Abstract
Cytotoxic T lymphocyte (CTL)–induced death triggered by the granule exocytosis pathway involves the perforin-dependent delivery of granzymes to the target cell. Gene targeting has shown that perforin is essential for this process; however, CTL deficient in the key granzymes A and B maintain the ability to kill their targets by granule exocytosis. It is not clear how granzyme AB−/− CTLs kill their targets, although it has been proposed that this occurs through perforin-induced lysis. We found that purified granzyme B or CTLs from wild-type mice induced classic apoptotic cell death. Perforin-induced lysis was far more rapid and involved the formation of large plasma membrane protrusions. Cell death induced by granzyme AB−/− CTLs shared similar kinetics and morphological characteristics to apoptosis but followed a distinct series of molecular events. Therefore, CTLs from granzyme AB−/− mice induce target cell death by a unique mechanism that is distinct from both perforin lysis and apoptosis.
Introduction
Cytotoxic T lymphocytes (CTLs) form a major part of the body's defense against viral infection and cancer by seeking out and killing infected or tumorigenic cells. CTLs kill their targets either by ligation of death receptors on the target cell or by granule exocytosis (for review see Waterhouse et al., 2004). During granule-induced cell death, perforin and granule-specific serine proteases (granzymes) are delivered to the target cell, where they induce cell death by pathways that have yet to be completely defined (Trapani and Smyth, 2002; Waterhouse and Trapani, 2002). Perforin is a key molecule in this process, as CTLs from perforin−/− mice are inefficient in killing target cells in vitro, and perforin−/− mice have a decreased ability to clear virus and diminished tumor surveillance manifested as increased susceptibility to spontaneous B cell lymphoma (Kagi et al., 1994; van den Broek et al., 1996; Lin et al., 1998; Smyth et al., 2000; Street et al., 2002). The primary role attributed to perforin is to ensure the correct trafficking of granzymes into the target cell (Peters et al., 1991; Browne et al., 1999); however, perforin has also been shown to induce direct lysis of the target cell (Ortaldo et al., 1992; Liu et al., 1995; Rochel and Cowan, 1997; Fraser et al., 2000; Wei et al., 2000; San Mateo et al., 2002).
Granzyme A has trypsinlike proteolytic specificity that induces cell death by cleaving a variety of substrates, including lamins, DNase within the SET complex, and PHAPII (for review see Lieberman and Fan, 2003). Granzyme B induces apoptotic cell death by cleaving the proapoptotic Bcl-2 family member Bid (Sutton et al., 2000; Pinkoski et al., 2001; Waterhouse et al., 2005) and the regulatory prodomain of caspase-3 (Martin et al., 1996; Sutton et al., 2003). Although somewhat less efficient than CTLs isolated from wild-type mice, CTLs from mice deficient in granzymes A and B (granzyme AB−/−) maintain their ability to kill many target cells by granule exocytosis (Simon et al., 1997; Mullbacher et al., 1999; Davis et al., 2001; Smyth et al., 2003), and, in contrast to perforin−/− mice, granzyme AB−/− mice develop normally, do not develop spontaneous malignancy, and clear many viruses normally. Granzymes C, K, and M have also been shown to induce cell death when delivered by perforin in vitro (MacDonald et al., 1999; Johnson et al., 2003; Kelly et al., 2004). Therefore, death induced by CTLs from granzyme AB−/− mice may be caused by perforin-mediated delivery of other death-inducing granzymes. CTLs of mice deficient in granzyme B have been reported to have reduced expression of granzyme C as a result of inadvertent silencing of its gene (Pham et al., 1996), and granzyme M is preferentially expressed by NK cells (Krenacs et al., 2003). Consistent with these observations, it has understandably been proposed that cell death induced by granzyme AB−/− CTLs occurs through direct perforin lysis (Simon et al., 1997).
The question of how CTLs kill their target cells in the absence of granzymes A and B is likely to be physiologically important in light of recent findings that individual activated lymphocytes within a population may express different combinations of perforin and granzymes or even no granzymes at all (Kelso et al., 2002). Indeed, in this study, up to 10% of perforin-positive lymphocytes did not express either granzyme A or B at day 3 after IL-2 activation. In addition, it has been proposed that the mechanism of target cell death (apoptosis or lysis) can subsequently influence the quality and quantity of the inflammatory response by modulating antigen processing and presentation (for review see Pinkoski et al., 2006).
The mechanism of death induced by granzyme AB−/− CTLs has been studied at a population level (Pardo et al., 2002, 2004); however, using these assays, it has not been possible to distinguish apoptosis from necrosis, lysis, or any alternate death mechanisms. Analysis of morphology remains one of the most decisive ways by which to distinguish the various forms of cell death. Time-lapse microscopy has previously been used to investigate the subcellular organization of polarized CTLs (Ludford-Menting et al., 2005) and to measure the kinetics of cell death induced by cytotoxic drugs (Goldstein et al., 2000a,b). Therefore, we have used time-lapse microscopy to study in real time the morphological and molecular parameters of single cells undergoing granule-induced death after attack by alloreactive CTLs. We found that wild-type CTLs induced apoptosis, but the absence of granzyme A and B resulted in a modified form of cell death that was distinct from both apoptosis or perforin-mediated lysis. These data show that death induced by CTLs from granzyme AB−/− mice cannot simply be attributed to perforin lysis; rather, death is likely to be initiated by other granule components. Furthermore, the unexpected resistance of the granzyme AB−/− animals to a variety of viral pathogens cannot simply be attributed to perforin.
Results
Purified granzyme B induces apoptosis, and purified perforin induces lysis
To characterize cell death induced by CTLs by time-lapse microscopy, it was first necessary to characterize cell death induced by purified perforin alone or perforin together with granzyme B. During apoptosis induced by cytotoxic drugs, cells initially undergo a period of intense ruffling of the plasma membrane known as blebbing, and phosphatidylserine (PS) becomes externalized from the inner leaflet of the plasma membrane to the outer leaflet (Martin et al., 1995). Such a cell would normally be rapidly phagocytosed in vivo; however, under typical cell culture conditions, the integrity of the plasma membrane is eventually lost, resulting in the rupture of the cell. PS externalization can be detected by binding of annexin V, and the loss of plasma membrane integrity can later be detected by the passive uptake of propidium iodide (PI; Goldstein et al., 2000a,b). This process is in contrast to necrosis, which involves rapid cell swelling and rupture of the plasma membrane in the absence of rounding and blebbing and results in simultaneous binding of annexin V and PI uptake. Cells undergoing early apoptosis stain with annexin V but exclude PI (annexin V+PI−), whereas necrotic cells or cells that have undergone late apoptotic changes stain with annexin V and PI.
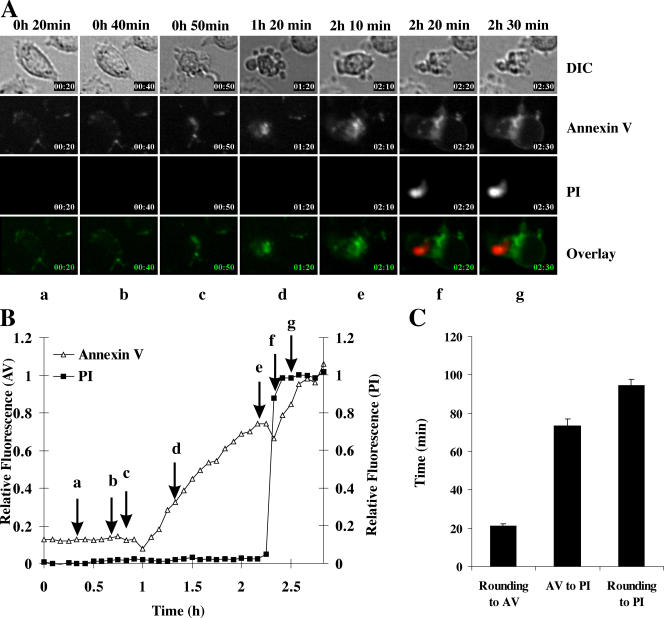
Granzyme B has been shown to induce apoptosis in a perforin-dependent manner (Shi et al., 1992), and purified perforin is believed to induce cell lysis (Podack and Konigsberg, 1984; Young et al., 1986). However, little is known about the kinetics and morphological features of death induced by these proteins. To investigate the kinetics of granzyme B–induced cell death, we used real-time imaging to follow annexin V binding and PI uptake in MS9II cells treated with 10 nM perforin (a concentration that does not cause cell death when applied to cells alone) and 25 nM granzyme B. Under these conditions, the majority of cells (∼70%) died within 4 h, and all showed typical signs of apoptosis (example provided in Fig. 1 A). Individual cells showed the initial morphological features of apoptosis (rounding/blebbing) in an asynchronous manner between 0.5 and 2 h and invariably followed a distinct sequence of events: (1) rounding and blebbing (seen at 50 min in Fig. 1 A, c); (2) PS exposure as detected by annexin V binding (at 1 h 20 min; Fig. 1 A, d); and (3) plasma membrane rupture as detected by PI uptake (2 h 20 min; Fig. 1 A, f). By calculating the relative intensity of each fluorochrome as a function of time, we determined when individual cells became positive for annexin V staining and permeable to PI (Fig. 1 B). In the example shown, the individual frames shown in Fig. 1 A correspond to the arrows (a–g) shown in Fig. 1 B. By studying multiple target cells in the population, we determined that the mean time from cell rounding/blebbing until it became annexin V positive was ∼20 min, and the mean duration from annexin V binding to the loss of plasma membrane integrity (PI staining) was ∼70 min (Fig. 1 C). Therefore, the mean total time for cell death, defined as commencing with the onset of morphological changes (rounding/blebbing) and ending with PI staining, was ∼90 min. Inevitably, this estimate did not include the time taken to achieve Bid cleavage, cytochrome c release, or caspase activation, as these parameters are known to occur extremely rapidly and precede rounding (Sutton et al., 2000; Pinkoski et al., 2001).
Figure 1.
Time-lapse microscopy of granzyme B–induced cell death. MS9II cells were washed in HBSS and treated with 10 nM perforin and 25 nM granzyme B. (A) Images of morphology, annexin V, and PI were obtained every 5 min. The cell maintains a normal morphology until 40 min (b), is rounded up, and blebbing occurs by 50 min (c). Increased annexin V staining is clearly evident by 1 h 20 min (d). The plasma membrane integrity was maintained until 2 h 10 min (e) but was lost by 2 h 20 min (f). By f, the annexin V staining is seen around large cellular protrusions (airbags) that define the swollen plasma membrane. The full video is presented in Video 1 (available at http://www.jcb.org/cgi/content/full/jcb.200510072/DC1). DIC, differential interference contrast. (B) Fluorescence of annexin V binding and PI relative to maximum fluorescence is presented for the cell depicted in A. The times at which the frames in A were captured are indicated by arrows. (C) The duration from rounding to annexin V binding and annexin V binding to PI uptake was calculated for individual cells and is presented as means ± SEM (error bars; n = 25). AV, annexin V.
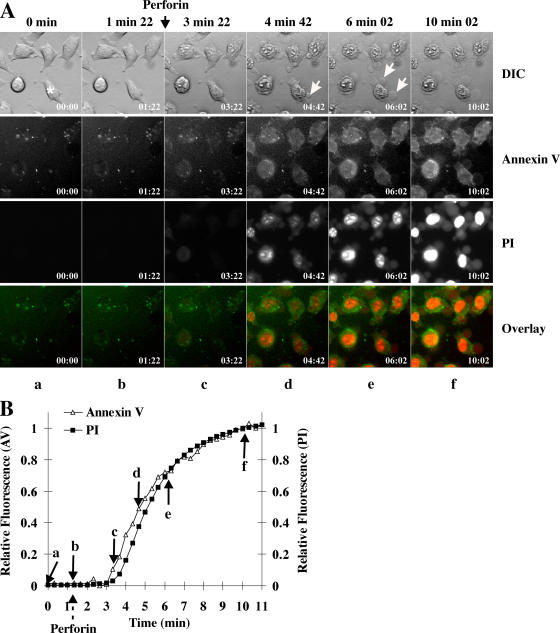
We also used the same methodology to investigate the characteristics of perforin-induced cell lysis by assaying annexin V binding and PI uptake in cells treated with sufficient purified perforin to induce direct lysis (80 nM) in the absence of granzyme B (Fig. 2). After perforin addition, the plasma membrane developed protrusions that rapidly expanded, and the cells simultaneously became permeable to PI and stained positive with annexin V (Fig. 2 A). Staining with each fluorochrome typically commenced 2–3 min after perforin addition and reached maximal levels by 10 min (Fig. 2 B). Interestingly, when the concentration of perforin was progressively reduced so that only 10–50% of the cells died, the changes in cell morphology and staining did not vary significantly (not depicted). In particular, the apoptotic changes seen in the presence of granzyme B were never observed; independent of the dosage used, purified perforin induced only cell lysis.
Figure 2.
Time-lapse microscopy of perforin-induced death in MS9II cells. MS9II cells were followed by time-lapse microscopy. (A) Images of morphology, annexin V, and PI were taken every 20 s. 80 nM perforin was added after the fifth frame (1 min 22 s). Plasma membrane protrusions are indicated by white arrows. Black arrow indicates the time of perforin addition. Asterisk indicates the cell analyzed in B. The full video is presented in Video 2 (available at http://www.jcb.org/cgi/content/full/jcb.200510072/DC1). (B) The fluorescence of annexin V and PI in the cell indicated by an asterisk in A was plotted as relative fluorescence compared with maximum fluorescence for that fluorochrome. Perforin addition is noted by the dashed arrow below the x axis. Arrows indicate the points at which the frames in A were recorded.
Intact wild-type CTLs induce apoptosis with similar kinetics to granzyme B
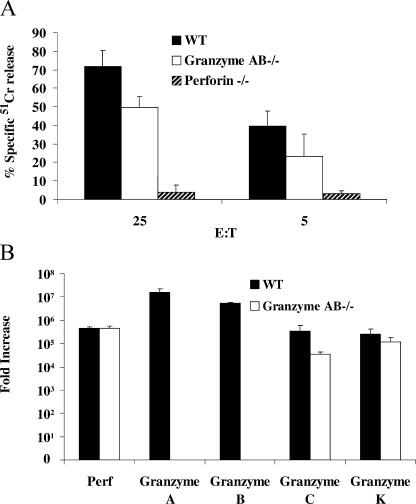
Using the aforementioned findings, we next turned to the question of how intact CTLs induce target cell death in the presence or absence of granzymes A and the B cluster (granzyme AB−/−). We raised alloreactive CD8+ mouse CTLs (H-2b anti–H-2k) that killed MS9II fibroblasts (H-2k) exclusively in a perforin-dependent manner (Fig. 3). CTLs from wild-type mice efficiently killed MS9II in a 4-h assay as indicated by release of preloaded 51Cr (Fig. 3 A). The CTLs of granzyme AB−/− mice were somewhat less potent than wild-type CTLs over the 4-h time frame (Fig. 3 A), but these CTLs maintained significant killing ability. CTLs derived from perforin−/− mice did not induce significant cell death (Fig. 3 A), and cytotoxicity was completely blocked by chelating calcium with EGTA (not depicted), which is consistent with a perforin-dependent cell death mechanism. Furthermore, the CTLs of gld mice that lack functional FasL were as efficient as wild type (not depicted), confirming that cell death in this assay was completely dependent on the granule pathway. Using RT-PCR, we showed that CTLs from granzyme AB−/− mice expressed comparable levels of perforin and granzyme K mRNA to CTLs isolated from wild-type mice but, as expected, were completely deficient in message for both granzymes A and B (Fig. 3 B). Consistent with a previous study (Pham et al., 1996), CTLs from granzyme AB−/− mice expressed mRNA for granzyme C; however, this was ∼10-fold less than that of wild-type mice. Therefore, the CTLs of granzyme AB−/− mice were capable of synthesizing both granzyme C and K as well as perforin.
Figure 3.
CTL-induced death of MS9II cells. Allogenic CTLs (H-2b anti–H-2k) were generated in a one-way mixed lymphocyte reaction from wild-type (WT), granzyme AB−/−, and perforin−/− mice. (A) MS9II cells preloaded with 100 μCi 51Cr were treated with CTLs from wild-type, granzyme AB−/−, and perforin−/− mice at the effector to target ratios indicated. The percentage of 51Cr released was assayed at 4 h. The data points shown are the means ± SD of data pooled from three independent assays each prepared in triplicate. (B) Real-time PCR for perforin and granzyme A, B, C, and K was performed on cDNA transcribed from mRNA isolated from WT and granzyme AB−/− mice. Each cDNA sample was assayed in duplicate. Data presented is the mean fold increase in message (calculated from the means of duplicate samples) relative to the level of granzyme C in naive T cells from wild-type mice. Data represents message amplified from CTLs of three mice tested independently in each case ± SD (error bars).
To characterize the morphology of death, we coincubated CTLs from wild-type mice with MS9II cells at effector/target ratios (2:1) that easily permitted us to identify CTLs (motile and small) from MS9II cells (large, flat, and adherent). We then followed the death by time-lapse microscopy (example presented in Fig. 4 A). We found that MS9II cells underwent changes that were virtually identical to those of cells killed by purified perforin and granzyme B. After conjugation, the target cells rapidly rounded and detached from the culture dish. The plasma membrane then became ruffled (blebbed), and, after a further period of time, the cell began to swell. A clear increase in annexin V binding was observed during the blebbing phase of death, but PI uptake was only observed when the cell had become swollen. The sequence of events (rounding, blebbing, annexin V binding, and PI uptake) was invariant for all of the cells examined. Using the same methodology described in Time-lapse microscopy to determine the relative fluorescence intensity of annexin V and PI, we could determine the specific timeline of events for MS9II cells killed by wild-type CTLs. For example, in the cell depicted in Fig. 4 (A and B), conjugation of the CTL was observed at 20 min, cell rounding was observed by 30 min, and annexin V binding was detectable by ∼1 h. The cell remained round and the plasma membrane remained intact until 1 h 45 min, after which the cell swelled and its outline became less distinct. PI uptake was detected at 1 h 50 min, which is coincident with cell swelling (Fig. 4, A and B). Pooled analysis of single cells (n = 33) revealed that the interval between rounding and annexin V binding was ∼30 min, whereas the duration between annexin V binding and PI uptake was ∼55 min (Fig. 4 C). Thus, the overall time of death (rounding to PI) was ∼90 min.
Figure 4.
Time-lapse microscopy of death induced by wild-type CTLs. CTLs isolated from wild-type mice were incubated with MS9II cells. (A) Images of morphology, annexin V binding, and PI uptake were taken sequentially every 5 min. At 20 min (a), the CTL (indicated by #) forms a synapse with the target (indicated by asterisks). By 30 min (b), the target cell is blebbing and rounding, and, by 55 min (d), the target cell is still blebbing but remains annexin V negative. By 1 h 10 min (e), the cell becomes annexin V positive but maintains plasma membrane integrity until 1 h 45 min (f). Plasma membrane integrity is lost, and the cell becomes PI positive by 1 h 50 min. The full video is presented in Video 3 (available at http://www.jcb.org/cgi/content/full/jcb.200510072/DC1. Arrow indicates the swollen plasma membrane. DIC, differential interference contrast. (B) Fluorescence of annexin V binding and PI relative to maximum fluorescence is presented for the cell depicted by the asterisk in A. The point at which frames (a–g) were recorded in A are shown on the time line by arrows. (C) The duration from rounding to annexin V binding and annexin V binding to PI uptake was calculated for individual cells and is presented as means ± SEM (error bars; n = 33).
Granzyme AB−/− CTL-induced cell death is distinct from classic apoptosis and perforin lysis
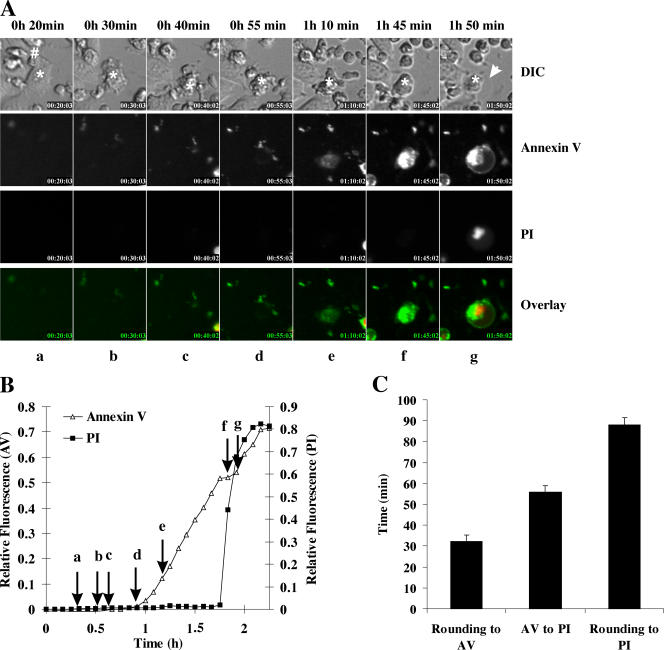
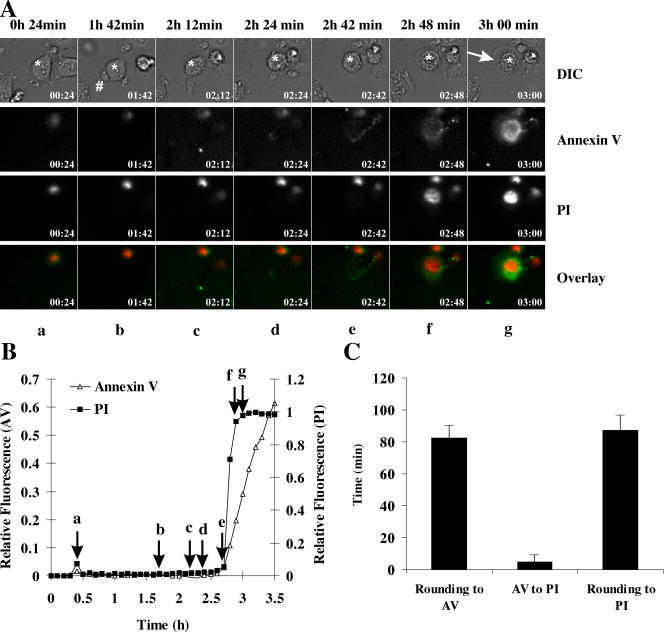
Similar to death induced by wild-type effector cells, CTLs from granzyme AB−/− mice induced rapid rounding and blebbing of the MS9II target cells followed eventually by plasma membrane swelling and rupture (example presented in Fig. 5 A). In contrast to death induced by wild-type CTLs, the onset of annexin V staining and PI uptake were virtually simultaneous during death induced by granzyme AB−/− CTLs (Fig. 5 B). In the example presented in Fig. 5 A, interaction between the CTL and target cell was first observed at 1 h 42 min; however, annexin V binding and PI uptake were not observed until 2 h 48 min (Fig. 5 B). When several target cells (n = 19) were analyzed in the same way, the overall time from cell rounding to annexin V binding was ∼90 min, but, in contrast to wild-type CTLs, the duration from annexin V binding to PI uptake was <5 min.
Figure 5.
Death induced by CTLs from granzyme AB−/− mice. CTLs isolated from granzyme AB−/− mice were incubated with MS9II cells. (A) Images of morphology, annexin V binding, and PI uptake were taken sequentially every 6 min. Frame a (24 min) shows the unconjugated target cell (indicated by an asterisk). By 1 h 42 min (b), the CTL (indicated by #) formed a synapse with the target (indicated by asterisks). By 2 h 12 min, (c) the target cell was blebbing and rounding, and, by 2 h 24 min (d), the target cell is fully round and still blebbing but remains annexin V and PI negative until 2 h 42 min (e). By 2 h 48 min (f), annexin V staining and PI uptake is evident. The cell continues swelling until 3 h (g). Arrow indicates the swollen plasma membrane. A montage of morphology images for each frame is presented in Fig. S1, and a full time-lapse video is presented in Video 4 (available at http://www.jcb.org/cgi/content/full/jcb.200510072/DC1). DIC, differential interference contrast. (B) Fluorescence of annexin V binding and PI relative to maximum fluorescence is presented for the cell depicted by the asterisk in A. The point at which frames (a–g) were recorded in A are shown on the time line by arrows. (C) The duration from rounding to annexin V binding and annexin V binding to PI uptake was calculated for individual cells and is presented as means ± SEM (error bars; n = 19).
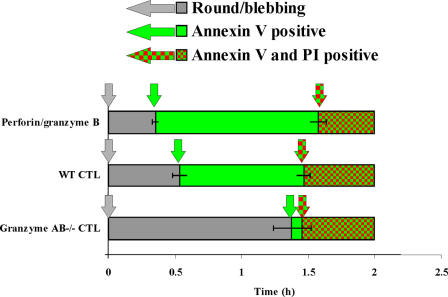
Using cell rounding as a common reference point, it was possible to compare the time course of CTL-induced cell death with apoptosis induced by granzyme B that was delivered by sublytic concentrations of purified perforin (Fig. 6). The execution phase (duration from rounding to PI uptake) of CTL-induced death (87.9 ± 3.6 min for wild-type CTLs and 87.4 ± 3.2 min for granzyme AB−/− CTLs) was very similar to that of purified perforin/granzyme B (94.6 ± 3.2 min). Therefore, in our model system, we found that regardless of whether death was induced by purified granzyme B/perforin, wild-type CTLs, or granzyme-deficient CTLs, the duration of death was ∼1.5 h in each case. During death induced by wild-type CTLs or granzyme B/perforin, the target cells became annexin V positive ∼30 min after rounding, and the cells remained annexin V positive but excluded PI for the following ∼60 min. During death induced by granzyme AB−/− CTLs, annexin V binding was not evident until ∼90 min and was almost concomitant with PI uptake. The major discrepancy between wild-type CTLs and granzyme AB−/− CTLs was that annexin positivity was markedly delayed during death induced by granzyme AB−/− CTLs so that the time during which the cells were annexin V+PI− was reduced virtually to zero. Because not all CTLs that came in contact with targets killed that target and some targets were contacted by numerous CTLs (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200510072/DC1), it was not possible to get an accurate measure of the initiation phase of apoptosis (i.e., duration from conjugation to rounding).
Figure 6.
Comparison of the timing of molecular events during granzyme B and CTL-induced cell death. Data from Figs. 1 C, 4 C, and 5 C were collated and presented as a function of time. T = 0 indicates time when rounding/blebbing started. Gray bars indicate times when cells were round and blebbing but neither annexin V nor PI positive. Green bars indicate times when the cells were round/blebbing and annexin V positive but PI negative. Red and green-checkered bars indicate when cells were PI positive. Arrows of the corresponding color indicate the time at which each event started. Error bars represent SEM.
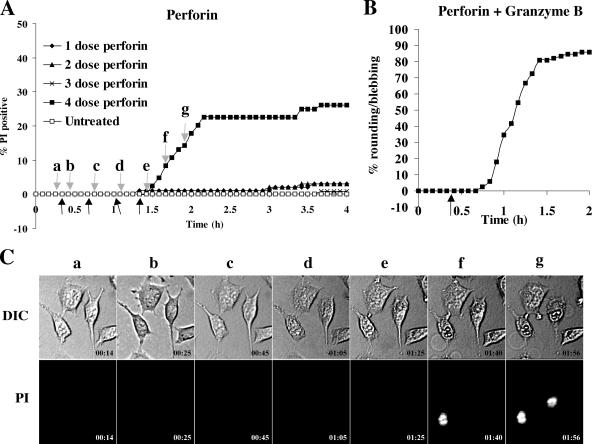
Although overall the morphology of cell death in response to granzyme AB−/− CTLs was strikingly similar to apoptosis, simultaneous staining of annexin V and PI was reminiscent of perforin lysis. Therefore, we considered that individual target cells might be attacked, in turn, by several CTLs (Fig. S2) delivering consecutive sublytic doses of perforin so that their cumulative effects would eventually kill the cell. To model this, we added multiple sublytic doses (5 nM) of perforin at 20-min intervals (Fig. 7). We found that at this concentration, a single addition of perforin induced death of <5% of cells after 4 h (Fig. 7 A). However, the same concentration plus granzyme B induced apoptosis in >80% of cells (Fig. 7 B). Two or three sequential doses of perforin every 20 min did not result in an increase in cell death, but four sequential doses induced death in ∼30% of cells. As three sequential doses of perforin did not induce more death than one single dose, the death observed after four doses represented a cumulative effect similar to what may occur if perforin was delivered by several CTLs. In contrast to death induced by granzyme AB−/− CTLs, the sequential addition of sublytic perforin did not result in rounding and blebbing (Fig. 7 C). Rather, this death involved formation of the very large membrane protrusions similar to that induced by single lytic doses of perforin (Fig. 2). These data indicated that death induced by granzyme AB−/− CTLs does not reflect multiple hits of sublytic perforin and more likely reflects the action of granule proteins delivered by perforin.
Figure 7.
Sequential addition of perforin induces cell lysis. MS9II cells were treated with 5 nM perforin between one (1 dose) and four times (4 dose) sequentially every 20 min (A) or with 5 nM perforin and 25 nM granzyme B (B). Black arrows indicate the times at which perforin was added. Gray arrows (a–g) in A indicate the times at which the images in C were taken. (C) Images of morphology or PI fluorescence were taken every 5 min. DIC, differential interference contrast.
Discussion
Much of our current knowledge of granule-mediated cell death by intact CTLs is derived from population-based studies that cannot easily distinguish between various death mechanisms (Sutton et al., 1997; Trapani et al., 1998; Davis et al., 2001). Therefore, relatively little is known about the nature of cell death induced by intact CTLs at a single cell level. Furthermore, the ability of CTLs from mice deficient in key granzymes A and B to kill target cells through the granule pathway has been documented in several experimental systems (Simon et al., 1997; Davis et al., 2001; Pardo et al., 2002, 2004; Smyth et al., 2003). However, it has not been possible to determine how the target cells were killed. It has recently been shown that individual CTLs in wild-type mice may contain perforin but neither granzyme A or B (Kelso et al., 2002). The killing observed by the granzyme AB−/− CTLs may, therefore, be caused by a specific subset of cells that are commonly present in populations of CTLs isolated from wild-type mice. As the nature of death (apoptosis, necrosis, lysis, or another alternate programmed cell death) can influence how the dying cell is presented to the immune system (Henson et al., 2001), the mode of death induced by granzyme AB−/− CTLs is likely to play a bona fide role in the immune response of wild-type animals to pathogens or tumor cells.
It has been proposed that the default pathway adopted by CTLs in the absence of most of the proapoptotic granzymes is perforin-induced lysis (Simon et al., 1997). In contrast, granzymes C, K, and M have been shown to induce the death of target cells when delivered by perforin. Therefore, death induced in the absence of granzymes A and B may be caused by various other death-inducing granzymes. We found that death induced by lytic concentrations of perforin was virtually immediate (plasma membrane rupture within 2–3 min and complete by ∼10 min) and involved the progressive swelling of plasma membrane protrusions followed within a few minutes by the loss of plasma membrane integrity. Cell rounding and detachment from the culture flask were notably absent. This is consistent with a recent study that also showed that perforin induced large “airbaglike” protrusions of the plasma membrane followed by plasma membrane rupture (Keefe et al., 2005). These protrusions were reported to be reversible when perforin was delivered at sublytic concentrations, probably as a result of plasma membrane repair by lysosomes (Keefe et al., 2005). In our model, we never observed large plasma membrane protrusions when we used sublytic concentrations of perforin alone or in combination with granzyme B. This may reflect cell type differences or may suggest that the formation of plasma membrane protrusions is not required for granzyme uptake. In contrast to perforin lysis, MS9II cells killed by granzyme B added with sublytic concentrations of perforin or by wild-type or granzyme AB−/− CTLs underwent more gradual death (taking ∼90 min), which was characterized by rapid rounding followed by a discrete period of plasma membrane blebbing before the eventual loss of plasma membrane integrity. Our thorough kinetic analysis of cell death at a single cell level has revealed that the morphology of death induced by either wild-type CTLs or CTLs from granzyme AB−/− mice was quite clearly distinct from cell lysis induced by purified perforin.
The rapidity of cell death induced by CTLs or perforin/granzyme B probably reflects the essential need to kill virus-infected cells as efficiently as possible to limit viral replication and spread. But strikingly, although the duration of target cell death induced by intact CTLs was ∼1.5 h in the presence or absence of granzyme A and the B cluster, the timing of specific molecular events differed somewhat. During apoptosis induced by granzyme B/perforin or the CTLs of wild-type mice, PS exposure occurred shortly after the target cell rounded up, and the cell maintained plasma membrane integrity over the following 45–60 min. In contrast, PS exposure was not evident shortly after rounding during death induced by granzyme AB−/− CTLs, and binding of annexin V to PS was only evident virtually at the same time as it lost plasma membrane integrity. Under our experimental conditions, it is not clear whether PS ever becomes exposed on the outer leaflet of the plasma membrane or whether the dying cell becomes permeable to annexin V. Regardless of the nature of annexin V binding, these data indicate that granzyme B and wild-type CTLs induce classic apoptosis, whereas granzyme AB−/− mice induce a novel form of programmed cell death that shares similar features with but is distinct from apoptosis. These findings are consistent with our previous study that showed that caspase-dependent DNA fragmentation, a classic hallmark of apoptotic cell death, was detected in cells killed by wild-type CTLs but was not detected in cells killed by granzyme AB−/− CTLs (Davis et al., 2001).
The physiological consequences of killing a target cell by apoptosis or by nonapoptotic cell death are unclear. It has been proposed that cells undergoing apoptosis are packaged and cleared by phagocytosis before plasma membrane rupture, avoiding the leakage of potentially toxic cytoplasmic material into the cellular milieu and reducing bystander cell damage. It has also been suggested that apoptotic cells may be presented to antigen-presenting cells in a different way than necrotic cells and that this may modulate the resultant immune response (for review see Henson et al., 2001). PS exposure is an important event in the phagocytosis of apoptotic cells, as macrophages deficient in PS receptor bind but do not ingest dying cells. As cells killed by granzyme AB−/− CTLs do not expose PS before plasma membrane rupture, it is possible that these cells would not be efficiently engulfed by macrophages and, thus, may be perceived by the immune system as having undergone necrosis. If the target cell under attack contains potentially viable virions, the outcome for the host might differ significantly. First, if viral particles were released extracellularly, the opportunity for spread to neighboring cells or for systemic infection is heightened. Second, the efficiency of viral uptake into antigen-presenting cells might well influence subsequent antigen presentation, chemotaxis, and the quality of the local inflammatory reaction. On the basis of our findings, we can speculate that one reason a virus might express a specific inhibitor of intrinsic apoptosis (e.g., IAPs) or granzyme B (e.g., the L4 100-kD protein of adenovirus) might be to influence the mechanism of cell death, thereby reducing the likelihood of phagocytosis and encouraging viral spread rather than significantly prolonging the life of the infected cell. It must be noted that it has not been determined whether cells killed by granzyme AB−/− CTLs can be recognized and cleared by phagocytic cells through a PS-independent mechanism.
It is intriguing to speculate on the molecular mechanisms responsible for the cell death induced by granzyme AB−/− CTLs. Several granzymes have been identified in mice in addition to granzymes A and B, including C, D, E, F, G, J, K, M, and N, of which granzyme C, K, and M have been shown to induce cell death when delivered by perforin (Trapani and Smyth, 1993; MacDonald et al., 1999; Johnson et al., 2003; Kelly et al., 2004). Granzyme M expression is restricted to natural killer cells (Trapani and Smyth, 1993; Sedelies et al., 2004). As our data is generated from CD8+ T cells, granzyme M is unlikely to be responsible for the death in our model. Granzyme AB−/− mice were generated by crossing granzyme A−/− mice with granzyme B−/− mice (Simon et al., 1997). As disrupting the granzyme B gene also resulted in the reduction in expression of multiple genes, including granzymes C, D, E, F, and G (Pham et al., 1996), we considered the possibility that granzyme C might be excluded as a cause of cell death. However, we found significant levels of granzyme C mRNA expressed in our alloreactive CTLs (10,000 times higher than that detected in naive T cells from wild-type mice). So, although granzyme C expression may be reduced in granzyme AB−/− mice, it cannot be excluded as causing the death of MS9II target cells in our model. We further showed that message for granzyme K is expressed at comparable levels in CTLs from granzyme AB−/− and wild-type mice. Therefore, the death induced by CTLs from granzyme AB−/− mice may be caused by either granzyme C, K, or other granule proteins for which a death-inducing ability has not been described.
The distinct specificity, alternate regulation, cell-specific expression profile, and species-to-species variation of granzymes suggest that each of the granzymes may play a very specific role in an immune response while collaborating to thwart most types of viral infection or cells undergoing oncogenic transformation. Understanding the mechanism by which granzyme AB−/− CTLs kill their targets will be important in developing our knowledge of how CTL-induced target cell killing helps prevent infection and may unmask the key granule constituents involved in maintaining the relatively healthy immune status of granzyme AB−/− mice compared with perforin-deficient mice.
Materials and methods
Materials
51Cr (as sodium dichromate) was purchased from GE Healthcare. Annexin V–FLUOS was obtained from Roche. Cell culture reagents were purchased from Invitrogen, and PI and all other chemicals were obtained from Sigma-Aldrich. Mouse perforin expressed in baculovirus-infected insect cells was purified essentially according to Liu et al. (1994). Human granzyme B was expressed and purified from Pichia pastoris as described previously (Sun et al., 1999).
Cell culture
The adherent mouse fibroblast cell line MS9II (derived from the C3H strain H-2k) was obtained from W. Sly (Washington University, St. Louis, MO) and cultured at 37°C in a humidified CO2 incubator in RPMI medium supplemented with 2 mM glutamine, 10% FBS, and 1 mM sodium pyruvate.
Isolation and culture of allogenic CTLs
C3H/J (H-2k) mice and C57BL/6 (H-2b) mice were maintained in specific pathogen-free conditions at The Walter and Eliza Hall Institute (Melbourne, Australia). Mice deficient in granzyme A and the granzyme B cluster (granzyme AB−/−; Simon et al., 1997; Mullbacher et al., 1999; Davis et al., 2001; Pardo et al., 2004) were obtained from M. Simon (Max-Planck-Institut für Immunbiologie, Freiburg, Germany; Simon et al., 1997) and maintained at the Peter MacCallum Cancer Centre (Melbourne, Australia). The mice were generated by backcrossing granzyme B cluster–deficient mice (generated on strain 129 embryonic stem cells; H-2b; Ebnet et al., 1995) for six generations with C57BL/6 mice and for a further two generations with granzyme A–deficient mice (generated from C57BL/6 embryonic stem cells; Heusel et al., 1994). Thus, the granzyme AB−/− mice were backcrossed a total of eight generations to C57BL/6.
Allogeneic CTLs were generated in a one-way mixed lymphocyte reaction in which splenocytes isolated from C57BL/6 or granzyme AB−/− mice were cocultured for 7 d in RPMI containing 10% FBS, 2 mM glutamine, 50 μM β-mercaptoethanol, 100 μM nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodiun pyruvate, and 50 U/ml rIL-2 with a similar number of lethally irradiated stimulator splenocytes from C3H/J mice (H-2k). In some experiments, the activated lymphocytes were restimulated with C3H/J splenocytes and cultured for a further 3–4 d. Phenotypic analysis by FACS demonstrated that the responder cells were >90% CD3+CD8+ T cells. The activation markers CD25, CD44, and CD69 did not vary significantly between the responder cell populations.
51Cr release assays for cytotoxicity
106 target cells were incubated with 75 μCi 51Cr in 100 μl of culture medium for 1 h at 37°C. Cells were washed three times in culture medium to remove the unincorporated 51Cr and resuspended at 2 × 105 cells/ml. CD8+ CTLs were incubated with the target cells at the ratios indicated, and the supernatant was harvested using a Skatron supernatant collection system (Molecular Devices). 51Cr released into the supernatant was detected using an automatic γ counter (Wallac Wizard 1470; PerkinElmer). In each case, the spontaneous release of radiolabel over the time of the assay was no higher than 10% of the total incorporated radioactivity. The percentage of 51Cr release was calculated by the following equation: [(cpm of 51Cr released from sample − cpm of 51Cr released from untreated cells/cpm of 51Cr released from cells treated with 1 M HCl − cpm released from untreated cells) × 100].
Time-lapse microscopy
MS9II cells were plated in 96-well culture plates and incubated overnight at 37°C in a humidified CO2 incubator. The plates were transferred to a temperature-controlled stage (Prior Proscan; GT Vision) maintained at 37°C on a microscope (IX-81; Olympus). PI was added to the cultures at 50 ng/ml, and annexin V–FLUOS was added at 2 μg/ml. Cells were exposed to an equal number of activated CD8+ CTLs and viewed using a 20× LCplanFL NA 0.40 lens (Olympus) for the times indicated. Images were captured at specified intervals using a CCD camera (model ORCA-ER; Hamamatsu) controlled by MetaMorph software (Universal Imaging Corp.). As the fluorescence intensities of annexin V–FLUOS and PI were significantly different, we used MetaMorph and Excel software (Microsoft) to plot the fluorescence reading for each frame relative to the maximum for that fluorochrome over the course of the experiment after subtraction of the background fluorescence reading from each. The maximum fluorescence plotted for each fluorophore was 1.0, and baseline fluorescence was zero.
RNA extraction, cDNA synthesis, and real-time PCR analysis
Splenic lymphocytes from a 7-d mixed lymphocyte reaction (>95% CD8+ T cells) were pelleted and resuspended in 1 ml TRIzol reagent (Invitrogen). RNA was extracted with 200 μl chloroform and precipitated with isopropanol. The RNA pellet was washed with 70% ethanol, resuspended in 40 μL RNA Storage Solution buffer (Ambion), and incubated at 60°C for 5 min. cDNA was synthesized using an Omniscript kit (QIAGEN) according to the manufacturer's instructions. In brief, cDNA mix (0.2 U/μL Omniscript reverse transcriptase [QIAGEN], 1× cDNA buffer, 0.5 mM each deoxynucleotide triphosphate, 1 μM oligo-dT [Promega], and 0.5 U/μL RNAsin [Invitrogen]) was added to 100 ng RNA and heated at 37°C for 1 h. cDNA encoding granzymes A, B, C, K, and perforin were quantified using real-time analysis, which was performed using an Assays-on-Demand TaqMan Gene Expression Assay kit (Applied Biosystems), and singleplex PCR was performed using TaqMan (FAM dye labeled) minor groove-binder probes. cDNA encoding the mitochondrial ribosomal protein L32 was quantified as an internal standard to provide a reference point for comparing mRNA levels for granzyme with perforin in naive and activated lymphocytes. Real-time PCR was performed using a thermocycler (ABI PRISM 7700; Applied Biosystems) with denaturation at 95°C for 2 min followed by 40 cycles of denaturation at 95°C for 10 s and synthesis for 30 s at 60°C.
Online supplemental material
Video 1 is a time-lapse video of the death of an MS9II cell induced by 10 nM perforin and 25 nM granzyme B as described in Fig. 1. Video 2 is a time-lapse video of the death of an MS9II cell induced by 80 nM perforin as described in Fig. 2. Video 3 is a time-lapse video of the death of an MS9II cell induced by a wild-type CTL as described in Fig. 4. Video 4 is a time-lapse video of the death of an MS9II cell induced by a granzyme AB−/− CTL as described in Fig. 5. Fig. S1 is a montage of a target cell killed by granzyme AB−/− CTLs as described in Fig. 5, and Fig. S2 is a montage of a target cell killed by granzyme AB−/− CTLs in which the target cell depicted is touched by several lymphocytes before rounding. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200510072/DC1.
Supplementary Material
Acknowledgments
We thank Sarah Ellis (Peter MacCallum Cancer Centre, Melbourne, Australia) for technical help.
This work was supported by grant funding from the National Health and Medical Research Council (NHMRC) of Australia to V.R. Sutton and J.A. Trapani. J.A. Trapani is a Senior Principal Research Fellow, and N.J. Waterhouse is the recipient of an RD Wright Career Award from NHMRC.
Abbreviations used in this paper: CTL, cytotoxic T lymphocyte; PI, propidium iodide; PS, phosphatidylserine.
References
- Browne, K.A., E. Blink, V.R. Sutton, C.J. Froelich, D.A. Jans, and J.A. Trapani. 1999. Cytosolic delivery of granzyme B by bacterial toxins: evidence that endosomal disruption, in addition to transmembrane pore formation, is an important function of perforin. Mol. Cell. Biol. 19:8604–8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J.E., M.J. Smyth, and J.A. Trapani. 2001. Granzyme A and B-deficient killer lymphocytes are defective in eliciting DNA fragmentation but retain potent in vivo anti-tumor capacity. Eur. J. Immunol. 31:39–47. [DOI] [PubMed] [Google Scholar]
- Ebnet, K., M. Hausmann, F. Lehmann-Grube, A. Mullbacher, M. Kopf, M. Lamers, and M.M. Simon. 1995. Granzyme A-deficient mice retain potent cell-mediated cytotoxicity. EMBO J. 14:4230–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, S.A., R. Karimi, M. Michalak, and D. Hudig. 2000. Perforin lytic activity is controlled by calreticulin. J. Immunol. 164:4150–4155. [DOI] [PubMed] [Google Scholar]
- Goldstein, J.C., R.M. Kluck, and D.R. Green. 2000. a. A single cell analysis of apoptosis. Ordering the apoptotic phenotype. Ann. NY Acad. Sci. 926:132–141. [DOI] [PubMed] [Google Scholar]
- Goldstein, J.C., N.J. Waterhouse, P. Juin, G.I. Evan, and D.R. Green. 2000. b. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2:156–162. [DOI] [PubMed] [Google Scholar]
- Henson, P.M., D.L. Bratton, and V.A. Fadok. 2001. The phosphatidylserine receptor: a crucial molecular switch? Nat. Rev. Mol. Cell Biol. 2:627–633. [DOI] [PubMed] [Google Scholar]
- Heusel, J.W., R.L. Wesselschmidt, S. Shresta, J.H. Russell, and T.J. Ley. 1994. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 76:977–987. [DOI] [PubMed] [Google Scholar]
- Johnson, H., L. Scorrano, S.J. Korsmeyer, and T.J. Ley. 2003. Cell death induced by granzyme C. Blood. 101:3093–3101. [DOI] [PubMed] [Google Scholar]
- Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K.J. Olsen, E.R. Podack, R.M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 369:31–37. [DOI] [PubMed] [Google Scholar]
- Keefe, D., L. Shi, S. Feske, R. Massol, F. Navarro, T. Kirchhausen, and J. Lieberman. 2005. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity. 23:249–262. [DOI] [PubMed] [Google Scholar]
- Kelly, J.M., N.J. Waterhouse, E. Cretney, K.A. Browne, S. Ellis, J.A. Trapani, and M.J. Smyth. 2004. Granzyme M mediates a novel form of perforin-dependent cell death. J. Biol. Chem. 279:22236–22242. [DOI] [PubMed] [Google Scholar]
- Kelso, A., E.O. Costelloe, B.J. Johnson, P. Groves, K. Buttigieg, and D.R. Fitzpatrick. 2002. The genes for perforin, granzymes A-C and IFN-gamma are differentially expressed in single CD8(+) T cells during primary activation. Int. Immunol. 14:605–613. [DOI] [PubMed] [Google Scholar]
- Krenacs, L., M.J. Smyth, E. Bagdi, T. Krenacs, L. Kopper, T. Rudiger, A. Zettl, H.K. Muller-Hermelink, E.S. Jaffe, and M. Raffeld. 2003. The serine protease granzyme M is preferentially expressed in NK-cell, gamma delta T-cell, and intestinal T-cell lymphomas: evidence of origin from lymphocytes involved in innate immunity. Blood. 101:3590–3593. [DOI] [PubMed] [Google Scholar]
- Lieberman, J., and Z. Fan. 2003. Nuclear war: the granzyme A-bomb. Curr. Opin. Immunol. 15:553–559. [DOI] [PubMed] [Google Scholar]
- Lin, M.T., D.R. Hinton, and S.A. Stohlman. 1998. Mechanisms of viral clearance in perforin-deficient mice. Adv. Exp. Med. Biol. 440:431–436. [DOI] [PubMed] [Google Scholar]
- Liu, C.C., P.M. Persechini, and J.D. Young. 1994. Characterization of recombinant mouse perforin expressed in insect cells using the baculovirus system. Biochem. Biophys. Res. Commun. 201:318–325. [DOI] [PubMed] [Google Scholar]
- Liu, C.C., P.M. Persechini, and J.D. Young. 1995. Perforin and lymphocyte-mediated cytolysis. Immunol. Rev. 146:145–175. [DOI] [PubMed] [Google Scholar]
- Ludford-Menting, M.J., J. Oliaro, F. Sacirbegovic, E.T. Cheah, N. Pedersen, S.J. Thomas, A. Pasam, R. Iazzolino, L.E. Dow, N.J. Waterhouse, et al. 2005. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 22:737–748. [DOI] [PubMed] [Google Scholar]
- MacDonald, G., L. Shi, C. Vande Velde, J. Lieberman, and A.H. Greenberg. 1999. Mitochondria-dependent and -independent regulation of granzyme B-induced apoptosis. J. Exp. Med. 189:131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S.J., C.P. Reutelingsperger, A.J. McGahon, J.A. Rader, R.C. van Schie, D.M. LaFace, and D.R. Green. 1995. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182:1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S.J., G.P. Amarante-Mendes, L. Shi, T.H. Chuang, C.A. Casiano, G.A. O'Brien, P. Fitzgerald, E.M. Tan, G.M. Bokoch, A.H. Greenberg, and D.R. Green. 1996. The cytotoxic cell protease granzyme B initiates apoptosis in a cell-free system by proteolytic processing and activation of the ICE/CED-3 family protease, CPP32, via a novel two-step mechanism. EMBO J. 15:2407–2416. [PMC free article] [PubMed] [Google Scholar]
- Mullbacher, A., P. Waring, R. Tha Hla, T. Tran, S. Chin, T. Stehle, C. Museteanu, and M.M. Simon. 1999. Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc. Natl. Acad. Sci. USA. 96:13950–13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortaldo, J.R., R.T. Winkler-Pickett, K. Nagashima, H. Yagita, and K. Okumura. 1992. Direct evidence for release of pore-forming protein during NK cellular lysis. J. Leukoc. Biol. 52:483–488. [DOI] [PubMed] [Google Scholar]
- Pardo, J., S. Balkow, A. Anel, and M.M. Simon. 2002. The differential contribution of granzyme A and granzyme B in cytotoxic T lymphocyte-mediated apoptosis is determined by the quality of target cells. Eur. J. Immunol. 32:1980–1985. [DOI] [PubMed] [Google Scholar]
- Pardo, J., A. Bosque, R. Brehm, R. Wallich, J. Naval, A. Mullbacher, A. Anel, and M.M. Simon. 2004. Apoptotic pathways are selectively activated by granzyme A and/or granzyme B in CTL-mediated target cell lysis. J. Cell Biol. 167:457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, P.J., J. Borst, V. Oorschot, M. Fukuda, O. Krahenbuhl, J. Tschopp, J.W. Slot, and H.J. Geuze. 1991. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 173:1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, C.T., D.M. MacIvor, B.A. Hug, J.W. Heusel, and T.J. Ley. 1996. Long-range disruption of gene expression by a selectable marker cassette. Proc. Natl. Acad. Sci. USA. 93:13090–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkoski, M.J., N.J. Waterhouse, J.A. Heibein, B.B. Wolf, T. Kuwana, J.C. Goldstein, D.D. Newmeyer, R.C. Bleackley, and D.R. Green. 2001. Granzyme B-mediated apoptosis proceeds predominantly through a Bcl-2-inhibitable mitochondrial pathway. J. Biol. Chem. 276:12060–12067. [DOI] [PubMed] [Google Scholar]
- Pinkoski, M.J., N.J. Waterhouse, and D.R. Green. 2006. Mitochondria, apoptosis and autoimmunity. Curr. Dir. Autoimmun. 9:55–73. [DOI] [PubMed] [Google Scholar]
- Podack, E.R., and P.J. Konigsberg. 1984. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J. Exp. Med. 160:695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochel, N., and J.A. Cowan. 1997. Dependence of the lytic activity of the N-terminal domain of human perforin on membrane lipid composition–implications for T-cell self-preservation. Eur. J. Biochem. 249:223–231. [DOI] [PubMed] [Google Scholar]
- San Mateo, L.R., M.M. Chua, S.R. Weiss, and H. Shen. 2002. Perforin-mediated CTL cytolysis counteracts direct cell-cell spread of Listeria monocytogenes. J. Immunol. 169:5202–5208. [DOI] [PubMed] [Google Scholar]
- Sedelies, K.A., T.J. Sayers, K.M. Edwards, W. Chen, D.G. Pellicci, D.I. Godfrey, and J.A. Trapani. 2004. Discordant regulation of granzyme H and granzyme B expression in human lymphocytes. J. Biol. Chem. 279:26581–26587. [DOI] [PubMed] [Google Scholar]
- Shi, L., R.P. Kraut, R. Aebersold, and A.H. Greenberg. 1992. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J. Exp. Med. 175:553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, M.M., M. Hausmann, T. Tran, K. Ebnet, J. Tschopp, R. ThaHla, and A. Mullbacher. 1997. In vitro- and ex vivo-derived cytolytic leukocytes from granzyme A x B double knockout mice are defective in granule-mediated apoptosis but not lysis of target cells. J. Exp. Med. 186:1781–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, M.J., K.Y. Thia, S.E. Street, D. MacGregor, D.I. Godfrey, and J.A. Trapani. 2000. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 192:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, M.J., S.E. Street, and J.A. Trapani. 2003. Cutting edge: granzymes A and B are not essential for perforin-mediated tumor rejection. J. Immunol. 171:515–518. [DOI] [PubMed] [Google Scholar]
- Street, S.E., J.A. Trapani, D. MacGregor, and M.J. Smyth. 2002. Suppression of lymphoma and epithelial malignancies effected by interferon γ. J. Exp. Med. 196:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J., C.H. Bird, M.S. Buzza, K.E. McKee, J.C. Whisstock, and P.I. Bird. 1999. Expression and purification of recombinant human granzyme B from Pichia pastoris. Biochem. Biophys. Res. Commun. 261:251–255. [DOI] [PubMed] [Google Scholar]
- Sutton, V.R., D.L. Vaux, and J.A. Trapani. 1997. Bcl-2 prevents apoptosis induced by perforin and granzyme B, but not that mediated by whole cytotoxic lymphocytes. J. Immunol. 158:5783–5790. [PubMed] [Google Scholar]
- Sutton, V.R., J.E. Davis, M. Cancilla, R.W. Johnstone, A.A. Ruefli, K. Sedelies, K.A. Browne, and J.A. Trapani. 2000. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J. Exp. Med. 192:1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, V.R., M.E. Wowk, M. Cancilla, and J.A. Trapani. 2003. Caspase activation by granzyme B is indirect, and caspase autoprocessing requires the release of proapoptotic mitochondrial factors. Immunity. 18:319–329. [DOI] [PubMed] [Google Scholar]
- Trapani, J.A., and M.J. Smyth. 1993. Killing by cytotoxic T cells and natural killer cells: multiple granule serine proteases as initiators of DNA fragmentation. Immunol. Cell Biol. 71:201–208. [DOI] [PubMed] [Google Scholar]
- Trapani, J.A., and M.J. Smyth. 2002. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2:735–747. [DOI] [PubMed] [Google Scholar]
- Trapani, J.A., D.A. Jans, P.J. Jans, M.J. Smyth, K.A. Browne, and V.R. Sutton. 1998. Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent. J. Biol. Chem. 273:27934–27938. [DOI] [PubMed] [Google Scholar]
- van den Broek, M.E., D. Kagi, F. Ossendorp, R. Toes, S. Vamvakas, W.K. Lutz, C.J. Melief, R.M. Zinkernagel, and H. Hengartner. 1996. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 184:1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse, N.J., and J.A. Trapani. 2002. CTL: caspases terminate life, but that's not the whole story. Tissue Antigens. 59:175–183. [DOI] [PubMed] [Google Scholar]
- Waterhouse, N.J., C.J. Clarke, K.A. Sedelies, M.W. Teng, and J.A. Trapani. 2004. Cytotoxic lymphocytes; instigators of dramatic target cell death. Biochem. Pharmacol. 68:1033–1040. [DOI] [PubMed] [Google Scholar]
- Waterhouse, N.J., K.A. Sedelies, K.A. Browne, M.E. Wowk, A. Newbold, V.R. Sutton, C.J. Clarke, J. Oliaro, R.K. Lindemann, P.I. Bird, et al. 2005. A central role for Bid in granzyme B-induced apoptosis. J. Biol. Chem. 280:4476–4482. [DOI] [PubMed] [Google Scholar]
- Wei, S., D.L. Gilvary, B.C. Corliss, S. Sebti, J. Sun, D.B. Straus, P.J. Leibson, J.A. Trapani, A.D. Hamilton, M.J. Weber, and J.Y. Djeu. 2000. Direct tumor lysis by NK cells uses a Ras-independent mitogen-activated protein kinase signal pathway. J. Immunol. 165:3811–3819. [DOI] [PubMed] [Google Scholar]
- Young, J.D., H. Hengartner, E.R. Podack, and Z.A. Cohn. 1986. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer activity. Cell. 44:849–859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.