Abstract
Cell migration is a rate-limiting event in skin wound healing. In unwounded skin, cells are nourished by plasma. When skin is wounded, resident cells encounter serum for the first time. As the wound heals, the cells experience a transition of serum back to plasma. In this study, we report that human serum selectively promotes epidermal cell migration and halts dermal cell migration. In contrast, human plasma promotes dermal but not epidermal cell migration. The on-and-off switch is operated by transforming growth factor (TGF) β3 levels, which are undetectable in plasma and high in serum, and by TGFβ receptor (TβR) type II levels, which are low in epidermal cells and high in dermal cells. Depletion of TGFβ3 from serum converts serum to a plasmalike reagent. The addition of TGFβ3 to plasma converts it to a serumlike reagent. Down-regulation of TβRII in dermal cells or up-regulation of TβRII in epidermal cells reverses their migratory responses to serum and plasma, respectively. Therefore, the naturally occurring plasma→serum→plasma transition during wound healing orchestrates the orderly migration of dermal and epidermal cells.
Introduction
It is estimated that each year >7 million people develop chronic nonhealing wounds, including pressure, leg, and diabetic ulcers and burns, in the United States. These wounds require long-term care that is labor intensive and costly. Delayed wound healing among the elderly in the United States, for instance, is estimated to cost >$9 billion each year (Wadman, 2005). Although tremendous efforts were made on the development of recombinant growth factors (GFs) and organotypic skin equivalents, the overall outcomes of GF treatments or the use of skin substitutes, such as xenografts, have not generated satisfactory cost-effective benefits (Boyce et al., 1995; Cross and Mustoe, 2003). Few of the GFs have ultimately received approvals from the Food and Drug Administration. Therefore, there is a pressing need to better understand the fundamentals of the skin wound-healing processes.
Skin wound healing is a complex process involving collaborative efforts of multiple types and lineages of skin cells, ECMs, and soluble GFs. Inflammation, reepithelialization, tissue formation, and tissue remodeling are proposed sequential events to heal skin wounds (Martin, 1997; Singer and Clark, 1999). Abnormalities in any of the events could result in nonhealing wounds or healed wounds with hypertrophic scars (Tredget et al. 1997). Throughout these processes, cell motility control is critical. The epidermal cells, largely keratinocytes, laterally migrate across the wound bed from the cut edge to resurface the wound in the process known as reepithelialization. The human dermal cells, including dermal fibroblasts (DFs) and dermal microvascular endothelial cells (HDMECs), move into the wound to produce and deposit large amounts of matrix proteins, to contract and remodel the wound, and to build new blood vessels. Thus, it is critical to understand what cells move into the wound first, second, or third and what mechanism orchestrates the order of the multitype skin cell motility during wound healing.
In unwounded skin, the resident skin cells are nourished by a filtrate of plasma. When skin is wounded, the resident cells in the wound encounter an acute transition from an initial stage of plasma to a stage of serum for the first time. As the wound heals and subsequent wound remodeling initiates, the resident cells experience a transition from plasma back to serum. In fact, the plasma→serum→plasma transition coincides with the classical phases of skin wound healing, as mentioned in the previous paragraph. There have been few studies that define the physiological function of this transition in the wound repair. In addition, the full ingredients in wound fluid may be more complex than those in plasma or serum. For instance, it should also contain released factors from inflammatory leukocytes and even from the resident skin cells (Coulombe, 2003). In particular, the inflammatory cells and factors have long been proposed to play important roles in the repair process. However, recent studies suggest that inflammation, which is a necessary mechanism of defense in adults, is not only dispensable for wound healing but rather harmful to the purposes of fast healing and less scaring. First, embryos, in which no inflammation takes place, heal wounds perfectly without a scar (Ferguson and O'Kane, 2004). Second, Smad3 and Pu.1 knockout mice cannot mount an inflammatory response; however, the reepithelialization and wound healing occur faster than their wild-type littermates and show less scaring (Ashcroft et al., 1999; Martin et al., 2003).
We recently reported that human serum, but not human plasma, promotes human keratinocyte (HK) migration (Henry et al., 2003). This suggested, for the first time, that the plasma to serum transition differentially regulates skin cell motility. In the present study, we studied the effects of plasma versus serum on the motility of three primary human skin cell types: DFs, HDMECs, and HKs. Our results suggest that the plasma→serum→plasma transition serves as a “traffic control” for the dermal and epidermal cell motility, in which TGFβ3 in serum acts as the “traffic controller” and the cell surface levels of type II TGFβ receptor (TβRII) operate as the “sensor” to determine the order of skin cell migration.
Results
Human plasma and serum have opposite effects on dermal and epidermal cell motility
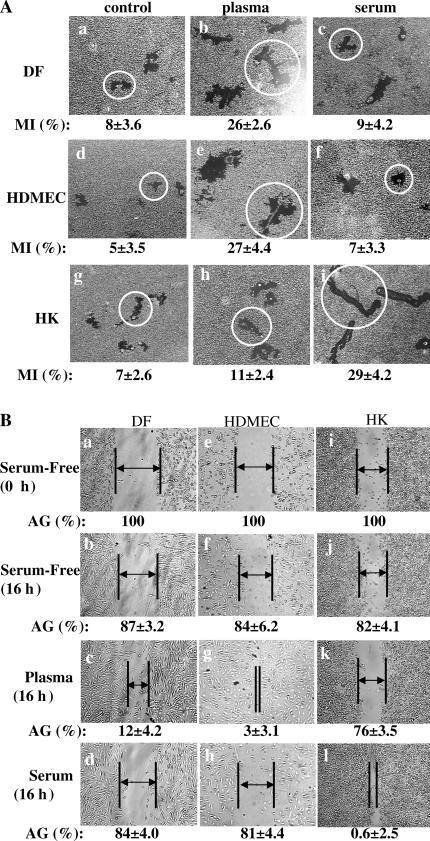
Human DFs, HDMECs, and HKs are the three major types of skin cells involved in wound healing. During the wound healing, either human plasma or serum represents the main source of soluble GFs in the wound fluid. Therefore, we studied the migratory responses of these three cell types to human plasma and serum. The individual cell motility-based colloidal gold motility assay (Albrecht-Buehler, 1977) and a modified in vitro wound-healing assay (Li et al., 2004b) were performed side by side. In the colloidal gold motility assay, cells are allowed to attach and migrate on an ECM-precoated colloidal gold surface in the presence of a cell proliferation inhibitor. As the cells move, they leave behind migration tracks that can be visualized under a microscope and added as a migration index (MI) by computer-assisted analysis (Woodley et al., 1988). As shown in Fig. 1 A, in the absence of plasma or serum, all three types of skin cells modestly migrated on collagen (a, d, and g) and generated MIs (see Materials and methods) from 4 to 8. This is consistent with our previous study reporting that ECMs alone can initiate cell migration in the total absence of soluble GFs (Li et al., 2004c). The addition of human plasma strongly stimulated DF and HDMEC migration (Fig. 1 A, b and e), generating MIs >25. The mean size migration tracks were marked with open circles. In contrast, human serum showed little stimulation of DF and HDMEC migration (Fig. 1 A, c and f) with MIs between 7 and 10. However, as previously described (Henry et al., 2003), plasma and serum showed opposite effects on HK migration. Plasma had a weak stimulation of HK migration (Fig. 1 A, h), with MIs of 9–11. In contrast, serum robustly stimulated HK migration, which left behind long and linear tracks (Fig. 1 A, i) with MIs of 28–30.
Figure 1.
Plasma and serum oppositely affect dermal and epidermal cell migration. HKs, DFs, and HDMECs were serum starved overnight and subjected to colloidal gold migration assays (A) and in vitro wound-healing assays (B). (A) Representative images of the colloidal gold migration assays under the following three conditions: (1) on a collagen matrix in the absence of any soluble GFs (a, d, and g); (2) on a collagen matrix in the presence of an optimal concentration (10% vol/vol) of human plasma (b, e, and h); and (3) on collagen matrix in the presence of an optimal concentration (10% vol/vol) of human serum (c, f, and l). The computer-assisted quantitative analyses of the migration tracks are shown as migration indices (MI). These experiments were repeated five times, and closely similar results were obtained. Average size migration tracks are marked with circles. (B) In vitro wound-healing assay was performed under the same conditions. The wound closures were photographed, and the average gaps (AGs) were quantitated as described previously (Li et al., 2004b). AGs are indicated by lines and arrows; some gaps were too close to insert an arrow. Similar results were reproduced in three independent experiments.
The results of the in vitro wound-healing assay, which measures the averaged and directional migration of a cell population, confirmed the findings. The unclosed distance in multiple wounded areas in each cell culture well was measured and quantified as an average gap (AG). As shown in Fig. 1 B, plasma but not serum stimulated wound closure by DFs (Fig. 1 B, c vs. d) and HDMECs (Fig. 1 B, g vs. h). In contrast, human serum but not plasma stimulated complete closure of the wounded area of HKs (Fig. 1 B, l vs. k). Therefore, the aforementioned data suggest that: (1) plasma contains a promotility factor for DFs and HDMECs but not for HKs; (2) as plasma converts to serum, serum acquires new factors that specifically stimulate HK migration; and (3) serum stops stimulation of the dermal cell migration by losing the plasma promotility factors, by gaining antimotility factors, or both. Therefore, we, investigated these possibilities.
TGFβ3 but not TGFβ1 or TGFβ2 in human serum determines the on and off of dermal versus epidermal cell migration
To identify the factors that determine the differential effect of plasma versus serum on dermal and epidermal cell motility, we focused on the possible antimotility factors of the dermal cells that should only be present in human serum. This approach was based on the assumption that serum should retain all the preexisting plasma factors in addition to acquired new factors during the plasma to serum transition. Therefore, we studied TGFβ, a well-characterized family of antigrowth and antimotility factors.
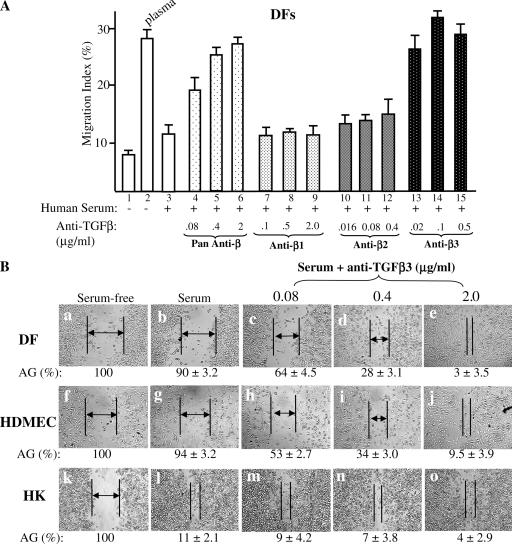
It is known that in human plasma, the levels of all three TGFβ isoforms are either low, such as TGFβ1, or undetectable, such as TGFβ2 and TGFβ3. In human serum, however, TGFβ1, TGFβ2, and TGFβ3 are all elevated to mean values of 30, 1–1.5, and 1–2 ng/ml, respectively (Assoian et al. 1983; Roberts, 1998; Grainger et al., 2000; Hering et al., 2001; Bellone et al. 2005). We tested whether depletion of the TGFβ function in serum would convert serum to plasma, regaining its promotility effect on the dermal cells. For simplicity, only the MIs of the colloidal gold cell migration experiments are presented. As shown in Fig. 2 A, plasma (bar 2) but not serum (bar 3) stimulated DF migration over the serum-free control (bar 1). However, the addition to serum of increasing amounts of pan–anti-TGFβ–neutralizing antibody, which neutralizes all three human TGFβ isoforms, completely converted the serum to a promotility stimulus of DF migration just like plasma (Fig. 2 A, bars 4–6 vs. bar 3).
Figure 2.
TGFβ3 but not TGFβ1 or TGFβ2 in human serum selectively blocks dermal cell migration. DFs, HDMECs, and HKs were serum starved overnight and subjected to migration assays on collagen in the absence or presence of plasma, serum, or serum with added increasing amounts of anti-TGFβ neutralizing antibodies. The four antibodies include a pan-antibody against all three TGFβ isoforms and three other antibodies specifically against the individual TGFβ1, TGFβ2, or TGFβ3. The amounts of these antibodies were predetermined to neutralize the same amounts of their corresponding TGFβ isoforms. (A) Anti-TGFβ3 antibody released the inhibition of serum in DF migration, whereas anti-TGFβ1 and anti-TGFβ2 antibodies did not. This experiment was repeated four times. Error bars represent SEM. (B) The addition of anti-TGFβ3 antibody to serum converted to a promotility agent for both DFs and HDMECs. The cells were subjected to in vitro wound-healing assays under either serum-free, serum, or serum with added anti-TGFβ3 neutralizing antibodies. After 16 h, the wound closures were photographed, and the AGs were quantitated as described previously (Li et al., 2004b). AGs are indicated by lines and arrows; some gaps were too close to insert an arrow. The results shown here were reproducible in two independent experiments.
To determine further which TGFβ isoform was specifically responsible for the inhibitory effect in serum, we made use of three isoform-specific neutralizing antibodies under the concentrations that block a similar amount of TGFβ1, TGFβ2, or TGFβ3 but show little cross-reacting activities. To our surprise, the anti-TGFβ1 antibody failed to eliminate the inhibitory effect of serum on DF migration even at 2.0 μg/ml, which is capable of blocking >50 ng/ml TGFβ1 (Fig. 2 A, bars 7–9 vs. bar 3). Just like the anti-TGFβ1 antibody, the addition of an anti-TGFβ2 antibody did not relieve the serum's inhibitory effect on DF migration (Fig. 2 A, bars 10–12 vs. bar 3). In contrast, the addition of an anti-TGFβ3 antibody completely converted serum to a plasmalike promotility stimulus for DFs (Fig. 2 A, bars 13–15 vs. bars 2 and 3).
The importance of TGFβ3 was confirmed by in vitro wound-healing assays. As shown in Fig. 2 B, the addition of increasing amounts of anti-TGFβ3 antibody to serum eliminated its inhibitory effect on DF (c–e vs. b) and HDMEC (h–j vs. g) migration in a dose-dependent manner, resulting in closure of the wounded areas. In contrast, the addition of the antibodies to serum had neither stimulatory nor inhibitory effects on the closure of the wounded HK cell monolayer (Fig. 2 B, m–o vs. l). Quantitation of the wound closures is shown below the corresponding images as AGs. These data indicate that it is the increased TGFβ3 after plasma to serum transition that selectively stops dermal cell migration.
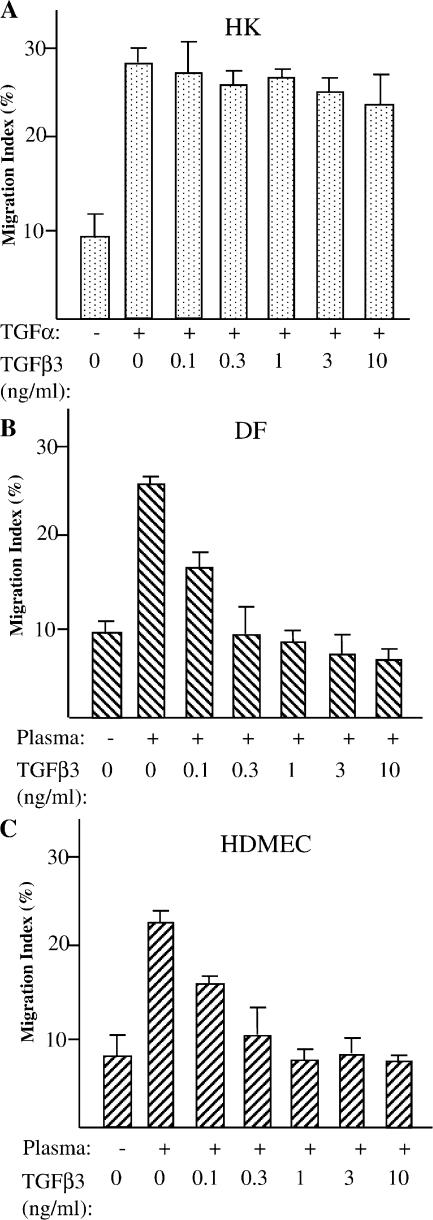
To gain a reciprocal support of this finding, we compared the effect of recombinant TGFβ3 on GF-induced migration of DFs, HDMECs, and HKs. As shown in Fig. 3, TGFα-stimulated HK migration (TGFα is used to stimulate HK migration because plasma does not stimulate HK migration and serum already contains TGFβ3) was not affected by the addition of TGFβ3 (Fig. 3 A). In contrast, plasma-stimulated migration of DFs and HDMECs was blocked by the addition of TGFβ3 in a dose-dependent manner (Fig. 3, B and C). Therefore, we conclude that along the steps of the plasma→serum→plasma transition during wound healing, TGFβ3 acts as an on-and-off switch to separate the migration of dermal cells from that of epidermal cells.
Figure 3.
TGFβ3 selectively inhibits dermal, but not epidermal, cell migration. DFs, HDMECs, and HKs were serum starved overnight and subjected to colloidal gold migration assays on collagen. The indicated concentrations of TGFβ3 were added to either TGFα- (stimulus of HK migration to replace serum that already contains TGFβ3) or plasma (stimulus of DF and HDMEC migration)-containing media. MIs of the migration are shown. The differences in MIs in HKs were statistically insignificant (P < 0.001). The differences in MIs in DFs and HDMECs were statistically significant (P < 0.005). These experiments were repeated four times, and reproducible results were obtained. Error bars represent SEM.
Differential expression of TβRs in epidermal versus dermal cells
To investigate the molecular basis by which the migratory responses of dermal and epidermal cells naturally divide them into either TGFβ-sensitive or TGFβ-insensitive cells, we examined the profiles of three TβRs—TβRI/ALK5, TβRII, and TβRIII/betaglycan—in DFs, HDMECs, and HKs as well as in melanocytes (MCs). As shown in Fig. 4 A, all four cell types expressed comparable levels of TβRI (a), with a similar level in HKs and DFs (lanes 1 and 3) and a slightly lower level in MCs and HDMECs (lanes 2 and 4). Among the four cell types, only DFs showed a detectable level of TβRIII/betaglycan (Fig. 4 A, e; lane 3). Anti–β-actin antibody blotting of the lower part of the same membranes (Fig. 4 A, b, d, and f) was used as the control for equal sample loading and the references for densitometry scan quantitation (ratio of TβR/β-actin). It can be seen that there is no apparent correlation between TβRI and TβRIII expression (note that HDMECs show no expression of TβRIII and yet are sensitive to TGFβ) and TGFβ sensitivity between the dermal and epidermal cells (the migration of MCs responds to plasma and serum just like HKs; not depicted). In contrast, there is a clear correlation between the levels of TβRII expression and the sensitivity of dermal and epidermal cell migration to TGFβ. The TGFβ-sensitive DFs and HDMECs exhibited 5–12-fold higher levels of TβRII than TGFβ-insensitive HKs and MCs (Fig. 4 A, c; lanes 3 and 4 vs. lanes 1 and 2) based on their anti–β-actin antibody blotting controls (Fig. 4 A, d).
Figure 4.
In vitro and in vivo human skin profiles of TβRs (I, II, and III) and their transmission of TGFβ-stimulated Smad2/3 activation. (A) Equal amounts of cell lysates of serum-starved HKs, MCs, DFs, and HDMECs were resolved in three SDS gels and subjected to Western blot analyses with antibodies against TβRI/ALK5 (a), TβRII (c), or TβRIII/betaglycan (e). Each lower part of the three membranes was blotted with an anti–β-actin antibody as a control (b, d, and f). The expression was quantitated by a densitometry scan, giving rise to a ratio of TβR band intensity over its corresponding β-actin band intensity. (B) Normal human skin sections were subjected to indirect immunofluorescence staining with the antibodies against TβRI (a), TβRII (f), TβRIII (k), DAPI (b, g, and l), rhodamine-conjugated phalloidin (c, h, and m), or corresponding IgG controls (see Materials and methods). The images show skin tissue distribution of the TβRs (a, f, and k), nuclei (b, g, and l), F-actin (c, h, and m), and all three stains merged (d, i, and n). A section of the anti-TβRI antibody staining (a, dotted box) was enlarged in the inset to visualize cellular levels of staining. Bar, 20 μm. (C) The cells were either untreated or treated with the indicated concentrations of TGFβ3 for 15 min, and equalized cell lysates (40 μg/lane) were subjected to Western blot analyses with either antiphospho-Smad2/3 (a, c, and e) or anti-Smad2/3 (b, d, and f) antibodies. (D) The cells were either untreated or treated with 2.5 ng/ml TGFβ3 for the indicated periods of time. Equalized cell lysates were subjected to Western blot analyses with either antiphospho-Smad2/3 (a, c, and e) or anti-Smad2/3 (b, d, and f) antibodies.
To confirm the TβR expression patterns in human skin, sections of frozen normal human skin were subjected to (1) indirect immunofluorescence staining with the corresponding anti-TβR antibodies, (2) DAPI staining (to strain the nuclei); and (3) phalloidin (to stain F-actin) or IgG control (control for anti-TβR antibodies). As shown in Fig. 4 B, the control IgG antibodies showed little staining in either the epidermal or the dermal areas (e, j, and o). In contrast, anti-TβRI antibody staining showed the expression of TβRI in both the epidermis and dermis (Fig. 4 B, a). This staining colocalized with phalloidin (membrane and cytosol) but not DAPI (nuclei), resulting in the yellow/orange-colored area when the images were merged (Fig. 4 B, d vs. b and c). Consistent with the Western blot data, the anti-TβRII antibody staining clearly showed stronger expression of the TβRII in the dermis than the epidermis (Fig. 4 B, f). The staining colocalized with phalloidin, resulting in a merged yellow/orange color in the dermis and less in the epidermis (Fig. 4 B, i). As expected, anti-TβRIII antibodies almost entirely stained the dermis (Fig. 4 B, k). No merged yellow/orange color was detected in the epidermis (Fig. 4 B, n vs. i and m).
We questioned whether the difference in TβR profiles affects the TGFβ-stimulated phosphorylation of Smad2/3 between dermal and epidermal cells. As shown in Fig. 4 C, the dose-dependent analyses showed little differences in TGFβ-induced Smad2/3 phosphorylation (ser-465/467) in all three cell types, in which the induced Smad2/3 phosphorylation could be clearly detected after stimulation with 0.1–0.5 ng/ml TGFβ3 (lanes 3 and 4). Moreover, as shown in Fig. 4 D, we also detected similar kinetics of TGFβ3-induced Smad2/3 phosphorylation in both epidermal (HKs) and dermal cells (DFs and HDMECs). Close analyses of the data from multiple experiments revealed a relatively sustained Smad2/3 phosphorylation in HKs and a transient Smad2/3 phosphorylation, which declined after 45 min of TGFβ3 stimulation in DFs and HDMECs (Fig. 4 D, c and e vs. a). Therefore, the insensitivity of HKs to the antimotility effects of human serum or TGFβ3 was not caused by a complete lack of or a significant difference in TGFβ3-stimulated Smad2/3 phosphorylation.
The lower TβRII level in epidermal cells selectively blocks the antimotility signal of human serum or TGFβ3
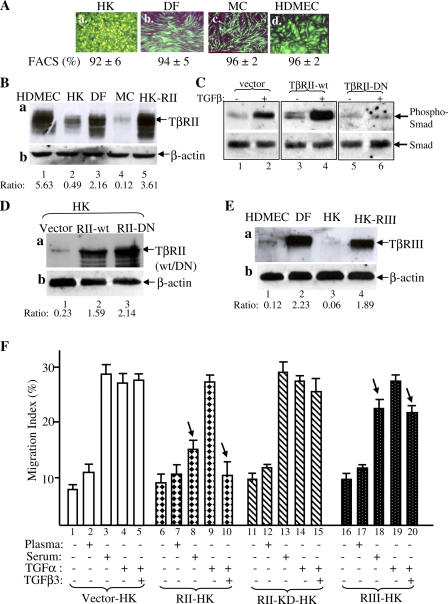
The critical question was whether the differences in TβR expression, especially TβRII, account for the differential migratory responses of the dermal and epidermal cells to plasma and serum. We took the following approaches: (1) to increase the TβR levels in epidermal cells to similar levels in the dermal cells and (2) to down-regulate the TβR expression in the dermal cells to similar levels seen in the epidermal cells. Then, we tested whether these changes would convert the TGFβ-insensitive epidermal cells to TGFβ-sensitive dermal cells and vice versa. To overexpress the TβR of interest, we chose to use lentiviral vector pRRLsinhCMV (Li et al., 2004a,b). Using the EGFP gene as the marker, as shown in Fig. 5 A, we provide evidence that this gene delivery system offers >90% gene transduction efficiency in all four human skin cell types.
Figure 5.
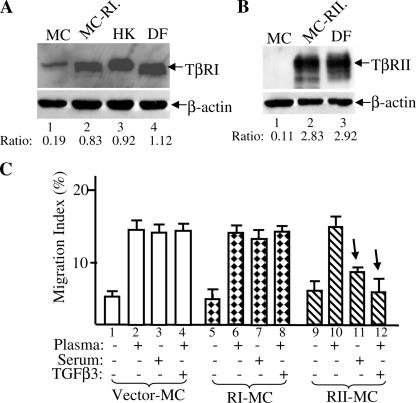
Up-regulation of TβRII converts epidermal HK motility to become TGFβ3 sensitive just like dermal cells. (A) Lentiviral infection offered >90% gene transduction efficiency in DFs, MCs, HDMECs, and HKs. 48 h after infection with pRRLsin-CMV-EGFP, the plates were analyzed under fluorescence microscopy and photographed. The cells were suspended by trypsin and subjected to FACS analyses for measurement of the gene transduction efficiency (percentage of green cells/total cells). (B) Elevated expression of TβRII in HKs after pRRLsin-TβRII infection (lane 5) in comparison with the endogenous TβRII expression in HKs (lane 2), DFs (lane 3), and HDMECs (lane 1). Equal amounts of cell extracts of the indicated cell types were blotted with anti-TβRII antibody (a) or anti–β-actin antibody (b). (C) TβRII-KD blocks TGFβ3-stimulated Smad2/3 phosphorylation in HKs. The HKs, infected with vector, wild-type TβRII, or TβRII-KD, were untreated or treated with 2.5 ng/ml TGFβ3 for 45 min and analyzed for the phosphorylation of Smad2/3 similar to the assays shown in Fig. 4 (C and D). (D) Elevated expression of TβRII-KD in HKs after pRRLsin-CMV–TβRII-KD infection (lane 3) in comparison with elevated wild-type TβRII expression (lane 2) and the endogenous TβRII expression in HKs (lane 1). (E) Elevated expression of TβRIII in HKs (lane 4 vs. lane 3) after pRRLsin-CMV-TβRIII infection in comparison with endogenous TβRIII expression in DFs (lane 2) and HDMECs (lane 1). (F) The aforementioned TβR-engineered HKs were tested for their migratory responses to plasma, serum, or TGFα with or without 0.5 ng/ml TGFβ3 in colloidal gold migration assays. MIs are shown. This experiment was repeated three times. Error bars represent SEM.
To study the role of TβRII, we focused on HKs, which showed a much lower level of TβRII than dermal cells, DFs, and HDMECs. As shown in Fig. 5 B, HKs express a lower level of TβRII than DFs or HDMECs (a; lane 2 vs. lanes 1 and 3). However, after pRRLsinhCMV-TβRII virus infection, the TβRII expression in HKs was increased to a similar level seen in DFs and HDMECs (Fig. 5 B, lane 5 vs. lanes 1 and 3). The kinase-defective TβRII mutant (TβRII-KD) was included as the control for specificity. As shown in Fig. 5 D, the exogenously expressed TβRII-KD (lane 3) was equivalent to the exogenously expressed wild-type TβRII (lane 2). As expected, overexpression of TβRII-KD but not wild-type TβRII blocked TGFβ3-stimulated Smad2/3 phosphorylation (Fig. 5 C, lanes 5 and 6 vs. lanes 3 and 4 and lanes 1 and 2). To study TβRIII, we infected HKs with pRRLsinhCMV-TβRIII virus. As shown in Fig. 5 E, the TβRIII expression in HKs was elevated from a nondetectable level in uninfected HKs (lane 3) to a similar level observed in DFs (lane 4 vs. lane 2). DFs are the only skin cell type expressing a detectable level of TβRIII.
These TβRII and TβRIII expression-equalized HKs were subjected to migration assays in response to plasma, serum, or TGFα in the absence or presence of TGFβ3. As shown in Fig. 5 F, migration of the parental HKs was unchanged by plasma (bar 2 vs. bar 1), dramatically stimulated by serum or TGFα (bars 3 and 4), but not blocked by the addition of TGFβ3 (bar 5 vs. bar 4). In contrast to the parental HKs, the TβRII-overexpressing HKs responded to serum as a strong antimotility reagent (Fig. 5 F, bar 8 vs. bar 3). Moreover, TGFβ3 completely blocked the TGFα-stimulated migration of these cells (Fig. 5 F, bar 10 vs. bar 9). However, overexpression of the TβRII-KD mutant did not show the same effect. The migration of TβRII-KD–HK cells, like the parental HKs, could still be stimulated by serum (Fig. 5 F, bar 13 vs. bar 3), and the TGFα-stimulated migration remained refractory to the presence of TGFβ3 (Fig. 5 F, bar 15 vs. bar 4).
The migration of TβRIII-overexpressing HKs was slightly inhibited by serum (Fig. 5 F, bar 18) in comparison with their parental counterpart (Fig. 5 F, bar 3; P < 0.001). TGFβ3 also partially blocked TGFα-driven migration of the cells (Fig. 5 F, bar 20 vs. bar 5; P < 0.005). It is possible that TβRIII overexpression allows HKs to have more access to the TGFβ3 that is bound to the exogenously overexpressed TβRIII. Based on our previous study that TGFβ selectively blocks the proliferation but not migration of HKs in response to GFs (Sarret et al., 1992), the aforementioned finding indicates that it is the lower TβRII level that determines HKs' sensitivity to the antiproliferation but not the antimotility signal of TGFβ3.
To further verify the key role of TβRII, we generated TβRII- as well as TβRI-overexpressing MCs because the parental MCs express the lowest or undetectable level of TβRI and TβRII among the four human skin cell types (Fig. 4 A). As shown in Fig. 6 A, the parental MCs expressed a lower level of TβRI (lane 1) than both HKs (lane 3) and DFs (lane 4). However, the pRRLsinhCMV-TβRI virus-infected MCs showed an increased expression of TβRI (Fig. 6 A, lane 2) similar to the TβRI expression in HKs and DFs. Similarly, as shown in Fig. 6 B, parental MCs expressed low or undetectable levels of TβRII (Fig. 6 B, lane 1). pRRLsinhCMV-TβRII virus infection increased the TβRII expression in MCs (Fig. 6 B, lane 2) to a similar level seen in DFs (Fig. 6 B, lane 3).
Figure 6.
Up-regulation of TβRII, but not TβRI, causes epidermal MC motility to become TGFβ3 sensitive. (A) Elevated expression of TβRI in MCs (lane 2 vs. lane 1) after pRRLsin-CMV-TβRI infection in comparison with endogenous TβRI expression in HKs (lane 3) and DFs (lane 4). (B) Elevated expression of TβRII in MCs (lane 2 vs. lane 1) after pRRLsin-CMV-TβRII infection in comparison with endogenous TβRII expression in DFs (lane 3). (C) Migration of MCs with vector control (bars 1–4), overexpressing TβRI (bars 5–8), or overexpressing TβRII (bars 9–12) in the presence of plasma, serum, or plasma plus 0.5 ng/ml TGFβ3. Error bars represent SEM.
We then tested the migratory response of these cells to plasma and serum. As shown in Fig. 6 C, parental MC migration was equally stimulated by plasma and serum (bars 2 and 3 vs. bar 1) and was not sensitive to TGFβ3 (bar 4). The increased expression of TβRI in MCs had no effect on cell migration in response to plasma (Fig. 6 C, bar 6), serum (Fig. 6 C, bar 7), or plasma plus TGFβ3 (Fig. 6 C, bar 8). In contrast, the migration of TβRII-overexpressing MCs became converted from TGFβ insensitive to TGFβ sensitive, in which serum strongly inhibited migration of the cells (Fig. 6 C, bar 11 vs. bar 3). In addition, TGFβ3 completely blocked the plasma-induced migration (Fig. 6 C, bar 12 vs. bar 4).
Higher TβRII levels make dermal cells sensitive to both antimotility and antiproliferation signals of human serum and TGFβ3
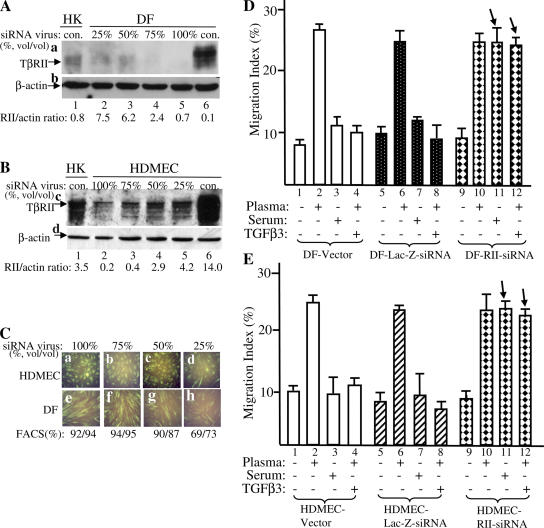
To reversely confirm the determining role for TβRII levels of expression, we wanted to down-regulate the endogenous TβRII expression in DFs and HDMECs to the similar level observed in HKs and tested whether these TβRII–down-regulated dermal cells would become refractory to the antimotility effect of serum and TGFβ3 just like the epidermal HKs and MCs. The FG-12 system, a lentiviral backbone carrying a short inhibitory RNA (siRNA) expression cassette (Qin et al., 2003), was used to transduce siRNA against human TβRII into DFs and HDMECs. To select the optimal virus titer, we infected DFs and HDMECs with various dilutions (vol/vol) of the original FG-12–TGFβ-RII–siRNA virus stock (∼4–7 × 106 transduction units/ml). As shown in Fig. 7 A, infection of DFs (a) with 50% of the original virus stock (lane 3) reduced the TβRII expression in DFs (lane 6) to the similar level observed in HKs (lanes 1). Similar results were obtained in HDMECs with 50% of the virus stock (Fig. 7 B, lane 4 vs. lane 1). The dilutions of the virus stocks did not cause any significantly compromised transduction efficiency. As shown in Fig. 7 C, the transduction efficiencies for 50% (1:1) or less dilutions were all >90%, as indicated by the coexpressed GFP for both HDMECs (a–c) and DFs (e–g). Only 25% (1:4) or higher diluted viruses showed a significant decrease in the transduction efficiency (Fig. 7 C, d and h).
Figure 7.
Down-regulation of TβRII causes dermal DF and HDMEC motility to become TGFβ3 insensitive just like the epidermal cells. DFs and HDMECs were infected with various dilutions (vol/vol) of the lentivirus (FG-12) carrying an siRNA against TβRII. After 48 h, the cells were first analyzed for down-regulation of the endogenous TβRII before being subjected to migration assays. (A) 50% dilution of the original virus stock decreases TβRII expression in DFs (a, lane 6) to the level similar to HKs (a, lane 3 vs. lane 1). (B) The same dilution of the virus stock decreases the TβRII expression in HDMECs (c, lane 6) to the similar level in HKs (c, lane 3 vs. lane 1). (C) Coexpressed GFP and FACS analyses show no significant reduction in gene transduction efficiency in HDMECs (a–c) or DFs (e–g) unless the virus stock is diluted to 25% or further (d and h). (D and E) DF and HDMEC cells 48 h after infection with 50% of the original virus stocks of vector alone, lac-Z–siRNA, and TβRII-siRNA were subjected to colloidal gold migration assays in the absence or presence of plasma, serum, and plasma plus TGFβ3. MIs are shown. These migration experiments were repeated three times. Error bars represent SEM.
Therefore, we chose 50% diluted virus stock to infect DFs and HDMECs for further migration assays. As shown in Fig. 7 D, in the vector- and control lac-Z–siRNA-infected DFs, plasma stimulated cell migration (bars 2 and 6), whereas serum and TGFβ3 inhibited cell migration (bars 3, 4, 7, and 8). However, in TβRII–down-regulated DFs, neither serum nor TGFβ3 was able to inhibit the cell migration (Fig. 7 D, bars 11 and 12 vs. bars 3 and 4). Instead, the cells responded to both plasma and serum as promotility agents (Fig. 7 D, bars 11 and 12). Similarly, in HDMECs, down-regulation of TβRII converted the cells from serum- and TGFβ3-sensitive cells to TGFβ3-insensitive cells just like epidermal HKs and MCs. As shown in Fig. 7 E, migration of the HDMEC–RII–siRNA cells became insensitive to the antimotility effect of serum or TGFβ3 (Fig. 7 E, bars 11 and 12 vs. bars 7 and 8). Moreover, both plasma (Fig. 7 E, bar 10) and serum (Fig. 7 E, bar 11) became promotility agents for the cells. In conjunction with our previous study reporting that TGFβ blocks both proliferation and migration of DFs (Sarret et al., 1992), these findings again indicate that the levels of TβRII determine the uptake of the antimotility signals, the antiproliferation signals, or both of TGFβ3.
Discussion
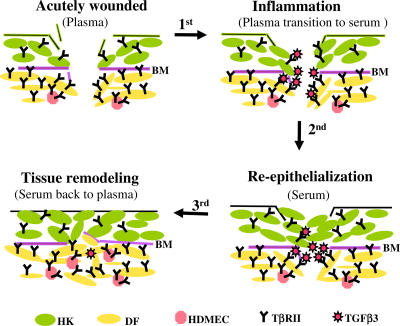
This study is a significant departure from previous wound-healing studies using human skin cells (for review see Li et al., 2004c). First, those studies used bovine pituitary extract (BPE) or FBS as the stimuli of human skin cell migration and proliferation. However, human skin cells are never in contact in real life with BPE or FBS. In fact, neither BPE nor FBS resembles human serum to differentially affect human dermal and epidermal cells. Second, because previous studies often focused on a single type of human skin cell or cell line, little could be learned about coordinated migration of the multitype skin cells, which are simultaneously present in the wound bed in vivo. Because of these new approaches, the results of this study unveil a previously unrecognized function for the naturally occurring plasma→serum→plasma transition along the classical four phases of skin wound healing. A schematic representation of the key findings is shown in Fig. 8. When skin is acutely wounded, whereas plasma is still not fully converted to serum, DFs and HDMECs may first migrate into the wound over provisional ECM. In contrast, the epidermal cell migration (i.e., reepithelialization) has yet to take place because of the lack of HK promotility activity in plasma (step 0). Then, the transition to serum quickly stops dermal cell migration by increasing the TGFβ3 level (first step) and, at the same time, promotes HK migration by newly acquired HK promotility factors, predominantly TGFα (second step; Li, et al., 2006). The selective inhibition of dermal cell migration by TGFβ3 from serum results from the higher TβRII expression levels in these cells, and, in contrast, the escape of inhibition by the epidermal cells is granted by either the lower (HKs) or undetectable (MCs) TβRII levels in the epidermal cells. After the wound heals, serum transforms back to plasma, which resumes DF and HDMEC migration into the newly healed wound for the final phase of wound healing, remodeling, and angiogenesis (third step). Therefore, this study has provided a mechanism by which the orderly migration of three major skin cell types take place during wound healing.
Figure 8.
A schematic representation of how the plasma→serum→plasma transitions coordinate the orderly skin cell migration during wound healing. Three major types of skin cells—HKs, DFs, and HDMECs—are shown here, as indicated by different colors. The dermal cells express higher levels of TβRII (symbolized by Y) than the epidermal cells. Therefore, the dermal cells are sensitive to the anti-promotility effect of TGFβ3 (red stars), whose concentration increases after the transition from plasma to serum in the wound bed. Contributions by other cell types and matrix components to skin wound healing are omitted for the sake of simplicity but not reality. The relative numbers and proportions of the various types of cells do not quantitatively reflect those in real human skin. BM, basement membrane.
The main TGFβ in human serum was thought to come from platelet degranulation, in which TGFβ1 is the only isoform present in the α granules of platelets (Assoian et al., 1983; Roberts and Sporn, 1996). However, human serum but not plasma was reported to contain TGFβ3 at a concentration range of 1–2 ng/ml (Grainger et al., 2000; Hering et al., 2001), which likely comes from the blood leukocytes (Assoian et al., 1987; Grotendorst et al., 1989; Nishimura et al., 1998). An increase in TGFβ2 in serum has also been reported (Bellone et al. 2005). Because of technical difficulties, there is still a lack of consensus in the exact levels of TGFβ in human plasma and serum (Roberts, 1998; Piek et al., 1999; Grainger et al., 2000). First, it is extremely difficult to collect platelet-free plasma and to completely prevent degranulation of the contaminated platelets because platelets tend to resuspend during plasma preparation (Assoian et al., 1984; Sporn and Roberts, 1990). Second, a wide range of methods for measuring TGFβ (mostly for TGFβ1) has been used. The bioassay-based measurements selectively measure the amount of the biologically active TGFβ, and, therefore, they provide data on the “operational” TGFβ (Gentry et al., 1988; Garrigue-Antar et al., 1995). On the other hand, the antibody-based ELISA assays detect the total amount of free TGFβ. In the latter case, during the dilution steps of these assays, some TGFβ peptides dissociate from their noncovalently bound complexes and become detectable. This dissociation could also cause nonlinear curves of the assays (Grainger et al., 2000). Third, because the half-life of an active TGFβ peptide is only 2–3 min, it is difficult to determine whether the detected amount of TGFβ represents the steady-state level or in vitro broken down level of TGFβ (Roberts, 1998; Piek et al., 1999). Finally, the wide variations on age, gender, or racial background of the selected donors for the human subjects also contributed to the variations in detected TGFβ.
The three mammalian TGFβ isoforms TGFβ1, TGFβ2, and TGFβ3 bind to and transmit signals via the heteromeric complex of TβRII and TβRI/activin receptor-like kinase (Alk) serine/threonine kinases (Miyazono, 2000; Derynck and Zhang, 2003; Shi and Massagué, 2003). However, the three mammalian TGFβ isoforms diverge significantly in their potency as growth inhibitors in vitro as a result of differences in receptor recognitions and binding to extracellular antagonists (Cheifetz et al., 1990; Goumans et al., 2002). In vascular endothelial cells and hematopoietic progenitor cells, TGFβ1 and TGFβ3 showed stronger growth-inhibitory effects than TGFβ2 (Ohta et al., 1987; Cheifetz et al., 1992). Roberts et al. (1990) showed that TGFβ3 was 10-fold more active than TGFβ2 in mesoderm induction in Xenopus laevis, whereas TGFβ1 had little effect. Ren et al. (1999) showed that TGFβ3 but not TGFβ1 and TGFβ2 induced the expression of presenilin-1, a familial Alzheimer's disease–linked gene, in neurons and astrocytes. Moreover, ex vivo wound-healing studies showed that TGFβ1 and TGFβ2 cause a fibrotic scarring response and that TGFβ3 elicits scar-free or regenerative healing responses (for review see Ferguson and O'Kane, 2004). However, discrepancies have also been reported (Wu et al., 1997; Shah et al., 1999).
Consistent with the divergence in vitro, knockout studies in mice showed that TGFβ1, TGFβ2, and TGFβ3 are not functionally redundant (for review see Roberts and Sporn, 1992; Attisano and Wrana, 1996; Dünker and Krieglstein, 2000). Unfortunately, skin wound-healing studies using these TGFβ knockout mice were compromised by severe developmental defects in the mice. TGFβ2 and TGFβ3 knockout mice die during or shortly after birth (Proetzel et al., 1995; Kaartinen et al., 1995; Sanford et al., 1997). While one half of the TGFβ1 knockout mice are born alive, they undergo early postnatal death (3 wk) as a result of a massive infiltration of inflammatory lymphocytes and macrophages onto several organs. The other half dies in utero because of defects in vasculogenesis and hematopoiesis (Shull et al., 1992; Kulkarni et al., 1993). Nonetheless, Koch et al. (2000) reported little impairment in wound healing in <10-d-old neonatal TGFβ1 knockout (Tgfβ1 −/−) mice and in 30-d-old Tgfβ1 −/− mice treated with rapamycin, an immune suppressant. In contrast, Crowe et al. (2000) reported a 1-wk delay in each of the major phases of wound healing in immunodeficient TGFβ1 knockout mice (Tgfβ−/−/Scid −/− mice; Crowe et al., 2000). The reason for the discrepancy is unclear.
Our study showed that the TβR profiles, especially the differences in the levels of TβRII, in three major human skin cell types as well as MCs determine their migratory responses to plasma or serum, respectively. How the quantitative differences in the TβRII levels are translated into the distinct signaling outcomes remain to be studied. We detected little differences in Smad2/3 activation between the dermal and epidermal cell types in response to various doses of TGFβ3. However, a difference in the kinetics of Smad2/3 activation between TGFβ-sensitive DFs and HDMECs and TGFβ-insensitive HKs was reproducibly observed. TGFβ3 appeared to stimulate a more sustained phosphorylation of Smad2/3 in the epidermal cells in comparison with the TGFβ3-stimulated kinetics of Smad2/3 phosphorylation in the dermal cells. Furthermore, we found that the difference in the kinetics of Smad2/3 activation was caused by the difference in TβRII levels between dermal and epidermal cells (unpublished data). Whether this difference accounts for the distinct physiologic responses of dermal versus epidermal cells remains to be studied. It is equally possible that TβRII might mediate the antimotility effect of TGFβ3 via a Smad-independent signaling pathway (Derynck and Zhang, 2003).
Materials and methods
Primary human neonatal DFs, HDMECs, HKs, and MCs were purchased from Clonetics. HKs were cultured in the EpiLife medium supplemented with the HKGS Kit (Cascade Biologics). MCs were maintained in Medium 154 supplemented with human MC growth supplement (Cascade Biologics). DFs were cultured in DME supplemented with 10% FBS. HDMECs were cultured in GF-supplemented Medium 131 (Cascade Biologics). Third or fourth passages of the cell cultures were used in all experiments. Human plasma and serum collected from a variety of donors were purchased from Sigma-Aldrich. Human recombinant TGFβ1, TGFβ2, and TGFβ3 were purchased from R&D Systems. Anti–human TGFβ (1, 2, and 3) pan-neutralizing antibody and neutralizing antibodies specifically against human TGFβ1, TGFβ2, or TGFβ3 were obtained from R&D Systems. Human TβR (I and II) cDNAs were obtained from T. Imamura and K. Miyazono (Japanese Foundation for Cancer Research, Tokyo, Japan). TβRIII cDNA was a gift from J. Massagué (Memorial Sloan-Kettering Cancer Center, New York, NY). TβRII-KD cDNA was a gift from K. Luo (University of California, Berkeley, Berkeley, CA). Antibodies against TβRI/Alk5, TβRII, and TβRIII/betaglycan were either gifts of J. Li (House Ear Institute, Los Angeles, CA) or were obtained from Cell Signaling Technology, Santa Cruz Biotechnology, Inc., and R&D Systems, respectively. DAPI and rhodamine-conjugated phalloidin were purchased from Sigma-Aldrich. The FG-12 system was provided by I. Chen (University of California, Los Angeles, Los Angeles, CA). Rat type I collagen was purchased from BD Biosciences. Antiphospho-Samd2 (ser-465/467) antibody (AB3849) was purchased from Chemicon International. Anti–β-actin antibody was obtained from Cell Signaling Technology, and the Plasmid Midi Kit was purchased from QIAGEN. XL-10 Gold ultracompetent cells were purchased from Stratagene. All other reagents and supplies, unless indicated, were obtained from VWR or Sigma-Aldrich.
Cell migration assay
The colloidal gold migration assay was performed as previously described (Li et al., 2004b).
Statistical analyses
The methodology to determine differences in MIs between experiments has been previously described (Chen et al., 1993). In brief, statistical analyses of the differences in MIs between triplicate sets of experimental conditions were performed using Microsoft Excel. Confirmation of a difference in migration as statistically significant requires rejection of the null hypothesis of no difference between mean MIs obtained from replicate sets at the P ≥ 0.05 levels with the t test.
Test and use of anti-TGFβ neutralizing antibodies
Based on the technical data provided by manufacturers, we performed titration experiments to obtain the highest concentration for each antibody that did not yet cross-react with the other two TGFβ isoforms. In addition, because the three antibodies' affinities (the ratio of antibody amount vs. antigen amount) toward their antigens are different, we obtained the concentrations (micrograms/milliliters) for all of the anti-TGFβ antibodies that neutralize a similar amount of their corresponding antigens (i.e., TGFβ1, TGFβ2, and TGFβ3). For example, 0.2 μg/ml of anti-TGFβ1, 0.08 μg/ml of anti-TGFβ2, and 0.1 μg/ml of anti-TGFβ3 all blocked the function of ∼5 ng/ml TGFβ1, TGFβ2, and TGFβ3, respectively, without cross-reactions. To achieve the maximum neutralizing effects, plasma- or serum-containing media were preincubated with the antibodies for 30 min before being added to migration wells.
cDNA subcloning, production of lentiviral stocks, and infection
The lentivirus-derived vector pRRLsinhCMV was inserted with cDNAs encoding EGFP (at EcoRV), human TβRI (at EcoRI and SacII), human TβRII (at BamHI), human TβRIII (at XbaI and EcoRI), and human TβRII-KD (at BamHI and EcoRI). For the PCR-amplified cDNA inserts (TβRIII and TGFβRII-KD), their DNA sequences in pRRLsinhCMV were confirmed by DNA sequencing. These constructs were used to cotransfect 293T cells together with packaging vectors pCMVΔR8.2 and pMDG as previously described (Chen et al. 2003). Typical viral titers were 1–7 × 106 transduction units/ml without concentration and 5 × 107–108 after ultracentrifugation using GFP as the marker directly measured by FACS analysis. Cell infection and infection efficiency, as monitored by EGFP expression, were performed as previously described (Li et al., 2004a). The cells were subjected to biochemical and cell migration experiments 48 h after infection. The images of GFP-expressing cells were analyzed and recorded by a fluorescence microscope (Axiovert 25; Carl Zeiss MicroImaging, Inc.) with an attached camera (type 12; model SC35; Olympus).
Measurement of transgene expression levels
Expression of TβR gene products were detected by immunoblotting the lysates of infected cells with antibodies against corresponding TβRs. The lower part of the same membranes were blotted with an anti–β-actin antibody as the control for equal sample loading. Selected autoradiographs with unsaturated exposure among the specific signals were used to assess fold increases (over their corresponding β-actin bands) by scanning densitometry. Means from three different exposures of each experiment were calculated against the independent gene product control as an averaged ratio (Li et al., 2004a).
Sequential staining of human skin tissue with antibodies, phalloidin, and DAPI
The detailed procedures are as previously described (Woodley et al., 2004). For immunostaining with anti-TβR antibodies, we used the diamond cutter to make a highly visible circle around the tissue on the back of the slides and then washed and air dried the slides. The tissue specimen on slides was fixed with ∼25 μl acetone for 5 min, washed with PBS, and blocked in 25 μl of the blocking reagent (1:10 dilution of normal goat serum, 0.05% Tween 20, 0.05 Triton X-100, and 1% BSA in PBS) for 60 min in a H2O humidified chamber. The slides were washed with PBS and incubated with primary antibodies for 60–120 min. We washed the slides three times (7 min each) in PBS, incubated with FITC-conjugated secondary antibody added directly onto the tissue area, and covered the slides to prevent light (for the rest of the procedures). Slides were washed in PBS twice and air dried. To continue with phalloidin staining, the tissue specimens were fixed with 4% formaldehyde in PBS for 15 min, washed with PBS twice, and permeabilized with 0.1% Triton X-100 in PBS for 5 min. After washing with PBS, the tissue specimen was blocked with 1% BSA in PBS for 30 min and incubated with 1 μg/ml rhodamine-labeled phalloidin in 1% BSA in PBS for 1 h. The slides were washed with PBS and air dried. To carry out the final staining with DAPI, we directly added prepared DAPI solution (1–2 μg/ml DAPI in 40% glycerol) to the tissue areas, mounted these with coverslips, and sealed them with nail polish. The results were analyzed under a microscope (100× at −20°C; TE-2000U Eclipse; Nikon) with sequential applications of the following fluorochromes: green (FITC), blue (DAPI), and red (phalloidin) in the absence of any imaging medium. The images were recorded in JPEG by an attached camera (TE-2000U; Nikon) using MetaMorph software (version-6.2rb; Universal Imaging).
siRNA, lentiviral infection delivery, and quantitation of transduction efficiency
We used the siRNA Selection Program as described previously to identify possible target sequences (Yuan et al., 2004). Six potential sites were selected and synthesized. The relative effectiveness of the synthetic and double-stranded siRNA in the down-regulation of PKCδ was measured by transfecting 293T cells and immunoblotting the cell lysates with corresponding anti-TβRII antibodies. The most potent one was then cloned into the lentiviral siRNA delivery vector FG-12 as previously described (Qin et al., 2003). The gene transduction efficiency was analyzed under a fluorescence microscope (Axioplan; Carl Zeiss MicroImaging, Inc.) with an MRm camera system (Axiocam; Carl Zeiss MicroImaging, Inc.) and by FACS analysis for a coexpressed GFP gene marker on the same vector. The selected siRNA sequence (sense) against human TβRII for FG-12 cloning was GACCUCAAGAGCUCCAAUA, which effectively down-regulated TβRII.
Acknowledgments
We thank the following scientists for reagents: Drs. T. Imamura and K. Miyazono (cDNAs of TβRI, TβRII, and TβRIII), Dr. J. Massagué (TβRIII cDNA), and K. Luo (TβRII-KD cDNA). We thank Dr. Yuanping Han for allowing us access to his fluorescence microscope and constant advice.
This study was supported by National Institutes of Health grants GM/AR67100-01 and GM066193-01 to W. Li and AR46538 to D.T. Woodley. The authors have no conflicting financial interests.
Abbreviations used in this paper: AG, average gap; BPE, bovine pituitary extract; DF, dermal fibroblast; GF, growth factor; HDMEC, human dermal microvascular endothelial cell; HK, human keratinocyte; MC, melanocyte; MI, migration index; siRNA, short inhibitory RNA; TβR, TGFβ receptor.
References
- Albrecht-Buehler, G. 1977. The phagokinetic tracks of 3T3 cells. Cell. 11:395–404. [DOI] [PubMed] [Google Scholar]
- Ashcroft, G.S., X. Yang, A.B. Glick, M. Weinsteil, J.L. Letterio, D.E. Mizel, M. Anzano, T. Greenwell-Wild, S.M. Wahl, C. Deng, and A.B. Roberts. 1999. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1:260–266. [DOI] [PubMed] [Google Scholar]
- Assoian, R.K., A. Komoriya, C.A. Meyers, D.M. Miller, and M.B. Sporn. 1983. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 258:7155–7160. [PubMed] [Google Scholar]
- Assoian, R.K., G.R. Grotendorst, D.M. Miller, and M.B. Sporn. 1984. Cellular transformation by coordinated action of three peptide growth factors from human platelets. Nature. 309:804–806. [DOI] [PubMed] [Google Scholar]
- Assoian, R.K., B.E. Fleurdelys, H.C. Stevenson, P.J. Miller, D.K. Madtes, E.W. Raines, R. Ross, and M.B. Sporn. 1987. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc. Natl. Acad. Sci. USA. 84:6020–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attisano, L., and J.L. Wrana. 1996. Signal transduction by members of the transforming growth factor-beta superfamily. Cytokine Growth Factor Rev. 7:327–339. [DOI] [PubMed] [Google Scholar]
- Bellone, G., C. Smirne, F.A. Mauri, E. Tonel, A. Carbone, A. Buffolino, L. Dughera, A. Robecchi, M. Pirisi, and G. Emanuelli. 2005. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol. Immunother. 11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce, S.T., M.J. Goretsky, D.G. Greenhalgh, D.G. Kagen, M.T. Rieman, and G.D. Warden. 1995. Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann. Surg. 222:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheifetz, S., H. Hernandez, H. Laiho, P. Ten Dijke, K.K. Iwata, and J. Massagué. 1990. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J. Biol. Chem. 265:20533–20538. [PubMed] [Google Scholar]
- Cheifetz, S., T. Bellon, C. Cales, S. Vera, C. Bernabeu, J. Massagué, and M. Letarte. 1992. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J. Biol. Chem. 267:19027–19030. [PubMed] [Google Scholar]
- Chen, J.D., J.P. Kim, K. Zhang, S. Varret, K.C. Wynn, R.H. Kramer, and D.T. Woodley. 1993. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp. Cell Res. 209:216–223. [DOI] [PubMed] [Google Scholar]
- Chen, M., W. Li, J. Fan, N. Kasahara, and D. Woodley. 2003. An efficient gene transduction system for studying gene function in primary human dermal fibroblasts and epidermal keratinocytes. Clin. Exp. Dermatol. 28:193–199. [DOI] [PubMed] [Google Scholar]
- Coulombe, P.A. 2003. Wound epithelialization: accelerating the pace of discovery. J. Invest. Dermatol. 121:219–230. [DOI] [PubMed] [Google Scholar]
- Cross, K.J., and T.A. Mustoe. 2003. Growth factors in wound healing. Surg. Clin. North Am. 83:531–545. [DOI] [PubMed] [Google Scholar]
- Crowe, M.J., T. Doetschman, and D.G. Greenhalgh. 2000. Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J. Invest. Dermatol. 115:3–11. [DOI] [PubMed] [Google Scholar]
- Derynck, R., and Y.E. Zhang. 2003. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 425:577–584. [DOI] [PubMed] [Google Scholar]
- Dünker, N., and K. Krieglstein. 2000. Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis. Eur. J. Biochem. 267:6982–6988. [DOI] [PubMed] [Google Scholar]
- Ferguson, M.W., and S. O'Kane. 2004. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 359:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigue-Antar, L., I. Barbieux, B. Lieubeau, O. Boisteau, and M. Gregoire. 1995. Optimisation of CCL64-based bioassay for TGF-beta. J. Immunol. Methods. 186:267–274. [DOI] [PubMed] [Google Scholar]
- Gentry, L.E., M.N. Lioubin, A.F. Purchio, and H. Marquardt. 1988. Molecular events in the processing of recombinant type 1 pre-pro-transforming growth factor beta to the mature polypeptide. Mol. Cell. Biol. 8:4162–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger, D.J., D.E. Mosedale, and J.C. Metcalfe. 2000. TGF-beta in blood: a complex problem. Cytokine Growth Factor Rev. 11:133–145. [DOI] [PubMed] [Google Scholar]
- Goumans, M.J., G. Valdimarsdottir, S. Itoh, A. Rosendahl, P. Sideras, and P. Ten Dijke. 2002. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 21:1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst, G.R., G. Smale, and D. Pencev. 1989. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J. Cell. Physiol. 140:396–402. [DOI] [PubMed] [Google Scholar]
- Henry, G., W. Li, W. Garner, and D.T. Woodley. 2003. Migration of human keratinocytes in plasma and serum and wound re-epithelialization. Lancet. 361:574–576. [DOI] [PubMed] [Google Scholar]
- Hering, S., F. Isken, J. Janott, C. Jost, A. Pommer, G. Muhr, H. Schatz, and A.F. Pfeiffer. 2001. Analysis of TGFbeta3 gene expression and protein levels in human bone and serum. Exp. Clin. Endocrinol. Diabetes. 109:107–115. [DOI] [PubMed] [Google Scholar]
- Kaartinen, V., J.W. Voncken, C. Shuler, D. Warburton, D. Bu, N. Heisterkamp, and J. Groffen. 1995. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet. 11:415–421. [DOI] [PubMed] [Google Scholar]
- Koch, R.M., N.S. Roche, W.T. Parks, G.S. Ashcroft, J.J. Letterio, and A.B. Roberts. 2000. Incisional wound healing in transforming growth factor-beta1 null mice. Wound Repair Regen. 8:179–191. [DOI] [PubMed] [Google Scholar]
- Kulkarni, A.B., C.G. Huh, D. Becker, A. Geiser, M. Lyght, K.C. Flanders, A.B. Roberts, M.B. Sporn, J.M. Ward, and S. Karlsson. 1993. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA. 90:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., J. Fan, M. Chen, S. Guan, D. Sawcer, G.M. Bokoch, and D.T. Woodley. 2004. a. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol. Biol. Cell. 15:294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., G. Henry, J. Fan, B. Bandyopadhyay, K. Pang, M. Garner, M. Chen, and D.T. Woodley. 2004. b. Signals that initiate, augment, and provide directionality for human keratinocyte motility. J. Invest. Dermatol. 123:622–633. [DOI] [PubMed] [Google Scholar]
- Li, W., J. Fan, M. Chen, and D.T. Woodley. 2004. c. Mechanisms of human skin cell motility. Histol. Histopathol. 19:1311–1324. [DOI] [PubMed] [Google Scholar]
- Li, Y., J. Fan, M. Chen, D.T. Woodley, and W. Li. 2006. Transforming growth factor-alpha in human serum is a major stimulus of human keratinocyte migration. J. Invest. Dermatol. In press. [DOI] [PubMed]
- Martin, P. 1997. Wound healing-aiming for perfect skin regeneration. Science. 276:75–81. [DOI] [PubMed] [Google Scholar]
- Martin, P., D. D'Souza, J. Martin, R. Grose, L. Cooper, R. Maki, and S.R. McKercher. 2003. Wound healing in the PU.1 null mouse–tissue repair is not dependent on inflammatory cells. Curr. Biol. 13:1122–1128. [DOI] [PubMed] [Google Scholar]
- Miyazono, K. 2000. Positive and negative regulation of TGF-beta signaling. J. Cell Sci. 113:1101–1119. [DOI] [PubMed] [Google Scholar]
- Nishimura, N., M. Harada-Shiba, S. Tajima, R. Sugano, T. Yamamura, Q.Z. Qiang, and A. Yamamoto. 1998. Acquisition of secretion of transforming growth factor-beta 1 leads to autonomous suppression of scavenger receptor activity in a monocyte-macrophage cell line, THP-1. J. Biol. Chem. 273:1562–1567. [DOI] [PubMed] [Google Scholar]
- Ohta, M., J.S. Greenberger, P. Anklesaria, A. Bassols, and J. Massagué. 1987. Two forms of transforming growth factor-beta distinguished by multipotential haematopoietic progenitor cells. Nature. 329:539–541. [DOI] [PubMed] [Google Scholar]
- Piek, E., C.H. Heldin, and P. Ten Dijke. 1999. Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J. 13:2105–2124. [PubMed] [Google Scholar]
- Proetzel, G., S.A. Pawlowski, M.V. Wiles, M. Yin, G.P. Boivin, P.N. Howles, J. Ding, M.W. Ferguson, and T. Doetschman. 1995. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat. Genet. 11:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, X.F., D.S. An, I.S. Chen, and D. Baltimore. 2003. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc. Natl. Acad. Sci. USA. 100:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, R.F., J.J. Lah, A. Diehlmann, E.S. Kim, D.B. Hawver, A.I. Levey, K. Beyreuther, and K.C. Flanders. 1999. Differential effects of transforming growth factor-beta(s) and glial cell line-derived neurotrophic factor on gene expression of presenilin-1 in human post-mitotic neurons and astrocytes. Neuroscience. 93:1041–1049. [DOI] [PubMed] [Google Scholar]
- Roberts, A.B. 1998. Molecular and cell biology of TGF-beta. Miner. Electrolyte Metab. 24:111–119. [DOI] [PubMed] [Google Scholar]
- Roberts, A.B., and M.B. Sporn. 1992. Differential expression of the TGF-beta isoforms in embryogenesis suggests specific roles in developing and adult tissues. Mol. Reprod. Dev. 32:91–98. [DOI] [PubMed] [Google Scholar]
- Roberts, A.B., and M.B. Sporn. 1996. Transforming growth factor-beta. In The Molecular and Cellular Biology of Wound Repair. R.A.F. Clark, editor. Plenum Press, New York. 275–308.
- Roberts, A.B., P. Kondaiah, F. Rosa, S. Watanabe, P. Good, D. Danielpour, N.S. Roche, M.L. Rebbert, I.B. Dawid, and M.B. Sporn. 1990. Mesoderm induction in Xenopus laevis distinguishes between the various TGF-beta isoforms. Growth Factors. 3:277–286. [DOI] [PubMed] [Google Scholar]
- Sanford, L.P., I. Ormsby, A.C. Gittenberger-de Groot, H. Sariola, R. Friedman, G.P. Boivin, E.L. Cardell, and T. Doetschman. 1997. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 124:2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret, Y., D.T. Woodley, K. Grigsby, K. Wynn, and E.J. O'Keefe. 1992. Human keratinocyte locomotion: the effect of selected cytokines. J. Invest. Dermatol. 98:12–16. [DOI] [PubMed] [Google Scholar]
- Shah, M., D. Revis, S. Herrick, R. Baillie, S. Thorgeirson, M. Ferguson, and A. Roberts. 1999. Role of elevated plasma transforming growth factor-beta1 levels in wound healing. Am. J. Pathol. 154:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y., and J. Massagué. 2003. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 113:685–700. [DOI] [PubMed] [Google Scholar]
- Shull, M.M., I. Ormsby, A.B. Kier, S. Pawlowski, R.J. Diebold, M. Yin, R. Allen, C. Sidman, G. Proetzel, D. Calvin, et al. 1992. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 359:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, A.J., and R.A. Clark. 1999. Cutaneous wound healing. N. Engl. J. Med. 341:738–746. [DOI] [PubMed] [Google Scholar]
- Sporn, M.B., and A.B. Roberts. 1990. The transforming growth factor-betas: past, present, and future. Ann. NY Acad. Sci. 593:1–6. [DOI] [PubMed] [Google Scholar]
- Tredget, E.E., B. Nedelec, P.G. Scott, and A. Ghahary. 1997. Hypertrophic scars, keloids, and contractures. The cellular and molecular basis for therapy. Surg. Clin. North Am. 77:701–730. [DOI] [PubMed] [Google Scholar]
- Wadman, M. 2005. Scar prevention: the healing touch. Nature. 436:1079–1080. [DOI] [PubMed] [Google Scholar]
- Woodley, D.T., P.M. Bachmann, and E.J. O'Keefe. 1988. Laminin inhibits human keratinocyte migration. J. Cell. Physiol. 136:140–146. [DOI] [PubMed] [Google Scholar]
- Woodley, D.T., D.R. Keene, T. Atha, Y. Huang, K. Lipman, W. Li, and M. Chen. 2004. Injection of recombinant human type VII collagen restores collagen function in dystrophic epidermolysis bullosa. Nat. Med. 10:693–695. [DOI] [PubMed] [Google Scholar]
- Wu, L., A. Siddiqui, D.E. Morris, D.A. Cox, S.I. Roth, and T.A. Mustoe. 1997. Transforming growth factor beta 3 (TGF beta 3) accelerates wound healing without alteration of scar prominence. Histologic and competitive reverse-transcription-polymerase chain reaction studies. Arch. Surg. 132:753–760. [DOI] [PubMed] [Google Scholar]
- Yuan, B., R. Latek, M. Hossbach, T. Tuschl, and F. Lewitter. 2004. siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucleic Acids Res. 32:W130–W134. [DOI] [PMC free article] [PubMed] [Google Scholar]