Abstract
Necrotic cell death is defined by distinctive morphological characteristics that are displayed by dying cells (Walker, N.I., B.V. Harmon, G.C. Gobe, and J.F. Kerr. 1988. Methods Achiev. Exp. Pathol. 13:18–54). The cellular events that transpire during necrosis to generate these necrotic traits are poorly understood. Recent studies in the nematode Caenorhabditis elegans show that cytoplasmic acidification develops during necrosis and is required for cell death (Syntichaki, P., C. Samara, and N. Tavernarakis. 2005. Curr. Biol. 15:1249–1254). However, the origin of cytoplasmic acidification remains elusive. We show that the alkalization of endosomal and lysosomal compartments ameliorates necrotic cell death triggered by diverse stimuli. In addition, mutations in genes that result in altered lysosomal biogenesis and function markedly affect neuronal necrosis. We used a genetically encoded fluorescent marker to follow lysosome fate during neurodegeneration in vivo. Strikingly, we found that lysosomes fuse and localize exclusively around a swollen nucleus. In the advanced stages of cell death, the nucleus condenses and migrates toward the periphery of the cell, whereas green fluorescent protein–labeled lysosomal membranes fade, indicating lysosomal rupture. Our findings demonstrate a prominent role for lysosomes in cellular destruction during necrotic cell death, which is likely conserved in metazoans.
Introduction
Previous studies implicate necrotic cell death in devastating human pathologies such as stroke and neurodegenerative diseases (Walker et al., 1988; Martin, 2001). In Caenorhabditis elegans, specific mutations in several genes that encode ion channel subunits and regulators trigger the degeneration of specific sets of neurons (for review see Syntichaki and Tavernarakis, 2003). Dying neurons exhibit macroscopic and ultrastructural characteristics that are reminiscent of the excitotoxic neuronal death that occurs during stroke in mammals (Hall et al., 1997; Lee et al., 1999; Nicotera et al., 1999). Thus, vertebrates and C. elegans share a death mechanism that involves the hyperactivation of ion channels. These observations are consistent with the hypothesis that a threshold of ion influx is needed to initiate the degenerative process.
Perturbation of cellular ionic homeostasis contributes decisively to necrotic neuronal death (Syntichaki and Tavernarakis, 2003). In addition to ion homeostasis, intracellular pH has emerged as an important modulator of necrosis in C. elegans. Cytoplasmic acidification develops during necrosis, whereas the vacuolar H+-ATPase, which is a pump that acidifies lysosomes, is required downstream of cytoplasmic calcium overload to promote necrotic cell death (Syntichaki et al., 2005). Interestingly, similar acidosis accompanies necrotic cell death after stroke in mammals (Sapolsky et al., 1996; Nicotera et al., 1999). Moreover, the examination of postmortem human brains associates neuronal pH alterations with several pathological and neurodegenerative states (Li et al., 2004). Investigations in both nematodes and mammals converge to implicate specific calpain and aspartyl proteases (cathepsins) in the execution of necrotic cell death (Syntichaki et al., 2002; Yoshida et al., 2002). Calpain proteases are normally dependent on calcium for activation, whereas aspartyl proteases require a highly acidic environment for full activity and are primarily confined to lysosomes and other acidic endosomal compartments (Ishidoh and Kominami, 2002; Goll et al., 2003). Studies in primates indicate that damage to the lysosomal membrane is inflicted enzymatically by activated calpains. Calpains localize to lysosomal membranes after the onset of ischemic episodes, with subsequent spillage of cathepsins to the cytoplasm (Yamashima et al., 2003). This observation led to the formulation of the “calpain–cathepsin hypothesis,” whereby the calcium-mediated activation of calpains results in the rupture of lysosomes and leakage of killer cathepsins that eventually dismantle the cell (Yamashima et al., 1998; Yamashima, 2000, 2004). Although these observations collectively indicate that lysosomes participate actively in the process of cell death, their contribution is poorly understood.
We examined the role of lysosomes in a well defined model of necrotic cell death in the nematode. We show that the alkalization of endosomal and lysosomal compartments protects against necrotic cell death that is induced by mutations in several ion channels, as well as by prolonged hypoxia. We investigated the effect of mutations that alter lysosome biogenesis in necrotic cell death and found that mutations resulting in the accumulation of large lysosomes exacerbate necrosis, whereas mutations that impair lysosome biogenesis are protective. Conditions that counterbalance intracellular acidification enhance suppression of neurodegeneration by aspartyl protease deficiency, indicating that aspartyl proteases are activated by low pH conditions, which develop during necrosis. By monitoring lysosomes during necrosis in vivo, we show that lysosomes coalesce around the nucleus and dramatically enlarge during the early and intermediate stages of necrosis, although, ultimately, lysosomal definition is lost. Together, these results point to a decisive role for lysosomes in the execution of necrotic cell death.
Results
Alkalization of endosomal and lysosomal compartments protects against necrotic cell death
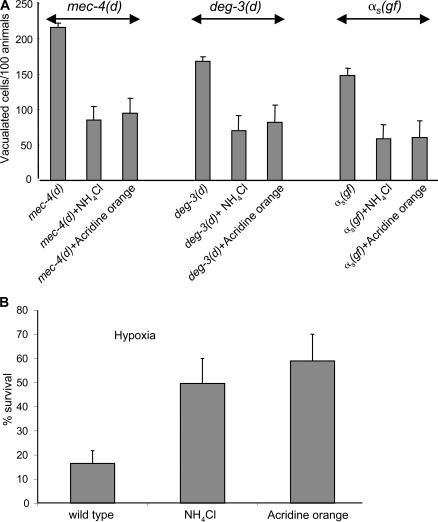
Recent data suggests that vacuolar H+-ATPase–mediated intracellular acidification is required downstream of cytoplasmic calcium overload to promote necrotic cell death, plausibly by enhancing the activity of the low pH–dependent proteases that dismantle the cell (Syntichaki et al., 2005). To emulate impaired lysosomal acidification in degenerating neurons, we treated animals expressing a neurotoxic gain-of-function (gf) mec-4(d) allele encoding a hyperactive ion channel subunit that is normally required for mechanosensation with NH4Cl and acridine orange. These lysotropic weak bases are known to accumulate in lysosomes and other acidic subcellular compartments, neutralizing their pH (Oka and Futai, 2000). Treatment ameliorated degeneration of the six touch receptor neurons of mec-4(d) mutant animals (Fig. 1 A). Similarly, cell death inflicted by the toxic deg-3(d) allele, encoding a hyperactive acetylcholine receptor calcium ion channel, or by overexpression of the hyperactivated Gαs(Q227L) variant (αs(gf)) was also suppressed after treatment with NH4Cl and acridine orange (Fig. 1 A). We assessed cell survival by scoring for expression of a touch receptor–specific mec-4∷GFP reporter fusion in adult animal neurons. Fluorescent neuron number increased after treatment with alkalizing agents in adult mec-4(d) mutant animals (309 ± 19 NH4Cl and 192 ± 13 acridine orange vs. 176 ± 11 fluorescent neurons in untreated mec-4(d) mutants; n = 100; P < 0.001, unpaired t test).
Figure 1.
Alkalization of endosomal compartments by weak bases protects against necrotic cell death. (A) Degenerating touch receptor neurons in mec-4(d) and PVC interneurons in deg-3(d) and αs (gf) in untreated animals or animals treated with NH4Cl and acridine orange. n > 150. P < 0.001, unpaired t test. (B) The effects of alkalizing agents on hypoxic death. The graph shows the percentage of animals that survived near-lethal treatment with sodium azide. n > 200. P < 0.001; unpaired t test. Error bars represent the SD of the mean.
Prolonged hypoxia, which is a condition of low oxygen availability that emerges during ischemia and stroke, induces necrotic cell death in the nematode (Scott et al., 2002). We examined the effect of alkalization in hypoxia-induced cell death. Nematodes were treated with sodium azide, which inhibits complex IV (cytochrome c oxidase) of the respiratory chain and simulates hypoxia. Treatment with NH4Cl and acridine orange reduced the hypoxic death of wild-type animals (Fig. 1 B).
We considered whether suppression of necrosis is an indirect effect of probable alterations in animal growth and development caused by alkalizing agents. We assayed developmental timing after egg hatching and past the L1 stage, which is where we assayed for cell death. We also assayed for animal locomotion, pharyngeal pumping, and defecation. Treatment with NH4Cl and acridine orange, at the concentrations and under the conditions used, does not result in any discernible defects in animal growth and development that could influence the course of necrotic cell death. We conclude that dependence on acidified intracellular compartments is a common denominator of necrotic cell death triggered by diverse stimuli.
Altered lysosomal biogenesis affects necrosis
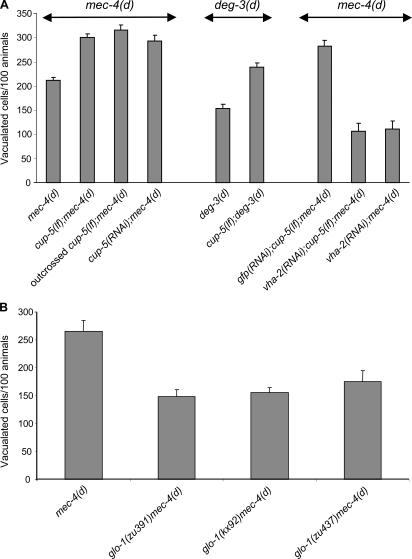
To further evaluate the lysosomal role in necrotic cell death, we examined mutants defective in lysosomal biogenesis. We examined necrosis in cup-5 loss-of-function (lf; ar465) mutant animals. Cells of cup-5(lf) mutants contain increased numbers of enlarged acidic lysosomes. cup-5 encodes the C. elegans mucolipin-1 homologue that is implicated in mucolipidosis type IV, which is a lysosomal storage disease that results in severe developmental neuropathology in humans (Fares and Greenwald, 2001; Hersh et al., 2002; Treusch et al., 2004). Neurodegeneration inflicted by mec-4(d) and deg-3(d) is exacerbated in cup-5(lf) mutants (Fig. 2 A). We confirmed the reduced cell survival by scoring for expression of a touch receptor–specific mec-4∷GFP reporter fusion in adult animal neurons. Fluorescent neuron number decreased in cup-5(lf);mec-4(d) double mutants, compared with mec-4(d) mutant animals (109 ± 16 cup-5(lf);mec-4(d) versus 176 ± 11 fluorescent neurons in mec-4(d) mutants; n = 150; P < 0.001, unpaired t test).
Figure 2.
Effect of lysosomal biogenesis mutants on necrotic cell death. (A) Neurodegeneration is enhanced in a cup-5(lf) genetic background, where the lysosomal system is expanded. This enhancement is suppressed by vacuolar H+-ATPase deficiency. Degenerating neurons in mec-4(d), cup-5(lf);mec-4(d), outcrossed cup-5(lf);mec-4(d), cup-5(RNAi);mec-4(d), deg-3(d), and cup-5(lf);deg-3(d) animals. Knockdown of cup-5 by RNAi also enhances mec-4(d)–induced neurodegeneration. n > 250. P < 0.005; unpaired t test. (B) Neurodegeneration is suppressed by three different glo-1 mutant alleles, where lysosomal biogenesis is defective. Degenerating neurons in mec-4(d), glo-1(zu391)mec-4(d), glo-1(kx92)mec-4(d), and glo-1(zu437)mec-4(d) animals. n > 350. P < 0.005, unpaired t test. Error bars represent the SD of the mean.
In addition, cup-5(lf) mutants showed increased sensitivity to hypoxia compared with wild type (16.5 ± 5.3% wild-type survival versus 5.3 ± 4.1% cup-5(lf) animal survival. n > 200; P < 0.005, unpaired t test). Knockdown of vha-2, which encodes for a subunit of the vacuolar H+-ATPase, by RNAi in cup-5(lf);mec-4(d) double mutants abolished cup-5(lf)–mediated enhancement of cell death (Fig. 2 A). This suggests that the enhanced cell death observed in cup-5(lf);mec-4(d) double mutants is caused by increased lysosome-mediated acidification. To confirm the effect of cup-5 deficiency on cell death, we outcrossed cup-5(lf) mutant animals in an effort to minimize the possibility that the effects on neurodegeneration we observed were caused by unlinked spurious mutations in the genetic background of the cup-5(lf) allele. Furthermore, we used RNAi to specifically target cup-5. Both outcrossed cup-5(lf) derivatives and RNAi-mediated knockdown of cup-5 resulted in enhanced mec-4(d)–induced neurodegeneration (Fig. 2 A).
In a reciprocal approach, we examined necrosis in glo-1(lf) mutants. The glo-1 gene encodes a predicted Rab GTPase that is similar to proteins implicated in the biogenesis of specialized lysosome-related organelles (Hermann et al., 2005). glo-1 mutant alleles were recovered in a screen aimed at identifying genes involved in the formation of birefringent gut granules, which are lysosome-related organelles (Hermann et al., 2005). glo-1(lf) mutants are defective in the biogenesis of lysosome-related gut granules, show little or no staining with lysosomal markers, and lack detectable expression of the vacuolar H+-ATPase subunits VHA-17 and VHA-11 in intestinal precursor cells (Hermann et al., 2005). We found that all three glo-1(lf) alleles ameliorate necrotic cell death triggered by mec-4(d) (Fig. 2 B). This suggests that the reduced number of lysosomes in touch receptor neurons of glo-1(lf)mec-4(d) double mutants results in reduced intracellular acidification and, consequently, in reduced necrotic cell death.
Suppression of necrosis by aspartyl protease deficiency is enhanced by conditions that impede lysosome-mediated intracellular acidification
Specific calpain and aspartyl proteases are implicated in the execution of necrotic cell death in both nematodes and mammals (Syntichaki et al., 2002; Yoshida et al., 2002), and the importance of calpain and aspartyl protease activation in acute cell injury and necrotic cell death triggered by calcium influx has been previously established (for review see Artal-Sanz and Tavernarakis, 2005). Calpain proteases become activated upon the abrupt increase of intracellular calcium that occurs in response to diverse necrosis-initiating stimuli, whereas aspartyl proteases function optimally under the highly acidic conditions present in the lumen of lysosomes and other acidic endosomal compartments (Ishidoh and Kominami, 2002; Goll et al., 2003).
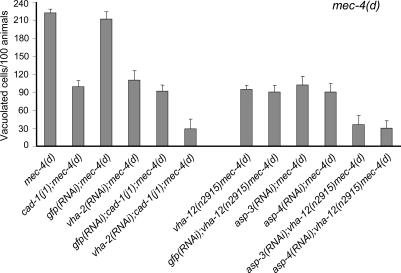
We assessed the effect of lysosome-mediated intracellular acidification on the requirement for aspartyl proteases in necrosis. cad-1(j1) mutants maintain aspartyl protease activity that is 90% lower than in wild-type animals (Jacobson et al., 1988). mec-4(d)–induced neurodegeneration is attenuated in cad-1(j1);mec-4(d) double-mutant strains (Syntichaki et al., 2002). RNAi-mediated knockdown of vha-2 diminishes cell death inflicted by mec-4(d) (Fig. 2 B and Fig. 3; Syntichaki et al., 2005). Cell death was further reduced in cad-1(j1);mec-4(d) mutant animals by RNAi-mediated knockdown of vha-2 (Fig. 3). Two aspartyl proteases, ASP-3 and -4, contribute the bulk of protease activity required for neurodegeneration inflicted by diverse genetic insults in C. elegans (Syntichaki et al., 2002). Similarly, knockdown of asp-3 or asp-4 by RNAi in vha-12(n2915)mec-4(d) double mutants augmented survival of the six receptor neurons, compared with single mec-4(d) mutants (Fig. 3). In contrast, reduced V-ATPase activity did not further enhance suppression of necrosis by calpain protease deficiency (Table I). We conclude that suppression of necrosis by aspartyl protease deficiency is enhanced by conditions that impede intracellular acidification.
Figure 3.
Suppression of necrosis by aspartyl protease deficiency is enhanced by conditions that impede intracellular acidification. The number of vacuolated touch receptor neurons per 100 L1 stage mec-4(d) animals. Bars represent the mean of three independent experiments. n > 300. Knockdown of both vacuolar H+-ATPase and aspartyl protease genes resulted in significantly more extended quenching of neurodegeneration than for any single gene. P < 0.001, unpaired t test. Efficacy of RNAi was assessed as described in Materials and methods. Error bars represent the SD of the mean.
Table I.
Calpain and V-ATPase activity in necrotic cell death
| Strain | Corpses |
|---|---|
| mec-4(d) | 205 ± 9 |
| gfp(RNAi);mec-4(d) | 193 ± 11 |
| clp-1(RNAi);mec-4(d) | 96 ± 18 |
| vha-12(n2915)mec-4(d) | 112 ± 9 |
| gfp(RNAi);vha-12(n2915)mec-4(d) | 101 ± 13 |
| clp-1(RNAi);vha-12(n2915)mec-4(d) | 89 ± 16 |
Suppression of necrosis by calpain protease deficiency is not further enhanced by reduced vacuolar H+-ATPase activity. Values represent vacuolated touch receptor neurons (± SD) per 100 animals at the L1 stage. n > 250.
We considered the contribution of additional cellular pH homeostasis mechanisms in necrosis. Two other major mechanisms have been implicated in cytoplasmic and subcellular organelle pH regulation; first, the sodium–hydrogen exchanger (NHX), and second, the cation transporter P-type ATPase. These mechanisms operate both on the plasma membrane and at the membranes of subcellular organelles, such as mitochondria, to facilitate proton trafficking and pH homeostasis. Multiple nhx genes are encoded in the C. elegans genome (more than 9 nhx isoforms; Nehrke and Melvin, 2002; http://www.wormbase.org). pmr-1 is the gene encoding the nematode P-type ATPase homologue. Knockdown of nhx-4, -5, and -9, which are three nhx isoforms expressed in neurons, did not alter the extent of neurodegeneration induced by mec-4(d) (137 ± 19 nhx-4(RNAi)mec-4(d), 134 ± 16 nhx-5(RNAi)mec-4(d), and 134 ± 21 nhx-9(RNAi);mec-4(d) versus 139 ± 14 vacuolated neurons in mec-4(d) mutants; n = 100). To address the potentially redundant function of these genes in the nervous system, we assayed cell death in animals in which all three isoforms were knocked down by RNAi. We did not observe significant suppression of necrosis in these animals (131 ± 28 nhx-9(RNAi); nhx-5(RNAi) nhx-4(RNAi)mec-4(d) versus 139 ± 14 vacuolated neurons in mec-4(d) mutants; n = 100.). Similarly knockdown of pmr-1 did not affect necrotic cell death (pmr-1(RNAi); mec-4(d): 141 ± 17, versus 139 ± 14 vacuolated neurons in mec-4(d) mutants; n = 100). Therefore, neurodegeneration is not affected by compromising these other, nonlysosomal pH homeostasis mechanisms.
Lysosomal fate during necrosis
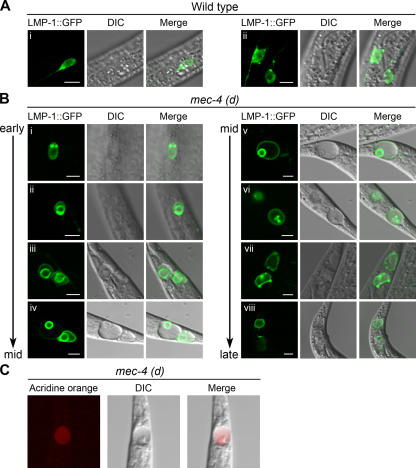
The importance of lysosomal membrane permeabilization in cell death has previously been established (Kroemer and Jaattela, 2005). Approaches combining electron microscopy and immunodetection show that calpains concentrate on lysosomal membranes during ischemic stroke in primates (Yamashima, 2000; Yamashima et al., 2003). However, information on lysosome fate and lysosomal system alterations during necrosis in vivo is lacking. We monitored the distribution and morphology of lysosomes in vivo during necrotic cell death in C. elegans. To visualize lysosomes and late endosomes, we fused GFP at the COOH terminus of LMP-1, which is the only C. elegans protein bearing a lysosomal targeting sequence (GYXXΦ; Φ, large hydrophobic amino acid residue) at its COOH terminus (Kostich et al., 2000). LMP-1 shows similarity to the vertebrate lysosome-associated membrane protein LAMP/CD68 (Kostich et al., 2000; Eskelinen et al., 2003), and it is widely used as a lysosomal marker (Treusch et al., 2004; Hermann et al., 2005; Nunes et al., 2005). We examined lysosomal distribution and morphology in touch receptor neurons of wild-type and mec-4(d) animals expressing an LMP-1∷GFP fusion.
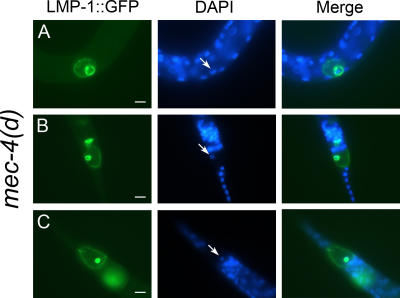
In the neurons of wild-type animals, lysosomes appear scattered throughout the cytoplasm (Fig. 4 A). In contrast, during the early stages of neurodegeneration, lysosomes enlarge and localize close to the nucleus (Fig. 4 B, i). As neurodegeneration progresses, lysosomes fuse to surround an internally vacuolated structure (Fig. 4 B, ii–viii). This encapsulated vacuole is likely the swollen nucleus of the dying neuron (Hall et al., 1997). To confirm the nuclear origin of the internal vacuole, we performed DAPI staining in mec-4(d) animals expressing the LMP-1∷GFP fusion protein. As shown in Fig. 5, LMP-1–labeled internal membranes are positive for DAPI staining. In agreement with our observation, elevation of cytosolic Ca2+ concentration can induce lysosomal fusion (Bakker et al., 1997). Consistent with previous studies (Hughes and August, 1982; Lippincott-Schwartz and Fambrough, 1986; Hermann et al., 2005), a portion of LMP-1 localizes to the plasma membrane (Fig. 4 B, ii–viii).
Figure 4.
Lysosomal morphology and distribution during neurodegeneration. (A) Confocal images of wild-type touch receptor neurons expressing a pmec-17 LMP-1∷GFP transgene. LMP-1∷GFP expression, differential interference contrast (DIC), and merged images are shown. Healthy neurons show a scattered and punctate pattern of lysosomal distribution. (i) Wild-type PVM touch receptor neuron. (ii) Wild-type ALM (left and right) touch receptor neurons. (B) Confocal images of PLM touch receptor neurons of mec-4(d) animals expressing a pmec-17LMP-1∷GFP transgene. During the early to middle (mid) stages of degeneration, lysosomes enlarge and appear to coalesce around a swollen nucleus (i–iv). Later on, the nucleus migrates to the periphery of the cell and condenses (iv–vi). At the late stage, no lysosome structure is evident and the vacuolated cell becomes diffusely fluorescent (vii and viii). (C) Acridine orange staining of a middle to late degenerating PLM touch receptor neuron. Acridine orange, DIC, and merged images are shown. Bars, 5 μm.
Figure 5.
DAPI staining of mec-4(d) animals expressing pmec-17 LMP-1∷GFP. (A) ALM touch receptor neuron. (B and C) PLM touch receptor neurons. Arrows point to DAPI-positive nuclei of the dying neurons. Bars, 5 μm.
Lysosomes remain confined around the distended nucleus during necrotic cell death (Fig. 4 B, ii–v). As neurodegeneration proceeds, the nucleus migrates to the periphery of the cell and condenses (Fig. 4 B, iv–vi). In the advanced stages of neurodegeneration, GFP intensity decreases and lysosomal definition is ultimately lost (Fig. 4 B, vii and viii). Interestingly, a decrease in LMP-1∷GFP immunoreactivity is associated with neuronal degeneration in mammals (Yamashima et al., 2003). Diffusion of the highly localized GFP staining during the late stages of neurodegeneration indicates lysosome rupture. To further confirm the loss of lysosomal integrity, we stained mec-4(d) animals with acridine orange, which is an acidophilic dye that distinctively stains lysosomes. Acridine orange accumulates diffusely in the cytoplasm of neurons during the late stages of necrosis, indicating extensive cytoplasmic acidification (Fig. 4 C). This observation, coupled with the loss of specific LMP-1∷GFP localization, suggests that lysosome rupture and the spillage of acidic lysosomal contents mediate cytoplasm acidification during necrosis.
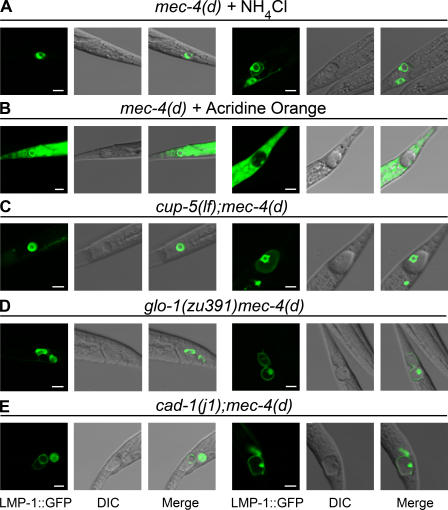
We investigated whether alkalizing treatments result in altered lysosomal fate. We examined the effects of acridine orange and NH4Cl treatment on LMP-1∷GFP distribution in mec-4(d) mutants. Although the number of unvacuolated cells with a wild-type pattern of lysosomal distribution increases, vacuolated neurons show the same pattern of lysosomal distribution as mec-4(d) animals (Fig. 6, A and B). Thus, reduced acidification does not affect lysosome distribution. Our observations are consistent with findings in animals deficient for the ATP-binding cassette transporter P-glycoprotein-2, which is also expressed in neurons. Acridine orange and Lysotracker red staining is reduced in animals lacking P-glycoprotein 2, indicating defective acidification. Nevertheless, LMP-1∷GFP distribution remains unchanged (Nunes et al., 2005). We further examined lysosomes in the different mutant genetic backgrounds that either enhance or suppress necrosis. We generated transgenic animals harboring LMP-1∷GFP in cup-5, glo-1, and the aspartyl protease–deficient cad-1 mutant background. The number of vacuolated neurons is decreased in glo-1 and cad-1 mutants and increased in cup-5 animals (Figs. 2 and 3). We find that lysosomal morphology and distribution is similar in neurons that do vacuolate and die (Fig. 6, C–E).
Figure 6.
Perturbation of lysosomal biogenesis, function, and neurodegeneration. Lysosomal morphology and distribution after treatment with alkalizing agents and in genetic backgrounds affecting lysosomal biogenesis and function. (A and B) Confocal images of PLM touch receptor neurons of mec-4(d) animals expressing a pmec-17LMP-1∷GFP reporter fusion, after alkalization of lysosomal compartments. (A) Images of degenerating neurons after treatment with 5 mM NH4Cl. (B) Degenerating neurons after treatment with 40 μM acridine orange. Nuclei can be seen because, at the concentration used, acridine orange stains DNA. (C–E) Confocal images of PLM touch receptor neurons of mec-4(d) animals under different genetic backgrounds that either suppress or enhance necrosis. Double mutants express the same pmec-17LMP-1∷GFP transgene. (C) Degenerating neurons of cup-5(lf);mec-4(d) mutants. Note that vacuoles appear larger. (D) Degenerating neurons of glo-1(zu391) mec-4(d) animals. (E) Images of degenerating neurons in cad-1(j1);mec-4(d) mutants. LMP-1∷GFP expression, differential interference contrast (DIC), and merged images are shown. (left) Early to middle stages of neurodegeneration. (right) Middle to late stages of neurodegeneration. Bars, 5 μm.
Discussion
In this study, we have examined the involvement of lysosomes in necrotic cell death using a well characterized model of neurodegeneration in C. elegans. We show that both genetic and pharmacological manipulations that affect lysosomal biogenesis and function modulate necrosis in the nematode. By following lysosome fate during neurodegeneration in vivo, we found that lysosomes fuse and localize exclusively around a swollen nucleus. In the advanced stages of cell death, GFP-labeled lysosomal membranes fade, indicating lysosomal rupture.
What is the cause of lysosomal rupture during necrosis? Interestingly, calcium, which is one of the major upstream death-initiating signals, has been implicated in this process (Zhao et al., 2005). Activated calcium-dependent calpain proteases have been found to localize to disrupted lysosomal membranes in hippocampal neurons of primates after acute ischemia (Yamashima, 2000, 2004), leading to the hypothesis that calpains compromise the integrity of lysosomal membranes and cause leakage of their acidic contents into the cytoplasm. Calpains become activated after the abrupt increase of intracellular calcium concentration that signals the initiation of necrosis (Syntichaki and Tavernarakis, 2002). Excessive calcium influx through several channels and transporter-mediated routes leads to intracellular calcium overload and concomitant cell death (Lipton and Nicotera, 1998; Nicotera and Bano, 2003). Sodium influx amplifies acute neuronal swelling and facilitates calcium entry through voltage-gated channels and the Na+/Ca2+ exchanger (Sattler and Tymianski, 2000).
Cell injury and death can also be induced by disturbances of calcium homeostasis in the ER (Mattson et al., 2000; Paschen, 2001). The ER is the major calcium storage compartment of the cell. Sequestration of calcium into the ER is mediated by the sarcoendoplasmic reticulum Ca2+-ATPase, and release back to the cytoplasm is controlled by ryanodine, and 1,4,5-inositol trisphosphate receptors (Carafoli, 2002). Within the ER, calcium binds to calcium-binding molecular chaperones such as calreticulin and calnexin (Michalak et al., 1999; Llewellyn et al., 2000). Under conditions of extreme cellular stress, ER calcium stores are rapidly mobilized, boosting the massive increase of intracellular calcium concentration, which signals cell demise (Ferri and Kroemer, 2001). Pharmacological treatments or genetic mutations that inhibit calcium release from the ER have a strong protective effect against necrotic cell death (Yu et al., 2000; Xu et al., 2001). In contrast, treatment with chemicals such as thapsigargin, which promotes the discharge of calcium from intracellular stores by specifically inhibiting the sarcoendoplasmic reticulum Ca2+-ATPase calcium pump, induces necrotic cell death (Takemura et al., 1989; Xu et al., 2001). We hypothesize that generalized osmotic destabilization of the cell during necrosis may also contribute to the bursting of lysosomes.
The exclusive confinement of lysosomes around the nucleus during neurodegeneration may reflect damaged microtubule or actin motors, which mediate the movement of lysosomal organelles (Burkhardt et al., 1997). It is known that calpain proteases contribute to cell death by cleaving essential cytoskeletal proteins of neuronal axons (for review see Artal-Sanz and Tavernarakis, 2005). Therefore, calpains may act on microtubules at early stages of neurodegeneration. However, we cannot rule out the possibility that lysosomes are specifically targeted to the periphery of the nucleus.
We observed that reduction of necrotic cell death by a drop in aspartyl protease activity is enhanced by conditions that counterbalance intracellular acidification. Such synergy between aspartyl proteases and pH indicates that aspartyl proteases become activated by low pH conditions, which develop during necrosis and facilitate cellular destruction. In addition to aspartyl proteases, other proteases that function optimally at low pH may become activated by acidification during necrosis. Such proteases have been implicated in both apoptotic and necrotic cell death (Ferri and Kroemer, 2001). We suggest that preventing acidification suppresses neurodegeneration, in part, by lowering the activity of these enzymes. Alternatively, aspartyl proteases and acidification may independently contribute to cell death. However, to discriminate between these alternatives requires complete elimination of aspartyl protease or vacuolar H+-ATPase activity, which results in embryonic lethality (Oka and Futai, 2000; Pujol et al., 2001; unpublished data).
The totality of our observations denotes an essential and general role for lysosomes in necrotic cell death induced by various insults. Our study is the first to monitor lysosomal alterations during necrosis in vivo, in any organism. Our findings uncovered novel aspects of the cellular changes that transpire during neurodegeneration in the nematode. Such information could be effectively used toward identifying candidate common intervention targets in an effort to battle numerous pathological conditions in humans. We envision that alterations of lysosomal biogenesis and function by genetic mutations or pharmacological treatments modify the susceptibility of neurons to necrosis. However, once a threshold is exceeded and cell death commences the sequence of events is essentially unaltered.
Materials and methods
Strains and genetics
We used standard procedures for C. elegans strain maintenance, crosses, and other genetic manipulations (Brenner, 1974). Nematodes were grown at 20°C. N2 was used as the wild-type strain. The following mutant alleles were used: mec-4(u231)X, which is referred to in the text as mec-4(d); deg-3(u662)V, which is referred to in the text as deg-3(d); nuIs5[pglr-1Gαs(Q227L)pgrl-1GFP], which is referred to in the text as αs (gf); arIs37[pmyo-3ssGFP]I;cup-5(ar465)III;dpy-20(e1282)IV, which is referred to in the text as cup-5(lf), cad-1(j1)II, glo-1(zu391)X, glo-1(Kx92)X, and glo-1(zu437)X. The glo-1 alleles were provided by G. Hermann (Lewis and Clark College, Portland, OR). The following double and triple mutants were used: cad-1(j1)II;mec-4(u231)X, vha-12(n2915)mec-4(u231)X, arIs37[pmyo-3ssGFP]I;cup-5(ar465)III;dpy-20(e1282)IV;mec-4(u231)X, arIs37- [pmyo-3ssGFP]I;cup-5(ar465)III;dpy-20(e1282)IV;deg-3(u662)V, glo-1(zu391)- mec-4(u231)X, glo-1(kx92)mec-4(u231)X, and glo-1(zu437)mec-4(u231)X.
Plasmids and RNAi
To generate pmec-17LMP-1∷GFP, we fused GFP at the COOH terminus of the C. elegans LMP-1 protein. The translational fusion includes the entire LMP-1 coding sequence lacking the stop codon, a Gly-Ser-Ser-Pro-Gly-Leu-Ala-Lys-Gly-Pro-Lys-Gly linker, and GFP. The resulting chimera was expressed in touch receptor neurons under the control of the mec-17 promoter. The plasmid carrying the reporter fusion was constructed in two steps. First, the mec-17 promoter was amplified from N2 genomic DNA with the primers 5′ CGGGATCCGAATCGTCTCACAACTGATCC 3′ and 5′ AACTGCAGGTGACTACTTGAGACCTG 3′. A 1,900-bp PstI–BamHI fragment was cloned into the promoterless gfp vector pPD95.77 (Fire et al., 1990). Second, the LMP-1 coding region was amplified from genomic DNA using the primers 5′ CGGGATCCGACGCTGGCATATCCTTGTCTC 3′ and 5′ CGGGATCCAATTGAACTATGTTGAAATCG 3′. A BamHI PCR fragment was cloned downstream of the mec-17 promoter on the pPD95.77 plasmid vector. For RNAi experiments, we used HT115(DE3) Escherichia coli bacteria, which were transformed with plasmids that direct the synthesis of double-stranded RNAs corresponding to the genes of interest; they were then fed to animals according to a previously described methodology (Kamath et al., 2001). For cup-5 RNAi, we used a 1.5-kb PCR-generated fragment derived from the cup-5 locus using the primers 5′ GGGGTACCCCATGATTTCAGATGTCTCGC 3′ and 5′ GGGGTACCCCGAATGCAAAGAATGAGAACG 3′. The primers used for nhx RNAi constructs are as follows: 5′ GCTCTAGACTCTTCACTGGCCTGTG 3′ and 5′ CCGCTCGAGATCAGTATGACTGCG 3′ for nhx-4, 5′ AACTGCAGTTATGGACGATATCAAC 3′ and 5′ CCGCTCGAGCCACAAACTTCAGCCAC 3′ for nhx-5, and 5′ GCTCTAGATGGTGTCCTGACTCTTC 3′ and 5′ CCGCTCGAGCTTCCACTCCAGACATC 3′ for nhx-9. For pmr-1 we used the following PCR primers: 5′ AACTGCAGATTGAAACACTGACATC 3′ and 5′ CCGCTCGAGTACCTGAAACATTCCG 3′. RNAi plasmids for vha-2, aspartyl proteases asp-3 and asp-4, and calpain clp-1 have been previously described (Syntichaki et al., 2002, 2005). We assayed the effectiveness of RNAi by monitoring the expression of full-length GFP reporter fusions. Plasmid vectors for C. elegans were provided by A. Fire (Stanford University School of Medicine, Stanford, CA).
Neurodegeneration assays
Degeneration of specific neuron sets in animals bearing deg-3(d), mec-4(d), and αs (gf) alleles was quantified as previously described (Syntichaki et al., 2002). For alkalization assays, we treated young adult animals with lysotropic alkalizing agents (5 mM NH4Cl and 40–150 μM acridine orange; Sigma-Aldrich) in liquid cultures supplemented with E. coli bacteria for 12 h at 20°C. Neurodegeneration was assayed in the progeny of treated animals at the L1 stage of development. To simulate death-inducing hypoxic conditions, we treated nematodes at the L4 stage of development with sodium azide (0.5 M for 30 min at 20°C; Sigma-Aldrich; adapted with modifications from Scott et al. [2002]). Statistical analysis of data was performed using Excel (Microsoft).
Microscopy
L1 stage mec-4(d) animals expressing LMP-1∷GFP were stained with 1 μg/ml DAPI for 15 min after methanol fixation. DAPI-stained animals were observed using a 40× objective (Plan-Neofluar; Carl Zeiss MicroImaging, Inc.), NA 0.75, and a 365 ± 12–nm band-pass excitation/397-nm long-pass emission filter set. A microscope was used, and pictures were taken using a camera (AxioPlan and AxioCam, respectively; both Carl Zeiss MicroImaging, Inc.). For LMP-1∷GFP imaging, animals were scanned with a 488-nm laser beam, under a confocal microscope (Radiance 2000; Bio-Rad Laboratories), using the LaserSharp 2000 software package (Bio-Rad Laboratories). Images of emission from individual PLM and ALM touch receptor neurons were acquired using a 515 ± 15–nm band-pass filter and a 40× Plan-Neofluar objective, NA 0.75. Acridine orange staining of necrotic cells was done by treating mec-4(d) early L1 larvae with 1 μM acridine orange for 20 min. To visualize stained cells, animals were scanned with a 543-nm laser beam. Images of emission from individual touch receptor neurons were acquired using a 590 ± 35–nm band-pass filter. Animals were mounted in a 2% agarose pad in M9 buffer containing 10 mM sodium azide and observed at room temperature. Bright field and epifluorescence images were merged using Photoshop (version 7.0.1; Adobe).
Acknowledgments
Some nematode strains used in this work were provided by the C. elegans Gene Knockout Project at the Oklahoma Medical Research Foundation (http://www.mutantfactory.ouhsc.edu/), which is part of the International C. elegans Gene Knockout Consortium, and the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. We thank A. Fire for plasmid vectors and G. Hermann for the glo-1(kx92) and glo-1(zu437) alleles.
This work was funded by grants from the European Union, the European Molecular Biology Organization (EMBO), and the Institute of Molecular Biology and Biotechnology. M. Artal-Sanz is supported by a Marie Curie postdoctoral fellowship (FP6-EIF). N. Tavernarakis is an EMBO Young Investigator.
References
- Artal-Sanz, M., and N. Tavernarakis. 2005. Proteolytic mechanisms in necrotic cell death and neurodegeneration. FEBS Lett. 579:3287–3296. [DOI] [PubMed] [Google Scholar]
- Bakker, A.C., P. Webster, W.A. Jacob, and N.W. Andrews. 1997. Homotypic fusion between aggregated lysosomes triggered by elevated [Ca2+]i in fibroblasts. J. Cell Sci. 110:2227–2238. [DOI] [PubMed] [Google Scholar]
- Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics. 77:71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt, J.K., C.J. Echeverri, T. Nilsson, and R.B. Vallee. 1997. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139:469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli, E. 2002. Calcium signaling: a tale for all seasons. Proc. Natl. Acad. Sci. USA. 99:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen, E.L., Y. Tanaka, and P. Saftig. 2003. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13:137–145. [DOI] [PubMed] [Google Scholar]
- Fares, H., and I. Greenwald. 2001. Regulation of endocytosis by CUP-5, the Caenorhabditis elegans mucolipin-1 homolog. Nat. Genet. 28:64–68. [DOI] [PubMed] [Google Scholar]
- Ferri, K.F., and G. Kroemer. 2001. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 3:E255–E263. [DOI] [PubMed] [Google Scholar]
- Fire, A., S.W. Harrison, and D. Dixon. 1990. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene. 93:189–198. [DOI] [PubMed] [Google Scholar]
- Goll, D.E., V.F. Thompson, H. Li, W. Wei, and J. Cong. 2003. The calpain system. Physiol. Rev. 83:731–801. [DOI] [PubMed] [Google Scholar]
- Hall, D.H., G. Gu, J. Garcia-Anoveros, L. Gong, M. Chalfie, and M. Driscoll. 1997. Neuropathology of degenerative cell death in Caenorhabditis elegans. J. Neurosci. 17:1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann, G.J., L.K. Schroeder, C.A. Hieb, A.M. Kershner, B.M. Rabbitts, P. Fonarev, B.D. Grant, and J.R. Priess. 2005. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol. Biol. Cell. 16:3273–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh, B.M., E. Hartwieg, and H.R. Horvitz. 2002. The Caenorhabditis elegans mucolipin-like gene cup-5 is essential for viability and regulates lysosomes in multiple cell types. Proc. Natl. Acad. Sci. USA. 99:4355–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, E.N., and J.T. August. 1982. Murine cell surface glycoproteins. Identification, purification, and characterization of a major glycosylated component of 110,000 daltons by use of a monoclonal antibody. J. Biol. Chem. 257:3970–3977. [PubMed] [Google Scholar]
- Ishidoh, K., and E. Kominami. 2002. Processing and activation of lysosomal proteinases. Biol. Chem. 383:1827–1831. [DOI] [PubMed] [Google Scholar]
- Jacobson, L.A., L. Jen-Jacobson, J.M. Hawdon, G.P. Owens, M.A. Bolanowski, S.W. Emmons, M.V. Shah, R.A. Pollock, and D.S. Conklin. 1988. Identification of a putative structural gene for cathepsin D in Caenorhabditis elegans. Genetics. 119:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath, R.S., M. Martinez-Campos, P. Zipperlen, A.G. Fraser, and J. Ahringer. 2001. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans Genome Biol. DOI: 10.1186/gb-2000-2-1-research0002. [DOI] [PMC free article] [PubMed]
- Kostich, M., A. Fire, and D.M. Fambrough. 2000. Identification and molecular-genetic characterization of a LAMP/CD68-like protein from Caenorhabditis elegans. J. Cell Sci. 113:2595–2606. [DOI] [PubMed] [Google Scholar]
- Kroemer, G., and M. Jaattela. 2005. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer. 5:886–897. [DOI] [PubMed] [Google Scholar]
- Lee, J.M., G.J. Zipfel, and D.W. Choi. 1999. The changing landscape of ischaemic brain injury mechanisms. Nature. 399:A7–14. [DOI] [PubMed] [Google Scholar]
- Li, J.Z., M.P. Vawter, D.M. Walsh, H. Tomita, S.J. Evans, P.V. Choudary, J.F. Lopez, A. Avelar, V. Shokoohi, T. Chung, et al. 2004. Systematic changes in gene expression in postmortem human brains associated with tissue pH and terminal medical conditions. Hum. Mol. Genet. 13:609–616. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., and D.M. Fambrough. 1986. Lysosomal membrane dynamics: structure and interorganellar movement of a major lysosomal membrane glycoprotein. J. Cell Biol. 102:1593–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton, S.A., and P. Nicotera. 1998. Calcium, free radicals and excitotoxins in neuronal apoptosis. Cell Calcium. 23:165–171. [DOI] [PubMed] [Google Scholar]
- Llewellyn, D.H., S. Johnson, and P. Eggleton. 2000. Calreticulin comes of age. Trends Cell Biol. 10:399–402. [DOI] [PubMed] [Google Scholar]
- Martin, L.J. 2001. Neuronal cell death in nervous system development, disease, and injury. Int. J. Mol. Med. 7:455–478. [PubMed] [Google Scholar]
- Mattson, M.P., F.M. LaFerla, S.L. Chan, M.A. Leissring, P.N. Shepel, and J.D. Geiger. 2000. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 23:222–229. [DOI] [PubMed] [Google Scholar]
- Michalak, M., E.F. Corbett, N. Mesaeli, K. Nakamura, and M. Opas. 1999. Calreticulin: one protein, one gene, many functions. Biochem. J. 344:281–292. [PMC free article] [PubMed] [Google Scholar]
- Nehrke, K., and J.E. Melvin. 2002. The NHX family of Na+-H+ exchangers in Caenorhabditis elegans. J. Biol. Chem. 277:29036–29044. [DOI] [PubMed] [Google Scholar]
- Nicotera, P., and D. Bano. 2003. The enemy at the gates. Ca2+ entry through TRPM7 channels and anoxic neuronal death. Cell. 115:768–770. [DOI] [PubMed] [Google Scholar]
- Nicotera, P., M. Leist, and L. Manzo. 1999. Neuronal cell death: a demise with different shapes. Trends Pharmacol. Sci. 20:46–51. [DOI] [PubMed] [Google Scholar]
- Nunes, F., M. Wolf, J. Hartmann, and R.J. Paul. 2005. The ABC transporter PGP-2 from Caenorhabditis elegans is expressed in the sensory neuron pair AWA and contributes to lysosome formation and lipid storage within the intestine. Biochem. Biophys. Res. Commun. 338:862–871. [DOI] [PubMed] [Google Scholar]
- Oka, T., and M. Futai. 2000. Requirement of V-ATPase for ovulation and embryogenesis in Caenorhabditis elegans. J. Biol. Chem. 275:29556–29561. [DOI] [PubMed] [Google Scholar]
- Paschen, W. 2001. Dependence of vital cell function on endoplasmic reticulum calcium levels: implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell Calcium. 29:1–11. [DOI] [PubMed] [Google Scholar]
- Pujol, N., C. Bonnerot, J.J. Ewbank, Y. Kohara, and D. Thierry-Mieg. 2001. The Caenorhabditis elegans unc-32 gene encodes alternative forms of a vacuolar ATPase a subunit. J. Biol. Chem. 276:11913–11921. [DOI] [PubMed] [Google Scholar]
- Sapolsky, R.M., J. Trafton, and G.C. Tombaugh. 1996. Excitotoxic neuron death, acidotic endangerment, and the paradox of acidotic protection. Adv. Neurol. 71:237–244. [PubMed] [Google Scholar]
- Sattler, R., and M. Tymianski. 2000. Molecular mechanisms of calcium-dependent excitotoxicity. J. Mol. Med. 78:3–13. [DOI] [PubMed] [Google Scholar]
- Scott, B.A., M.S. Avidan, and C.M. Crowder. 2002. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science. 296:2388–2391. [DOI] [PubMed] [Google Scholar]
- Syntichaki, P., and N. Tavernarakis. 2002. Death by necrosis: uncontrollable catastrophe, or is there order behind the chaos? EMBO Rep. 3:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syntichaki, P., and N. Tavernarakis. 2003. The biochemistry of neuronal necrosis: rogue biology? Nat. Rev. Neurosci. 4:672–684. [DOI] [PubMed] [Google Scholar]
- Syntichaki, P., K. Xu, M. Driscoll, and N. Tavernarakis. 2002. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature. 419:939–944. [DOI] [PubMed] [Google Scholar]
- Syntichaki, P., C. Samara, and N. Tavernarakis. 2005. The vacuolar H+-ATPase mediates intracellular acidification required for neurodegeneration in C. elegans. Curr. Biol. 15:1249–1254. [DOI] [PubMed] [Google Scholar]
- Takemura, H., A.R. Hughes, O. Thastrup, and J.W. Putney Jr. 1989. Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J. Biol. Chem. 264:12266–12271. [PubMed] [Google Scholar]
- Treusch, S., S. Knuth, S.A. Slaugenhaupt, E. Goldin, B.D. Grant, and H. Fares. 2004. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proc. Natl. Acad. Sci. USA. 101:4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, N.I., B.V. Harmon, G.C. Gobe, and J.F. Kerr. 1988. Patterns of cell death. Methods Achiev. Exp. Pathol. 13:18–54. [PubMed] [Google Scholar]
- Xu, K., N. Tavernarakis, and M. Driscoll. 2001. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron. 31:957–971. [DOI] [PubMed] [Google Scholar]
- Yamashima, T. 2000. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog. Neurobiol. 62:273–295. [DOI] [PubMed] [Google Scholar]
- Yamashima, T. 2004. Ca2+-dependent proteases in ischemic neuronal death: a conserved ‘calpain-cathepsin cascade’ from nematodes to primates. Cell Calcium. 36:285–293. [DOI] [PubMed] [Google Scholar]
- Yamashima, T., Y. Kohda, K. Tsuchiya, T. Ueno, J. Yamashita, T. Yoshioka, and E. Kominami. 1998. Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: a novel strategy for neuroprotection based on ‘calpain-cathepsin hypothesis’. Eur. J. Neurosci. 10:1723–1733. [DOI] [PubMed] [Google Scholar]
- Yamashima, T., A.B. Tonchev, T. Tsukada, T.C. Saido, S. Imajoh-Ohmi, T. Momoi, and E. Kominami. 2003. Sustained calpain activation associated with lysosomal rupture executes necrosis of the postischemic CA1 neurons in primates. Hippocampus. 13:791–800. [DOI] [PubMed] [Google Scholar]
- Yoshida, M., T. Yamashima, L. Zhao, K. Tsuchiya, Y. Kohda, A.B. Tonchev, M. Matsuda, and E. Kominami. 2002. Primate neurons show different vulnerability to transient ischemia and response to cathepsin inhibition. Acta Neuropathol. (Berl.). 104:267–272. [DOI] [PubMed] [Google Scholar]
- Yu, G., R. Zucchi, S. Ronca-Testoni, and G. Ronca. 2000. Protection of ischemic rat heart by dantrolene, an antagonist of the sarcoplasmic reticulum calcium release channel. Basic Res. Cardiol. 95:137–143. [DOI] [PubMed] [Google Scholar]
- Zhao, H.F., X. Wang, and G.J. Zhang. 2005. Lysosome destabilization by cytosolic extracts, putative involvement of Ca(2+)/phospholipase C. FEBS Lett. 579:1551–1556. [DOI] [PubMed] [Google Scholar]