Abstract
A population of fast muscle fibers from aging mice is dependent on external Ca2+ to maintain tetanic force during repeated contractions. We hypothesized that age-related denervation in muscle fibers plays a role in initiating this contractile deficit, and that prevention of denervation by IGF-1 overexpression would prevent external Ca2+-dependent contraction in aging mice. IGF-1 overexpression in skeletal muscle prevents age-related denervation, and prevented external Ca2+-dependent contraction in this work. To determine if the effects of IGF-1 overexpression are on muscle or nerve, aging mice were injected with a tetanus toxin fragment-C (TTC) fusion protein that targets IGF-1 to spinal cord motor neurons. This treatment prevented external Ca2+-dependent contraction. We also show evidence that injections of the IGF-1-TTC fusion protein prevent age-related alterations to the nerve terminals at the neuromuscular junctions. We conclude that the slow age-related denervation of fast muscle fibers underlies dependence on external Ca2+ to maintain tetanic force in a population of muscle fibers from senescent mice.
Keywords: Aging, skeletal muscle, excitation-contraction coupling, insulin-like growth factor-I
Introduction
Aging leads to contractile deficits in skeletal muscle fibers such as decreased absolute and specific force (Brooks and Faulkner, 1988, Gonzalez, et al., 2000, Lindle, et al., 1997) resulting from decreased muscle mass and excitation-contraction (EC) uncoupling, respectively, among other factors (Delbono, 2003, Lexell, 1995, Renganathan, et al., 1997, Wang, et al., 2000). In addition, we have previously reported that aging causes a population of fast muscle fibers to become dependent on extracellular Ca2+ to maintain tetanic force (Payne, et al., 2004). An age-related denervation process in fast skeletal muscle fibers (Kadhiresan, et al., 1996) is thought to underlie EC uncoupling, and thus, decreased specific force (Delbono, 2003, Payne and Delbono, 2004). Overexpression of insulin-like growth factor-1 (IGF-1) exclusively in skeletal muscle prevents EC uncoupling (Renganathan, et al., 1998, Wang, et al., 2002) and almost completely maintains skeletal muscle fiber specific force (Gonzalez, et al., 2003) in aged mice. Additionally, overexpression of IGF-1 in skeletal muscle prevents denervation of fast muscle fibers in aged mice (Messi and Delbono, 2003). To investigate whether muscle-derived IGF-1 exerted its benefits to aging muscles via direct effects on the muscle fibers, maintenance of muscle fiber innervation, or both, we designed a human IGF-1/tetanus toxin fragment C (TTC) fusion protein (hIGF-1-TTC). We have previously shown that intramuscular injection of hIGF-1-TTC into aging mice attenuated denervation of fast muscle fibers and prevented specific force decline, indicating that innervation state plays an important role in maintenance of function in aging muscle fibers (Payne, et al., 2006). In this work, we test the hypothesis that both IGF-1 overexpression in muscle and targeting IGF-1 to motor neurons (via hIGF-1-TTC injection) prevent age-related increased dependence on extracellular Ca2+ to maintain tetanic force.
Methods
Animals and Injections
DBA (dilute brown agouti, National Institute on Aging/Harlan Sprague Dawley, Indianapolis, IN) and FVB (Friend virus B, our colony) mice were housed at Wake Forest University School of Medicine and all procedures were approved by the Animal Care and Use Committee. Old (23–24 months old) control FVB mice and old transgenic FVB (S1S2) mice, which overexpress hIGF-1 exclusively in skeletal muscle (Coleman, et al., 1995), were used to examine the effects of muscle hIGF-1 overexpression on external Ca2+-dependent contraction. Old control FVB and DBA mice were used to examine the effects of hIGF-1-TTC injections on external Ca2+-dependent contraction. Mice aged 18 months old were injected with 10 μl saline, hIGF-1 (1μg; 0.1 μg/μl in PBS), TTC (10μg; 1 μg/μl in PBS), or hIGF-1-TTC (10μg; 1 μg/μl in PBS) into the anterior compartment of the right lower leg (tibialis anterior, TA; extensor digitorum longus, EDL) every 28 days for three injections. The injection interval was chosen for two reasons: 1) to limit the exposure of the muscle to the protein in order to minimize potential direct effects on the muscle and 2) the hIGF-1-TTC fusion protein is detectable in the spinal cord for 21 days post-injection (Payne, et al., 2006). TTC was chosen as the carrier for hIGF-1 based on its rapid uptake from muscle and subsequent retrograde axonal delivery to spinal cord motor neurons (Coen, et al., 1997, Fishman and Carrigan, 1987, Fishman and Savitt, 1989, Miana-Mena, et al., 2002). Indeed, in a previous report, our laboratory has shown that the hIGF-1-TTC protein undergoes quick retrograde axonal transport to the spinal cord following intramuscular injection (Payne, et al., 2006). Animals were sacrificed by cervical dislocation when 20–21 months old, at 14–28 days past the third and final injection.
Single Intact Muscle Fiber Contraction
At time of sacrifice, flexor digitorum brevis (FDB) or injected EDL muscles were carefully dissected and pinned into a Petri dish lined with Sylgard (Dow Corning, Auburn, MI) in a Ca2+-containing physiological solution, consisting of (in mM): 121 NaCl, 5 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.4 NaH2PO4, 24 NaHCO3, and 5.5 glucose, continuously bubbled with 95/5% O2/CO2 to maintain pH 7.4. All contraction experiments were carried out at room temperature (21–22°C). Single intact fiber dissection followed procedures previously published (Gonzalez, et al., 2000, Lannergren and Westerblad, 1987). Following dissection, tendons of single intact fibers were attached placed in custom-made micro-clips, and these clips were connected to a force transducer and a moveable arm for length control. Fibers were adjusted to optimum length (LO) by using single twitches. Once at LO, fibers were stimulated with 350-ms trains of 0.5-ms square wave pulses at 20 V, using frequencies varying from 50 to 100 Hz. The stimulation frequency that elicited maximum force was used for the remainder of the experiment. Single intact FDB fibers underwent two prolonged contractile sequences (reference trial and test trial) consisting of tetanic contractions every 10 sec for 25 min (150 total tetanic contractions). The physiological solution was perfused during the entire reference trial. During the test trial, Ca2+-free solution was perfused from min 5-15 (contractions 31–90) as was previously published (Payne, et al., 2004). The Ca2+-free solution consisted of (in mM) 121 NaCl, 5 KCl, 2.3 MgCl2, 0.4 NaH2PO4, 24 NaHCO3, and 5.5 glucose, and was bubbled continuously with 95/5% O2/CO2 to maintain pH 7.4. Force decline was assessed during the perfusion of Ca2+-free solution and compared to any force decline that occurred during the same time frame (minutes 5–15) of the reference trial. The contraction experiments in single intact EDL fibers followed a modified version of the FDB experiments. This modification was implemented to improve the survival of EDL fibers – longer fibers (e.g., EDL) are more difficult to dissect and mount than shorter fibers (e.g., FDB). Briefly, EDL fibers underwent repeated tetanic contractions (200 ms trains, 0.5 ms square wave pulses, 20 V, 100–120 Hz) every 10 seconds for 30 minutes (180 total tetanic contractions), replacing physiological solution with Ca2+-free solution from minute 10 to minute 20 (contractions 61–120). Minutes 0–10 served as the “reference” portion, while minutes 10–20 served as the “test” portion of this single trial. Force decline was assessed during minutes 0–10 and during minutes 10–20 (the perfusion of Ca2+-free solution). If force fell below measurable levels before the full 10-minute perfusion of Ca2+-free solution, physiological solution was returned right away (see Figure 3C-E). Up to 15 minutes of recovery were allowed post-trial for both fiber types, with tetanic force measured once every 5 minutes. Dependence on external Ca2+ in both FDB and EDL fibers was determined by the percent force decline elicited by the Ca2+-free solution compared to force decline in physiological solution. Only experiments in which force recovered to at least 90% of baseline were included for analysis.
Figure 3. NMJs in injected TA muscles from old FVB and DBA mice.

Combined silver-cholinesterase staining of longitudinal cryosections of TA muscles from old mice injected with saline (A), TTC (B), hIGF-1 (C), and hIGF-1-TTC (D).
Cholinesterase-staining procedure
Injected TA muscles from aged mice were removed, pinned into embedding molds at resting length, covered with OCT, and quickly frozen in isopentane cooled with dry ice. Longitudinal sections (30 μm thick) were cut with a cryostat to perform a modified combined stain for motor nerve terminals and cholinesterase at the neuromuscular junctions (NMJs) (Pestronk and Drachman, 1978). This procedure used bromoindoxyl acetate dye staining for cholinesterase and silver-gold impregnation for nerve terminals. Sections were placed on a slide in a drop of 3% disodium EDTA to prevent contracture and air dried at 37°C. For cholinesterase-staining, slides were immersed in 20% sodium sulfate to prevent shrinkage of sections. Sections were incubated for 8–10 min in the staining solution described previously (Pestronk and Drachman, 1978). For nerve-staining, sections were dehydrated to prevent loss from slides and treated subsequently as described previously (Pestronk and Drachman, 1978).
Microsome preparation and immunoblot for dihydropyridine receptor (DHPR) α1 subunit
Microsomes from mouse hind limb skeletal muscles (quadriceps and TA; n=5 and 7 animals from saline and hIGF-1-TTC injected, respectively; ~1g total muscle) were prepared as described (Inui, et al., 1988, Saito, et al., 1984) with modifications as previously published by our laboratory (Payne, et al., 2004). Briefly, muscles were finely cut, and were homogenized with a blender homogenizer (Kinematica) in a homogenization buffer containing 5 mM imidazol (pH 7.4) and 300 mM sucrose with complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). The homogenate was centrifuged at 5000 g for 15 min and the supernatants were filtered through four layers of cheesecloth and centrifuged at 15 000 g 15 min, then the second supernatants were centrifuged at 100 000 g for 90 min. Microsome pellets were resuspended in 1% digitonin buffer. After the measurement of protein concentration by BCA protein assay, samples were mixed with 1 × sample buffer. All samples were boiled for 2–3 min. Protein samples (50 μg protein) were then loaded into each lane, separated on 10% denaturing SDS-PAGE gels, and transferred to nitrocellulose membranes. Immunoblot procedures for DHPRα1 subunit also followed previously published procedures (Payne, et al., 2004), but with the IIF7 rabbit anti-mouse DHPRα1 subunit as the primary antibody (generously provided by Dr. Kevin Campbell, University of Iowa, Iowa City, IA, USA). Immunoblots were performed in quadruplicate. After film exposure, optical density X blot area was set relative to control (saline injected).
Statistical analysis
All data are presented as means ± s.e.m. Data from contraction experiments were analyzed with two-way repeated measures ANOVA, with Tukey’s multiple comparisons test applied post hoc. NMJ pre-terminal data were analyzed with ANOVA and Tukey’s test. Immunoblot data were analyzed with a Mann-Whitney rank sum test.
Results
Muscle IGF-1 overexpression prevents external Ca2+-dependent contraction in aged mouse muscle
Single intact FDB muscle fibers were dissected from aged control FVB or transgenic S1S2 mice and subjected to repeated tetanic contractions in both physiological and Ca2+-free solutions. We have previously shown this contraction protocol causes minimal force decline in FDB fibers from young and old mice in physiological solution. In Ca2+-free solution, force does not decline in fibers from young mice, while force declines greatly in a population of fibers from old mice in the Ca2+-free solution (Payne, et al., 2004). The population of fibers from old mice in which force declines while contracting in Ca2+-free solution were termed “Old Affected,” while fibers that exhibit no force decline in Ca2+-free solution were termed “Old Unaffected” (Payne, et al., 2004). In these experiments, muscle fibers from aged mice displayed minimal force decline during the reference trial but dependence on external Ca2+ to maintain tetanic force, as indicated by 34 ± 4.7% force decline during the test trial (Figure 1A). Specifically, force declined dramatically in Ca2+-free solution in 8 fibers (75 ± 3.5% force decline, range 33 to >99%), while no significant force decline was detected in 11 fibers from old control mice (Old Affected vs. Old Unaffected, respectively, Figure 1B). However, fibers from old transgenic mice are not dependent on extracellular Ca2+ to maintain tetanic force (Figure 1A and B). Figure 1C shows the contraction protocol for an “Old Affected” fiber during which force declines by >99% in Ca2+-free solution during the test trial, then recovers almost completely by the end of the experiment. Figure 1D shows the same contraction protocol for a fiber from an old transgenic mouse. Of 14 fibers used from old transgenic mice, force decline of >10% during the test trial was found in only 1 fiber (~23% decline). These data indicate that hIGF-1 overexpression in muscle rescues muscle fibers in old mice from this contractile deficit.
Figure 1. IGF-1 overexpression in skeletal muscle prevents force decline in Ca2+-free solution.

A, peak force in FDB fibers from old control mice (n=19) significantly declined during the test trial compared to the reference trial (*** p<0.001) and compared to old transgenic (n=14; ### p<0.001). Peak force in fibers from old transgenic mice did not decline in the test trial (p>0.05). B, two populations of fibers in old control mice: no force decline during test trial (Old Unaffected, n=11, p>0.05) and significant force decline during the test trial (Old Affected, n=8, *** p<0.001 vs. reference trial, ### p<0.001 vs. Old Unaffected and Old Transgenic). C and D, time course of reference (left traces) and test (right traces) trials, as well as a single recovery tetanus 5 min post-trial (far right traces), for representative FDB fibers from Old Affected (C) and from Old Transgenic (D). Top bars show solution changes.
IGF-1 targeted to spinal cord motor neurons prevents external Ca2+-dependent contraction in aged muscle fibers
We have previously shown that hIGF-1-TTC injection targets delivery of hIGF-1 to spinal cord motor neurons and prevents age-related decline in specific force in EDL fibers (Payne, et al., 2006). In this work, mice aged 17–18 months received three injections of saline, TTC, hIGF-1, or hIGF-1-TTC into the EDL and TA muscles (see Methods). These muscles were selected due to the ability to reliably inject the superficially located anterior leg muscle compartment and the difficulty of reliably injecting the FDB muscle located deep within the plantar surface of the foot. EDL muscles were removed at 20–21 months of age, and single intact fibers dissected for contraction experiments in physiological and Ca2+-free solutions. Figure 2A shows that, like FDB fibers, EDL fibers from aged mice are dependent on the presence of external Ca2+ ions to maintain tetanic force. Monthly injections of saline, TTC, or hIGF-1 did not prevent external Ca2+-dependence of intact single EDL fibers from aged mice, as fibers from these groups of mice displayed tetanic force decline in Ca2+-free solution. Similar to FDB fibers, a population of EDL fibers in these three groups was found to be dependent on external Ca2+ to maintain tetanic force (saline, 6 of 9 fibers; TTC, 4 of 8 fibers; hIGF-1, 6 of 7 fibers; Figure 2B). Monthly injections of hIGF-1-TTC prevents significant force decline in Ca2+-free solution in EDL fibers from aged mice (Figure 2A and B). Figure 2C-F shows the contraction protocol from single intact EDL fibers injected with saline, TTC, hIGF-1, and hIGF-1-TTC, respectively. The data shown in Figure 2C-E are from the “Old Affected” population of saline, TTC, and hIGF-1 injected EDL fibers.
Figure 2. IGF-1 targeted to spinal cord motor neurons prevents skeletal muscle force decline in Ca2+-free solution.

A, peak force in EDL fibers from old mice injected with saline, TTC, and hIGF-1 declined in Ca2+-free solution compared to physiological solution (** p<0.01, * p<0.05). Injection of hIGF-1-TTC in old mice prevented EDL fiber force decline in Ca2+-free solution compared to physiological solution (p>0.05) and compared to saline and hIGF-1 injected fibers (# p<0.05). Force decline in TTC injected fibers tended to be greater than hIGF-1-TTC injected fibers in Ca2+-free solution (p=0.75). B, Unaffected and Affected populations of fibers in saline, TTC, and hIGF-1 injected fibers. Force declined in Ca2+-free solution for all Affected fibers compared to physiological solution, compared to unaffected fibers, and compared to hIGF-1-TTC injected fibers (*** p<0.001). C, D, E, and F, time courses of contraction protocol for representative Affected EDL fibers injected with saline (C), TTC (D), hIGF-1 (E), and a representative fiber injected with hIGF-1-TTC (F). Top bars show solution changes. Far right trace, recovery tetanus 5 min post-trial.
IGF-1 targeted to spinal cord motor neurons prevents neuromuscular junction atrophy and fragmentation in aged muscle fibers
hIGF-1 overexpression in muscle preserves both NMJ pre- and post-terminal size and morphology (Messi and Delbono, 2003). Recently we showed that injections of hIGF-1-TTC into TA muscles of aging mice also preserved NMJ post-terminal size and morphology (Payne, et al., 2006). To examine whether hIGF-1-TTC injections had similar effects on the pre-terminal in aged muscle, we used a combined silver-cholinesterase stain to visualize NMJs in TA muscles from aged mice injected with saline, TTC, hIGF-1, or hIGF-1-TTC. This technique displays the cholinesterase-containing endplate as a well demarcated transparent blue zone, against which the black silver-stained nerve terminals stand out clearly (Messi and Delbono, 2003, Pestronk and Drachman, 1978).
Muscle sections from each group (n=2 muscles per group, 1 muscle per mouse; n=16 sections per muscle, 32 sections per group; n=112, 126, 83, and 141 NMJs from saline, TTC, hIGF-1, and hIGF-1-TTC, respectively) were analyzed for the following five parameters: (1) percentage of cholinesterase-stained zones (CSZ) exhibiting nerve terminal branching, (2) number of nerve branches at the CSZ, (3) number of nerve branch points within the CSZ, (4) percentage of terminals exhibiting sprouting outside the CSZ, and (5) endplate area as outlined by the cholinesterase stain. Figure 3A-D illustrates NMJs corresponding to aged mouse TA muscles injected with saline, TTC, hIGF-1, or hIGF-1-TTC, respectively. Compared to young mice, NMJs in old mice display reduced percentage of CSZs with nerve terminal branching, reduced average number of branches in CSZs, reduced number of branch points in CSZs, increased sprouting of nerve terminals outside the CSZ, and reduced CSZ area (Messi and Delbono, 2003). In this work, we find that ~70% of nerve terminals exhibit branching within the CSZ in aged muscle from saline, TTC, and hIGF-1 injected mice (Figures 3A–C and 4A). However, 95% of nerve terminals exhibit branching within the CSZ in muscles from mice injected with hIGF-1-TTC (Figures 3D and 4A). This is associated with an increased number of nerve terminal branches within the CSZ in hIGF-1-TTC injected muscles compared to saline, TTC, and hIGF-1 injected muscles (Figure 4B). Similarly, injection of hIGF-1-TTC results in a greater number of nerve terminal branch points within the CSZ compared to other groups (Figure 4C). We previously showed that hIGF-1 overexpression in muscle increases the percentage of terminals exhibiting sprouting outside the CSZ in both young and old animals, suggesting a trophic effect of muscle IGF-1 on the nerve (Messi and Delbono, 2003). Here, we show that IGF-1 targeted to the nerve via intramuscular hIGF-1-TTC injections also results in a several-fold increase in nerve sprouting in aged mouse muscle (Figure 4D; 39% of terminals vs. 14%, 13%, and 11% in saline, TTC, and hIGF-1 groups). Figures 3 and 4E show that hIGF-1-TTC injections also prevented age-related reduction of the presynaptic terminal area (indicated by the blue CSZ), while injections of saline, TTC, or hIGF-1 did not.
Figure 4. Analysis of NMJs using combined silver-cholinesterase stain.
NMJs presynapses in TA muscle from old mice injected with saline, TTC, hIGF-1, or hIGF-1-TTC. Results are presented as mean ± SEM, except for A and D, which are expressed as mean values. All values measured for NMJs in muscles injected with hIGF-1-TTC are larger than other groups (** p ≤ 0.003; *** p<0.001).
IGF-1 targeted to motor neurons increases DHPRα1 subunit protein in aged mouse skeletal muscle
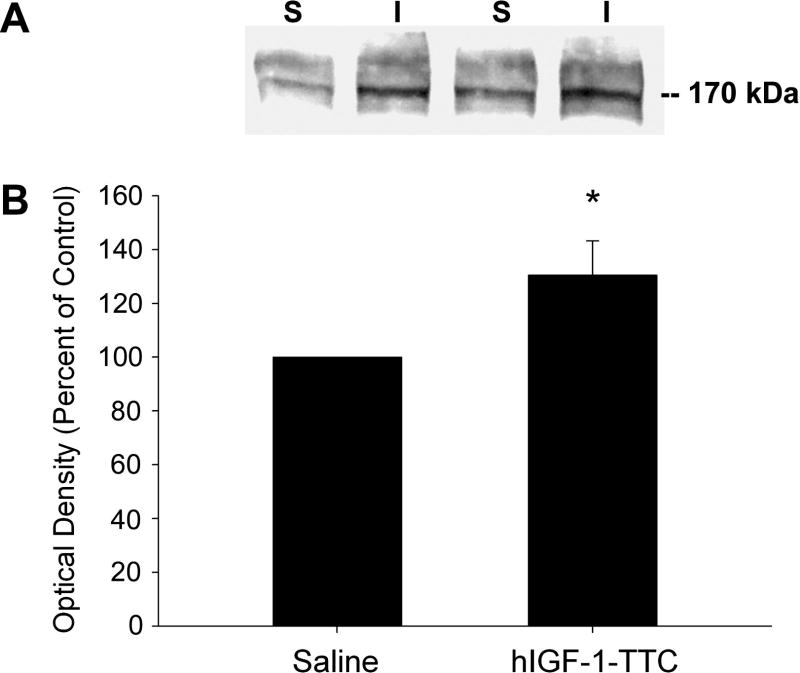
A major contributor to specific force decline in aging muscle is the decrease in DHPRα1 subunit expression (Delbono, et al., 1995, Gonzalez, et al., 2000, Gonzalez, et al., 2003, Wang, et al., 2000); and IGF-1 overexpression exclusively in skeletal muscle prevents both DHPRα1 subunit and specific force decline (Gonzalez, et al., 2003, Renganathan, et al., 1998, Renganathan, et al., 1997, Wang, et al., 2002, Wang, et al., 1999, Zheng, et al., 2002). We have recently reported that IGF-1 targeted to motor neurons with hIGF-1-TTC injections increased specific force of single intact EDL fibers from aged mice (Payne, et al., 2006). We have hypothesized that slow age-related denervation of muscle fibers contributes to the age-related decline in DHPR expression, and thus, specific force decline (Delbono, 2003, Payne and Delbono, 2004). Here, we now show evidence that targeting IGF-1 to motor neurons via intramuscular hIGF-1-TTC injections increases DHPRα1 subunit expression in aged muscle compared to saline-injected muscles (~27% increase, Figure 5B, p=0.05). Figure 5A shows a representative immunoblot from pooled quadriceps and TA muscles (n=5 and 7 animals from saline and hIGF-1-TTC injected groups, respectively; ~1g total muscle).
Figure 5. IGF-1 targeted to motor neurons increases DHPRα1 subunit protein levels in muscles of aged mice.
A, Representative immunoblots following membrane isolation from hind limb muscles (quadriceps and TA) from old mice injected with either saline (S) or hIGF-1-TTC (I) reveal higher levels of DHPRα1 subunit in hIGF-1-TTC injected muscles compared to saline injected. B, Analysis of immunoblot optical density reveals increased DHPRα1 subunit (~27%) in hIGF-1-TTC injected muscles compared to saline injected muscles (* p=0.05).
Discussion
This work reports that: 1) increasing external Mg2+ to maintain membrane charge screening in the absence of external Ca2+ does not prevent force decline in muscle fibers from aged mice, 2) overexpression of hIGF-1 exclusively in skeletal muscle of mice prevents age-related dependence on external Ca2+ to maintain tetanic force in single intact FDB muscle fibers, 3) force declines in a large population of EDL muscle fibers from aged mice in the absence of external Ca2+, similar to FDB fibers, 4) targeted delivery of hIGF-1 to spinal cord motor neurons via intramuscular hIGF-1-TTC fusion protein injection prevents age-related dependence on external Ca2+ to maintain tetanic force, 5) hIGF-1-TTC injections promote maintenance of muscle fiber innervation, and 6) targeted delivery of hIGF-1 to spinal cord motor neurons via intramuscular hIGF-1-TTC injection increases DHPRα1 subunit protein expression in aged mouse skeletal muscle.
IGF-1 and external Ca2+-dependent skeletal muscle contraction
We previously reported that a population of fast muscle fibers from aging mice requires the presence of external Ca2+ to maintain tetanic force (Payne, et al., 2004). In that report and in this work, we chose to use the single intact fiber contraction technique to study the dependence on external Ca2+ for several reasons. First, the contraction protocol is prolonged, and therefore requires an in vitro muscle preparation that can reproduce mechanical responses for many hours. This is possible with single intact fibers, whereas whole muscle deteriorates with repeated stimulation. Second, the perfusion solution is changed twice during the trial, requiring fibers to be exposed to the solution quickly. A single intact fiber is very quickly exposed, whereas many fibers in a large multi-fiber preparation, such as a whole muscle, are not exposed quickly, if at all, to new solutions. Furthermore, the lack of exposure to solutions is a primary reason whole muscles deteriorate quickly compared to single intact fibers. Third, our laboratory has previously reported that IGF-1 overexpression in muscle and injection of the hIGF-1-TTC fusion protein can increase specific force in fibers from aged mice (Gonzalez, et al., 2003, Payne, et al., 2006). The single intact fiber technique offers the advantage of more precise measurement of cross-sectional area, optimum length, and specific force; whereas the variety of fibers within a whole muscle with differing mechanical properties as well as structural differences in young and old muscles makes measurement of cross-sectional area, optimum length, and specific force more difficult (Gonzalez, et al., 2000, Sugi and Tsuchiya, 1998). Fourth, as described in our previous report, muscle fibers from aged mice exhibit two populations (external Ca2+-dependent and –independent). Therefore, in order to identify an external Ca2+-dependent fiber and measure its force decline in Ca2+-free solution, the fiber must be isolated from surrounding fibers.
In this work, we show tetanic force declines in a population of FDB fibers from aged mice when in Ca2+-free solution, in agreement with our previous report (Payne, et al., 2004). In addition, we show that muscle hIGF-1 overexpression prevents this decline in fibers from old transgenic mice. Muscle IGF-1 overexpression prevents several deleterious effects of aging, including muscle mass and specific force decline (Barton-Davis, et al., 1998, Gonzalez, et al., 2003). The site of action for the effects of elevated muscle IGF-1 has been postulated to be: 1) the muscle itself, increasing DHPR expression and function through IGF-1 receptor signaling (Renganathan, et al., 1998, Wang, et al., 2002, Zheng, et al., 2002), or 2) the motor neuron, via paracrine trophic effects, preserving innervation and thus function of aged muscle fibers (Barton-Davis, et al., 1998, Messi and Delbono, 2003). These data from transgenic mice indicate that elevated IGF-1 prevents this type of contractile deficit in aged muscle fibers, but does not elucidate the site of action of the IGF-1.
To examine the role of IGF-1 in preventing external Ca2+-dependent contraction in aged muscle fibers, we injected hIGF-1-TTC into EDL muscles of aged animals and subjected fibers to the same Ca2+-free solution during contraction experiments. These data show that aged EDL fiber force declines in Ca2+-free solution, similar to aged FDB fibers. While monthly injections of saline, TTC, and hIGF-1 were not able to prevent external Ca2+-dependent contraction, monthly injections of hIGF-1-TTC completely prevented force decline in Ca2+-free solution. Whether this population of fibers is dependent on the mere presence of external Ca2+ in the medium or on Ca2+ influx into the cell is unknown at this time. It is known that excessive Ca2+ entry into cells can lead to a host of problems ranging from decreased force production to overt cellular damage and tissue disrepair (see (Gissel, 2005) for review). It seems unlikely that the force decline in fibers from aged mice reported here and previously (Payne, et al., 2004) is due to damage from excessive Ca2+ entry, given that the force decline occurs in Ca2+-free solution. Whether excess Ca2+entry plays a role in other age-related muscle problems (e.g., atrophy) requires further investigation.
We previously showed that hIGF-1-TTC binds to and is internalized by spinal cord motor neurons in vitro and in vivo. We also showed that monthly hIGF-1-TTC injections prevented specific force decline in aged single intact EDL fibers (Payne, et al., 2006). The current data indicating increased DHPRα1 subunit protein expression following hIGF-1-TTC injections provides a potential mechanism to explain maintenance of specific force following the fusion protein injections, as well as further evidence for neurogenic control of EC coupling in aging muscle. Prevention of age-related DHPR and specific force decline and prevention of external Ca2+-dependent contraction by targeting IGF-1 to motor neurons indicates age-related denervation plays an important role in both problems and lends strong support to the neurogenic hypothesis of EC uncoupling in aging muscle (Delbono, 2003, Payne and Delbono, 2004). Prevention of these age-related deficits with hIGF-1-TTC injections also indicates that IGF-1 overexpression in muscle exerts at least part of its benefit through paracrine effects on muscle fiber innervation.
IGF-1 and muscle innervation in aged mice
To confirm that hIGF-1-TTC maintains innervation of aging muscle fibers, we previously showed that monthly hIGF-1-TTC injections attenuated age-related atrophy and disorganization of the NMJ post-terminal (Payne, et al., 2006), similar to the findings produced by muscle IGF-1 overexpression (Messi and Delbono, 2003). We examined the effects of the hIGF-1-TTC fusion protein on the NMJ pre-terminal in this work. Intramuscular injection of saline, TTC, or hIGF-1 into aged mice did not prevent decreases in NMJ pre-terminal size, percent of branching terminals, or number of branches and branch points per NMJ normally found in aged muscle (Messi and Delbono, 2003, Robbins and Nakashiro, 1993). Additionally, injection of saline, TTC, or hIGF-1 did not prevent increased percentage of NMJs exhibiting extraterminal sprouting. However, hIGF-1-TTC injections almost completely prevented decline in NMJ pre-terminal size, percent of branching terminals, and number of branches and branch points. Further, hIGF-1-TTC injections led to a several-fold increase in nerve terminal sprouting outside the CSZ compared to other groups. These data are similar to findings in aged mice overexpressing hIGF-1 in muscle (Messi and Delbono, 2003), and agree with previous findings illustrating elevated IGF-1 causes neurite outgrowth in motor neurons (Caroni and Grandes, 1990, Caroni, et al., 1994). In combination with data on NMJ post-terminal (Payne, et al., 2006), these data provide further support for the trophic effects of hIGF-1-TTC on aging spinal cord motor neurons. Age-related skeletal muscle denervation is a phenomenon shown in both animals (Kadhiresan, et al., 1996, Larsson and Ansved, 1995, Wang, et al., 2005) and humans (Campbell, et al., 1973, Doherty, et al., 1993, McNeil, et al., 2005, Morse, et al., 2004). Although a long way from clinical trials, the findings presented herein suggest that similar types of treatments (i.e., targeting spinal cord motor neurons with IGF-1 or other growth factors) may be useful in preventing age-related motor neuron loss in humans, also.
The percentage of fast muscle fibers from aged mice exhibiting dependence on external Ca2+ to maintain tetanic force is very similar to the percentage of fast fibers exhibiting signs of full or partial denervation as measured previous in our laboratory (~50%) (Wang, et al., 2005). This knowledge, taken together with previous findings that muscle IGF-1 overexpression prevents age-related denervation (Messi and Delbono, 2003) and the current finding that muscle IGF-1 overexpression prevents external Ca2+-dependent contraction, suggests that age-related denervation contributes to dependence of aged muscle fibers on external Ca2+ ions to maintain tetanic force. The findings that targeting IGF-1 to spinal cord motor neurons prevents both external Ca2+-dependent contraction and denervation of muscle fibers ((Payne, et al., 2006) and this work) further support this hypothesis.
The dependence of a population of fibers on external Ca2+ to maintain tetanic force, and the prevention of this phenomenon, may not be the most relevant findings to aging in this work. After all, a fully denervated fiber will not contract in vivo. It is likely that a “partially denervated fiber” (Wang, et al., 2005) still contracts, but its exact function is unknown at this time. Partially denervated fibers represent up to 35% of fibers in aged muscle (Wang, et al., 2005), and we believe this diminished or altered state of innervation to be important in the external Ca2+-dependent contraction phenomenon. Indeed, dependence on external Ca2+ to maintain tetanic force has been shown in muscles that have undergone nerve damage and repair (Louboutin, et al., 1996, 1997, Pereon, et al., 1997). The recognition that denervation may be the underlying cause of, specifically, age-related external Ca2+ dependence is important because it is a physiological indicator of the extension of muscle fiber denervation in aged muscle (i.e., ~50% of muscle fibers).
Conclusions
While the exact molecular mechanism of external Ca2+-dependent contraction in aged muscle fibers is still unknown, our data strongly suggest that age-related denervation plays a key role in initiating this alteration in EC coupling mode. Potential mechanisms may include: alterations to key proteins like the DHPR or its subunits (Sheridan, et al., 2003); or other junctional proteins which may play a role in EC coupling such as MG29 in whose absence muscle cells exhibit external Ca2+-dependent contraction (Pan, et al., 2002), or JP-45 which interacts directly with the DHPR (Anderson, et al., 2003). Alternatively, the mechanism for external Ca2+-dependent contraction may be due to potential age-related changes in processes such as store-operated Ca2+ entry (SOCE) (Kurebayashi and Ogawa, 2001) or excitation-coupled Ca2+ entry (ECCE) (Cherednichenko, et al., 2004). One or both of these processes may play a role in the dependence on external Ca2+ to maintain tetanic force in muscle fibers from aged mice by compensatory replenishment or maintenance of intracellular Ca2+ stores in aged mice during repeated contractions.
The role of IGF-1 decline is undergoing intense study as an underlying cause for age-related degenerative problems in a variety of tissues, including bone fractures, cognitive decline, and cardiac/vascular dysfunction in addition to skeletal muscle. Accordingly, research is therefore examining roles of IGF-1 replacement or replenishment as potential treatment for age-related disease and degeneration (Groban, et al., 2006, Schmidmaier, et al., 2006, Sonntag, et al., 2005). We and others have previously shown that IGF-1 overexpression in skeletal muscle of aging mice prevents deleterious effects of age on skeletal muscle and the neuromuscular system as a whole (Barton-Davis, et al., 1998, Gonzalez, et al., 2003, Musaro, et al., 2001, Renganathan, et al., 1998, Wang, et al., 2002, Zheng, et al., 2002). Since gene therapy is possibly far off in the future, transgenic humans are not feasible at this time. Therefore, this work, along with our initial report on hIGF-1-TTC (Payne, et al., 2006) seek to offer alternative approaches for delivery of IGF-1 to the aging neuromuscular system for prevention or, at least, attenuation of the deleterious effects of aging on muscle structure and function.
Acknowledgments
The present study was supported by grants from the National Institutes of Health/National Institute on Aging (AG13934 and AG15820) and the Muscular Dystrophy Association to Osvaldo Delbono, and by the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AA, Treves S, Biral D, Betto R, Sandona D, Ronjat M, Zorzato F. The novel skeletal muscle sarcoplasmic reticulum JP-45 protein. Molecular cloning, tissue distribution, developmental expression, and interaction with alpha 1.1 subunit of the voltage-gated calcium channel. J Biol Chem. 2003;278(41):39987–39992. doi: 10.1074/jbc.M305016200. [DOI] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A. 1998;95(26):15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry. 1973;36(2):174–182. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol. 1990;110(4):1307–1317. doi: 10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Schneider C, Kiefer MC, Zapf J. Role of muscle insulin-like growth factors in nerve sprouting: suppression of terminal sprouting in paralyzed muscle by IGF-binding protein 4. J Cell Biol. 1994;125(4):893–902. doi: 10.1083/jcb.125.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Hurne AM, Fessenden JD, Lee EH, Allen PD, Beam KG, Pessah IN. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci U S A. 2004;101(44):15793–15798. doi: 10.1073/pnas.0403485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen L, Osta R, Maury M, Brulet P. Construction of hybrid proteins that migrate retrogradely and transynaptically into the central nervous system. Proc Natl Acad Sci U S A. 1997;94(17):9400–9405. doi: 10.1073/pnas.94.17.9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270(20):12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2(1):21–29. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- Delbono O, O’Rourke KS, Ettinger WH. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol. 1995;148(3):211–222. doi: 10.1007/BF00235039. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993;74(2):868–874. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- Fishman PS, Carrigan DR. Retrograde transneuronal transfer of the C-fragment of tetanus toxin. Brain Res. 1987;406(1–2):275–279. doi: 10.1016/0006-8993(87)90792-x. [DOI] [PubMed] [Google Scholar]
- Fishman PS, Savitt JM. Transsynaptic transfer of retrogradely transported tetanus protein-peroxidase conjugates. Exp Neurol. 1989;106(2):197–203. doi: 10.1016/0014-4886(89)90094-0. [DOI] [PubMed] [Google Scholar]
- Gissel H. The role of Ca2+ in muscle cell damage. Ann N Y Acad Sci. 2005;1066:166–180. doi: 10.1196/annals.1363.013. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Messi ML, Delbono O. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol. 2000;178(3):175–183. doi: 10.1007/s002320010025. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Messi ML, Zheng Z, Delbono O. Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J Physiol. 2003;552(Pt 3):833–844. doi: 10.1113/jphysiol.2003.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW, Sonntag WE. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61(1):28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- Inui M, Wang S, Saito A, Fleischer S. Junctional and longitudinal sarcoplasmic reticulum of heart muscle. Methods Enzymol. 1988;157:100–106. doi: 10.1016/0076-6879(88)57072-6. [DOI] [PubMed] [Google Scholar]
- Kadhiresan VA, Hassett CA, Faulkner JA. Properties of single motor units in medial gastrocnemius muscles of adult and old rats. J Physiol. 1996;493(Pt 2):543–552. doi: 10.1113/jphysiol.1996.sp021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533(Pt 1):185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannergren J, Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol. 1987;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol. 1995;45(5):397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50 doi: 10.1093/gerona/50a.special_issue.11. Spec No 11–16. [DOI] [PubMed] [Google Scholar]
- Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997;83(5):1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Fichter-Gagnepain V, Noireaud J. Effects of external calcium on contractile responses in rat extensor digitorum longus muscles after sciatic nerve injury at birth. Muscle Nerve. 1996;19(11):1421–1428. doi: 10.1002/(SICI)1097-4598(199611)19:11<1421::AID-MUS6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Fichter-Gagnepain V, Noireaud J. Long-term external calcium dependence of autotransplanted and sliced extensor digitorum longus muscle contractility. Muscle Nerve. 1997;20(8):1032–1034. doi: 10.1002/(sici)1097-4598(199708)20:8<1032::aid-mus15>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31(4):461–467. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- Messi ML, Delbono O. Target-derived trophic effect on skeletal muscle innervation in senescent mice. J Neurosci. 2003;23(4):1351–1359. doi: 10.1523/JNEUROSCI.23-04-01351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miana-Mena FJ, Roux S, Benichou JC, Osta R, Brulet P. Neuronal activity-dependent membrane traffic at the neuromuscular junction. Proc Natl Acad Sci U S A. 2002;99(5):3234–3239. doi: 10.1073/pnas.052023599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV. Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. Eur J Appl Physiol. 2004;92(1–2):219–226. doi: 10.1007/s00421-004-1056-y. [DOI] [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27(2):195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol. 2002;4(5):379–383. doi: 10.1038/ncb788. [DOI] [PubMed] [Google Scholar]
- Payne AM, Delbono O. Neurogenesis of excitation-contraction uncoupling in aging skeletal muscle. Exerc Sport Sci Rev. 2004;32(1):36–40. doi: 10.1097/00003677-200401000-00008. [DOI] [PubMed] [Google Scholar]
- Payne AM, Zheng Z, Gonzalez E, Wang ZM, Messi ML, Delbono O. External Ca(2+)-dependent excitation--contraction coupling in a population of ageing mouse skeletal muscle fibres. J Physiol. 2004;560(Pt 1):137–155. doi: 10.1113/jphysiol.2004.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AM, Zheng Z, Messi ML, Milligan CE, Gonzalez E, Delbono O. Motor neurone targeting of IGF-1 prevents specific force decline in ageing mouse muscle. J Physiol. 2006;570(Pt 2):283–294. doi: 10.1113/jphysiol.2005.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereon Y, Sorrentino V, Dettbarn C, Noireaud J, Palade P. Dihydropyridine receptor and ryanodine receptor gene expression in long-term denervated rat muscles. Biochem Biophys Res Commun. 1997;240(3):612–617. doi: 10.1006/bbrc.1997.7712. [DOI] [PubMed] [Google Scholar]
- Pestronk A, Drachman DB. A new stain for quantitative measurement of sprouting at neuromuscular junctions. Muscle Nerve. 1978;1(1):70–74. doi: 10.1002/mus.880010110. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Delbono O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J Membr Biol. 1997;157(3):247–253. doi: 10.1007/s002329900233. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Delbono O. Overexpression of IGF-1 exclusively in skeletal muscle prevents age-related decline in the number of dihydropyridine receptors. J Biol Chem. 1998;273(44):28845–28851. doi: 10.1074/jbc.273.44.28845. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Schwartz R, Delbono O. Overexpression of hIGF-1 exclusively in skeletal muscle increases the number of dihydropyridine receptors in adult transgenic mice. FEBS Lett. 1997;417(1):13–16. doi: 10.1016/s0014-5793(97)01225-8. [DOI] [PubMed] [Google Scholar]
- Robbins N, Nakashiro S. Connections among plasticity, regeneration, and aging at the neuromuscular junction. Adv Neurol. 1993;59:47–52. [PubMed] [Google Scholar]
- Saito A, Seiler S, Chu A, Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidmaier G, Lucke M, Schwabe P, Raschke M, Haas NP, Wildemann B. Collective review: bioactive implants coated with poly(D, L-lactide) and growth factors IGF-I, TGF-beta1, or BMP-2 for stimulation of fracture healing. J Long Term Eff Med Implants. 2006;16(1):61–69. doi: 10.1615/jlongtermeffmedimplants.v16.i1.70. [DOI] [PubMed] [Google Scholar]
- Sheridan DC, Carbonneau L, Ahern CA, Nataraj P, Coronado R. Ca2+-dependent excitation-contraction coupling triggered by the heterologous cardiac/brain DHPR beta2a-subunit in skeletal myotubes. Biophys J. 2003;85(6):3739–3757. doi: 10.1016/S0006-3495(03)74790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4(2):195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Sugi H, Tsuchiya T. Muscle Mechanics I: Intact single muscle fibers. In: Sugi H, editor. Current Methods in Muscle Physiology. Advantages, Problems and Limitations. Osford University Press; New York: 1998. pp. 3–31. [Google Scholar]
- Wang ZM, Messi ML, Delbono O. L-Type Ca(2+) channel charge movement and intracellular Ca(2+) in skeletal muscle fibers from aging mice. Biophys J. 2000;78(4):1947–1954. doi: 10.1016/S0006-3495(00)76742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZM, Messi ML, Delbono O. Sustained overexpression of IGF-1 prevents age-dependent decrease in charge movement and intracellular Ca(2+) in mouse skeletal muscle. Biophys J. 2002;82(3):1338–1344. doi: 10.1016/S0006-3495(02)75489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZM, Messi ML, Renganathan M, Delbono O. Insulin-like growth factor-1 enhances rat skeletal muscle charge movement and L-type Ca2+ channel gene expression. J Physiol. 1999;516(Pt 2):331–341. doi: 10.1111/j.1469-7793.1999.0331v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZM, Zheng Z, Messi ML, Delbono O. Extension and magnitude of denervation in skeletal muscle from ageing mice. J Physiol. 2005;565(Pt 3):757–764. doi: 10.1113/jphysiol.2005.087601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Wang ZM, Delbono O. Insulin-like growth factor-1 increases skeletal muscle dihydropyridine receptor alpha 1S transcriptional activity by acting on the cAMP-response element-binding protein element of the promoter region. J Biol Chem. 2002;277(52):50535–50542. doi: 10.1074/jbc.M210526200. [DOI] [PubMed] [Google Scholar]