Abstract
Protection from ultraviolet (UV) irradiation is a fundamental issue for living organisms. Although melanin's critical role in the protection of basal keratinocytes is well understood, other factors remain essentially unknown. We demonstrate that up-regulation of squamous cell carcinoma antigen-1 (SCCA1) suppresses c-Jun NH2-terminal kinase-1 (JNK1) and thus blocks UV-induced keratinocyte apoptosis. We found that serpin SCCA1 is markedly elevated in the top layers of sun-exposed or UV-irradiated epidermis. UV-induced apoptosis was significantly decreased when SCCA was overexpressed in 3T3/J2 cells. It was significantly increased when SCCA was down-regulated with small interfering RNA in HaCaT keratinocytes. A search for SCCA-interacting molecules showed specific binding with phosphorylated JNK. Interestingly, SCCA1 specifically suppressed the kinase activity of JNK1. Upon exposure of keratinocytes to UV, SCCA1 was bound to JNK1 and transferred to the nucleus. Involucrin promoter–driven SCCA1 transgenic mice showed remarkable resistance against UV irradiation. These findings reveal an unexpected serpin function and define a novel UV protection mechanism in human skin.
Introduction
Squamous cell carcinoma antigen (SCCA) was first discovered as a marker of squamous cell carcinomas in the cervix (Kato and Torigoe, 1977). Cloning of the SCCA gene demonstrated that SCCA belongs to the serpin superfamily of serine proteinase inhibitors (Suminami et al., 1991). However, it soon became apparent that SCCA is a cross-class inhibitor and that its target molecules include cysteine proteinases such as cathepsin L and papain (Takeda et al., 1995; Schick et al., 1998). Recent work has revealed the presence of tandemly aligned homologous genes. The telomeric DNA segment contains a gene that was cloned by Suminami et al. (1991) and has since been designated SCCA1 (Schneider et al., 1995). The centromeric gene, which is 92% identical at the nucleic acid level, was named SCCA2. Interestingly, SCCA2 inhibits chymotrypsin and its relatives, as is expected for a serpin (Schick et al., 1997). SCCAs are also expressed in psoriatic epidermis (Takeda et al., 2002), which is where abnormal proliferation and aberrant differentiation are characteristic features.
The epidermis is the outermost tissue, whose primary role is to form a barrier against hostile environmental factors, including UV, and it consists of four kinds of cells (i.e., cornified, granular, spinous, and basal cells). Although the epidermis has effective countermeasures against UV irradiation, its protective mechanisms, other than melanin, are still unknown.
We show that SCCA1 is a specific endogenous inhibitor of c-Jun-NH2-terminal kinase-1 (JNK1) and acts to protect UV-exposed keratinocytes from apoptotic cell death.
Results and discussion
SCCAs are up-regulated in UV-irradiated and sun-exposed skin
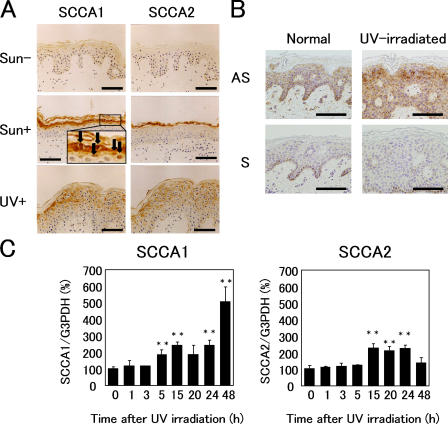
During studies to analyze the localization of SCCAs in normal and diseased skin, we observed strong up-regulation of SCCAs in sun-exposed epidermis. In normal, sun-protected skin, SCCAs were only weakly stained in the upper epidermis (Fig. 1 A). When the cheeks and eyelids of 22–84-yr-old subjects were examined, all the skin tissues showed marked elevation of SCCAs, although SCCA1 was predominant. Interestingly, some of the nuclei in sun-exposed epidermis were heavily stained, as was the cytoplasm (Fig. 1 A, inset). A study involving UV irradiation of the buttocks of healthy volunteers confirmed strong induction of SCCA1 in the spinous to granular layers of the irradiated skin. An in situ hybridization study showed that SCCA1 mRNA is weakly detectable in normal epidermis (Fig. 1 B). UV irradiation caused strong induction of SCCA1 mRNA in the top layers of the epidermis. In cultured neonatal human keratinocytes (NHK), quantitative PCR analysis showed induction of both SCCAs, but a larger amount of SCCA1 mRNA is synthesized in the later stage, after UV irradiation (Fig. 1 C). This is presumably the reason why the overall production of SCCA1 is much higher than that of SCCA2 in sun-exposed skin.
Figure 1.
SCCA is up-regulated by UV irradiation in vivo and in vitro. (A) Immunostaining for SCCA1 and SCCA2 in sun-protected buttock skin, sun-exposed (cheek), and UV-irradiated buttock skin (two minimal erythema doses of radiation; biopsy taken after 48 h). The inset shows nuclear localization of SCCA1 at high magnification. Arrows indicate heavy SCCA1 staining in the nuclei. (B) In situ mRNA hybridization of SCCA1. The sense probe did not show any positive reaction. The dark brown color seen in basal cells is caused by melanin. (C) Quantitative real-time PCR analysis of SCCA1 and SCCA2 mRNA levels in cultured human keratinocytes. Values given are SCCA1 or SCCA2 mRNA levels normalized to the amount of G3PDH. Error bars represent the mean of five wells ± SD. **, P < 0.01. Bars, 100 μm.
SCCAs play a key role in UV-induced cell death
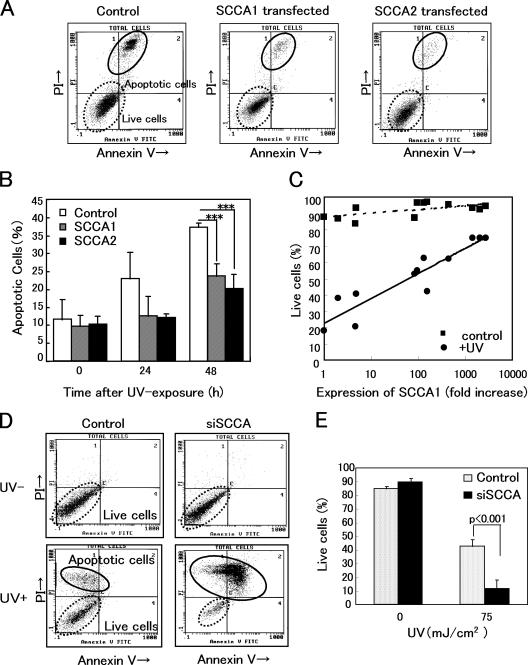
In mouse, serpin b3a is considered the mouse orthologue of human SCCA because of its strong inhibition of chymotrypsin (Ray et al., 2005). Semiquantitative PCR analysis showed that mouse epidermis expressed serpin b3a (mouse SCCA), although 3T3/J2, which is a mouse fibroblast cell line, did not produce mouse SCCA (Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200508064/DC1). To investigate the photobiological role of SCCA, we stably transfected SCCA cDNAs into 3T3/J2; clones were established under G418 selection. Each clone expressed only one transfected gene, SCCA1 or SCCA2 (Fig. S1 B). FACS analyses demonstrated that 3T3/J2 cells undergo apoptosis when exposed to UV at doses >30 mJ/cm2 (Fig. 2 A). At 30 mJ/cm2, 37.2 ± 1.3% of the mock-transfected cells (control) died within 48 h (Fig. 2 B). When each transfected pool was used, SCCA1- or SCCA2-transfected cells showed resistance to UV-induced apoptosis (P < 0.001) and only 23.8 ± 3.3% or 19.9 ± 4.4% of transfected cells died after UV irradiation, respectively. There was no significant difference in antiapoptotic activity between SCCA1 and SCCA2. We established 12 individual clones for SCCA1 and examined the effect of UV irradiation. Only 20.7% of the minimally expressing cells survived at 50 mJ/cm2, whereas 74.7% of the maximally expressing cells (2,772-fold expression) survived in the same condition (Fig. 2 C). Clearly, SCCA1-expressing clones showed a strong correlation between the expression levels and the suppression of UV-induced apoptosis (r = 0.734). We then examined the effect of the suppression of SCCAs using small interfering RNA (siRNA). An H1 promoter-driven siRNA sequence directed against homologous sites of SCCA1 and SCCA2 was introduced into HaCaT cells. We used the HaCaT cell line because it was derived from spontaneous transformation of human adult keratinocytes (Boukamp et al., 1988) and synthesizes relatively high levels of both SCCAs under proliferative conditions. A stably transfected cell line was established (small interfering SCCA [siSCCA]/HaCaT), which showed >90% suppression of both SCCA1 and SCCA2 mRNA levels, compared with the control siRNA (targeted to GFP mRNA) as judged by quantitative PCR (Fig. S1 C). Western blot analysis showed that SCCA protein levels were also decreased to approximately one-tenth of the control in siSCCA/HaCaT (Fig. S1 D). The siSCCA- and control siRNA-expressing cells did not show any changes by FACS analyses (Fig. 2 D). However, UV irradiation caused a significant decrease of cell survival in siSCCA/HaCaT cells (Fig. 2, D and E). Under conditions where 42.8 ± 5.0% of control cells survived, only 11.8 ± 6.3% of siSCCA/HaCaT cells survived (P < 0.001). Overall, the results suggest that SCCA1 and SCCA2 can similarly suppress UV-induced apoptosis and that the expression levels of SCCA regulate the antiapoptotic activity under UV irradiation. The antiapoptotic activity may be independent of proteinase-inhibitory activity because these molecules possess highly specific inhibition profiles for cysteine and serine proteinases, respectively. Furthermore, SCCA1 may play a primary role because of its abundance after the UV induction.
Figure 2.
Overexpression or down-regulation of SCCAs significantly affected UV-induced apoptosis. (A) FACS analyses of apoptotic cells using SCCA1- or SCCA2-transfected 3T3/J2 cells, 48 h after UV irradiation (30 mJ/cm2). Cells were stained with FITC-conjugated Annexin V and propidium iodide. (B) Analyses of five experiments are summarized. (C) 12 clones whose expression levels of SCCA1 mRNA distributed from 1 to 2,772-fold were established. Using these clones, the effects of UV irradiation were examined. Cells were harvested 48 h after UV irradiation (50 mJ/cm2) and FACS analyses were performed. The antiapoptotic activity correlated with SCCA1 expression. r = 0.734. (D) Using pSilencer vector, an siRNA construct targeted to a homologous sequence of SCCAs was stably transfected into HaCaT keratinocytes. Typical FACS analyses of nonirradiated (UV−) and UV-irradiated (UV+) siSCCA/HaCaT cells were shown. Apoptotic cells were analyzed 48 h after UV irradiation (75 mJ/cm2). (E) Statistical analyses of five experiments. Error bars represent the mean of five wells ± SD.
SCCA1 binds with phosphorylated JNK1 and is transferred into the nucleus after UV irradiation
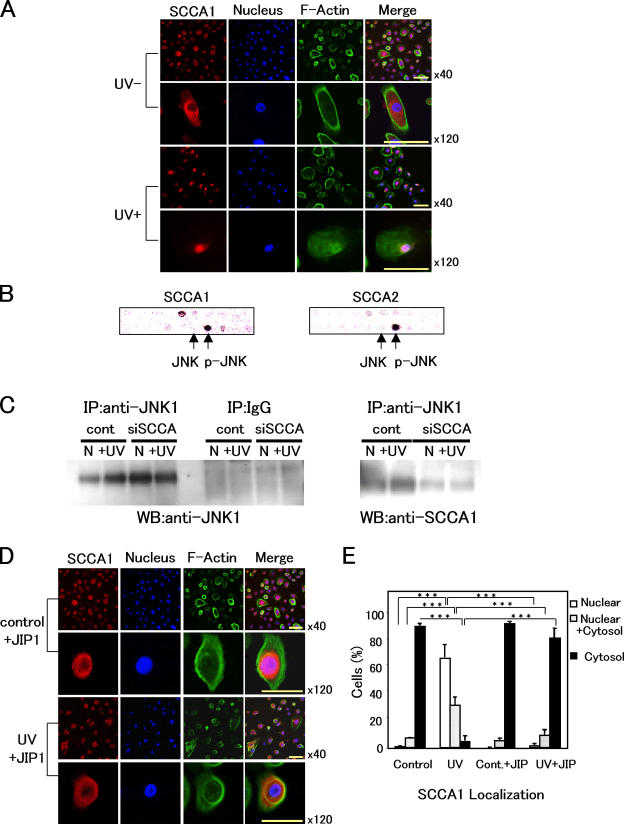
To determine the intracellular localization of SCCAs in NHK, we used laser confocal microscopy. As shown in Fig. 3 A, SCCA1 was present exclusively in the cytoplasm of the proliferating keratinocytes. After UV irradiation at 50 mJ/cm2, SCCA1 was translocated into the nucleus and cytoplasmic localization was greatly reduced at 24 h. Because the SCCAs do not contain known nuclear translocation signals, it is likely that SCCAs are transported to the nucleus via binding with a target molecule.
Figure 3.
SCCA1 binds with JNK1 and translocates into nucleus after UV irradiation. (A) Localization of SCCA1 before and 24 h after UV irradiation (50 mJ/cm2). NHK cells were stained for SCCA1, nucleus, and F-actin with (UV+) or without UV treatment (UV−). (B) Interaction of SCCAs with p-JNK. HSC-4 cell extract was applied to a signal transduction antibody array, followed by incubation with HRP-conjugated antibody against either SCCA1 or SCCA2. Black spots indicate positive binding to p-JNK. Arrows indicate the spots representing antibodies to JNK and p-JNK. (C) HaCaT cell extracts transfected with control pSilencer vector or siSCCA were immunoprecipitated with anti-JNK1 antibody, followed by blotting with indicated antibodies. Normal rabbit IgG was used as a nonspecific control (middle). Effects of UV irradiation were also examined (N, not irradiated; +UV, 5 min after 100 mJ/cm2 UV irradiation). (D) Effects of JIP1 peptide on SCCA1 localization. NHK cells were cultured in the presence of 5 μM of JIP1 peptide and stained for SCCA1, nucleus, and F-actin with (UV+, 50 mJ/cm2) or without (UV−) UV treatment. (E) Statistical analysis of SCCA1 translocation. In each case (i.e., control [nontreated], UV-irradiated [UV], control + JIP1 peptide [5 μM; Cont. + JIP], and UV-irradiated [50 mJ/cm2] + JIP1 peptide [5 μM; UV + JIP]), a minimum of 300 cells were counted three times. Error bars represent the mean of three experiments ± SD. ***, P < 0.001. Bars, 20 μm.
To look for SCCA-binding molecules, we used a signal transduction antibody array. This screening resulted in the identification of JNK as a protein that binds to both SCCA1 and SCCA2 (Fig. 3 B). Interestingly, the binding of SCCAs was observed only with active JNK (p-JNK), but not with inactive JNK. Anti-p38, ERK1, and ERK2 antibodies were also present on the membrane, but showed a negative result (unpublished data). Interaction between SCCA1 and JNK1 was confirmed with immunoprecipitation. Interestingly, SCCA1 was detected in the precipitates prepared with anti-JNK1 antibody (Fig. 3 C), indicating the binding of SCCA1 to JNK1 within the cell. SCCA1 precipitated with anti-JNK1 antibody was markedly reduced in siSCCA/HaCaT.
We examined the UV responsiveness of JNK1 in cultured NHK cells because growing evidence suggests that JNK1 is responsible for UV-induced apoptotic cell death in other cell types (Butterfield et al., 1997; Tong et al., 2001; Hochedlinger et al., 2002). JNK1 was found in the cytoplasm, as well as in the nucleus, of proliferating NHK cells (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200508064/DC1). After UV irradiation, JNK1 accumulated into the nucleus (77.9 ± 9.4%; Fig. S2, A and B).
To investigate whether translocation of SCCAs is caused by binding with JNK1, we used a peptide inhibitor of JNKs, TAT-containing JNK-interacting protein-1 (JIP1) peptide (Bonny et al., 2001; Barr et al., 2002). First, we confirmed that in the presence of 5 μM of JIP1 peptide nuclear translocation of JNK1 was no longer observed, even after 50 mJ/cm2 UV irradiation. JNK1 remained in the cytoplasm of most NHK cells (exclusively cytoplasmic, 65.4 ± 11.5%; cytoplasmic and nuclear, 31.3 ± 8.9%) after UV irradiation (Fig. S2 B). JIP1 control peptide had no effect on the localization of JNK1 (Fig. S2 C). We then analyzed the effect of JIP1 peptide on SCCA1 localization. SCCA1, which was present mostly in the cytoplasm of control keratinocytes (92.5 ± 1.5%), promptly accumulated in the nucleus after UV irradiation (confined nuclear, 63.6 ± 9.4%; peripheral nuclear, 32.0 ± 7.0%; Fig. 3, D and E). JIP1-negative control peptide had no effect on JNK or SCCA1 localization (Fig. S2 C). JIP1 peptide alone did not affect the localization of SCCA1. Interestingly, in the presence of 5 μM of JIP1 peptide, SCCA1 remained in the cytoplasm of UV-irradiated cells (81.1 ± 8.5%; Fig. 3, D and E). The fact that JIP1 peptide inhibited translocation of SCCA1 into the nucleus after UV irradiation strongly suggests that SCCA1 requires direct or indirect association with JNK1 for the translocation. Our results indicate that the interaction of SCCA1 with the complex may be involved in the UV-induced JNK activation.
SCCA1 specifically inhibits kinase activity of JNK1
Gene disruption studies have revealed that JNK1, but not JNK2, plays a key role in UV-induced apoptosis (Hochedlinger et al., 2002). Therefore, we investigated the effect of SCCA1 on JNK1 kinase activity. Because c-Jun is a well-known substrate of JNK, we used in vitro kinase assays to examine whether c-Jun phosphorylation by JNK1 is affected by SCCA1. In the presence of SCCA1, c-Jun phosphorylation was significantly reduced (Fig. 4 A). Inhibition was nearly parallel with the active JNK1 concentration and was observed at a uniform rate with increasing amounts of active JNK1 (Fig. 4 B), suggesting stoichiometric interaction between SCCA1 and p-JNK1. We also confirmed that SCCA1 did not show any suppression on the kinase activities of other MAPKs, p38α (Fig. 4 C), ERK1, or ERK2 (Fig. 4 D).
Figure 4.
SCCA1 specifically inhibits kinase activity of JNK1. (A) Inhibition of JNK1 kinase activity by SCCA1. An in vitro kinase pull-down assay of the phosphorylation of c-Jun by JNK1 was performed using an SAPK/JNK assay kit. A constant amount of recombinant SCCA1 was incubated with various concentrations of active JNK1, and changes in c-Jun phosphorylation were analyzed with Western blots. (B) Quantitative analysis of the results shown in A. Representative data from five independent experiments are shown. Each band was scanned and the relative intensity was measured with or without SCCA1 (control) and plotted against the amount of p-JNK1. (C) Effects of SCCA1 on p38α kinase activity. Various concentrations of p38α MAPK were incubated with or without 10 times the excess amount of SCCA1, and changes in ATF2 phosphorylation were analyzed with Western blot using anti–phospho-ATF2 antibody. (D) Effects of SCCA1 on ERK1 and ERK2 kinase activity. Kinase activities of ERK1 or ERK2 with or without 20 ng SCCA1 were measured usinga MAPK (ERK1/2) activity assay kit. (E) Changes in c-Jun phosphorylation in HaCaT cells. Extracts from the control or siSCCA/HaCaT cells were obtained 0, 5, and 15 min after UV irradiation (100 mJ/cm2), and levels of c-Jun or phospho-c-Jun were compared with immunoblots. Densitmetric analysis of the ratio between c-Jun and phospho-c-Jun from three independent assays was also shown. Error bars represent the mean of five experiments ± SD. (F) Effects of SCCA knockdown on ERK1/2, p38α, and MAPKAPK2 after UV irradiation. The same extracts used in E were immunoblotted for each form of kinases.
Interestingly, SCCA1 altered the phosphorylation of c-Jun in siSCCA/HaCaT keratinocytes (Fig. 4 E). Down-regulation of SCCA1 resulted in marked increase of phospho-c-Jun, and UV irradiation further enhanced the levels of c-Jun phosphorylation. On the other hand, down-regulation of SCCA1 did not show any detectable changes in either the protein or phosphorylation levels of ERK1/2, p38α, and its primary substrate, MAPK-activated protein kinase-2 (MAPKAPK2; Fig. 4 F; Rouse et al., 1994). p38α is also known to respond to UV stress (Kim et al., 2005). After UV irradiation, phosphorylated p38α increased its intensity at 5–15 min in control HaCaT and siSCCA/HaCaT cell extracts, whereas the p38α protein remained at the same level. Similar changes were observed for MAPKAPK2. These results suggest that the kinase activity of JNK1 is specifically regulated by SCCA1 that is also in the cell. It is well known that death enzyme caspases play essential roles in apoptotic cell death. It might be possible that suppression of UV-induced apoptosis by SCCA1 could include inhibition of certain caspases because SCCA1 is a strong inhibitor of cysteine proteinases. We tested this possibility and confirmed that even a 50-fold excess of SCCA1 had no effect on the activities of caspase-1–10 (Fig. S3 B, available at http://www.jcb.org/cgi/content/full/jcb.200508064/DC1), although an equimolar amount of SCCA1 strongly inhibited a typical cysteine proteinase, known as papain (Fig. S3 A). Thus, in the UV-signaling pathway, it is suggested that the antiapoptotic property of SCCA1 depends on the inhibition of JNK1.
SCCA1 transgenic mouse shows remarkable resistance to UV irradiation
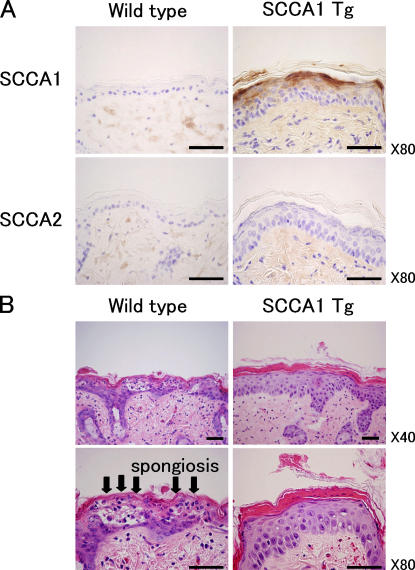
To examine the physiological importance of SCCA up-regulation in the epidermis, we generated transgenic mice with a hairless phenotype (HR-1) that overexpress SCCA1 driven from the involucrin promoter (Carroll et al., 1993). We chose involucrin promoter because UV irradiation induced strong up-regulation of SCCA1 mRNA in the upper epidermis (Fig. 1 B). Immunostaining of skin sections revealed strong expression of SCCA1 in the upper epidermis of the transgenic mice, but not in the skin of wild-type HR-1 mice (Fig. 5 A). When 200 mJ/cm2 UV was irradiated twice on the backs of mice, the epidermis of wild-type HR-1 mice was seriously damaged, as seen in loss of the granular layer and spongiosis in the suprabasal layer (Fig. 5 B). Surprisingly, the same dose of UV irradiation did not cause any degeneration in the epidermis of SCCA1-overexpressing mice, although the epidermis of the transgenic mice showed hypertrophic and parakeratotic changes. These results clearly reveal that SCCA1 plays a critical role in the UV protection mechanism in the skin.
Figure 5.
Effect of UV irradiation on involucrin promoter-driven SCCA1 transgenic mice. (A) Immunostaining of SCCAs in wild-type and transgenic epidermis. Paraffin-embedded skin sections were stained with antibodies to SCCA1 or SCCA2. (B) Histological appearance of wild-type and transgenic mice 24 h after UV irradiation (200 mJ/cm2/d for 2 d). Wild-type (HR-1) mice demonstrated degeneration of the epidermis, including spongiosis (arrows) in the suprabasal layer. Involucrin-SCCA1 transgenic mice did not show any notable changes in UV-irradiated skin. Bars, 50 μm.
UV irradiation profoundly damages living organisms, which have had to evolve effective countermeasures. It is well known that UVB (290–320 nm) has the most toxic effect on chromosomal DNA. Basal cells, which are replicating cells, are physically protected with melanin, which forms a supranuclear melanin cap to shield the nucleus against UV irradiation. Furthermore, keratinocytes as well as melanocytes produce the potent antiapoptotic factors bcl-2 and bcl-xL (Gillardon et al., 1999; Taylor et al., 1999). Deficiency of these survival factors sensitizes keratinocytes to apoptotic stimuli, including UV irradiation. However, UV irradiation induces down-regulation of bcl-2 expression (Isoherranen et al., 1999). On the contrary, SCCA1 is up-regulated in the upper epidermis with UV irradiation. Considering the importance of reproductive basal cells and barrier-forming cornified cells, the middle to top layers of spinous and granular cells are thought to represent transient states that bridge the early and terminal differentiation of keratinocytes. We have identified a novel UV protection mechanism that will function to protect the middle to top layers of epidermis. Observations from bullous diseases such as pemphigus vulgaris clearly demonstrate the importance of maintaining these layers. Our study suggests that under UVB exposure SCCAs are highly synthesized in spinous and granular layers and suppress UV-induced cell death via the inhibition of JNK1. This is supported by our current study on the SCCA mutant mice, showing that up-regulation of SCCA1 in top layers of epidermis was enough to block death-promoting UV stress. It would be beneficial for humans because this mechanism is not functioning in replicating basal cells, but is working for the differentiated spinous and granular cells, which are destined to be shed after cornification.
Materials and methods
In situ hybridization
An SCCA1-specific sequence was PCR amplified from exon 8 (642–1,001 nt) and cloned into pCRII vector. Complementary RNA probes were prepared using DIG RNA labeling mix (Roche). HRP-conjugated antidigoxigenin antibody (DakoCytomation) was used for visualization.
Establishment of stably transfected cell lines
SCCA1 and SCCA2 cDNAs cloned from a psoriatic cDNA library (Takeda et al., 2002) were subcloned into pTarget vector and transfected into 3T3/J2 cells using Lipofectamine Plus (Invitrogen). After 4 wk of culture in 500 μg/ml of G418, 12 colonies were isolated for SCCA1 and established as SCCA-expressing cell lines. Levels of SCCA expression were determined with Taqman PCR (Fig. S1). Expression levels among 12 colonies varied from 1–2,772-fold.
Vector-based siRNA
A double-stranded oligonucleotide was designed corresponding to a common sequence of human SCCAs (5′-AAGCCAACACCAAGTTCATGT-3′) to allow formation of the hairpin structure in the expressed oligo-RNA, cloned into the pSilencer vector (Ambion), and transfected into human keratinocyte cell line HaCaT cells. siRNA against GFP mRNA (Ambion) was used as a control. Transfection was performed with Lipofectamine 2000 (Invitrogen) and stable cell lines were established in hygromycin-B media for 4–6 wk.
Cell culture and UV irradiation
HaCaT, HSC-4, and 3T3/J2 cells were cultured in DME (Invitrogen) supplemented with 10% FBS. NHK was cultured with EpiLife (Kurabo). Cells were irradiated with UVB at 60–70% confluency using a transilluminator (model TOREX FL205-E-30/DMR; Toshiba Medical Supply) emitting UVB at 0–100 mJ/cm2.
FACS analysis
The cells were trypsinized and stained using the Annexin V-FITC and propidium iodide double-staining method (Immunotech). The cells were analyzed on a FACS Coulter EPICS XL-MCL (Beckman Coulter) with acquisition of a total of 10,000 events/samples to ensure adequate data.
Preparation of recombinant SCCA1
SCCA1 cDNA was subcloned into pQE-30 (QIAGEN) with a poly-histidine tag (6× His). His-tagged SCCA1 was expressed in Escherichia coli stain BL21 (DE3) cells and purified on Ni2+-nitrilotriacetate resin (QIAGEN) and a Mono-Q column (GE Healthcare).
Antibody array
To screen protein–protein interactions, a Signal Transduction AntibodyArray (Hypomatorix, Inc.) was used. The extract from a squamous cell carcinoma cell line, HSC-4, showed the highest expression of both SCCAs among NHK, HaCaT, and other SCCA cell lines. The cells were irradiated with 50 mJ/cm2 UVB were lysed and extracted with 1% Triton X-100 extraction buffer containing 15 mM Tris-HCl, pH 7.5, 120 mM NaCl, 25 mM KCl, 2 mM EGTA, 2 mM EDTA, 0.1 mM DTT, 80 μM bestatin, and 10 μM pepstatin. After incubation with the extract, the antibody array membrane was blotted with HRP-conjugated anti-SCCA1 or -SCCA2 mAb and detected with ECL Plus kit (GE Healthcare).
MAPK assays and immunoblots
Kinase activity of JNK was determined using a stress-activated protein kinase (SAPK)/JNK assay kit (Cell Signaling Technology). Inhibition of JNK kinase activity by SCCA1 was assessed using phosphorylated-JNK1a1/SAPK1c (Upstate Biotechnology). Phosphorylated JNK, c-Jun fusion beads, and SCCA1 were incubated with gentle rocking overnight at 4°C. The phosphorylated c-Jun was separated by SDS-PAGE and probed with anti–phospho-c-Jun antibody (1:1,000 dilution). ERK1 and ERK2 kinase activities were measured using MAPK (ERK1/2) activity assay kit (Chemicon International, Inc.). Kinase activity of p38α was assessed using active p38α and GST-tagged ATF2 (19–96; Upstate Biotechnology) as a substrate. Antibodies to ERK1/2 (Chemicon International, inc.), p-ERK1/2 (Biosource International), p38α (Upstate Biotechnology), p-p38α, p-ATF2, MAPKAPK2, and phosphorylated MAPKAPK2 (Cell Signaling Technology) were used for immunoblots.
Involucrin-SCCA1 transgenic mice
SCCA1 cDNA was fused to the involucrin promoter (L.B. Taichman, State University of New York, Stony Brook, NY; Carroll et al., 1993) and transgenic mice were generated using BDF1 mice. To obtain the hairless phenotype, the involucrin-SCCA1 transgenic mice were crossed with HR-1 mice, and SCCA1+/+ mice were obtained after three passages. UVB irradiation was done with a transilluminator at 200 mJ/cm2/day for 2 d. Skin specimens were taken the following day and stained with hematoxylin and eosin or immunostained with anti-SCCA1 or -SCCA2 mAb using MoMap kit (Ventana Medical Systems, Inc.).
Immunohistochemical analysis and acquisition of images
Anti-SCCA1 and -SCCA2 mAbs and rabbit anti-JNK1 antibody (Santa Cruz Biotechnology, Inc.) were used. Alexa Fluor 555–, 488–, and 546–conjugated secondary antibodies (Invitrogen) were used for immunofluorescence detection. Nuclei were stained with DAPI (Invitrogen). Actin filaments were visualized with Alexa Fluor 488–conjugated phalloidin (Invitrogen). Fluorescence images were collected using a microscope (model BX51WI; Olympus) equipped with a confocal system (Radiance 2100; BioRad Laboratories) at 21°C. UPlanApo 20×/0.70 ∞/0.17, UPlanApo 40×/0.85 ∞/0.11–0.23, and PlanApo 60×/1.00 WLSM ∞/0.17 were used.
Online supplemental material
Fig. S1 shows establishment of SCCA-overexpressing (A) and knockdown (B) cell lines. Strong suppression of the SCCA mRNA and protein in the knockdown cells was shown in B. Fig. S2 shows localization of JNK1 in cultured NHK cells before and after UV irradiation (A). Comparison of cell numbers in cytoplasmic or nuclear localization of JNK1 before and after UV irradiation was summarized in B. Effects of the JIP1 control peptide (C) after UV irradiation were shown. Fig. S3 shows effect of SCCA1 on papain and various caspase activities. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200508064/DC1.
Supplementary Material
Acknowledgments
We thank Dr. L.B. Taichman for involucrin promoter, A. Maeda for her excellent technical assistance in cell culture and Western blot analysis, and Dr. P.F. Goetinck (Massachusetts General Hospital-Harvard Medical School, Charlestown, MA) for valuable discussion and for critical reading of the manuscript.
K. Kadoya's present address is The Burnham Institute, La Jolla, CA 92037.
Abbreviations used in this paper: JIP1, JNK-interacting protein-1; JNK, c-Jun NH2-terminal kinase; MAPKAPK2, MAPK-activated protein kinase-2; NHK, neonatal human keratinocytes; SCCA, squamous cell carcinoma antigen; SAPK, stress-activated protein kinase; siRNA, small interfering RNA; siSCCA, small interfering SCCA.
References
- Barr, R.K., T.S. Kendrick, and M.A. Bogoyevitch. 2002. Identification of the critical features of a small peptide inhibitor of JNK activity. J. Biol. Chem. 277:10987–10997. [DOI] [PubMed] [Google Scholar]
- Bonny, C., A. Oberson, S. Negri, C. Sauser, and D.F. Schorderet. 2001. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 50:77–82. [DOI] [PubMed] [Google Scholar]
- Boukamp, P., R.T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N.E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield, L., B. Storey, L. Maas, and L.E. Heasley. 1997. c-Jun NH2-terminal kinase regulation of the apoptotic response of small cell lung cancer cells to ultraviolet radiation. J. Biol. Chem. 272:10110–10116. [DOI] [PubMed] [Google Scholar]
- Carroll, J.M., K.M. Albers, J.A. Garlick, R. Harrington, and L.B. Taichman. 1993. Tissue- and stratum-specific expression of the human involucrin promoter in transgenic mice. Proc. Natl. Acad. Sci. USA. 90:10270–10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillardon, F., I. Moll, M. Meyer, and T.M. Michaelidis. 1999. Alterations in cell death and cell cycle progression in the UV-irradiated epidermis of bcl-2-deficient mice. Cell Death Differ. 6:55–60. [DOI] [PubMed] [Google Scholar]
- Hochedlinger, K., E.F. Wagner, and K. Sabapathy. 2002. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene. 21:2441–2445. [DOI] [PubMed] [Google Scholar]
- Isoherranen, K., I. Sauroja, C. Jansen, and K. Punnonen. 1999. UV irradiation induces downregulation of bcl-2 expression in vitro and in vivo. Arch. Dermatol. Res. 291:212–216. [DOI] [PubMed] [Google Scholar]
- Kato, H., and T. Torigoe. 1977. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 40:1621–1628. [DOI] [PubMed] [Google Scholar]
- Kim, A.L., J.M. Labasi, Y. Zhu, X. Tang, K. McClure, C.A. Gabel, M. Athar, and D.R. Bickers. 2005. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J. Invest. Dermatol. 124:1318–1325. [DOI] [PubMed] [Google Scholar]
- Ray, R., M. Choi, Z. Zhang, G.A. Silverman, D. Askew, and A.B. Mukherjee. 2005. Uteroglobin suppresses SCCA gene expression associated with allergic asthma. J. Biol. Chem. 280:9761–9764. [DOI] [PubMed] [Google Scholar]
- Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A.R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 78:1027–1037. [DOI] [PubMed] [Google Scholar]
- Schick, C., Y. Kamachi, A.J. Bartuski, S. Cataltepe, N.M. Schechter, P.A. Pemberton, and G.A. Silverman. 1997. Squamous cell carcinoma antigen 2 is a novel serpin that inhibits the chymotrypsin-like proteinases cathepsin G and mast cell chymase. J. Biol. Chem. 272:1849–1855. [DOI] [PubMed] [Google Scholar]
- Schick, C., P.A. Pemberton, G.P. Shi, Y. Kamachi, S. Cataltepe, A.J. Bartuski, E.R. Gornstein, D. Bromme, H.A. Chapman, and G.A. Silverman. 1998. Cross-class inhibition of the cysteine proteinases cathepsins K, L, and S by the serpin squamous cell carcinoma antigen 1: a kinetic analysis. Biochemistry. 37:5258–5266. [DOI] [PubMed] [Google Scholar]
- Schneider, S.S., C. Schick, K.E. Fish, E. Miller, J.C. Pena, S.D. Treter, S.M. Hui, and G.A. Silverman. 1995. A serine proteinase inhibitor locus at 18q21.3 contains a tandem duplication of the human squamous cell carcinoma antigen gene. Proc. Natl. Acad. Sci. USA. 92:3147–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suminami, Y., F. Kishi, K. Sekiguchi, and H. Kato. 1991. Squamous cell carcinoma antigen is a new member of the serine protease inhibitors. Biochem. Biophys. Res. Commun. 181:51–58. [DOI] [PubMed] [Google Scholar]
- Takeda, A., T. Yamamoto, Y. Nakamura, T. Takahashi, and T. Hibino. 1995. Squamous cell carcinoma antigen is a potent inhibitor of cysteine proteinase cathepsin L. FEBS Lett. 359:78–80. [DOI] [PubMed] [Google Scholar]
- Takeda, A., D. Higuchi, T. Takahashi, M. Ogo, P. Baciu, P.F. Goetinck, and T. Hibino. 2002. Overexpression of serpin squamous cell carcinoma antigens in psoriatic skin. J. Invest. Dermatol. 118:147–154. [DOI] [PubMed] [Google Scholar]
- Taylor, J.K., Q.Q. Zhang, B.P. Monia, E.G. Marcusson, and N.M. Dean. 1999. Inhibition of Bcl-xL expression sensitizes normal human keratinocytes and epithelial cells to apoptotic stimuli. Oncogene. 18:4495–4504. [DOI] [PubMed] [Google Scholar]
- Tong, T., W. Fan, H. Zhao, S. Jin, F. Fan, P. Blanck, I. Alomo, B. Rajasekaran, Y. Liu, N.J. Holbrook, and Q. Zhan. 2001. Involvement of the MAP kinase pathways in induction of GADD45 following UV radiation. Exp. Cell Res. 269:64–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.