIN90 phosphorylation and interaction with Nck by ERK and cell adhesion. J. Biol. Chem. 278:52116–52123. [DOI] [PubMed] [Google Scholar]
- Manser, E., T. Leung, H. Salihuddin, Z.S. Zhao, and L. Lim. 1994. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 367:40–46. [DOI] [PubMed] [Google Scholar]
- Manser, E., T.H. Loo, C.G. Koh, Z.S. Zhao, X.Q. Chen, L. Tan, I. Tan, T. Leung, and L. Lim. 1998. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell. 1:183–192. [DOI] [PubMed] [Google Scholar]
- Michaelson, D., J. Silletti, G. Murphy, P. D'Eustachio, M. Rush, and M.R. Philips. 2001. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 152:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels, F., G.G. Habets, J.C. Stam, R.A. van der Kammen, and J.G. Collard. 1995. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 375:338–340. [DOI] [PubMed] [Google Scholar]
- Mott, H.R., D. Nietlispach, K.A. Evetts, and D. Owen. 2005. Structural analysis of the SH3 domain of beta-PIX and its interaction with alpha-p21 activated kinase (PAK). Biochemistry. 44:10977–10983. [DOI] [PubMed] [Google Scholar]
- Nalbant, P., L. Hodgson, V. Kraynov, A. Toutchkine, and K.M. Hahn. 2004. Activation of endogenous Cdc42 visualized in living cells. Science. 305:1615–1619. [DOI] [PubMed] [Google Scholar]
- Nishiya, N., W.B. Kiosses, J. Han, and M.H. Ginsberg. 2005. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat. Cell Biol. 7:343–352. [DOI] [PubMed] [Google Scholar]
- Nobes, C.D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 81:53–62. [DOI] [PubMed] [Google Scholar]
- Olofsson, B. 1999. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell. Signal. 11:545–554. [DOI] [PubMed] [Google Scholar]
- Price, L.S., J. Leng, M.A. Schwartz, and G.M. Bokoch. 1998. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell. 9:1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, L.S., M. Langeslag, J.P. ten Klooster, P.L. Hordijk, K. Jalink, and J.G. Collard. 2003. Calcium signaling regulates translocation and activation of Rac. J. Biol. Chem. 278:39413–39421. [DOI] [PubMed] [Google Scholar]
- Reeder, M.K., I.G. Serebriiskii, E.A. Golemis, and J. Chernoff. 2001. Analysis of small GTPase signaling pathways using p21-activated kinase mutants that selectively couple to Cdc42. J. Biol. Chem. 276:40606–40613. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., M.A. Schwartz, K. Burridge, R.A. Firtel, M.H. Ginsberg, G. Borisy, J.T. Parsons, and A.R. Horwitz. 2003. Cell migration: integrating signals from front to back. Science. 302:1704–1709. [DOI] [PubMed] [Google Scholar]
- Rossman, K.L., C.J. Der, and J. Sondek. 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6:167–180. [DOI] [PubMed] [Google Scholar]
- Schmidt, M.H., B. Chen, L.M. Randazzo, and O. Bogler. 2003. SETA/CIN85/Ruk and its binding partner AIP1 associate with diverse cytoskeletal elements, including FAKs, and modulate cell adhesion. J. Cell Sci. 116:2845–2855. [DOI] [PubMed] [Google Scholar]
- Schmoranzer, J., G. Kreitzer, and S.M. Simon. 2003. Migrating fibroblasts perform polarized, microtubule-dependent exocytosis towards the leading edge. J. Cell Sci. 116:4513–4519. [DOI] [PubMed] [Google Scholar]
- Sells, M.A., A. Pfaff, and J. Chernoff. 2000. Temporal and spatial distribution of activated Pak1 in fibroblasts. J. Cell Biol. 151:1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling, S.H., K.L. Binns, G.A. Wayman, S.M. Davee, S.H. Ong, T. Pawson, and J.D. Scott. 2002. The WRP component of the WAVE-1 complex attenuates Rac-mediated signalling. Nat. Cell Biol. 4:970–975. [DOI] [PubMed] [Google Scholar]
- Tolias, K.F., A.D. Couvillon, L.C. Cantley, and C.L. Carpenter. 1998. Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex. Mol. Cell. Biol. 18:762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, C.E., M.C. Brown, J.A. Perrotta, M.C. Riedy, S.N. Nikolopoulos, A.R. McDonald, S. Bagrodia, S. Thomas, and P.S. Leventhal. 1999. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J. Cell Biol. 145:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hennik, P.B., J.P. ten Klooster, J.R. Halstead, C. Voermans, E.C. Anthony, N. Divecha, and P.L. Hordijk. 2003. The C-terminal domain of Rac1 contains two motifs that control targeting and signaling specificity. J. Biol. Chem. 278:39166–39175. [DOI] [PubMed] [Google Scholar]
- Wang, B., J.X. Zou, B. Ek-Rylander, and E. Ruoslahti. 2000. R-Ras contains a proline-rich site that binds to SH3 domains and is required for integrin activation by R-Ras. J. Biol. Chem. 275:5222–5227. [DOI] [PubMed] [Google Scholar]
- Watanabe, T., J. Noritake, and K. Kaibuchi. 2005. Regulation of microtubules in cell migration. Trends Cell Biol. 15:76–83. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer, C.M., and E. Salmon. 1999. Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr. Opin. Cell Biol. 11:61–67. [DOI] [PubMed] [Google Scholar]
- Xu, J., F. Wang, A. Van Keymeulen, P. Herzmark, A. Straight, K. Kelly, Y. Takuwa, N. Sugimoto, T. Mitchison, and H.R. Bourne. 2003. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 114:201–214. [DOI] [PubMed] [Google Scholar]
- Zhang, J., J. Guo, I. Dzhagalov, and Y.W. He. 2005. An essential function for the calcium-promoted Ras inactivator in Fcgamma receptor-mediated phagocytosis. Nat. Immunol. 6:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z.S., E. Manser, X.Q. Chen, C. Chong, T. Leung, and L. Lim. 1998. A conserved negative regulatory region in alphaPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol. Cell. Biol. 18:2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z.S., E. Manser, and L. Lim. 2000. a. Interaction between PAK and nck: a template for Nck targets and role of PAK autophosphorylation. Mol. Cell. Biol. 20:3906–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z.S., E. Manser, T.H. Loo, and L. Lim. 2000. b. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell. Biol. 20:6354–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]