Abstract
The conversion of an epithelial cell to a mesenchymal cell is critical to metazoan embryogenesis and a defining structural feature of organ development. Current interest in this process, which is described as an epithelial–mesenchymal transition (EMT), stems from its developmental importance and its involvement in several adult pathologies. Interest and research in EMT are currently at a high level, as seen by the attendance at the recent EMT meeting in Vancouver, Canada (October 1–3, 2005). The meeting, which was hosted by The EMT International Association, was the second international EMT meeting, the first being held in Port Douglas, Queensland, Australia in October 2003. The EMT International Association was formed in 2002 to provide an international body for those interested in EMT and the reverse process, mesenchymal–epithelial transition, and, most importantly, to bring together those working on EMT in development, cancer, fibrosis, and pathology. These themes continued during the recent meeting in Vancouver.
Discussion at the Vancouver meeting spanned several areas of research, including signaling pathway activation of EMT and the transcription factors and gene targets involved. Also covered in detail was the basic cell biology of EMT and its role in cancer and fibrosis, as well as the identification of new markers to facilitate the observation of EMT in vivo. This is particularly important because the potential contribution of EMT during neoplasia is the subject of vigorous scientific debate (Tarin, D., E.W. Thompson, and D.F. Newgreen. 2005. Cancer Res. 65:5996–6000; Thompson, E.W., D.F. Newgreen, and D. Tarin. 2005. Cancer Res. 65:5991–5995).
Defining epithelial–mesenchymal transition (EMT)
Historically, epithelial and mesenchymal cells have been identified on the basis of their unique visual appearance and the morphology of the multicellular structures they create (Shook and Keller, 2003). A typical epithelium is a sheet of cells, often one cell thick, with individual epithelial cells abutting each other in a uniform array. Regularly spaced cell–cell junctions and adhesions between neighboring epithelial cells hold them tightly together and inhibit the movement of individual cells away from the epithelial monolayer. Internal adhesiveness allows an epithelial sheet to enclose a three-dimensional space and provide it with structural definition and mechanical rigidity. The epithelial sheet itself is polarized, meaning that the apical and basal surfaces are likely to be visually different, adhere to different substrates, or have different functions. Mesenchymal cells, on the other hand, generally exhibit neither regimented structure nor tight intracellular adhesion. Mesenchymal cells form structures that are irregular in shape and not uniform in composition or density. Adhesions between mesenchymal cells are less strong than in their epithelial counterparts, allowing for increased migratory capacity. Mesenchymal cells also have a more extended and elongated shape, relative to epithelial cells, and they possess front-to-back leading edge polarity. Unlike epithelia, the irregular structure of mesenchyme does not allow for rigid topological specialization. Moreover, mesenchymal migration is mechanistically different from epithelial movement. Epithelial cells move as a sheet en block, whereas mesenchymal migration is considerably more dynamic. Mesenchymal cells move individually and can leave part of the trailing region behind. Elizabeth Hay (Harvard University, Boston, MA), who first described the EMT (Hay, 2005), illustrated the fundamental differences of such movement in embryogenesis (subtle/controlled) and tumorigenesis (aggressive/uncontrolled) to define the distinct EMT mechanisms at the EMT conference.
Turning an epithelial cell into a mesenchymal cell requires alterations in morphology, cellular architecture, adhesion, and migration capacity. Commonly used molecular markers for EMT include increased expression of N-cadherin and vimentin, nuclear localization of β-catenin, and increased production of the transcription factors such as Snail1 (Snail), Snail2 (Slug), Twist, EF1/ZEB1, SIP1/ZEB2, and/or E47 that inhibit E-cadherin production. Phenotypic markers for an EMT include an increased capacity for migration and three-dimensional invasion, as well as resistance to anoikis/apoptosis. A summary of common EMT markers is listed in Table I. Importantly, these developmental regulators can induce EMT in a nondevelopmental context and thereby have an important role in cancer and fibrosis.
Table I.
EMT markers
| Proteins that increase in abundance |
| N-cadherin |
| Vimentin |
| Fibronectin |
| Snail1 (Snail) |
| Snail2 (Slug) |
| Twist |
| Goosecoid |
| FOXC2 |
| Sox10 |
| MMP-2 |
| MMP-3 |
| MMP-9 |
| Integrin αvβ6 |
| Proteins that decrease in abundance |
| E-cadherin |
| Desmoplakin |
| Cytokeratin |
| Occludin |
| Proteins whose activity increases |
| ILK |
| GSK-3β |
| Rho |
| Proteins that accumulate in the nucleus |
| β-catenin |
| Smad-2/3 |
| NF-κβ |
| Snail1 (Snail) |
| Snail2 (Slug) |
| Twist |
| In vitro functional markers |
| Increased migration |
| Increased invasion |
| Increased scattering |
| Elongation of cell shape |
| Resistance to anoikis |
Signaling pathways in EMT
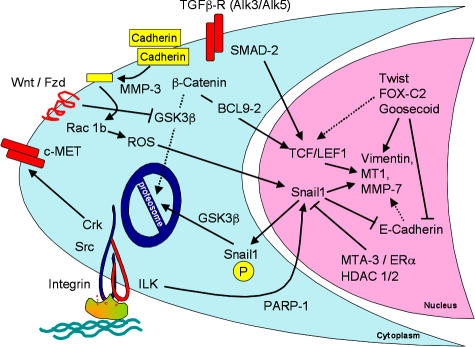
Much of the meeting highlighted signaling pathways that regulate or mediate the EMT, focusing both on refinement and extension of known pathways, but also on the discovery of new regulators and novel pathways (Fig. 1).
Figure 1.
Signaling events during EMT. The major signaling events that were reported in the meeting are summarized. Cleavage of E-cadherin (yellow) by MMP-3 resulted in activation of Snail1 through ROS. Snail1 localization to the nucleus is controlled by phosphorylation of a nuclear export motif and a proteosomal degradation motif, which are each phosphorylatable by GSK-3β. An ILK-responsive element in the Snail1 promoter binds PARP-1. Snail1 expression is inhibited by the MTA3–NuRD chromosomal rearrangement complex, acting downstream of the activated estrogen receptor. Repression of E-cadherin by Snail1, Twist, or other repressors leads indirectly to expression of vimentin and other mesenchymal gene products, partly because of β-catenin/Tcf–Lef1 activation. FOX-C2, as well as SIP1, can also directly activate mesenchymal gene expression. Translocation of β-catenin to the nucleus requires BCL9-2, which itself can induce EMT. Abundance of β-catenin is regulated by phosphorylation-dependent proteosomal degradation, unless GSK-3β is silenced through Wnt signaling. TGF-β is known to activate this canonical Wnt pathway, but TGF-β also directly activates the Tcf–Lef1 transcription complex through tyrosine phosphorylation of SMAD-2. The c-Met receptor tyrosine kinase, through the Crk adaptor, also stimulates EMT.
One of the first cell surface receptors identified that was able to stimulate scattering of epithelial cells was the Met receptor tyrosine kinase. Activation of Met by its ligand, hepatocyte growth factor, enhances the migration of multiple cell lines in vitro, and scattering of cultured multicystic dysplastic kidney cells is a classical EMT assay. Morag Park (McGill University, Montreal, Quebec, Canada) reported that transgenic mice expressing wild-type or active variants of Met under the control of the mouse mammary tumor virus promoter develop nodal and ductal hyperplasia and spontaneous mammary tumors, albeit with a long latency period (∼1.5 yr). Park suggested that Met cooperates with the Her2/neu oncogene in activating EMT, and that the Crk family of SH2 and SH3 adaptor proteins are critical in Met-mediated EMT. Crk proteins are highly expressed in human breast tumors, and Park reported that small interfering RNA (siRNA) ablation of Crk inhibits Met-dependent cell migration and EMT.
Although the Met receptor-mediated signaling results in cell scattering, it has not been made clear whether Met signaling also has a more permanent effect on the expression or localization of some of the effectors of EMT, such as E-cadherin and β-catenin. Recent work by Walter Birchmeier (Max Delbruck Center, Berlin, Germany) suggests that Met also regulates intracellular localization of β-catenin. β-Catenin has a dual role in the EMT; it enhances cell–cell adhesion when bound to cadherin complexes in adherens junctions and also functions as a transcriptional coactivator upon entry into the nucleus (van Es et al., 2003). The ability of β-catenin to enhance cadherin-dependent adhesion depends on β-catenin binding to α-catenin and on α-catenin binding to the cadherin (Chu et al., 2004). Phosphorylation of β-catenin residue Y142 prevents α-catenin interaction and enhances the binding of β-catenin to BCL9-2, which is the vertebrate homologue of the Drosophila melanogaster legless gene (Brembeck et al., 2004). Interaction of β-catenin with BCL9-2 enhances nuclear accumulation of both proteins, simultaneously decreasing cadherin-mediated adhesion and activating catenin target gene transcription. Ectopic BCL9-2 expression is sufficient to induce EMT in cultured cells, and siRNA-mediated BCL9-2 inactivation drives the reverse mesenchymal–epithelial transition (MET). Birchmeier reported that Y142 can be phosphorylated by the Met tyrosine kinase, indicating the existence of an EMT activation pathway where Met induces β-catenin nuclear translocation by enhancing BCL9-2 interaction. This pathway satisfactorily links these two well known EMT regulators.
Interestingly, Pez/PTPN14, which is a tyrosine phosphatase that is frequently mutated in colorectal tumors (Wang et al., 2004), induces Snail1 expression and can also activate cell migration (Yeesim Khew-Goodall, Hanson Institute, Adelaide, Australia). Pez can dephosphorylate β-catenin on tyrosine residues that regulate its interaction with the adherens junction complex, suggesting that Pez mutations contribute to EMT by preventing cytoplasmic β-catenin–cadherin interaction and enhancing its nuclear translocation. However, Pez overexpression in MDCK and MDA-MB468 cells was shown to be sufficient to cause EMT, and knockdown in zebrafish causes multiple developmental abnormalities, including aberrant pigmentation and craniofacial deformation. These defects are broadly consistent with dysfunctional neural crest EMT in the absence of Pez.
Cancer-relevant insights into EGF signaling were provided by Erik Thompson (University of Melbourne, Melbourne, Australia), who has identified EGF as a novel EMT inducer in human breast cancer, as measured by EGF's ability to decrease E-cadherin and increase vimentin production in PMC42 cells. Interestingly, EMT may influence the response of certain cancers to EGF receptor (EGFR)–targeted therapeutics. John Haley (OSI Pharmaceuticals, Melville, NY) presented data showing that the sensitivity of nonsmall cell lung cancer cell lines to erlotinib, which is an EGFR-targeted monoclonal antibody, did not correlate with EGFR levels, but rather depended on their EMT status, with those having undergone EMT showing resistance (Thomson et al., 2005).
An interesting and novel aspect of EGFR signaling was presented by Mien-Chie Hung (The University of Texas MD Anderson Cancer Center, Houston, TX), who reported that EGFR, which is a transmembrane receptor tyrosine kinase, complexes with the STAT3 transcription factor in the nucleus and can be immunoprecipitated from the EGF-responsive iNos promoter (Lo et al., 2005a). The role that promoter-complexed EGFR has in EMT is uncertain, but high nuclear EGFR is associated with a poor prognosis in breast carcinoma (Lo et al., 2005b). The observation that a transmembrane receptor is found in functional promoter complexes in the nucleus was one of the meeting's most surprising observations, and it will be of great interest to characterize the topological and structural mechanisms through which a membrane receptor enters the nucleus and activates transcription (Giri et al., 2005).
TGF-β is a major regulator of EMT and has been implicated in skin cancer development (Zavadil and Bottinger, 2005). Jiri Zavadil (New York University School of Medicine, New York, NY) reported that TGF-β activates EMT through Smad-3–dependent activation of the HEY1 gene, a member of the Hairy/Enhancer-of-split family of transcriptional repressors. Zavadil used extensive gene expression profiling to identify HEY1 targets that are important in EMT induction (Zavadil et al., 2004). He reported on the profiling of EMT in the following three different contexts: HaCaT human keratinocyte EMT in response to TGF-β, mouse model of aristolochic acid nephropathy, and human kidney-proximal tubule cells. Satisfyingly, one of these targets is Dishevelled 2 (DVL2), which is a gene that regulates EMT by repressing the production of Notch, GSK3β, and β-catenin. Another HEY1 target seen in all three systems was the polycomb family histone methyltransferases EZH1/EZH2, suggesting that TGF-β–activated EMT could be controlled through structural histone modification. Other TGF-β targets include integrins β4 and α6. Richard Bates (University of Massachusetts, Worcester, MA) reported that the integrin αvβ6 is up-regulated during colon cancer development and highly expressed in metastatic samples (Bates, 2005).
Christopher Gebeshuber showed that TGF-β induced Smad-2 tyrosine phosphorylation and that TGF-β–induced EMT was blocked upon expression of nonphosphorylatable Smad-2 mutant, the expression of which inhibited metastases formation. Gebeshuber also reported that this mutant had a reduced ability to interact with the Tcf–Lef1 transcription factor. This suggests that tyrosine phosphorylation of Smad-2 may potentiate Tcf–Lef1 interaction and stimulate both EMT and metastatic induction. Ali Nawshad (University of Nebraska, Lincoln, NE) and Elizabeth Hay reported a similar noncanonical role for TGF-β in the EMT of mouse palatal epithelial seam and kidney-proximal tubule cells. They reported that Smad-2/4 repressed E-cadherin transcription through Tcf–Lef1 (Masszi et al., 2004; Nawshad et al., 2005).
One of the functions of TGF-β is to stimulate expression of ECM proteins. Do ECM proteins initiate EMT? Andre Menke (University of Ulm, Ulm, Germany) showed that extracellular collagen that is deposited during a fibrotic disease can be an initiator of EMT. Menke reported that pancreatic cancer cell lines cultured on collagen I have a reduced capacity to cluster E-cadherin at points of cell–cell contact and have a more mesenchyme-like morphology. Menke postulated an EMT pathway where collagen induces both the recruitment of FAK to cadherin adhesion complexes and the phosphorylation of β-catenin. Phosphorylated β-catenin then translocates to the nucleus, activating EMT target genes. Conceptually, this may be similar to work by Mina Bissell describing the capacity of mechanical forces or the shape of the cell to initiate EMT.
Regulating Snail1
The Snail1 transcriptional repressor is a key EMT regulator (Barrallo-Gimeno and Nieto, 2005). There was much interest in signaling pathways converging on Snail1 production, stability, and intracellular localization. Derek Radisky (Mayo Clinic, Jacksonville, FL) reported that matrix metalloproteinase-3 (MMP-3) activates Snail1 production in mammary cells. MMP-3 is expressed in many primary breast tumors, induces mammary carcinogenesis in transgenic mice, and causes an in vitro EMT in mouse mammary cells (Lochter et al., 1997; Sternlicht et al., 1999). Radisky reported that MMP-3 activates EMT by inducing the production of an alternatively spliced variant of Rac1, which is a small GTPase that regulates cell migration through control of actin polymerization (Burridge and Wennerberg, 2004). This splice variant, termed Rac1b, activates the mitochondrial production of reactive oxygen species (ROS), which subsequently activates Snail1 production (Radisky et al., 2005). However, the mechanism by which MMP-3 stimulates alternative splicing, or how the Rac1 variant activates ROS, is unclear. Snail genes can be considered regulators of cell survival, adhesion, and migration, and the triggering of the EMT is just one of the mechanisms they use to promote cell movement (Barrallo-Gimeno and Nieto, 2005). Pierre Savagner (Batiment de Recherche en Cancerologie, Montpellier, France) reported that Snail2-deficient mice show delayed mammary gland tubule growth, and precocious branching morphogenesis similar to that seen in the mammary gland lacking P-cadherin, which is a cadherin that is selectively expressed in myoepithelial cells (Radice et al., 1997). Snail2-deficient mammary gland retained normal smooth muscle actin-staining myoepithelial cells. These cells lack P-cadherin, suggesting that Snail2 controls a progenitor-like phenotype in the mammary gland through P-cadherin.
Several investigators reported new insights into the control of Snail1 expression. Shoukat Dedhar (University of British Columbia, Vancouver, British Columbia, Canada) reported that integrin-linked kinase (ILK) activates Snail1 expression. Using proteomic approaches, Dedhar and coworkers made the surprising finding that ILK-mediated induction of Snail1 transcription maps to a portion of the Snail1 promoter that is bound by poly-ADP-ribose polymerase 1 (PARP-1). PARP-1 regulates transcription by modifying chromatin structure and through interaction with other transcription factors (Kim et al., 2005). ILK activation promotes PARP-1 binding to the Snail1 promoter, whereas siRNA ILK knockdown and drug inhibition of ILK activity prevents PARP-1 from binding to the promoter. siRNA knockdown of PARP-1 in mesenchymally transformed PC-3 cells inhibited Snail1 expression and stimulated E-cadherin expression, suggesting the novel idea that PARP-1 itself is an important factor in EMT control. It is unclear whether direct phosphorylation of PARP-1 by ILK controls its ability to interact with the Snail1 promoter. Inhibiting ILK activity with the small molecular inhibitor QLT0267 inhibited production of urokinase type plasminogen activator and the invasion of MDA-MB231 breast cancer cells (Nancy Dos Santos, University of British Columbia, Vancouver, British Columbia, Canada).
Anna Bagnato (Regina Elena Cancer Institute, Rome, Italy) also reported that endothelin 1 induced EMT in ovarian carcinomas in in vitro and in vivo cells through a phosphoinositide 3 kinase– and ILK-mediated signaling pathway, leading to glycogen synthase kinase-3β (GSK-3β) inhibition, Snail and β-catenin stabilization, and transcriptional programs that control repression of E-cadherin. Inhibition of the endothelin A receptor reversed the EMT, suppressed ILK and Snail1 expression, and restored E-cadherin expression. Snail1 represses E-cadherin expression by binding to three independent E-boxes in the cadherin promoter. Snail1 prevents E-cadherin expression through at least two pathways, one dependent on class I histone deacetylases and the other independent of it (Antonio Garcia de Herreros, Universitat Pompeu Fabra, Barcelona, Spain).
Snail transcription is regulated by the estrogen receptor (ER; Paul Wade, National Institute of Environmental Health Sciences, Research Triangle Park, NC). ER is an EMT inhibitor and is critical in maintaining the epithelial status of normal breast cells. Wade reported that MTA3, which is a component of the Mi-2–NuRD transcriptional repressor complex, is an ER-responsive gene, and its expression correlates well with ER expression in primary breast tissue samples. Wade reported that MTA3 binds to the Snail1 promoter and inhibits Snail1 transcription (Fujita et al., 2003). Because expression of the ER is a marker for good breast cancer prognosis, the observation that ER is an EMT inhibitor provides further evidence in support of a role for EMT in oncogenesis.
Snail1 levels can also be controlled posttranslationally, and Garcia de Herreros and Hung both reported that Snail1 is a phosphoprotein. Garcia de Herreros reported that Snail1 phosphorylation prevents its nuclear accumulation and inhibits its ability to activate EMT (Dominguez et al., 2003). Hung reported that Snail1 is phosphorylated by GSK-3β on two distinct motifs. Phosphorylation of two serines in the first motif directs Snail1 ubiquitination and proteolytic destruction. Phosphorylation of four serines on the second motif directs nuclear export. Mutation of all six GSK-3β phosphorylation sites increased the half-life of the Snail1 protein and ensured that it was constitutively nuclear. Consistent with a role for Snail1 phosphorylation in EMT, expression of Snail1 that could not be phosphorylated caused a loss of E-cadherin production and an EMT-like morphological change in human tumor lines (Zhou et al., 2004). Jim Woodgett (Samuel Lunenfeld Research Institute, Toronto, Ontario, Canada) described an important role for GSK-3β in controlling embryonic stem cell differentiation and the maintenance of pluripotency.
EMT in embryogenesis and adults
During embryogenesis, the neural crest develops from a small portion of the dorsal neural tube (Huang and Saint-Jeannet, 2004; Newgreen and McKeown, 2005). After an EMT, neural crest cells migrate away from the neural tube and differentiate into bone, smooth muscle, peripheral neurons and glia, and melanocytes. Don Newgreen (Murdoch Children's Research Institute, Melbourne, Australia) reported that the Sox transcription factors control this EMT and subsequent migration. Using an electroporation system that delivers Sox genes to cells on one side of the neural tube in living chicken embryos, Newgreen reported that ectopic expression of Sox-8, -9, or -10 was sufficient to induce EMT and activate migration away from the neural tube while suppressing terminal differentiation. This migratory capacity was conferred to all cells of the neural tube, indicating that Sox expression was overriding inhibitory signals that normally restrict neural tube EMT to cells of the neural crest.
Nelly Auersperg (University of British Columbia, Vancouver, British Columbia, Canada) provided evidence that EMT occurs in the ovaries of adult women. The mature mammalian ovary is enveloped by the ovarian surface epithelium (OSE), and the bulk of ovarian carcinomas arise from these cells. As a result of wound repair after egg extrusion, OSE cells are trapped in the ovarian follicle or stroma of postovulatory ovaries. Dr. Auersperg presented evidence showing that normal human OSE cells have a strong propensity to undergo EMT in vitro and in vivo in response to growth factor stimulation and alteration in their extracellular matrix. Auersperg suggested that normal OSE trapped within the ovary may undergo EMT as a means of maintaining ovarian homeostasis.
Cell adhesion and EMT
A defining feature of EMT is a reduction in E-cadherin levels and a concomitant production of N-cadherin. Cadherins are transmembrane proteins whose homotypic interaction between neighboring cells creates adherens junctions (Gumbiner, 2005). Alteration of cadherin-based adhesion has a key role in modulating development and organogenesis. At the cell membrane, cadherin proteins are found as homodimers tethered to the actin cytoskeleton by a multiprotein complex that includes α-, β-, and p120-catenin.
To characterize the physical forces underlying cadherin-based adhesion, Jean-Paul Thiery (Institut Pasteur, Paris, France) reported on an elegant system designed to measure the force necessary to separate two cells that are adhered solely to each other (Chu et al., 2004, 2005). Thiery reported that the development of intercellular adhesion by N- or E-cadherin is a two-step process. The first step relies on interactions between the cadherins on the surface of adjacent cells. This interaction takes 30 s to develop and requires a force of ∼10 nanoNewtons to break apart. The second step, which takes up to 30 min to maximize, strengthens the initial interaction and requires ∼200 nanoNewtons to separate it. This strengthening depends on Rac- and Cdc42-mediated induction of actin polymerization, presumably to anchor the cell surface cadherins to the cytosol. Thiery also reported that four times more force is required to separate adhesions between E-cadherin molecules compared with N-cadherin ones. In addition, there is no detectable interaction strength between E- and N-cadherin. This supports the current EMT paradigm, where the presence of E-cadherin in epithelial cells allows for greater cell–cell adhesive strength compared with that of the N-cadherin–expressing mesenchyme. Moreover, the minimal adhesive interaction between E- and N-cadherin would be predicted to allow an N-cadherin–expressing cell to migrate through a layer of E-cadherin–expressing cells.
Alpha Yap (University of Queensland, Brisbane, Australia) reported evidence that E-cadherin clustering at cell–cell junction sites requires dynamic microtubules. Yap reported visual evidence that the plus ends of microtubules terminate in E-cadherin puncta and that agents that block dynamic plus ends inhibit the ability of cells to concentrate cadherin at cell–cell contacts. This suggests that the actin and microtubule cytoskeletons both serve to anchor E-cadherin adhesions. This would contrast cadherin adhesions to integrin-containing focal adhesions because microtubule association with focal adhesions triggers their disassembly (Ezratty et al., 2005).
Mina Bissell (Lawrence Berkeley National Laboratory, Berkeley, CA) described data suggesting that cell shape changes brought about by the destruction of the basement membrane cause EMT. She then described a model of branching morphogenesis of the mammary gland and showed data to support a transient EMT at the tip of the branching structures. This was demonstrated by the activation of the vimentin promoter (visualized by a GFP reporter) at the branch tip. Bissell went on to describe studies that provided an understanding of how branching structures are created. She used engineered matrices and biomaterials to show that the architecture of the created vessel in collagen gels can determine where and how branches are created. Although the role of cell geometry in growth (Folkman and Moscona, 1978; Chen et al., 1997), apoptosis (Chen et al., 1997), and metabolic regulation (Bissell et al., 1977) has been known for decades, the molecular pathways that link cell shape to these events, and also to EMT, are only now beginning to be elucidated (Weaver et al., 2002; Paszek et al., 2005; Radisky et al., 2005). The orientation of a cell to its growth substrate may also regulate EMT. Marcia McCoy and Calvin Roskelley (University of British Columbia, Vancouver, British Columbia, Canada) reported that overexpression or mislocalization of the apical marker podocalyxin destabilized cell polarity in vitro, which may explain why podocalyxin overexpression is an independent marker of in vivo breast carcinoma progression (Somasiri et al., 2004).
EMT in cancer
The occurrence of EMT during tumor progression allows benign tumor cells (i.e., ones that are noninvasive and nonmetastatic) to acquire the capacity to infiltrate surrounding tissue and to ultimately metastasize to distant sites. The pathological staging of tumors supports this paradigm. The most compelling evidence for the involvement of EMT in oncogenesis is the ability of multiple EMT regulators to enhance tumor formation and/or metastasis (Thiery, 2002). For example, expression of Snail1 increases the aggressiveness of experimentally induced breast tumors, and high Snail1 expression correlates with an increased risk of tumor relapse and poor survival rates in human breast cancer (Moody et al., 2005). Loss of E-cadherin is a hallmark of metastatic carcinoma (Cavallaro and Christofori, 2004), and proteomic analysis of breast cancer reveals that circulating mammary tumor cells, or those found as micrometastases, show evidence of mesenchymal conversion (Willipinski-Stapelfeldt et al., 2005). The EMT meeting added to the growing list of EMT regulators that control some aspect of oncogenesis, which includes MMP-3, BCL9–2, EGFR, Met, Goosecoid, Kaiso, TGF-β, FOXC2, GSK-3β, Smad-3, Pez, Snail1, Snail2, and ILK (Table I).
However, there remains some controversy in the cancer community, particularly among pathologists, as to whether the transformation of a normal cell into a cancerous cell or a noninvasive tumor into a metastatic tumor is truly an EMT (Tarin et al., 2005). Skepticism about the role of EMT in cancer stems from the apparent rarity of the EMT–like morphological changes that are observed in primary tumor sections, and also from the observation that metastases appear histologically similar to the primary tumor from which they are derived. Of central importance, therefore, is the direct visualization of EMT during tumor progression. Garcia de Herreros used a new Snail1 antibody that is suitable for mouse and human immunohistochemistry (EC3) to show that Snail1 protein is expressed specifically at the invading front of colorectal tumors. Snail antibodies have been difficult to use in immunohistochemistry, and Karl-Friedrich Becker (Technical University of Munich, Munich, Germany) used another new Snail1 antibody (Sn9H2; Rosivatz et al., 2005) to demonstrate nuclear Snail1 in gastric, mammary, and endometrial tumors. Richard Bates reported that integrin αvβ6 is specifically expressed at the invading edge of colorectal cancer xenografts. Thomas Brabletz (University of Erlangen, Erlangen, Germany) reported that tumor cells at the invading edge of colorectal carcinomas have nuclear β-catenin and loss of E-cadherin. Nuclear localization of β-catenin is frequently used as an EMT marker, and nuclear β-catenin is a marker for a poor prognosis in colorectal cancer. The ability of EMT markers to identify a subset of tumor cells raises the possibility that EMT could be associated with the maintenance of cancer stem cells. Brabletz reported that invading cells with nuclear β-catenin also express the stem cell markers hTert and survivin, possibly implicating EMT in cancer stem cell maintenance (Brabletz et al., 2005). The presence of EMT markers at the tumor–host interface, but not in the bulk tumor, is strong evidence that EMT occurs during tumor development and that it regulates invasiveness and tumor aggressiveness.
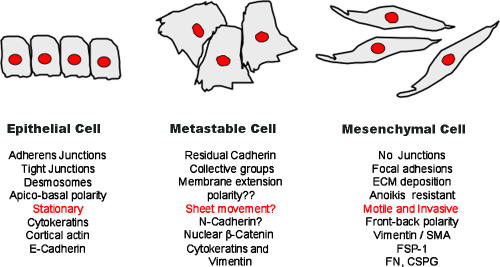
The histological similarity of secondary, metastasis-derived tumors to the primary tumor indicates that EMT-mediated metastatic development must be followed by a reverse MET to allow colonization of secondary sites. Brabletz reported that metastases derived from tumors originally expressing nuclear β-catenin were found to reexpress E-cadherin, and their β-catenin became cytoplasmic, which is suggestive of a MET (Brabletz et al., 2001). Similarly, Christine Chaffer (Bernard O'Brien, Institute of Microsurgery, Melbourne, Australia) reported that variants of the metastatic T24/TSU-Pr1 bladder carcinoma line that were selected for enhanced metastatic potential have more epithelial markers (E-cadherin and keratins) than their less metastatic counterparts, but continue to express some mesenchymal markers (vimentin and MMPs). This ability of cells to express attributes of both epithelial and mesenchymal phenotypes was referred to by Savagner as a “metastable phenotype” (Fig. 2). Consistent with this idea, Savanger reported that Rac distribution can be found with both epithelial-like (adherens junctions) and mesenchyme-like (lamellopodia) patterns during the migration of cohesive epithelial cells, and probably during tumor invasion as well. Metastability is consistent with the expression of stem cell markers in colorectal cells undergoing EMT and suggests that such plasticity may be found in progenitor cells in various organs. This plasticity could also be an explanation for the difficulty in observing EMT in cancer development; acquisition of mesenchymal characteristics may be transitory and undergo a reversal during later tumorigenesis.
Figure 2.
The metastable cell phenotype. Several studies have identified a hybrid cell showing both epithelial and mesenchymal traits. These cells are summarized here, in conjunction with their epithelial and mesenchymal counterparts. The term metastable was introduced at the meeting by Pierre Savagner, who showed evidence of epithelial and mesenchymal Rac localization within the same cells. Similar scenarios of hybrid cells were shown by Chaffer (metastasis-derived T24 human bladder carcinoma cells) and Thompson (EGF-treated PMC42 human breast cancer cells). Coexpression of mixed lineage traits within the same cell may be consistent with the stem cell–like profiles reported by Brabletz in colon carcinoma cells at the invasive front.
Robert Weinberg (Whitehead Institute, Cambridge, MA) reported that three transcription factors regulating developmental EMT—Twist, Goosecoid, and FOXC2—have important roles in metastasis. Each of these gene products enhances metastasis in experimental mouse models and is highly expressed in primary human tumors and metastases. Twist is a basic helix-loop-helix transcription factor that was originally identified as a D. melanogaster EMT activator (Castanon and Baylies, 2002). Weinberg reported that Twist expression is sufficient to induce an in vitro EMT in breast cells and that Twist inactivation inhibits metastasis development in vivo (Yang et al., 2004). Goosecoid is a homeobox transcriptional repressor that marks the Spemann organizer in vertebrate gastrulation and is one of the first identified regulators of embryological patterning (De Robertis et al., 2001). Both Twist and Goosecoid regulate FOXC2, which is a transcription factor of the FOX family of forkhead helix-turn-helix DNA-binding proteins that regulates EMT and organ development in multiple tissues (Carlsson and Mahlapuu, 2002). Twist, Goosecoid, and Snail1 all repress E-cadherin and induce FOXC2; they also enhance cell migration in vitro and metastatic potential in vivo. It is not yet known whether these three genes regulate individual or overlapping pathways of EMT and metastases. Importantly, FOXC2 also directly up-regulated mesenchymal gene transcription, rather than causing an EMT through E-cadherin repression.
Frans van Roy and Geert Berx (Ghent University, Ghent, Belgium) reported on the identification of a series of novel target genes of the E-cadherin repressors Snail1 and SIP1/ZEB2 that control the establishment of junctional complexes, intermediate filament networks, and the actin cytoskeleton (De Craene et al., 2005). They also showed some direct effects on mesenchymal factor transcription via these pathways. Christine Gilles (University of Liege, Liege, Belgium) reported that vimentin transcription was activated by SIP1/ZEB2, as well as a Tcf– β-catenin complex.
EMT in fibrosis
The accumulation of fibroblasts, excess collagen, and other matrix components at sites of chronic inflammation lead to scar tissue formation and progressive tissue injury. These fibroblasts derive from the bone marrow, but also arise from an EMT of cells at injury sites (Kalluri and Neilson, 2003; Neilson, 2005). EMT is likely involved in the progressive fibrotic diseases of the heart, lung, liver, and kidney.
Eric Neilson (Vanderbilt University, Nashville, TN) presented work using fibroblast-specific protein 1 (FSP1) as a marker for EMT that occurs during fibrosis (Iwano et al., 2002). FSP1-positive cells appear during kidney fibrosis and in IgA nephropathy; increased expression of FSP1 correlates with the prognosis and extent of fibrosis (Nishitani et al., 2005). The ablation of FSP1 cells attenuates fibrosis and collagen deposition, indicating a causal role for these cells in fibrotic disease (Iwano et al., 2001). Kidney FSP1-positive cells derive from two sources; from the bone marrow and from an EMT at sites of renal fibrosis (Iwano et al., 2002). Inactivation of FSP1 with a LacZ “knock in” mouse produced fibroblasts that were less motile in wound healing assays and had impaired angiogenesis in an aortic ring outgrowth model. Neilson also introduced studies on the FSP1 promoter and reported the identification a new zinc finger protein, fibroblast transcription factor 1, which binds in the FSP1 promoter. Fibroblast transcription factor 1 also up-regulates Twist and Snail1 and suppresses β-catenin, E-cadherin, and ZO-1 during EMT, indicating that it may be a key regulator of the EMT transcriptome.
Raghu Kalluri (Harvard University, Boston, MA) introduced the novel concept of endothelial–mesenchymal transition, which is probably an important process in TGF-β1–mediated cardiac fibrosis. Kalluri also reported that an inhibitor of TGF-β signaling, bone-morphogenic protein 7 (BMP7), could inhibit cardiac fibrosis in two mouse models of this disease. BMP7 belongs to the BMP family of TGF-β growth factors, and has a specific role as a morphogen during liver development. Kalluri also discussed the functional interconnection between EMT and angiogenesis, suggesting that angiogenesis inhibition could be therapeutic for fibrosis as well as cancer. Michael Zeisberg (Harvard University, Boston, MA) reported that BMP7 can inhibit fibroblast migration and prevent fibrotic disease in mouse models of liver fibrosis.
Emerging concepts and future directions of EMT
The detection of EMT in vivo during disease progression in adult organisms remains one of the central challenges of EMT physiology. Pioneering work by Iwano et al. (2002) established that fibrosis involves EMT, and this approach has been extended to include the formation of metastatic tumor cells (Xue et al., 2003). Evidence of EMT markers at the leading edge of invading tumors was provided by Bates (integrin αvβ6), Garcia de Herreros (using a new Snail1 antibody), and Brabletz (nuclear β-catenin), and these new findings were some of the highlights of the meeting, strongly suggesting an important role for EMT in driving tumor invasion and metastasis.
Because it is now possible to visualize the movement and morphology of individual tumor cells in real-time in a living animal (Condeelis and Segall, 2003), the examination of EMT in real-time is a possibility for the future. The detailed molecular studies of many investigators at the EMT meetings will hopefully provide additional markers for this task (Table I). These markers may allow further investigation into the role of metastability in cancer. Metastability indicates the existence of cells with features of both epithelial and mesenchymal cells. This concept is consistent with the sequential steps of junctional dissolution that were described by Thiery (Thiery and Huang, 2005) and is gaining momentum through the accumulation of evidence in favor of such hybrid states. The predominantly epithelial, yet somewhat mesenchymal, phenotype of highly aggressive and metastatic bladder cancer cells presented by Chaffer reinforces the potential of many cancer cells for plastic differentiation. In addition, Savagner showed evidence of both epithelial and mesenchymal patterning of Rac in epithelial cells that were induced to migrate. The importance of MET or other partial loss of mesenchymal markers in the successful growth of metastases could add further opportunities for therapies that block metastases. The possibility that softer boundaries exist between epithelial and mesenchymal tumor cells and the possibility of hybrid cells may help explain the current lack of robust clinical evidence for EMT as a metastasis mediator (Tarin et al., 2005).
Most importantly, the meeting witnessed the emergence of EMT as a target for drug development in cancer and fibrosis. For example, BMP7 mimetics antagonize TGF-β–driven EMT in fibrotic kidney and heart and inhibit disease development. In addition, small molecule ILK inhibitors inhibit Snail1 production, induce E-cadherin expression, and inhibit invasion. Also discussed at the meeting was the possibility that angiogenesis, EMT, fibrosis, and cancer have common regulatory pathways and that the angiogenesis inhibition may be useful in both fibrosis and cancer. The involvement of ILK in angiogenesis, EMT, fibrosis, and cancer suggest that ILK inhibition may be one useful therapy. In addition, EMT could be used as a functional screen for novel anticancer agents, a strategy that led to the identification of motuporamine (Calvin Roskelley). Motuporamine was derived from a library of marine invertebrate compounds and inhibits in vitro invasion and migration by activating the Rho GTPase and stimulating actin stress fiber formation. Continued identification of new EMT inhibitors holds the promise of novel cancer and fibrosis treatment options.
We anticipate considerable progress in this field in the year leading up to the 2007 EMT meeting, which is planned to take place in Montpellier, France (http://www.mtci.com.au/temtia.html), building on the current exponential trend of EMT observations in numerous cellular systems of physiological and pathophysiological importance.
Acknowledgments
We thank all those whose presentations we summarized for reviewing the appropriate text and for their permission to report unpublished work. The EMT 2005 meeting was convened by Shoukat Dedhar and Raghu Kalluri and with an International Committee comprised of Mina Bissell, Elizabeth Hay, Kohei Miyazono, Suresh Mohla, Donald Newgreen, Pierre Savagner, Jean-Paul Thiery, Erik Thompson, and Robert Weinberg.
Jonathan Lee is supported by operating grants from the National Cancer Institute of Canada, the Canadian Breast Cancer Research Alliance, and the U.S. Army Medical Research and Materiel Command (DAMD17-03-1-0671). Shoukat Dedhar is supported by grants from the National Cancer Institute of Canada, the Canadian Breast Cancer Research Alliance, and the Canadian Institutes for Health Research. The work in Raghu Kalluri's laboratory is supported by grants from National Institutes of Health (DK62987, DK61688, DK55001, and AA13913). Erik Thompson acknowledges support from the Victorian Breast Cancer Research Consortium and the U.S. Army Medical Research and Materiel Command (DAMD17-03-1-0416).
Abbreviations used in this paper: BMP7, bone-morphogenic protein 7; EGFR, EGF receptor; EMT, epithelial–mesenchymal transition; ER, estrogen receptor; FSP1, fibroblast-specific protein 1; GSK, glycogen synthase kinase; ILK, integrin-linked kinase; MET, mesenchymal–epithelial transition; MMP, matrix metalloproteinase; OSE, ovarian surface epithelium; PARP-1, poly-ADP-ribose polymerase 1; ROS, reactive oxygen species; siRNA, small interfering RNA.
References
- Barrallo-Gimeno, A., and M.A. Nieto. 2005. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 132:3151–3161. [DOI] [PubMed] [Google Scholar]
- Bates, R.C. 2005. Colorectal cancer progression: integrin alphavbeta6 and the epithelial-mesenchymal transition (EMT). Cell Cycle. 4:1350–1352. [DOI] [PubMed] [Google Scholar]
- Bissell, M.J., D. Farson, and A.S. Tung. 1977. Cell shape and hexose transport in normal and virus-transformed cells in culture. J. Supramol. Struct. 6:1–12. [DOI] [PubMed] [Google Scholar]
- Brabletz, T., A. Jung, S. Reu, M. Porzner, F. Hlubek, L.A. Kunz-Schughart, R. Knuechel, and T. Kirchner. 2001. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA. 98:10356–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz, T., A. Jung, S. Spaderna, F. Hlubek, and T. Kirchner. 2005. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat. Rev. Cancer. 5:744–749. [DOI] [PubMed] [Google Scholar]
- Brembeck, F.H., T. Schwarz-Romond, J. Bakkers, S. Wilhelm, M. Hammerschmidt, and W. Birchmeier. 2004. Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev. 18:2225–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge, K., and K. Wennerberg. 2004. Rho and Rac take center stage. Cell. 116:167–179. [DOI] [PubMed] [Google Scholar]
- Carlsson, P., and M. Mahlapuu. 2002. Forkhead transcription factors: key players in development and metabolism. Dev. Biol. 250:1–23. [DOI] [PubMed] [Google Scholar]
- Castanon, I., and M.K. Baylies. 2002. A Twist in fate: evolutionary comparison of Twist structure and function. Gene. 287:11–22. [DOI] [PubMed] [Google Scholar]
- Cavallaro, U., and G. Christofori. 2004. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer. 4:118–132. [DOI] [PubMed] [Google Scholar]
- Chen, C.S., M. Mrksich, S. Huang, G.M. Whitesides, and D.E. Ingber. 1997. Geometric control of cell life and death. Science. 276:1425–1428. [DOI] [PubMed] [Google Scholar]
- Chu, Y.S., O. Eder, W.A. Thomas, I. Simcha, F. Pincet, A. Ben-Ze'ev, E. Perez, J.P. Thiery, and S. Dufour. 2005. Prototypical type-I E-cadherin and type-II cadherin-7 mediate very distinct adhesiveness through their extracellular domain. J. Biol. Chem. 281:365–373. [DOI] [PubMed] [Google Scholar]
- Chu, Y.S., W.A. Thomas, O. Eder, F. Pincet, E. Perez, J.P. Thiery, and S. Dufour. 2004. Force measurements in E-cadherin–mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J. Cell Biol. 167:1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis, J., and J.E. Segall. 2003. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer. 3:921–930. [DOI] [PubMed] [Google Scholar]
- De Craene, B., B. Gilbert, C. Stove, E. Bruyneel, F. van Roy, and G. Berx. 2005. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 65:6237–6244. [DOI] [PubMed] [Google Scholar]
- De Robertis, E.M., O. Wessely, M. Oelgeschlager, B. Brizuela, E. Pera, J. Larrain, J. Abreu, and D. Bachiller. 2001. Molecular mechanisms of cell–cell signaling by the Spemann-Mangold organizer. Int. J. Dev. Biol. 45:189–197. [PMC free article] [PubMed] [Google Scholar]
- Dominguez, D., B. Montserrat-Sentis, A. Virgos-Soler, S. Guaita, J. Grueso, M. Porta, I. Puig, J. Baulida, C. Franci, and A. Garcia de Herreros. 2003. Phosphorylation regulates the subcellular location and activity of the snail transcriptional repressor. Mol. Cell. Biol. 23:5078–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty, E.J., M.A. Partridge, and G.G. Gundersen. 2005. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7:581–590. [DOI] [PubMed] [Google Scholar]
- Folkman, J., and A. Moscona. 1978. Role of cell shape in growth control. Nature. 273:345–349. [DOI] [PubMed] [Google Scholar]
- Fujita, N., D.L. Jaye, M. Kajita, C. Geigerman, C.S. Moreno, and P.A. Wade. 2003. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 113:207–219. [DOI] [PubMed] [Google Scholar]
- Giri, D.K., M. Ali-Seyed, L.Y. Li, D.F. Lee, P. Ling, G. Bartholomeusz, S.C. Wang, and M.C. Hung. 2005. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol. Cell. Biol. 25:11005–11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner, B.M. 2005. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6:622–634. [DOI] [PubMed] [Google Scholar]
- Hay, E.D. 2005. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev. Dyn. 233:706–720. [DOI] [PubMed] [Google Scholar]
- Huang, X., and J.P. Saint-Jeannet. 2004. Induction of the neural crest and the opportunities of life on the edge. Dev. Biol. 275:1–11. [DOI] [PubMed] [Google Scholar]
- Iwano, M., A. Fischer, H. Okada, D. Plieth, C. Xue, T.M. Danoff, and E.G. Neilson. 2001. Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Mol. Ther. 3:149–159. [DOI] [PubMed] [Google Scholar]
- Iwano, M., D. Plieth, T.M. Danoff, C. Xue, H. Okada, and E.G. Neilson. 2002. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri, R., and E.G. Neilson. 2003. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 112:1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M.Y., T. Zhang, and W.L. Kraus. 2005. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 19:1951–1967. [DOI] [PubMed] [Google Scholar]
- Lo, H.W., S.C. Hsu, M. Ali-Seyed, M. Gunduz, W. Xia, Y. Wei, G. Bartholomeusz, J.Y. Shih, and M.C. Hung. 2005. a. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 7:575–589. [DOI] [PubMed] [Google Scholar]
- Lo, H.W., W. Xia, Y. Wei, M. Ali-Seyed, S.F. Huang, and M.C. Hung. 2005. b. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res. 65:338–348. [PubMed] [Google Scholar]
- Lochter, A., A. Srebrow, C.J. Sympson, N. Terracio, Z. Werb, and M.J. Bissell. 1997. Misregulation of stromelysin-1 expression in mouse mammary tumor cells accompanies acquisition of stromelysin-1-dependent invasive properties. J. Biol. Chem. 272:5007–5015. [DOI] [PubMed] [Google Scholar]
- Masszi, A., L. Fan, L. Rosivall, C.A. McCulloch, O.D. Rotstein, I. Mucsi, and A. Kapus. 2004. Integrity of cell–cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: role for beta-catenin. Am. J. Pathol. 165:1955–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody, S.E., D. Perez, T.C. Pan, C.J. Sarkisian, C.P. Portocarrero, C.J. Sterner, K.L. Notorfrancesco, R.D. Cardiff, and L.A. Chodosh. 2005. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 8:197–209. [DOI] [PubMed] [Google Scholar]
- Nawshad, A., D. Lagamba, A. Polad, and E.D. Hay. 2005. Transforming growth factor-beta signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 179:11–23. [DOI] [PubMed] [Google Scholar]
- Neilson, E.G. 2005. Setting a trap for tissue fibrosis. Nat. Med. 11:373–374. [DOI] [PubMed] [Google Scholar]
- Newgreen, D.F., and S.J. McKeown. 2005. Neural crest cells migration. In Rise and Fall of Epithelial Phenotype: Concepts of Epithelial-Mesenchymal Transition. P. Savagner, editor. Landes Bioscience, Texas. 29–39.
- Nishitani, Y., M. Iwano, Y. Yamaguchi, K. Harada, K. Nakatani, Y. Akai, T. Nishino, H. Shiiki, M. Kanauchi, Y. Saito, and E.G. Neilson. 2005. Fibroblast-specific protein 1 is a specific prognostic marker for renal survival in patients with IgAN. Kidney Int. 68:1078–1085. [DOI] [PubMed] [Google Scholar]
- Paszek, M.J., N. Zahir, K.R. Johnson, J.N. Lakins, G.I. Rozenberg, A. Gefen, C.A. Reinhart-King, S.S. Margulies, M. Dembo, D. Boettiger, et al. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell. 8:241–254. [DOI] [PubMed] [Google Scholar]
- Radice, G.L., M.C. Ferreira-Cornwell, S.D. Robinson, H. Rayburn, L.A. Chodosh, M. Takeichi, and R.O. Hynes. 1997. Precocious mammary gland development in P-cadherin–deficient mice. J. Cell Biol. 139:1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky, D.C., D.D. Levy, L.E. Littlepage, H. Liu, C.M. Nelson, J.E. Fata, D. Leake, E.L. Godden, D.G. Albertson, M.A. Nieto, et al. 2005. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 436:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosivatz, E., K.F. Becker, E. Kremmer, C. Schott, K. Blechschmidt, H. Hofler, and M. Sarbia. 2005. Expression and nuclear localization of Snail, an E-cadherin repressor, in adenocarcinomas of the upper gastrointestinal tract. Virchows Arch. 17:1–11. [DOI] [PubMed] [Google Scholar]
- Shook, D., and R. Keller. 2003. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech. Dev. 120:1351–1383. [DOI] [PubMed] [Google Scholar]
- Somasiri, A., J.S. Nielsen, N. Makretsov, M.L. McCoy, L. Prentice, C.B. Gilks, S.K. Chia, K.A. Gelmon, D.B. Kershaw, D.G. Huntsman, et al. 2004. Overexpression of the anti-adhesin podocalyxin is an independent predictor of breast cancer progression. Cancer Res. 64:5068–5073. [DOI] [PubMed] [Google Scholar]
- Sternlicht, M.D., A. Lochter, C.J. Sympson, B. Huey, J.P. Rougier, J.W. Gray, D. Pinkel, M.J. Bissell, and Z. Werb. 1999. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 98:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin, D., E.W. Thompson, and D.F. Newgreen. 2005. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 65:5996–6000. [DOI] [PubMed] [Google Scholar]
- Thiery, J.P. 2002. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2:442–454. [DOI] [PubMed] [Google Scholar]
- Thiery, J.P., and R. Huang. 2005. Linking epithelial-mesenchymal transition to the well-known polarity protein Par6. Dev. Cell. 8:456–458. [DOI] [PubMed] [Google Scholar]
- Thompson, E.W., D.F. Newgreen, and D. Tarin. 2005. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 65:5991–5995. [DOI] [PubMed] [Google Scholar]
- Thomson, S., E. Buck, F. Petti, G. Griffin, E. Brown, N. Ramnarine, K.K. Iwata, N. Gibson, and J.D. Haley. 2005. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 65:9455–9462. [DOI] [PubMed] [Google Scholar]
- van Es, J.H., N. Barker, and H. Clevers. 2003. You Wnt some, you lose some: oncogenes in the Wnt signaling pathway. Curr. Opin. Genet. Dev. 13:28–33. [DOI] [PubMed] [Google Scholar]
- Wang, Z., D. Shen, D.W. Parsons, A. Bardelli, J. Sager, S. Szabo, J. Ptak, N. Silliman, B.A. Peters, M.S. van der Heijden, et al. 2004. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science. 304:1164–1166. [DOI] [PubMed] [Google Scholar]
- Weaver, V.M., S. Lelievre, J.N. Lakins, M.A. Chrenek, J.C. Jones, F. Giancotti, Z. Werb, and M.J. Bissell. 2002. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willipinski-Stapelfeldt, B., S. Riethdorf, V. Assmann, U. Woelfle, T. Rau, G. Sauter, J. Heukeshoven, and K. Pantel. 2005. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin. Cancer Res. 11:8006–8014. [DOI] [PubMed] [Google Scholar]
- Xue, C., D. Plieth, C. Venkov, C. Xu, and E.G. Neilson. 2003. The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res. 63:3386–3394. [PubMed] [Google Scholar]
- Yang, J., S.A. Mani, J.L. Donaher, S. Ramaswamy, R.A. Itzykson, C. Come, P. Savagner, I. Gitelman, A. Richardson, and R.A. Weinberg. 2004. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 117:927–939. [DOI] [PubMed] [Google Scholar]
- Zavadil, J., and E.P. Bottinger. 2005. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774. [DOI] [PubMed] [Google Scholar]
- Zavadil, J., L. Cermak, N. Soto-Nieves, and E.P. Bottinger. 2004. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 23:1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B.P., J. Deng, W. Xia, J. Xu, Y.M. Li, M. Gunduz, and M.C. Hung. 2004. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 6:931–940. [DOI] [PubMed] [Google Scholar]