Abstract
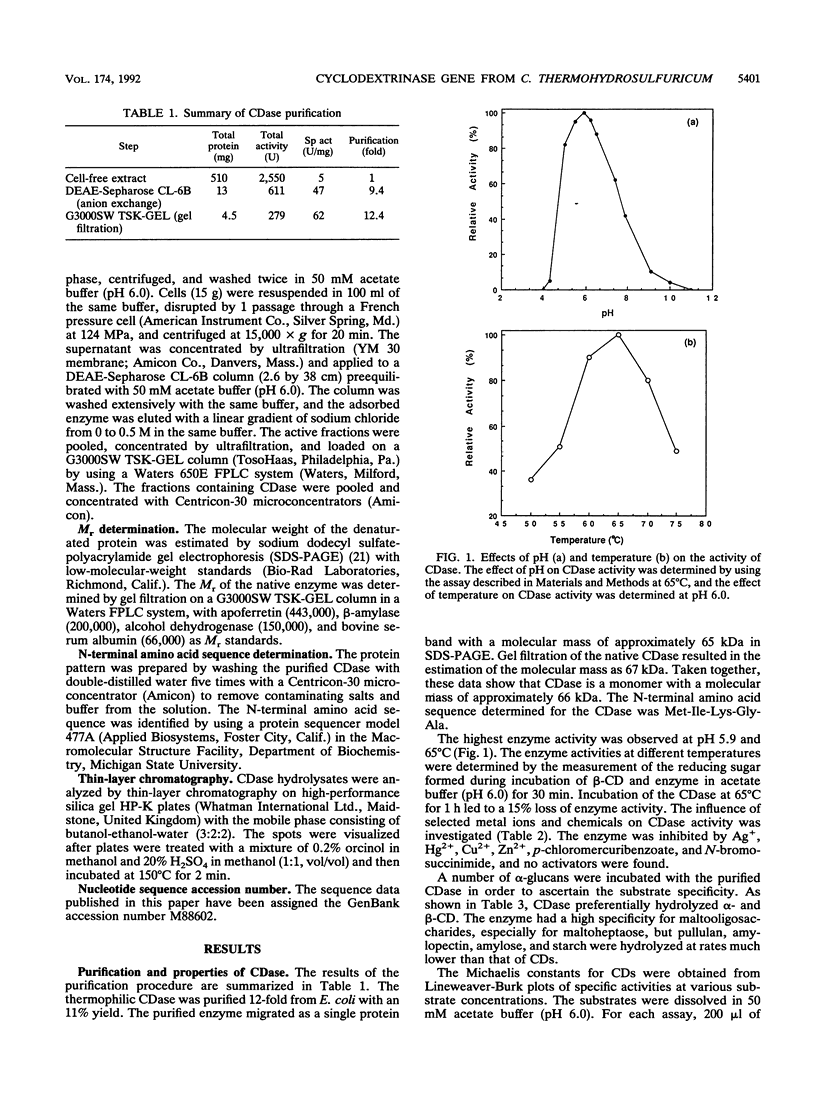
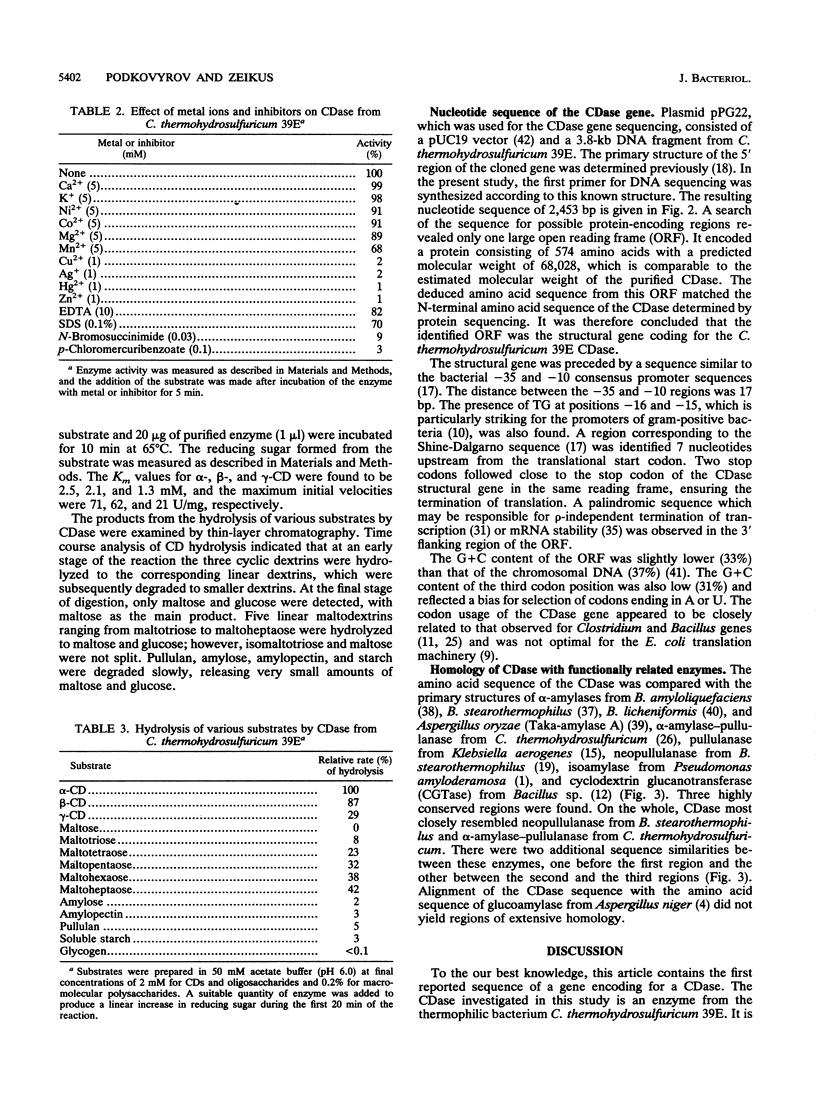
Clostridium thermohydrosulfuricum 39E, a gram-positive thermophilic anaerobic bacterium, produced a cyclodextrin (CD)-degrading enzyme, cyclodextrinase (CDase) (EC 3.2.1.54). The enzyme was purified to homogeneity from Escherichia coli cells carrying a recombinant multicopy plasmid that contained the gene encoding for thermophilic CDase. The purified enzyme was a monomer with an M(r) of 66,000 +/- 2,000. It showed the highest activity at pH 5.9 and 65 degrees C. The enzyme hydrolyzed alpha-, beta-, and gamma-CD and linear maltooligosaccharides to yield maltose and glucose. The Km values for alpha-, beta-, and gamma-CD were 2.5, 2.1, and 1.3 mM, respectively. The rates of hydrolysis for polysaccharides (starch, amylose, amylopectin, and pullulan) were less than 5% of the rate of hydrolysis for alpha-CD. The entire nucleotide sequence of the CDase gene was determined. The deduced amino acid sequence of CDase, consisting of 574 amino acids, showed some similarities with those of various amylolytic enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender H. A bacterial glucoamylase degrading cyclodextrins. Partial purification and properties of the enzyme from a Flavobacterium species. Eur J Biochem. 1981 Apr;115(2):287–291. doi: 10.1111/j.1432-1033.1981.tb05236.x. [DOI] [PubMed] [Google Scholar]
- Boel E., Hansen M. T., Hjort I., Høegh I., Fiil N. P. Two different types of intervening sequences in the glucoamylase gene from Aspergillus niger. EMBO J. 1984 Jul;3(7):1581–1585. doi: 10.1002/j.1460-2075.1984.tb02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buisson G., Duée E., Haser R., Payan F. Three dimensional structure of porcine pancreatic alpha-amylase at 2.9 A resolution. Role of calcium in structure and activity. EMBO J. 1987 Dec 20;6(13):3909–3916. doi: 10.1002/j.1460-2075.1987.tb02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePinto J. A., Campbell L. L. Purification and properties of the cyclodextrinase of Bacillus macerans. Biochemistry. 1968 Jan;7(1):121–125. doi: 10.1021/bi00841a016. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier T., Cole S. T. Characterization of a bacteriocinogenic plasmid from Clostridium perfringens and molecular genetic analysis of the bacteriocin-encoding gene. J Bacteriol. 1986 Dec;168(3):1189–1196. doi: 10.1128/jb.168.3.1189-1196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves M. C., Rabinowitz J. C. In vivo and in vitro transcription of the Clostridium pasteurianum ferredoxin gene. Evidence for "extended" promoter elements in gram-positive organisms. J Biol Chem. 1986 Aug 25;261(24):11409–11415. [PubMed] [Google Scholar]
- Grépinet O., Chebrou M. C., Béguin P. Nucleotide sequence and deletion analysis of the xylanase gene (xynZ) of Clostridium thermocellum. J Bacteriol. 1988 Oct;170(10):4582–4588. doi: 10.1128/jb.170.10.4582-4588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Sugimoto T., Amemura A., Harada T. A Pseudomonas intracellular amylase with high activity on maltodextrins and cyclodextrins. Biochim Biophys Acta. 1975 May 23;391(1):96–108. doi: 10.1016/0005-2744(75)90156-4. [DOI] [PubMed] [Google Scholar]
- Katsuragi N., Takizawa N., Murooka Y. Entire nucleotide sequence of the pullulanase gene of Klebsiella aerogenes W70. J Bacteriol. 1987 May;169(5):2301–2306. doi: 10.1128/jb.169.5.2301-2306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriki T., Imanaka T. Nucleotide sequence of the neopullulanase gene from Bacillus stearothermophilus. J Gen Microbiol. 1989 Jun;135(6):1521–1528. doi: 10.1099/00221287-135-6-1521. [DOI] [PubMed] [Google Scholar]
- Kuriki T., Takata H., Okada S., Imanaka T. Analysis of the active center of Bacillus stearothermophilus neopullulanase. J Bacteriol. 1991 Oct;173(19):6147–6152. doi: 10.1128/jb.173.19.6147-6152.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Kusunoki M., Harada W., Kakudo M. Structure and possible catalytic residues of Taka-amylase A. J Biochem. 1984 Mar;95(3):697–702. doi: 10.1093/oxfordjournals.jbchem.a134659. [DOI] [PubMed] [Google Scholar]
- McConnell D. J., Cantwell B. A., Devine K. M., Forage A. J., Laoide B. M., O'Kane C., Ollington J. F., Sharp P. M. Genetic engineering of extracellular enzyme systems of Bacilli. Ann N Y Acad Sci. 1986;469:1–17. doi: 10.1111/j.1749-6632.1986.tb26480.x. [DOI] [PubMed] [Google Scholar]
- Melasniemi H., Paloheimo M., Hemiö L. Nucleotide sequence of the alpha-amylase-pullulanase gene from Clostridium thermohydrosulfuricum. J Gen Microbiol. 1990 Mar;136(3):447–454. doi: 10.1099/00221287-136-3-447. [DOI] [PubMed] [Google Scholar]
- Moseley M. H., Keay L. Purification and characterization of the amylase of B. subtilis NRRL B3411. Biotechnol Bioeng. 1970 Mar;12(2):251–271. doi: 10.1002/bit.260120207. [DOI] [PubMed] [Google Scholar]
- Nakamura A., Haga K., Ogawa S., Kuwano K., Kimura K., Yamane K. Functional relationships between cyclodextrin glucanotransferase from an alkalophilic Bacillus and alpha-amylases. Site-directed mutagenesis of the conserved two Asp and one Glu residues. FEBS Lett. 1992 Jan 13;296(1):37–40. doi: 10.1016/0014-5793(92)80398-z. [DOI] [PubMed] [Google Scholar]
- Oguma T., Kikuchi M., Mizusawa K. Purification and some properties of cyclodextrin-hydrolyzing enzyme from Bacillus sphaericus. Biochim Biophys Acta. 1990 Oct 12;1036(1):1–5. doi: 10.1016/0304-4165(90)90205-b. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Saha B. C., Mathupala S. P., Zeikus J. G. Purification and characterization of a highly thermostable novel pullulanase from Clostridium thermohydrosulfuricum. Biochem J. 1988 Jun 1;252(2):343–348. doi: 10.1042/bj2520343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B. C., Zeikus J. G. Characterization of thermostable cyclodextrinase from Clostridium thermohydrosulfuricum 39E. Appl Environ Microbiol. 1990 Sep;56(9):2941–2943. doi: 10.1128/aem.56.9.2941-2943.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. J., Ames G. F., Smith N. H., Robinson E. C., Higgins C. F. Repetitive extragenic palindromic sequences: a major component of the bacterial genome. Cell. 1984 Jul;37(3):1015–1026. doi: 10.1016/0092-8674(84)90436-7. [DOI] [PubMed] [Google Scholar]
- Suetsugu N., Koyama S., Takeo K., Kuge T. Kinetic studies on the hydrolyses of alpha-, beta-, and gamma-cyclodextrins by Taka-amylase A. J Biochem. 1974 Jul;76(1):57–63. doi: 10.1093/oxfordjournals.jbchem.a130559. [DOI] [PubMed] [Google Scholar]
- Takkinen K., Pettersson R. F., Kalkkinen N., Palva I., Söderlund H., Käriäinen L. Amino acid sequence of alpha-amylase from Bacillus amyloliquefaciens deduced from the nucleotide sequence of the cloned gene. J Biol Chem. 1983 Jan 25;258(2):1007–1013. [PubMed] [Google Scholar]
- Tsukagoshi N., Furukawa M., Nagaba H., Kirita N., Tsuboi A., Udaka S. Isolation of a cDNA encoding Aspergillus oryzae Taka-amylase A: evidence for multiple related genes. Gene. 1989 Dec 14;84(2):319–327. doi: 10.1016/0378-1119(89)90506-4. [DOI] [PubMed] [Google Scholar]
- Wiegel J., Ljungdahl L. G., Rawson J. R. Isolation from soil and properties of the extreme thermophile Clostridium thermohydrosulfuricum. J Bacteriol. 1979 Sep;139(3):800–810. doi: 10.1128/jb.139.3.800-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zhang H., Scholl R., Browse J., Somerville C. Double stranded DNA sequencing as a choice for DNA sequencing. Nucleic Acids Res. 1988 Feb 11;16(3):1220–1220. doi: 10.1093/nar/16.3.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]



