Abstract
αMβ2 integrins mediate phagocytosis of opsonized particles in a process controlled by RhoA, Rho kinase, myosin II, Arp2/3, and actin polymerization. αMβ2, Rho, Arp2/3, and F-actin accumulate underneath bound particles; however, the mechanism regulating Rho function during αMβ2-mediated phagocytosis is poorly understood. We report that the binding of C3bi-opsonized sheep red blood cells (RBCs) to αMβ2 increases Rho-GTP, but not Rac-GTP, levels. Deletion of the cytoplasmic domain of β2, but not of αM, abolished Rho recruitment and activation, as well as phagocytic uptake. Interestingly, a 16–amino acid (aa) region in the membrane-proximal half of the β2 cytoplasmic domain was necessary for activating Rho. Three COOH-terminal residues (aa 758–760) were essential for β2-induced accumulation of Rho at complement receptor 3 (CR3) phagosomes. Activation of Rho was necessary, but not sufficient, for its stable recruitment underneath bound particles or for uptake. However, recruitment of active Rho was sufficient for phagocytosis. Our data shed light on the mechanism of outside-in signaling, from ligated integrins to the activation of Rho GTPase signaling.
Introduction
Integrins are adhesion receptors that connect components of the extracellular matrix or molecules borne by cells or microbial pathogens to intracellular signaling pathways (Hynes, 2002; Boyle and Finlay, 2003). Integrins are subjected to bidirectional signaling; ligand binding can be regulated by inside-out signaling, and it also triggers a variety of downstream pathways, including the physical association of integrins to the actin cytoskeleton and cytoskeletal remodeling (e.g., during focal adhesion formation and cell motility; Ridley et al., 2003; Blystone, 2004; Campbell and Ginsberg, 2004). Signaling to and from integrins is mainly regulated by the short cytoplasmic tails of α and β subunits, which lack catalytic activity. In contrast to the well conserved cytoplasmic domains of β subunits, α subunit cytoplasmic domains share few sequence similarities (Hynes, 2002), which has led to the idea that the two integrin tails play distinct roles and to the suggestion that α tails could confer specificity to integrins sharing identical β chains (Liu et al., 1999; Weber et al., 1999). α and/or β cytoplasmic tails have been involved in several aspects of integrin biology and function (e.g., inside-out signaling, localization to focal adhesions, and cell adhesion; Lu and Springer, 1997; Keely et al., 1999; Weber et al., 1999; Fagerholm et al., 2004). It is thought that both inside-out and outside-in signaling are regulated through the binding of cytoskeletal and regulatory proteins to integrin cytoplasmic regions. Over 20 such proteins have been described to date (Calderwood et al., 2000; Liu and Ginsberg, 2000; Zamir and Geiger, 2001; Tadokoro et al., 2003). Small GTP-binding proteins also play crucial roles in integrin function; Rap1 activity controls inside-out activation of β1, β2, and β3 integrins (Caron, 2003; Bos, 2005), whereas Rho family proteins control integrin signaling to the cytoskeleton (Arthur et al., 2002). However, the molecular mechanisms underlying the cross-talk between integrins and small GTPases remain unclear.
β2 integrins, which are mutated in type-1 leukocyte adhesion deficiency patients, control the majority of immune cell functions, including cell migration, immunological synapse formation, and phagocytosis (Hogg and Bates, 2000). Accordingly, immune cells isolated from mice that were deficient in one or more β2 integrins manifested a range of defects in cytoskeletal-based functions (Rosenkranz and Mayadas, 1999). The αMβ2 integrin (which is also known as complement receptor 3 [CR3] or CD11b/CD18) is the main phagocytic receptor for particles opsonized with the complement fragment C3bi; as such, it plays a major role in the phagocytosis of microorganisms and apoptotic cells (Gasque, 2004). αM and β2 are both needed for the efficient binding and ingestion of C3bi-opsonized particles (Caron and Hall, 1998), and, similar to αLβ2 (Tominaga et al., 1993; Sebzda et al., 2002; Tohyama et al., 2003; Vielkind et al., 2005), αMβ2 function is regulated by small GTP-binding proteins (Caron et al., 2000). Interestingly, unlike other modes of uptake, αMβ2-dependent phagocytosis relies exclusively on Rho function (Wiedemann et al., 2005). RhoA, but not Rac1, accumulates at sites of particle binding, where it colocalizes with F-actin. A dominant-negative allele of Rho (N19), overexpression of a kinase-dead Rho kinase mutant, or treatment with the Rho kinase inhibitor Y-27632 blocks the recruitment of the actin nucleator/organizer Arp2/3 complex at nascent phagosomes, whereas blockers of Rac and Cdc42 function have no effect (Caron and Hall, 1998; May et al., 2000; Olazabal et al., 2002). RhoA and Rho kinase activities are thus specifically required at phagosomes to drive Arp2/3-dependent actin polymerization and uptake. These results also indicate a striking similarity between the signaling pathways elicited during αMβ2-driven phagocytosis and other processes that are mediated by integrins, Rho, and Rho kinase (Liu et al., 2002; McMullan et al., 2003; Ridley et al., 2003; Smith et al., 2003; Worthylake and Burridge, 2003). Nonetheless, the mechanisms involved in Rho regulation downstream of integrin ligation are totally unknown.
We show that the binding of C3bi-opsonized targets to αMβ2 activates Rho, but not Rac, in macrophages and αMβ2-transfected Cos-7 cells. Moreover, the cytoplasmic domain of β2, but not of αM, controls Rho function during phagocytosis. Remarkably, two distinct regions within the β2 cytoplasmic tail control Rho activation and stable recruitment. Finally, we show that recruitment of active Rho to nascent phagosomes is sufficient to induce αMβ2-dependent uptake.
Results
Binding of C3bi-opsonized RBCs to αMβ2 increases Rho-GTP levels
αMβ2-mediated phagocytosis requires Rho, but not Rac, activity (Caron and Hall, 1998). To investigate if the levels of active, GTP-bound Rho increase during phagocytosis, we performed pull-down experiments using the Rho-binding domain of rhotekin (RBD) fused to GST, as previously described (Sander et al., 1999). J774.A1 macrophages were pretreated with the phorbol ester PMA, to activate αMβ2 binding ability through inside-out signaling (Caron et al., 2000), and challenged with C3bi-opsonized sheep red blood cells (RBCs). At different time points, cells were lysed and levels of active Rho were determined. Progression of phagocytosis correlated with a transient increase in GTP-loaded Rho, peaking 20 min after RBC challenge. A significant increase (up to sevenfold) in GTP-Rho occurred only after stimulation with both PMA and RBCs. The levels of endogenous active Rac1, as determined in the p21-activated kinase (PAK)–CRIB pull-down assay (Sander et al., 1999; Patel et al., 2002), did not change significantly during phagocytosis (Fig. 1 A). These results show that αMβ2 ligation by C3bi-opsonized particles (i.e., outside-in signaling) leads to a specific increase in Rho activity.
Figure 1.
αMβ2 ligation specifically increases Rho activity in phagocytes. (A) Control (C) or activated (PMA) J774.A1 macrophages were challenged with either medium alone or with C3bi-opsonized RBC (RBC). Active Rho and Rac were precipitated and analyzed as described in Materials and methods. (top) Kinetic analysis of Rho activation. (bottom) Quantification of the variations in Rho (open bars) and Rac (shaded bars) GTP-levels at the 20-min time point, expressed as a ratio of GTP versus total levels of Rho and Rac in cell lysates. This ratio was arbitrarily set to 1 in the control condition (challenge with medium alone). (B) Effect of expressing αM and β2 alone, together, or in combination with N19Rho-GFP on RBC attachment (open bars) and phagocytosis (shaded bars) in Cos-7 cells. (C) Rho activation in αMβ2-transfected Cos-7 cells challenged for 20 min with medium alone (−) or RBC (+) and analyzed as described in A. (top) Representative example. (bottom) Quantification of the variations in Rho-GTP levels, with the ratio of Rho-GTP to total Rho observed in the absence of RBC set arbitrarily to 1. (A–C) Data shown are the mean ± SEM of at least three independent experiments.
To characterize the mechanism of Rho regulation during CR3-mediated phagocytosis, we turned to Cos-7 cells, which do not contain any endogenous αM or β2 and offer a robust alternative system for the study of phagocytosis, as shown by several studies (Rabb et al., 1993; Downey et al., 1999; May et al., 2000). As shown in Fig. 1 B, cells expressing αM bound RBCs poorly, and expression of β2 alone conferred no binding ability. However, as previously reported (Caron and Hall, 1998), coexpression of αM and β2 induced efficient binding and phagocytosis. PMA treatment increases RBCs binding by twofold in transfected COS7 cells, as it does in macrophages and neutrophils. This is not because of increased αMβ2 expression, but, rather, because of the activation of regulators of inside-out signaling such as PKC and Rap1 (Caron et al. 2000; unpublished data). Nevertheless, there are clearly enough active integrins in transfected COS cells to bind and zipper around RBC, thereby allowing binding and phagocytosis in the absence of PMA. Coexpression of dominant-negative Rho decreased uptake by 70%, but had no effect on RBC binding (Fig. 1 B). Furthermore, phagocytosis is also accompanied by an increase in the levels of GTP-Rho in transfected Cos-7 cells (Fig. 1 C). No changes in Rac-GTP levels were detected in these cells.
The cytoplasmic domain of β2 is essential for αMβ2-mediated phagocytosis
A mutagenesis strategy was undertaken to identify which regions of αMβ2 are responsible for signaling to Rho and phagocytosis. We deleted 14 (αMΔ1139) or 23 (αMΔ1130) aa of the αM cytoplasmic domain or deleted the whole β2 cytoplasmic domain (β2Δ724; Table I) and cotransfected each mutant with its wild-type (wt) counterpart (Fig. 2). At steady-state, αMΔ1139/β2 and αMβ2Δ724 were expressed at wt levels, as determined by flow cytometry; however, αMΔ1130β2 did not reach the plasma membrane (unpublished data). The membrane-proximal 10 residues of αM, but not the β2 cytoplasmic tail, are thus important in directing heterodimerization and/or surface expression of the αMβ2 integrin.
Table I.
aa sequence of the cytoplasmic domains of the αM and β2 constructs used in this study
| Construct | aa sequence |
|---|---|
| β2 | KALIH LSDLR EYRRF EKEKL KSQWN NDNPL FKSAT TTVMN PKFAE S |
| β2Δ724 | K |
| β2Δ732 | KALIH LSDL |
| β2Δ742 | KALIH LSDLR EYRRF EKEK |
| β2Δ747 | KALIH LSDLR EYRRF EKEKL KSQW |
| β2Δ755 | KALIH LSDLR EYRRF EKEKL KSQWN NDNPL FK |
| β2Δ767 | KALIH LSDLR EYRRF EKEKL KSQWN NDNPL FKSAT TTVMN PKFA |
| β2F766A | KALIH LSDLR EYRRF EKEKL KSQWN NDNPL FKSAT TTVMN PKAAE S |
| β2AAA | KALIH LSDLR EYRRF EKEKL KSQWN NDNPL FKSAAAAVMN PKFAE S |
| β2ΔN | KSAT TTVMN PKFAE S |
| αM | KLGFF KRQYK DMMSE GGPPG AEPQ |
| αMΔ1130 | K |
| αMΔ1139 | KLGFF KRQYK |
Figure 2.
The β2 cytoplasmic domain is required for αMβ2-mediated phagocytosis. Cos-7 cells were cotransfected with wt or mutant αM and β2 constructs as indicated, and then challenged with RBC, processed for immunofluorescence, and scored, as described in Materials and methods. The binding (open bars) and phagocytosis (shaded bars) indices were related to the values obtained for wt αMβ2. Data shown are the mean ± SEM of at least three independent experiments.
We next compared the capacity of wt and mutant αMβ2 integrins to bind and internalize C3bi-opsonized RBC. The combination αMΔ1139/β2 led to control levels of binding and uptake. In contrast, deleting the cytoplasmic domain of β2 (β2Δ724) increased binding, as described previously for αLβ2 (Bleijs et al., 2001), but completely abolished phagocytosis (Fig. 2). The β2 cytoplasmic domain is therefore necessary for αMβ2-mediated uptake.
The cytoplasmic domain of β2 controls the recruitment and activation of Rho during αMβ2 phagocytosis
Accumulation of RhoA at nascent phagosomes can be visualized by expressing a tagged wild-type RhoA construct (Caron and Hall, 1998). In cells expressing full-length αMβ2, Rho-GFP was enriched at 60 ± 11% of the phagosomes (Fig. 3, A and B). Interestingly, when coexpressed with wt β2, αMΔ1139 was as able to recruit Rho around RBC as wt αM. In contrast, deletion of the β2 cytoplasmic tail abolished Rho recruitment, reducing it to the background levels observed with GFP alone (Fig. 3 B). Thus, Rho recruitment to early phagosomes during CR3-mediated phagocytosis is controlled by the cytoplasmic domain of β2.
Figure 3.
The β2 cytoplasmic tail is required for Rho recruitment and activation. (A) Cos-7 cells were transfected as indicated, challenged for 30 min with RBC, and processed for confocal microscopy as described in Materials and methods. Images represent the merged image of the GFP and RBC channels, and arrows indicate typical staining patterns. Bar, 10 μm. (B) Quantification of the recruitment of GFP and Rho-GFP to bound RBC. (C) Cos-7 cells were transfected as indicated and challenged with RBC for 20 min at 37°C, and the levels of GTP-bound and total Rho were determined using the rhotekin pull-down assays and related as described in Fig. 1. Results are normalized to the control value of αMβ2-transfected cells challenged with medium alone (set to 1). (B and C) Data shown are the mean ± SEM of at least three independent experiments.
Rho proteins are thought to cycle between an active GTP-bound, membrane-bound state and an inactive cytosolic state, which suggests that the recruitment we observe might result from RBC-induced (outside-in) activation of transfected RhoA and local enrichment underneath RBC. To determine which of the cytoplasmic domains of αMβ2 controlled Rho activation during phagocytosis, we performed rhotekin pull-down assays in cells transfected with the truncation mutants. None of the cytoplasmic deletions influenced Rho-GTP levels in resting cells (Fig. 3 C, open bars). When coexpressed with αMΔ1139, β2 was still able to increase the levels of active Rho in response to RBC (Fig. 3 C, shaded bars). Strikingly, deletion of the β2 cytoplasmic tail abrogated the RBC-induced increase in GTP-Rho, strongly suggesting that the β2 cytoplasmic domain controls both αMβ2-induced Rho activation and phagocytosis.
A 16-aa acid region in the cytoplasmic domain of β2 is essential for Rho activation during αMβ2 phagocytosis
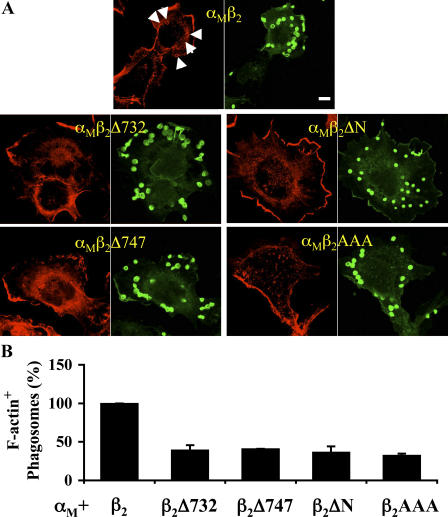
Additional truncations and point mutations (Table I) were engineered into the β2 cytoplasmic tail to analyze in detail the regions required for phagocytosis. Ftlow cytometry analysis showed that all the combinations of β2 mutant chains with wt αM were expressed at the cell surface at the same levels as wt β2, except β2F766A and β2AAA, which were consistently expressed at 80 and 50% of the control levels (unpublished data). Interestingly, only three mutants (β2Δ732, β2Δ767, and β2ΔN) showed binding indices similar or superior to wt values. This suggests that, apart from β2AAA, whose reduced binding ability (Fig. 4 A) correlated with reduced surface expression, all other mutations influence integrin activation and/or recycling. Importantly, the phagocytic capacity of Cos-7 cells transfected with all but two β2 mutants was low (Fig. 4 B), indicating a possible link between β2 mutation and the abrogation of Rho function. The exceptions were β2Δ767, which behaved like wt not only for particle binding but also for phagocytosis, and β2F766A, which was still able to engulf bound particles efficiently. Interestingly, the ability of β2 mutants to mediate phagocytosis was correlated with their ability to trigger the accumulation of F-actin at sites of particle binding (Fig. 5).
Figure 4.
Abilities of mutant β2 chains to bind/phagocytose RBC and activate Rho. Cos-7 cells were cotransfected with wt αM and mutant β2 constructs as indicated, then challenged for 30 min with RBCs. The effect of expressing different β2 constructs together with wt αM on the attachment (A) and phagocytosis (B) of RBC was analyzed. Results are expressed relative to the values obtained for wt αMβ2 (arbitrarily set to 100%). (C) After challenge with RBC for 20 min at 37°C, transfected Cos-7 cells were subjected to rhotekin pull-down assays. The fold-increase in Rho activity was calculated as for Fig. 1, with the ratio of GTP versus total Rho set to 1 for each β2 construct in control conditions (i.e., in the absence of RBC). (A–C) Data shown are the mean ± SEM of at least three independent experiments.
Figure 5.
Abilities of mutant β2 chains to remodel the actin cytoskeleton locally. Cos-7 cells were transfected as indicated, challenged for 30 min with RBC, stained for RBC (green) and F-actin (red), and analyzed by confocal microscopy as described in Materials and methods. The percentage of bound RBC showing a discrete local enrichment in F-actin was scored. (A) Representative examples; arrows indicate typical F-actin enrichment patterns. Bar, 10 μm. (B) Quantification, expressed relative to F-actin enrichment observed for wt αMβ2 (arbitrarily set at 100%). Data shown are the mean ± SEM of at least three independent experiments.
We next determined whether any of these β2 mutants affected RBC-induced Rho activation. Importantly, none of the mutants influenced Rho-GTP levels in resting cells (unpublished data). The deletion mutants β2Δ747 and β2Δ755 induced significant Rho activation after phagocytic challenge (Fig. 4 C). In contrast, β2Δ732 was unable to increase Rho-GTP levels in response to RBC. To confirm the requirement of the 732–747 region of β2 in Rho activation, we engineered a mutant (β2ΔN) lacking the NH2-terminal half of the cytoplasmic tail (residues 724–755), thereby missing the putative RhoA activation domain. β2ΔN was also unable to increase Rho-GTP levels upon RBC binding. Together, these results strongly suggest that the 732–747 region of the β2 tail is necessary to promote Rho activation during αMβ2-mediated phagocytosis, but not sufficient to trigger actin polymerization and uptake.
Threonines 758–760 are essential for β2-mediated Rho recruitment during CR3-mediated phagocytosis
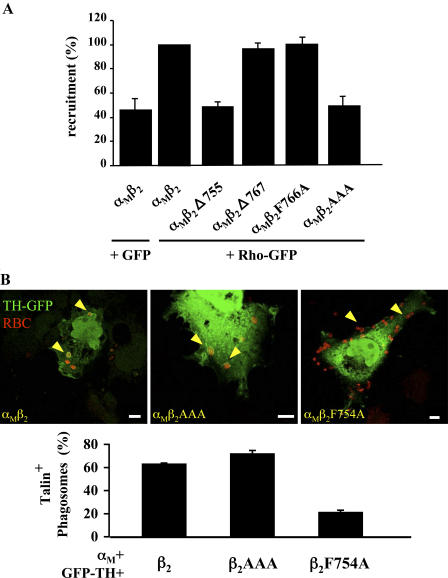
We next investigated the impact of β2 mutations on Rho recruitment to nascent phagosomes using confocal microscopy. COOH-terminal deletions of the β2 cytoplasmic domain had varying impacts on Rho recruitment (Fig. 6 A), suggesting that one or more aa within the 756–767 region of β2 are required for a detectable accumulation of Rho at phagosomes. Interestingly, several residues within this 12-aa region of β2 are conserved in other β chains and proposed to regulate integrin function. In particular, a cluster of threonine residues (758–760) is phosphorylated upon phorbol ester stimulation of leukocytes (Valmu and Gahmberg, 1995) and required for the modulation of leukocyte adhesiveness (Hibbs et al., 1991b; Peter and O'Toole, 1995). Also, mutations in the conserved Asn-Pro-Xaa-Tyr/Phe motif (NPxΦ, with Φ at positions 754 and 766 in human β2) perturb the interaction of integrins with binding partners and integrin signaling (Liu and Ginsberg, 2000; Sirim et al., 2001; Calderwood et al., 2002). Thus, we engineered mutants β2F766A and β2AAA (threonines 758–760 mutated to alanine) and studied their impact on the recruitment of Rho-GFP to forming phagosomes. Flow cytometry analysis revealed that the β2F766A mutant was expressed at 80% of the control value at steady-state. This mutant also showed a markedly reduced ability to bind opsonized RBC (Fig. 4 A), but phagocytosis (Fig. 4 B) and Rho recruitment (Fig. 6 A) were both measurable. However, although the β2AAA mutant was still able to bind particles, it was unable to recruit Rho to nascent phagosomes. In contrast, β2AAA showed wt binding to a GFP–talin head fragment whose interaction with the β2 integrin depends on residue F754 (Fig. 6 B). We concluded that the threonine residues at position 758–760 are necessary for Rho recruitment during CR3-mediated phagocytosis.
Figure 6.
The β2-dependent recruitment of Rho is controlled by residues 758–760. Cos-7 cells were transfected as indicated, challenged for 30 min with RBC, and processed for confocal microscopy as described in Materials and methods. (A) Quantification of the recruitment of GFP and Rho-GFP to bound RBC, which was expressed relative to the Rho recruitment observed for wt αMβ2 (arbitrarily set at 100%). (B) Cells were transfected with integrin constructs, along with a GFP-tagged fragment containing the β2-binding, FERM-domain of talin (TH, talin head). (top) Representative examples, with RBC in red and arrows indicating typical talin enrichment patterns. (bottom) Quantification of the percentage of bound RBC that are positive for GFP-talin head. (A and C) Data shown are the mean ± SEM of at least three independent experiments.
Recruitment of active Rho to nascent phagosomes is sufficient to induce CR3-mediated phagocytosis
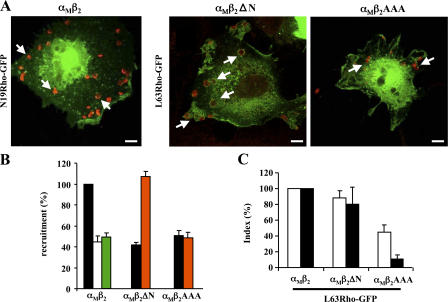
Despite being unable to detectably recruit Rho, β2Δ755 and β2AAA were still able to up-regulate Rho activity upon RBC binding (Fig. 4 C and not depicted). This suggests that Rho activation and visible accumulation at sites of particle binding are independent steps and that Rho activation precedes its stable recruitment. In line with this, inactive (N19) Rho is not recruited beneath RBC bound to αMβ2 (Fig. 7). To obtain further evidence of the mechanism involved, we analyzed the recruitment of a constitutively active form of Rho (L63) in cells cotransfected with αM and β2 with either wt, AAA, or ΔN (lacking the Rho activation domain but comprising the three threonine residues). Although β2ΔN cannot activate Rho (Fig. 4 C), coexpression with L63Rho allowed the recruitment of GFP-tagged, active Rho underneath RBC. However, when the experiment was repeated with β2AAA, L63Rho was no longer recruited to nascent phagosomes (Fig. 7, A and B), confirming our hypothesis that Rho is locally recruited to forming phagosomes in its active, GTP-bound form, in a manner that is dependent on threonines 758–760.
Figure 7.
Presence of active Rho at nascent phagosomes is sufficient to induce phagocytosis. (A) Cos-7 cells were cotransfected with wt αM, various β2 constructs, and either GFP or GFP-tagged Rho alleles as indicated, then challenged for 30 min with RBC, stained for RBC (red) and examined by confocal microscopy. Photographs represent the merged image of the GFP and RBC channels. Arrows indicate typical staining patterns. Bar, 10 μm. (B) Recruitment of GFP (open bars), N19 (green bars), L63 (orange bars), and wtRho (shaded bars) after normalization to the value obtained with wt αMβ2 and wtRho (which was arbitrarily set at 100%). (C) Effect of expressing L63Rho on RBC binding (open bars) and phagocytosis (shaded bars). Only cells positive for both β2 and GFP were scored. Results are expressed relative to the values obtained for wt αMβ2 and L63Rho-GFP (arbitrarily set to 100%). (B and C) Data shown are the mean ± SEM of at least three independent experiments.
To investigate the consequences of the local recruitment of active Rho on phagocytic uptake, we analyzed the capacity of β2ΔN and β2AAA to internalize RBC when coexpressed with wt αM and L63Rho-GFP. As shown in Fig. 7 C, coexpression of L63Rho rescued the phagocytic defect of β2ΔN-expressing cells without affecting their ability to bind RBC or the phagocytic ability of αMβ2-expressing cells. Therefore, expression of active Rho completely bypassed the need for the 732–747 region, which is normally necessary for Rho activation, actin polymerization, and phagocytosis (Fig. 4, B and C; and Fig. 5), and allowed both Rho accumulation and RBC uptake by cells expressing the internally truncated mutant. In contrast, cells expressing β2AAA were not rescued for phagocytosis by L63Rho-GFP (Fig. 7 C). Together, these results indicate that threonines 758–760 control the local accumulation of active Rho, which is sufficient to affect CR3-mediated phagocytosis.
Discussion
The αMβ2 integrin, which is mainly expressed in macrophages and neutrophils, mediates phagocytosis of microbes and apoptotic cells. αMβ2-mediated phagocytosis requires Rap1, RhoA, and Rho kinase activity and is dependent on the Arp2/3 complex and actin polymerization (Caron and Hall, 1998; Caron et al., 2000). Because of its spatially localized signaling, phagocytosis offers an ideal system to understand how extracellular (particularly integrin) ligands regulate the function of small GTP-binding proteins to remodel the actin cytoskeleton. We show that outside-in signaling from the αMβ2 integrin requires the cytoplasmic tail of β2, but not αM. Specifically, two distinct subregions within the β2 tail control the recruitment and activation of RhoA. Interestingly, Rho recruitment to sites of particle binding was dependent on prior activation of the G protein and sufficient for phagocytosis. Our data shed light on the mechanisms by which integrin ligation is coupled to the activation of small GTPase function.
Unlike other modes of phagocytosis or bacterial invasion, αMβ2 uptake is dependent on RhoA signaling, but does not require Rac or Cdc42 activity (Wiedemann et al., 2005). Until now, the underlying mechanism had remained unclear. We show that the levels of active, GTP-bound RhoA increase transiently during αMβ2 phagocytosis, whereas the levels of active Rac show insignificant variation, suggesting that ligation of αMβ2 is sufficient to activate RhoA specifically. In line with this, antibody cross-linking of αMβ2 in J774.A1 macrophages also leads to RhoA activation (unpublished data). Whereas αMβ2 is so far the only known phagocytic receptor that is exclusively coupled to the activity of the RhoA protein, several studies have previously linked integrin signaling to the up-regulation of Rho activity. For example, a transient up-regulation of RhoA activity was described in human neutrophils freshly plated on immobilized anti-β2 antibodies (Dib et al., 2001). Although integrin-mediated spreading is associated to Rac1 activation (Price et al., 1998; del Pozo et al., 2000), integrin ligation or overexpression have already been linked to increased RhoA activity in a variety of cell types (Danen et al., 2002; Miao et al., 2002; Zhou and Kramer, 2005).
Mutagenesis revealed that the cytoplasmic domain of β2 is the main regulator of αMβ2 during outside-in signaling to phagocytosis. Maximal cytoplasmic deletions in αM that were compatible with surface expression did not alter any of the heterodimer properties that we examined (such as particle binding and phagocytosis and Rho recruitment and activation). In contrast, the phagocytic function of αMβ2 was critically dependent on the integrity of the β2 cytoplasmic domain. Truncation of the entire β2 tail increased the association of C3bi-opsonized RBC, as previously described (Bleijs et al., 2001), which is consistent with the idea that disruption of intrachain interactions between α and β cytoplasmic domains results in constitutively active integrins (Lu et al., 2001). Except for β2AAA, for which reduced surface expression correlated with reduced binding, our results suggest that mutations that decrease RBC binding significantly result from an impact of these mutations on integrin inside-out activation or recycling. Indeed, in αMβ2 and other integrins, several of the residues between 732 and 766 have been involved in talin binding and activation by Rap1, two important mechanisms in inside-out activation (Garcia-Alvarez et al., 2003; Tadokoro et al., 2003; unpublished data) and in recycling or stimulated internalization (Rabb et al., 1993; Vignoud et al., 1997; Gawaz et al., 2001).
Remarkably, deletion of the entire β2 tail abolished both RhoA recruitment and activation and also abrogated phagocytosis. Added to the fact that blocking Rho function inhibits RBC uptake without affecting binding, this strongly suggests that Rho activity is specifically required during outside-in signaling from αMβ2. This seems to conflict with several papers claiming that RhoA is needed for β2 activation in lymphocytes and thymocytes (Laudanna et al., 1996; Giagulli et al., 2004; Vielkind et al., 2005). Although it is conceivable that RhoA could regulate either inside-out or outside-in signaling for different integrins or in different cell types, it seems more likely that the discrepancy comes from a different understanding of the readouts used in these studies. Indeed, adhesion to ligand-coated surfaces is not simply a consequence of pure inside-out signaling, but rather a reflection of both inside-out and outside-in signaling. Nevertheless, our results show clearly that the intracellular domain of the β integrin chain controls RhoA activation.
β subunits consist of a large extracellular domain, a transmembrane segment, and a relatively short cytoplasmic tail (20–70 aa, 46 in human β2, except for the much longer β4 integrin). Truncation of the intracellular region of β1, β2, and β3 abrogates their ability to localize to focal adhesions, and prevents activation of signaling molecules and downstream cytoskeletal remodeling (Solowska et al., 1989; Marcantonio et al., 1990; Hibbs et al., 1991a; Leong et al., 1995; Ylanne et al., 1995). Reciprocally, isolated β1 and β3 tails are sufficient to activate focal adhesion kinase and can regulate cell cycle progression and actin cytoskeleton assembly (Tahiliani et al., 1997; Belkin and Retta, 1998; David et al., 1999). Although there is no crystal structure available for any isolated integrin cytoplasmic domain, several groups used nuclear magnetic resonance to investigate the three-dimensional organization of αIIbβ3 intracellular domains (Vinogradova et al., 2002; Weljie et al., 2002; Garcia-Alvarez et al., 2003). We engineered our truncated β2 mutants based on their results, which suggested that, in both tails, the membrane-proximal regions were α-helical and interacted with each other, whereas the more distal regions are disordered in aqueous environment. Interestingly, we found that two distinct cytosolic regions within β2 control RhoA activation and recruitment to forming phagosomes. The region controlling Rho activation maps to the helical domain, whereas Rho recruitment is controlled by the TTT motif within the disordered region. Importantly, Rho activation can occur independently of a measurable recruitment. However, the reverse is not true, as nascent phagosomes are negative for RhoA enrichment unless Rho can be activated. There is no Rho recruitment in cells expressing wt αMβ2 and N19RhoA or in cells coexpressing wt RhoA and a β2 mutant harboring an internal deletion of the Rho activation domain, therefore suggesting that only active RhoA can accumulate where RBCs bind. Therefore, there might be a structural basis for the mechanisms underlying Rho recruitment and activation. We also speculate that recruitment of active Rho may play a role in stabilizing the disordered domain of β2 and possibly of other β chains.
How could Rho recruitment and activation be coordinately regulated? We describe a specific, transient up-regulation of RhoA activity upon challenge with RBC. The simplest explanation is that, as has been proposed for bacterial invasion, FcγR-mediated phagocytosis, and various extracellular stimuli, particle binding triggers the local activation of guanine-nucleotide exchange factor molecules (Patel et al., 2002; Rossman et al., 2005; Wiedemann et al., 2005). Because N19Rho, which is thought to titrate endogenous exchange factors, is not recruited to nascent phagosomes, our data might suggest that Rho is activated away from ligated integrins. However, it is hard to envisage how the membrane-proximal RhoA activation domain could direct activation of the GTPase at a distance. The alternative possibility is that RhoA is activated locally. We are currently investigating the possible role of guanine-nucleotide exchange factors, RhoGDI, and, indirectly, GTPase-activating proteins in Rho activation during αMβ2 phagocytosis. To explain the local activation of RhoA in the absence of detectable recruitment, we favor the hypothesis that our experimental approach does not allow us to visualize the interaction between overexpressed RhoA and the truncated mutants (e.g., β2Δ747). This could happen either because the TTT motif is necessary to stabilize the interaction of β2 with active RhoA, with a regulator of Rho activation, or, finally, because a crucial regulator of Rho recruitment to the truncated mutants is missing in our overexpression setting.
We also found that three threonine residues in the β2 cytosolic region governed the recruitment of active Rho to nascent phagosomes. Interestingly, the corresponding three-residue sequence, which is flanked by two NPxY/F motifs (Calderwood, 2004), has been associated to the regulation of the adhesive function of β1, β2, β3, and β7 integrins. However, whether this short sequence is involved in inside-out or outside-in signaling has remained controversial (Hayashi et al., 1990; Hibbs et al., 1991b; Balzac et al., 1993; Peter and O'Toole, 1995; Wennerberg et al., 1998; Calderwood et al., 2001). Our results strongly suggest that residues 758–760 regulate the recruitment of active Rho to the integrin complex in response to ligand binding (outside-in signaling). Phorbol esters were shown to induce phosphorylation of these threonine residues in β2 (Valmu and Gahmberg, 1995; Fagerholm et al., 2002). As PKC was shown to interact with β1 tails (Ng et al., 1999), it is tempting to imagine a regulatory mechanism whereby PKC would interact with β2 and phosphorylate the three threonine residues, allowing the docking of active RhoA (binding either directly or indirectly). Although PKC activity is required for αMβ2 phagocytosis (Newman et al., 1991), a role upstream of Rho is hard to reconcile with the observation that mutations in the putative PKC-binding sites on β2 (Parsons et al., 2002) do not affect Rho recruitment in our model system (unpublished data). Moreover, the β1–PKC interaction has been connected to increased motility rather than increased adhesion (Ng et al., 1999). Alternatively, it is possible that another as yet unidentified serine/threonine kinase mediates the phosphorylation of these threonine residues. Importantly, several binding partners, including ICAP-1, filamin, and 14-3-3 proteins interact in a phosphorylation-dependent manner with the TTT region of various β chains (Valmu et al., 1999; Stroeken et al., 2000; Calderwood et al., 2001; Degani et al., 2002; Fagerholm et al., 2002). Unfortunately, none of these molecules has been implicated in αMβ2-mediated phagocytosis. Interestingly, however, they are all related to small GTPase signaling (Ohta et al., 1999; Bellanger et al., 2000; Degani et al., 2002; Ueda et al., 2003); 14-3-3 and filamin offer the most direct connection with RhoA signaling, respectively through Rho kinase and binding/activation, via LIM-kinase-induced phosphorylation of cofilin (Arber et al., 1998; Yang et al., 1998; Gohla and Bokoch, 2002). Whether any of these proteins are involved in the stable recruitment of Rho to β2 integrins during phagocytosis will form the basis of future studies.
In conclusion, we show that ligand binding to αMβ2 specifically activates Rho, but not Rac. Rho function is regulated in two steps, which are governed by distinct regions of the β2 cytoplasmic tail. Whereas a membrane-proximal α-helical region controls the activation of Rho, its detectable recruitment involves a distal TTT motif. We demonstrate that stable enrichment of activated Rho at the plasma membrane is controlled by the threonines 758–760 in β2-cytoplasmic domain and is sufficient to promote αMβ2-dependent phagocytosis, a process known to require, successively, Rho kinase, myosin light chain kinase, myosin II, the Arp2/3 complex, and actin polymerization. Because of the conservation of signaling pathways downstream of integrin β chains, we propose that similar mechanisms operate in related cellular activities, e.g., rear detachment during cell motility.
Materials and methods
Reagents
RBCs were purchased from TCS Biosciences, Ltd., anti–sheep erythrocyte IgM antibodies were purchased from Cedarlane Laboratories, Ltd., and gelatin veronal buffer and C5-deficient serum were obtained from Sigma-Aldrich.
The antibodies used in this study were mouse anti-Rac1 (clone 23A8; Upstate Biotechnology), anti-GFP (JL-8; CLONTECH Laboratories, Inc.), anti-myc (9E10; Santa Cruz Biotechnology, Inc), anti-β2 (BD Biosciences), and rabbit anti-RhoA (119; Santa Cruz Biotechnology, Inc). Conjugated secondary antibodies were obtained from either Jackson ImmunoResearch Laboratories (fluorescence) or GE Healthcare (Western blots).
DNA constructs
Eukaryotic expression vectors (pRK5) encoding human wt β2, wt αM, and myc-tagged N19Rho constructs were previously described (Caron and Hall, 1998), as were pGEX-2T vectors encoding the RBD and the Rac/Cdc42-binding domain of PAK-CRIB (Patel et al., 2002; Dutt et al., 2004). The pRC/CMV plasmid encoding the β2Δ732 mutant was provided by Y. van Kooyk (VU University Medical Center, Amsterdam, Netherlands).
GFP-tagged small GTPase constructs were generated by subcloning wt, N19, and L63Rho from pGEXRho (Self and Hall, 1995) or pRK5-myc into pEGFP-C1 (CLONTECH Laboratories, Inc.). GFP-talin head was a gift of D.R. Critchley (University of Leicester, Leicester, UK).
αM mutants were engineered via PCR by introducing a premature stop codon within the cytosolic tail at positions 1130 (αMΔ1130) and 1139 (αMΔ1139) using pRK5-αM as a template; the sense primer 5′-GCTCTAGACTCTCGAGTACGTGCCACACC-3′, which incorporates an internal XhoI site; and the antisense primers 5′-CTTGAAAAGCTTCTA CTTGTACAGCGCGGCGGT-3′ for αMΔ1130 and 5′-TTCACTAAGCTTCTACTTGTATTGCCGCTTGAA-3′ for αMΔ1139. β2 mutants were generated in an analogous manner. Because of unfavorable restriction sites, some required initial subcloning of an Xbal–HindIII fragment of pRK5-β2 into pUC19. Mutants were cloned by replacing a SacII–HindIII fragment of pUC19-β2 with different SacII–HindIII PCR fragments, which were amplified using pUC19-β2 as a template, the sense primer 5′-GCTCTAGAACCCCGCGGCGTGTTGAGTG-3′, an incorporated internal SacII site, and one of the following antisense primers: 5′-CAGGTGAAGCTTCTACTTCCAGATGACCAGCAG-3′ (β2Δ724); 5′-CCACTGAAGCTTCTACTTCTCCTTCTCAAAGCG-3′ (β2Δ742); 5′-CGTCGTAAGCTTCTACTTGAAAAGGGGATTATC-3′ (β2Δ755); 5′-CCCCTAAAGCTTCTAACTCTCAGCAAACTTGGGGTTCATGACCGTCGTGGTGGCGCTCTTCCAGATGACCAG-3′ (β2ΔN); 5′-CCCCTAAAGCTTCTAACTCTCAGCAAACTTGGGGTTCATGACCGCCGCGGCGGCGCTCTTGAAAAG-3′ (β2AAA).
The β2Δ767, β2Δ747, and β2F766A mutants were constructed by PCR using wt β2 as a template, the sense primer 5′-GGGGGGTCTAGAATGCTGGGCCTGCGCCCCCCACTG-3′, which incorporates an internal XbaI site, and the following antisense primers: for β2Δ767, 5′-GGGGGGAAGCTTCTAAGCAAACTTGGGGTTCAT-3′; for β2Δ747, 5′-GGGGGGAAGCTTCTA CCACTGGGACTTGAGCTTCTC-3′; and for β2F766A, 5′-GGGGGGAAGCTTCTAACTCTCAGCAGCCTTGGGGTTCAT-3′. The aa sequences of the cytoplasmic tails of all αM and β2 mutants used in this study are given in Table I.
Cell culture and transfection
Cells from the murine macrophage J774.A1 and Cos-7 cell lines, which were obtained from the American Type Culture Collection, were maintained, seeded, and transfected as previously described (Caron and Hall, 1998). For pull-down assays, 9 μg DNA was used to transfect 3 × 105 cells on a 10-cm dish, using SuperFect (QIAGEN) as recommended by the manufacturer. After 4 h, fresh medium was added for 16 h and cells were then serum starved overnight.
Flow cytometry
Transfected Cos-7 cells were washed with cold wash buffer (0.5% BSA and 0.02% azide in PBS) and stained to detect surface β2, using mouse monoclonal and FITC-conjugated goat anti–mouse antibodies successively. The relative fluorescence of gated Cos-7 cells was analyzed using a FACSCalibur analyzer (Becton Dickinson).
Phagocytic challenge
C3bi-opsonized RBCs were essentially prepared and used to challenge phagocytes as previously described (Caron and Hall 1998), using 0.5 and 20 μl of fresh RBC per coverslip/per dish for immunofluorescence and/or pull-down assays, respectively. For efficient binding and phagocytosis of C3bi-opsonized erythrocytes, macrophages require preactivation (Wright and Jong, 1986; Caron et al., 2000); we routinely pretreated J774.A1 cells with 150 ng/ml PMA in buffered serum-free DME for 15 min at 37°C. PMA-treated cells were washed with warm PBS to remove unbound RBC and fixed in cold 4% paraformaldehyde for 10 min at 4°C.
Immunofluorescence
Transfected Cos-7 cells were first stained for surface β2. For differential staining of adherent and internalized C3bi-opsonized RBC, cells were incubated with Texas red–conjugated goat anti–rabbit antibodies, permeabilized with 0.2% Triton X-100, and incubated with FITC-conjugated goat anti–rabbit antibodies. Coverslips were finally mounted in Mowiol (Calbiochem) containing p-phenylenediamine (Sigma-Aldrich) as an antifading reagent and analyzed using a fluorescence microscope. Only β2-positive cells were analyzed; internalized particles (which were red) were easily distinguishable from extracellular RBCs (which were yellow), a property that was used to score phagocytosis. The binding and phagocytic indices are defined as the number of targets bound to and engulfed by 100 phagocytes, respectively. The recruitment of Rho during CR3-mediated phagocytosis was studied by scoring local enrichment in the GFP signal at sites of RBC binding by confocal microscopy (model LSM510; Carl Zeiss MicroImaging, Inc.). A minimum of 20 transfected cells were analyzed per condition. The percentage of bound RBC showing a discrete local enrichment in GFP signal was scored. Phagosomes were scored as positive when at least a quarter of the underlying/surrounding area showed significant GFP enrichment compared with the neighboring areas. All of the overexpressed GFP constructs showed a low level of plasma membrane localization.
GST pull-down assays
Rhotekin RBD and PAK-CRIB fused to GST were prepared as previously described (Ren et al., 1999; Sander et al., 1999). J774A.1 or Cos-7 cell lysates were incubated for 45 min at 4°C with 15 μg of a 50% slurry of glutathione Sepharose 4B beads coupled to GST-RBD-rhotekin or GST-PAK-CRIB to precipitate GTP-RhoA or GTP-Rac. Beads were washed three times in cold lysis buffer (10% glycerol, 1% NP-40, 50 mM Tris, pH 7.6, 200 mM NaCl, 2.5 mM MgCl2, 1 mM PMSF, and protease inhibitor cocktail). Equal volumes of beads and total lysates were analyzed by SDS-PAGE and Western blotting, which were performed as previously described (Patel et al., 2002). Intensities of the RhoA and Rac1 signals were determined by densitometric analysis and related to the levels of total RhoA or Rac1 in the lysates, using the ImageJ software.
Acknowledgments
We thank Alan Hall for his critical reading of the manuscript.
This research is supported by grants from the Biotechnology and Biological Sciences Research Council (28/C18637) and the Wellcome Trust (068556/Z/02/Z).
Abbreviations used in this paper: CR3, complement receptor 3; PAK, p21-activated kinase; RBC, sheep red blood cell; RBD, Rho-binding domain of rhotekin; wt, wild-type.
References
- Arber, S., F.A. Barbayannis, H. Hanser, C. Schneider, C.A. Stanyon, O. Bernard, and P. Caroni. 1998. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 393:805–809. [DOI] [PubMed] [Google Scholar]
- Arthur, W.T., N.K. Noren, and K. Burridge. 2002. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol. Res. 35:239–246. [DOI] [PubMed] [Google Scholar]
- Balzac, F., A.M. Belkin, V.E. Koteliansky, Y.V. Balabanov, F. Altruda, L. Silengo, and G. Tarone. 1993. Expression and functional analysis of a cytoplasmic domain variant of the β1 integrin subunit. J. Cell Biol. 121:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin, A.M., and S.F. Retta. 1998. beta1D integrin inhibits cell cycle progression in normal myoblasts and fibroblasts. J. Biol. Chem. 273:15234–15240. [DOI] [PubMed] [Google Scholar]
- Bellanger, J.M., C. Astier, C. Sardet, Y. Ohta, T.P. Stossel, and A. Debant. 2000. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat. Cell Biol. 2:888–892. [DOI] [PubMed] [Google Scholar]
- Bleijs, D.A., G.C. van Duijnhoven, S.J. van Vliet, J.P. Thijssen, C.G. Figdor, and Y. van Kooyk. 2001. A single amino acid in the cytoplasmic domain of the beta 2 integrin lymphocyte function-associated antigen-1 regulates avidity-dependent inside-out signaling. J. Biol. Chem. 276:10338–10346. [DOI] [PubMed] [Google Scholar]
- Blystone, S.D. 2004. Integrating an integrin: a direct route to actin. Biochim. Biophys. Acta. 1692:47–54. [DOI] [PubMed] [Google Scholar]
- Bos, J.L. 2005. Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 17:123–128. [DOI] [PubMed] [Google Scholar]
- Boyle, E.C., and B.B. Finlay. 2003. Bacterial pathogenesis: exploiting cellular adherence. Curr. Opin. Cell Biol. 15:633–639. [DOI] [PubMed] [Google Scholar]
- Calderwood, D.A. 2004. Integrin activation. J. Cell Sci. 117:657–666. [DOI] [PubMed] [Google Scholar]
- Calderwood, D.A., S.J. Shattil, and M.H. Ginsberg. 2000. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J. Biol. Chem. 275:22607–22610. [DOI] [PubMed] [Google Scholar]
- Calderwood, D.A., A. Huttenlocher, W.B. Kiosses, D.M. Rose, D.G. Woodside, M.A. Schwartz, and M.H. Ginsberg. 2001. Increased filamin binding to beta-integrin cytoplasmic domains inhibits cell migration. Nat. Cell Biol. 3:1060–1068. [DOI] [PubMed] [Google Scholar]
- Calderwood, D.A., B. Yan, J.M. de Pereda, B.G. Alvarez, Y. Fujioka, R.C. Liddington, and M.H. Ginsberg. 2002. The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 277:21749–21758. [DOI] [PubMed] [Google Scholar]
- Campbell, I.D., and M.H. Ginsberg. 2004. The talin-tail interaction places integrin activation on FERM ground. Trends Biochem. Sci. 29:429–435. [DOI] [PubMed] [Google Scholar]
- Caron, E. 2003. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J. Cell Sci. 116:435–440. [DOI] [PubMed] [Google Scholar]
- Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 282:1717–1721. [DOI] [PubMed] [Google Scholar]
- Caron, E., A.J. Self, and A. Hall. 2000. The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr. Biol. 10:974–978. [DOI] [PubMed] [Google Scholar]
- Danen, E.H., P. Sonneveld, C. Brakebusch, R. Fassler, and A. Sonnenberg. 2002. The fibronectin-binding integrins α5β1 and αvβ3 differentially modulate RhoA–GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J. Cell Biol. 159:1071–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, F.S., P.E. Zage, and E.E. Marcantonio. 1999. Integrins interact with focal adhesions through multiple distinct pathways. J. Cell. Physiol. 181:74–82. [DOI] [PubMed] [Google Scholar]
- Degani, S., F. Balzac, M. Brancaccio, S. Guazzone, S.F. Retta, L. Silengo, A. Eva, and G. Tarone. 2002. The integrin cytoplasmic domain–associated protein ICAP-1 binds and regulates Rho family GTPases during cell spreading. J. Cell Biol. 156:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo, M.A., L.S. Price, N.B. Alderson, X.D. Ren, and M.A. Schwartz. 2000. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19:2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib, K., F. Melander, and T. Andersson. 2001. Role of p190RhoGAP in beta 2 integrin regulation of RhoA in human neutrophils. J. Immunol. 166:6311–6322. [DOI] [PubMed] [Google Scholar]
- Downey, G.P., R.J. Botelho, J.R. Butler, Y. Moltyaner, P. Chien, A.D. Schreiber, and S. Grinstein. 1999. Phagosomal maturation, acidification, and inhibition of bacterial growth in nonphagocytic cells transfected with FcgammaRIIA receptors. J. Biol. Chem. 274:28436–28444. [DOI] [PubMed] [Google Scholar]
- Dutt, P., A.B. Jaffe, K.D. Merdek, A. Hall, and D. Toksoz. 2004. Galphaz inhibits serum response factor-dependent transcription by inhibiting Rho signaling. Mol. Pharmacol. 66:1508–1516. [DOI] [PubMed] [Google Scholar]
- Fagerholm, S., N. Morrice, C.G. Gahmberg, and P. Cohen. 2002. Phosphorylation of the cytoplasmic domain of the integrin CD18 chain by protein kinase C isoforms in leukocytes. J. Biol. Chem. 277:1728–1738. [DOI] [PubMed] [Google Scholar]
- Fagerholm, S.C., T.J. Hilden, and C.G. Gahmberg. 2004. P marks the spot: site-specific integrin phosphorylation regulates molecular interactions. Trends Biochem. Sci. 29:504–512. [DOI] [PubMed] [Google Scholar]
- Garcia-Alvarez, B., J.M. de Pereda, D.A. Calderwood, T.S. Ulmer, D. Critchley, I.D. Campbell, M.H. Ginsberg, and R.C. Liddington. 2003. Structural determinants of integrin recognition by talin. Mol. Cell. 11:49–58. [DOI] [PubMed] [Google Scholar]
- Gasque, P. 2004. Complement: a unique innate immune sensor for danger signals. Mol. Immunol. 41:1089–1098. [DOI] [PubMed] [Google Scholar]
- Gawaz, M., F. Besta, J. Ylanne, T. Knorr, H. Dierks, T. Bohm, and W. Kolanus. 2001. The NITY motif of the beta-chain cytoplasmic domain is involved in stimulated internalization of the beta3 integrin A isoform. J. Cell Sci. 114:1101–1113. [DOI] [PubMed] [Google Scholar]
- Giagulli, C., E. Scarpini, L. Ottoboni, S. Narumiya, E.C. Butcher, G. Constantin, and C. Laudanna. 2004. RhoA and zeta PKC control distinct modalities of LFA-1 activation by chemokines: critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity. 20:25–35. [DOI] [PubMed] [Google Scholar]
- Gohla, A., and G.M. Bokoch. 2002. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr. Biol. 12:1704–1710. [DOI] [PubMed] [Google Scholar]
- Hayashi, Y., B. Haimovich, A. Reszka, D. Boettiger, and A. Horwitz. 1990. Expression and function of chicken integrin β1 subunit and its cytoplasmic domain mutants in mouse NIH 3T3 cells. J. Cell Biol. 110:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs, M.L., H. Xu, S.A. Stacker, and T.A. Springer. 1991. a. Regulation of adhesion of ICAM-1 by the cytoplasmic domain of LFA-1 integrin beta subunit. Science. 251:1611–1613. [DOI] [PubMed] [Google Scholar]
- Hibbs, M.L., S. Jakes, S.A. Stacker, R.W. Wallace, and T.A. Springer. 1991. b. The cytoplasmic domain of the integrin lymphocyte function-associated antigen 1 β subunit: sites required for binding to intercellular adhesion molecule 1 and the phorbol ester–stimulated phosphorylation site. J. Exp. Med. 174:1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg, N., and P.A. Bates. 2000. Genetic analysis of integrin function in man: LAD-1 and other syndromes. Matrix Biol. 19:211–222. [DOI] [PubMed] [Google Scholar]
- Hynes, R.O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell. 110:673–687. [DOI] [PubMed] [Google Scholar]
- Keely, P.J., E.V. Rusyn, A.D. Cox, and L.V. Parise. 1999. R-Ras signals through specific integrin α cytoplasmic domains to promote migration and invasion of breast epithelial cells. J. Cell Biol. 145:1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanna, C., J.J. Campbell, and E.C. Butcher. 1996. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 271:981–983. [DOI] [PubMed] [Google Scholar]
- Leong, L., P.E. Hughes, M.A. Schwartz, M.H. Ginsberg, and S.J. Shattil. 1995. Integrin signaling: roles for the cytoplasmic tails of alpha IIb beta 3 in the tyrosine phosphorylation of pp125FAK. J. Cell Sci. 108:3817–3825. [DOI] [PubMed] [Google Scholar]
- Liu, S., S.M. Thomas, D.G. Woodside, D.M. Rose, W.B. Kiosses, M. Pfaff, and M.H. Ginsberg. 1999. Binding of paxillin to alpha4 integrins modifies integrin-dependent biological responses. Nature. 402:676–681. [DOI] [PubMed] [Google Scholar]
- Liu, S., and M.H. Ginsberg. 2000. Paxillin binding to a conserved sequence motif in the alpha 4 integrin cytoplasmic domain. J. Biol. Chem. 275:22736–22742. [DOI] [PubMed] [Google Scholar]
- Liu, S., W.B. Kiosses, D.M. Rose, M. Slepak, R. Salgia, J.D. Griffin, C.E. Turner, M.A. Schwartz, and M.H. Ginsberg. 2002. A fragment of paxillin binds the alpha 4 integrin cytoplasmic domain (tail) and selectively inhibits alpha 4-mediated cell migration. J. Biol. Chem. 277:20887–20894. [DOI] [PubMed] [Google Scholar]
- Lu, C., J. Takagi, and T.A. Springer. 2001. Association of the membrane proximal regions of the alpha and beta subunit cytoplasmic domains constrains an integrin in the inactive state. J. Biol. Chem. 276:14642–14648. [DOI] [PubMed] [Google Scholar]
- Lu, C.F., and T.A. Springer. 1997. The alpha subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1. J. Immunol. 159:268–278. [PubMed] [Google Scholar]
- Marcantonio, E.E., J.L. Guan, J.E. Trevithick, and R.O. Hynes. 1990. Mapping of the functional determinants of the integrin beta 1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, R.C., E. Caron, A. Hall, and L.M. Machesky. 2000. Involvement of the Arp2/3 complex in phagocytosis mediated by FcgammaR or CR3. Nat. Cell Biol. 2:246–248. [DOI] [PubMed] [Google Scholar]
- McMullan, R., S. Lax, V.H. Robertson, D.J. Radford, S. Broad, F.M. Watt, A. Rowles, D.R. Croft, M.F. Olson, and N.A. Hotchin. 2003. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr. Biol. 13:2185–2189. [DOI] [PubMed] [Google Scholar]
- Miao, H., S. Li, Y.L. Hu, S. Yuan, Y. Zhao, B.P. Chen, W. Puzon-McLaughlin, T. Tarui, J.Y. Shyy, Y. Takada, et al. 2002. Differential regulation of Rho GTPases by beta1 and beta3 integrins: the role of an extracellular domain of integrin in intracellular signaling. J. Cell Sci. 115:2199–2206. [DOI] [PubMed] [Google Scholar]
- Newman, S.L., L.K. Mikus, and M.A. Tucci. 1991. Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J. Immunol. 146:967–974. [PubMed] [Google Scholar]
- Ng, T., D. Shima, A. Squire, P.I. Bastiaens, S. Gschmeissner, M.J. Humphries, and P.J. Parker. 1999. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 18:3909–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta, Y., N. Suzuki, S. Nakamura, J.H. Hartwig, and T.P. Stossel. 1999. The small GTPase RalA targets filamin to induce filopodia. Proc. Natl. Acad. Sci. USA. 96:2122–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazabal, I.M., E. Caron, R.C. May, K. Schilling, D.A. Knecht, and L.M. Machesky. 2002. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr. Biol. 12:1413–1418. [DOI] [PubMed] [Google Scholar]
- Parsons, M., M.D. Keppler, A. Kline, A. Messent, M.J. Humphries, R. Gilchrist, I.R. Hart, C. Quittau-Prevostel, W.E. Hughes, P.J. Parker, and T. Ng. 2002. Site-directed perturbation of protein kinase C-integrin interaction blocks carcinoma cell chemotaxis. Mol. Cell. Biol. 22:5897–5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, J.C., A. Hall, and E. Caron. 2002. Vav regulates activation of Rac but not Cdc42 during FcgammaR-mediated phagocytosis. Mol. Biol. Cell. 13:1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter, K., and T.E. O'Toole. 1995. Modulation of cell adhesion by changes in alpha L beta 2 (LFA-1, CD11a/CD18) cytoplasmic domain/cytoskeleton interaction. J. Exp. Med. 181:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, L.S., J. Leng, M.A. Schwartz, and G.M. Bokoch. 1998. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell. 9:1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabb, H., M. Michishita, C.P. Sharma, D. Brown, and M.A. Arnaout. 1993. Cytoplasmic tails of human complement receptor type 3 (CR3, CD11b/CD18) regulate ligand avidity and the internalization of occupied receptors. J. Immunol. 151:990–1002. [PubMed] [Google Scholar]
- Ren, X.D., W.B. Kiosses, and M.A. Schwartz. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J., M.A. Schwartz, K. Burridge, R.A. Firtel, M.H. Ginsberg, G. Borisy, J.T. Parsons, and A.R. Horwitz. 2003. Cell migration: integrating signals from front to back. Science. 302:1704–1709. [DOI] [PubMed] [Google Scholar]
- Rosenkranz, A.R., and T.N. Mayadas. 1999. Leukocyte-endothelial cell interactions—lessons from knockout mice. Exp. Nephrol. 7:125–136. [DOI] [PubMed] [Google Scholar]
- Rossman, K.L., C.J. Der, and J. Sondek. 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6:167–180. [DOI] [PubMed] [Google Scholar]
- Sander, E.E., J.P. ten Klooster, S. van Delft, R.A. van der Kammen, and J.G. Collard. 1999. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147:1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebzda, E., M. Bracke, T. Tugal, N. Hogg, and D.A. Cantrell. 2002. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat. Immunol. 3:251–258. [DOI] [PubMed] [Google Scholar]
- Self, A.J., and A. Hall. 1995. Measurement of intrinsic nucleotide exchange and GTP hydrolysis rates. Methods Enzymol. 256:67–76. [DOI] [PubMed] [Google Scholar]
- Sirim, P., L. Zeitlmann, B. Kellersch, C.S. Falk, D.J. Schendel, and W. Kolanus. 2001. Calcium signaling through the beta 2-cytoplasmic domain of LFA-1 requires intracellular elements of the T cell receptor complex. J. Biol. Chem. 276:42945–42956. [DOI] [PubMed] [Google Scholar]
- Smith, A., M. Bracke, B. Leitinger, J.C. Porter, and N. Hogg. 2003. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J. Cell Sci. 116:3123–3133. [DOI] [PubMed] [Google Scholar]
- Solowska, J., J.L. Guan, E.E. Marcantonio, J.E. Trevithick, C.A. Buck, and R.O. Hynes. 1989. Expression of normal and mutant avian integrin subunits in rodent cells. J. Cell Biol. 109:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroeken, P.J., E.A. van Rijthoven, E. Boer, D. Geerts, and E. Roos. 2000. Cytoplasmic domain mutants of beta1 integrin, expressed in beta 1-knockout lymphoma cells, have distinct effects on adhesion, invasion and metastasis. Oncogene. 19:1232–1238. [DOI] [PubMed] [Google Scholar]
- Tadokoro, S., S.J. Shattil, K. Eto, V. Tai, R.C. Liddington, J.M. de Pereda, M.H. Ginsberg, and D.A. Calderwood. 2003. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 302:103–106. [DOI] [PubMed] [Google Scholar]
- Tahiliani, P.D., L. Singh, K.L. Auer, and S.E. LaFlamme. 1997. The role of conserved amino acid motifs within the integrin beta3 cytoplasmic domain in triggering focal adhesion kinase phosphorylation. J. Biol. Chem. 272:7892–7898. [DOI] [PubMed] [Google Scholar]
- Tohyama, Y., K. Katagiri, R. Pardi, C. Lu, T.A. Springer, and T. Kinashi. 2003. The critical cytoplasmic regions of the alphaL/beta2 integrin in Rap1-induced adhesion and migration. Mol. Biol. Cell. 14:2570–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga, T., K. Sugie, M. Hirata, N. Morii, J. Fukata, A. Uchida, H. Imura, and S. Narumiya. 1993. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J. Cell Biol. 120:1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, K., Y. Ohta, and H. Hosoya. 2003. The carboxy-terminal pleckstrin homology domain of ROCK interacts with filamin-A. Biochem. Biophys. Res. Commun. 301:886–890. [DOI] [PubMed] [Google Scholar]
- Valmu, L., and C.G. Gahmberg. 1995. Treatment with okadaic acid reveals strong threonine phosphorylation of CD18 after activation of CD11/CD18 leukocyte integrins with phorbol esters or CD3 antibodies. J. Immunol. 155:1175–1183. [PubMed] [Google Scholar]
- Valmu, L., T.J. Hilden, G. van Willigen, and C.G. Gahmberg. 1999. Characterization of beta2 (CD18) integrin phosphorylation in phorbol ester-activated T lymphocytes. Biochem. J. 339:119–125. [PMC free article] [PubMed] [Google Scholar]
- Vielkind, S., M. Gallagher-Gambarelli, M. Gomez, H.J. Hinton, and D.A. Cantrell. 2005. Integrin regulation by RhoA in thymocytes. J. Immunol. 175:350–357. [DOI] [PubMed] [Google Scholar]
- Vignoud, L., C. Albiges-Rizo, P. Frachet, and M.R. Block. 1997. NPXY motifs control the recruitment of the alpha5beta1 integrin in focal adhesions independently of the association of talin with the beta1 chain. J. Cell Sci. 110:1421–1430. [DOI] [PubMed] [Google Scholar]
- Vinogradova, O., A. Velyvis, A. Velyviene, B. Hu, T. Haas, E. Plow, and J. Qin. 2002. A structural mechanism of integrin alpha(IIb)beta(3) “inside-out” activation as regulated by its cytoplasmic face. Cell. 110:587–597. [DOI] [PubMed] [Google Scholar]
- Weber, K.S., L.B. Klickstein, and C. Weber. 1999. Specific activation of leukocyte beta2 integrins lymphocyte function-associated antigen-1 and Mac-1 by chemokines mediated by distinct pathways via the alpha subunit cytoplasmic domains. Mol. Biol. Cell. 10:861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weljie, A.M., P.M. Hwang, and H.J. Vogel. 2002. Solution structures of the cytoplasmic tail complex from platelet integrin alpha IIb- and beta 3-subunits. Proc. Natl. Acad. Sci. USA. 99:5878–5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg, K., R. Fassler, B. Warmegard, and S. Johansson. 1998. Mutational analysis of the potential phosphorylation sites in the cytoplasmic domain of integrin beta1A. Requirement for threonines 788-789 in receptor activation. J. Cell Sci. 111:1117–1126. [DOI] [PubMed] [Google Scholar]
- Wiedemann, A., J. Lim, and E. Caron. 2005. Small GTP-binding proteins and the control of phagocytic uptake. In Molecular Mechanisms of Phagocytosis. C. Rosales, editor, Springer Verlag, New York. pp. 72–84.
- Worthylake, R.A., and K. Burridge. 2003. RhoA and ROCK promote migration by limiting membrane protrusions. J. Biol. Chem. 278:13578–13584. [DOI] [PubMed] [Google Scholar]
- Wright, S.D., and M.T. Jong. 1986. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J. Exp. Med. 164:1876–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, N., O. Higuchi, K. Ohashi, K. Nagata, A. Wada, K. Kangawa, E. Nishida, and K. Mizuno. 1998. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 393:809–812. [DOI] [PubMed] [Google Scholar]
- Ylanne, J., J. Huuskonen, T.E. O'Toole, M.H. Ginsberg, I. Virtanen, and C.G. Gahmberg. 1995. Mutation of the cytoplasmic domain of the integrin beta 3 subunit. Differential effects on cell spreading, recruitment to adhesion plaques, endocytosis, and phagocytosis. J. Biol. Chem. 270:9550–9557. [DOI] [PubMed] [Google Scholar]
- Zamir, E., and B. Geiger. 2001. Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 114:3583–3590. [DOI] [PubMed] [Google Scholar]
- Zhou, H., and R.H. Kramer. 2005. Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J. Biol. Chem. 280:10624–10635. [DOI] [PubMed] [Google Scholar]