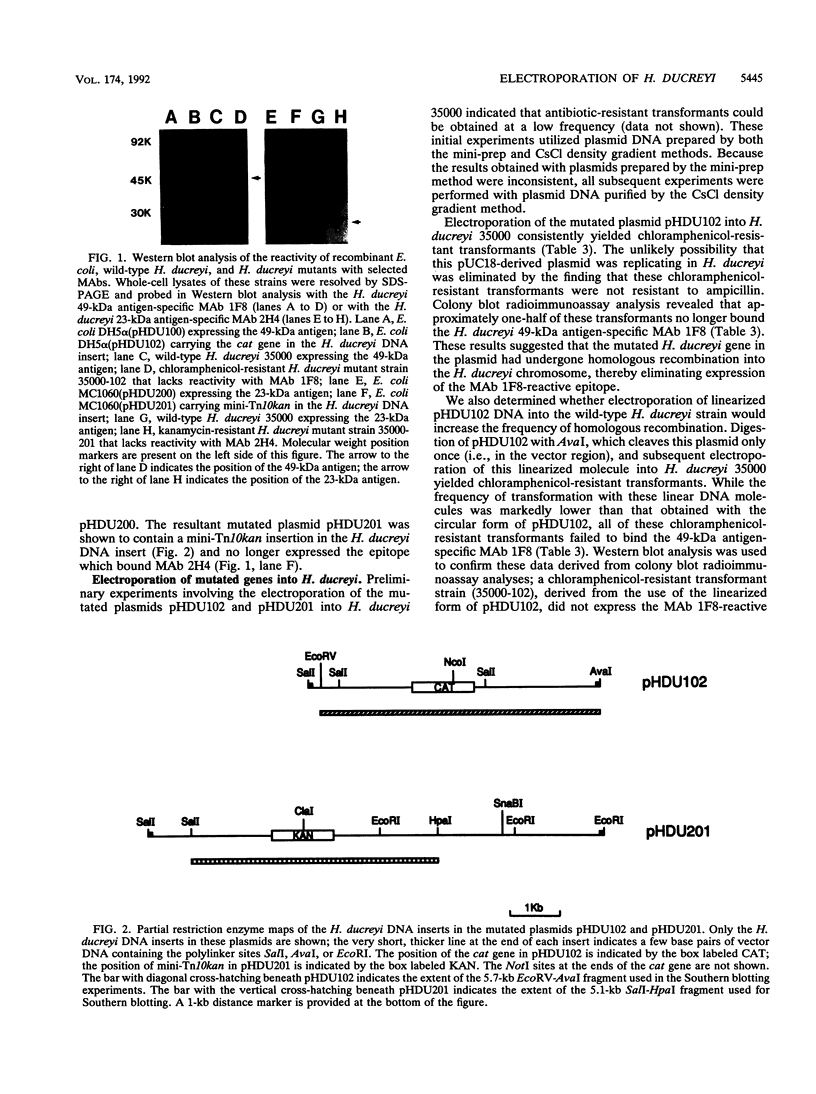
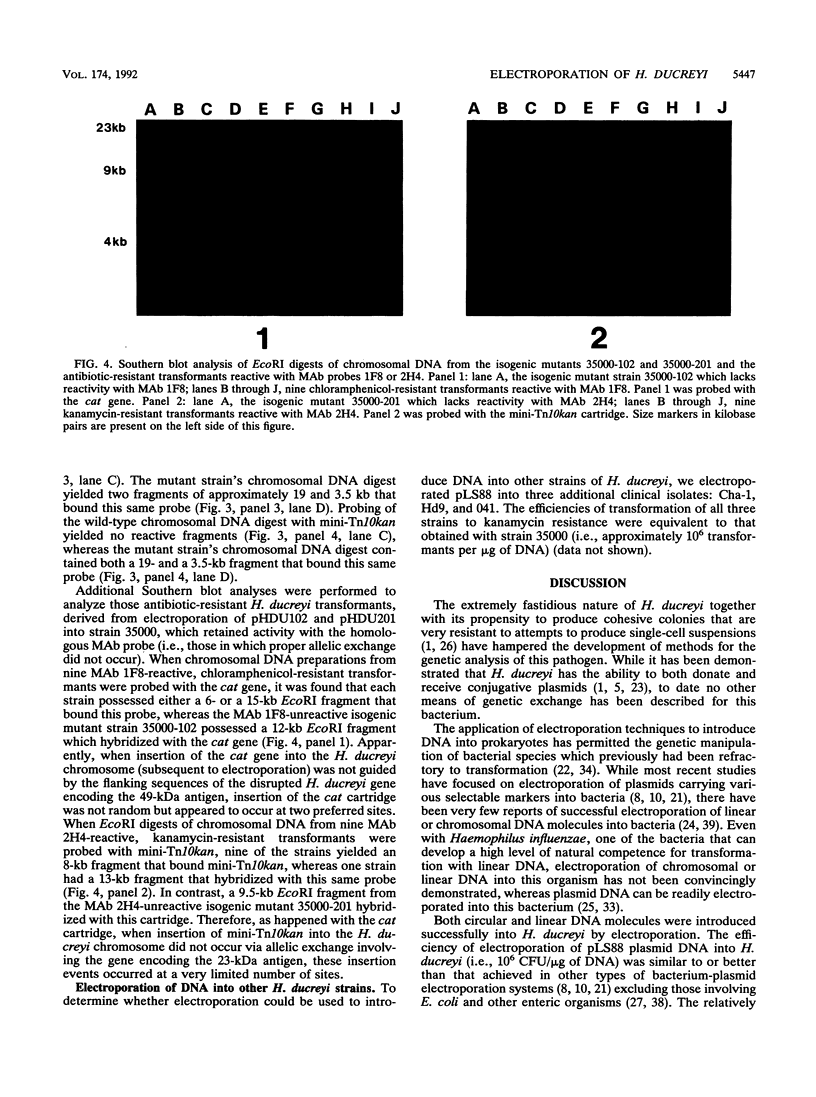
Abstract
Little is known about the genetics of Haemophilus ducreyi, the etiologic agent of chancroid. To develop a method for constructing isogenic mutants of this organism that could be utilized in pathogenesis-related studies, electroporation techniques were evaluated as a means of introducing DNA into this organism. Electroporation of the plasmid shuttle vector pLS88 into H. ducreyi yielded approximately 10(6) antibiotic-resistant transformants per microgram of plasmid DNA. Studies of the feasibility of moving mutated genes into H. ducreyi were initiated by using NotI linker insertion and mini-Tn10kan mutagenesis techniques to introduce insertion mutations into cloned H. ducreyi genes encoding cell envelope antigens. In the former case, a gene encoding chloramphenicol acetyltransferase was then inserted into the NotI linker site created in the cloned H. ducreyi gene. The recombinant Escherichia coli strains containing these mutated plasmids no longer expressed the homologous H. ducreyi cell envelope antigens, as evidenced by their lack of reactivity with monoclonal antibody probes for these H. ducreyi proteins. Subsequent electroporation of both circular and linearized forms of plasmids carrying these mutated H. ducreyi genes into the homologous wild-type strain of H. ducreyi yielded antibiotic-resistant transformants which also lacked reactivity with the cell envelope antigen-specific monoclonal antibodies. Southern blot analysis confirmed that homologous recombination had occurred in these monoclonal antibody-unreactive transformants, resulting in the replacement of the wild-type allele with the mutated allele. Allelic exchange was most efficient when linear DNA molecules were used for electroporation. These results indicate that electroporation methods can be utilized to construct isogenic mutants of H. ducreyi.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L. Biology of Haemophilus ducreyi. Microbiol Rev. 1989 Dec;53(4):377–389. doi: 10.1128/mr.53.4.377-389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J. L., Maclean I., Ronald A. R., Albritton W. L. Plasmid-mediated ampicillin resistance in Haemophilus ducreyi. Antimicrob Agents Chemother. 1979 Feb;15(2):294–299. doi: 10.1128/aac.15.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conchas R. F., Carniel E. A highly efficient electroporation system for transformation of Yersinia. Gene. 1990 Mar 1;87(1):133–137. doi: 10.1016/0378-1119(90)90505-l. [DOI] [PubMed] [Google Scholar]
- Diver J. M., Bryan L. E., Sokol P. A. Transformation of Pseudomonas aeruginosa by electroporation. Anal Biochem. 1990 Aug 15;189(1):75–79. doi: 10.1016/0003-2697(90)90046-c. [DOI] [PubMed] [Google Scholar]
- Finn G. Y., Karim Q. N., Easmon C. S. The production and characterisation of rabbit antiserum and murine monoclonal antibodies to Haemophilus ducreyi. J Med Microbiol. 1990 Mar;31(3):219–224. doi: 10.1099/00222615-31-3-219. [DOI] [PubMed] [Google Scholar]
- Greenblatt R. M., Lukehart S. A., Plummer F. A., Quinn T. C., Critchlow C. W., Ashley R. L., D'Costa L. J., Ndinya-Achola J. O., Corey L., Ronald A. R. Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS. 1988 Feb;2(1):47–50. doi: 10.1097/00002030-198802000-00008. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Loftus T. A. Monoclonal antibodies reactive with all strains of Haemophilus ducreyi. Infect Immun. 1984 Apr;44(1):196–198. doi: 10.1128/iai.44.1.196-198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., So M., McCarthy B. J. In vitro mutagenesis of a circular DNA molecule by using synthetic restriction sites. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6012–6016. doi: 10.1073/pnas.75.12.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauc L., Goodgal S. H. Introduction of transposon Tn916 DNA into Haemophilus influenzae and Haemophilus parainfluenzae. J Bacteriol. 1989 Dec;171(12):6625–6628. doi: 10.1128/jb.171.12.6625-6628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Hansen E. J. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect Immun. 1986 Jan;51(1):69–79. doi: 10.1128/iai.51.1.69-79.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F., Schurig G. G., Boyle S. M. Electroporation of a suicide plasmid bearing a transposon into Brucella abortus. Microb Pathog. 1990 Nov;9(5):363–368. doi: 10.1016/0882-4010(90)90070-7. [DOI] [PubMed] [Google Scholar]
- Latif A. S., Katzenstein D. A., Bassett M. T., Houston S., Emmanuel J. C., Marowa E. Genital ulcers and transmission of HIV among couples in Zimbabwe. AIDS. 1989 Aug;3(8):519–523. doi: 10.1097/00002030-198908000-00006. [DOI] [PubMed] [Google Scholar]
- Marcus H., Ketley J. M., Kaper J. B., Holmes R. K. Effects of DNase production, plasmid size, and restriction barriers on transformation of Vibrio cholerae by electroporation and osmotic shock. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):149–154. doi: 10.1111/j.1574-6968.1990.tb04139.x. [DOI] [PubMed] [Google Scholar]
- McIntyre D. A., Harlander S. K. Genetic transformation of intact Lactococcus lactis subsp. lactis by high-voltage electroporation. Appl Environ Microbiol. 1989 Mar;55(3):604–610. doi: 10.1128/aem.55.3.604-610.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol P. J., Albritton W. L., Ronald A. R. Characterization of ampicillin resistance plasmids of Haemophilus ducreyi and Neisseria gonorrhoeae with regard to location of origin of transfer and mobilization by a conjugative plasmid of Haemophilus ducreyi. J Bacteriol. 1983 Oct;156(1):437–440. doi: 10.1128/jb.156.1.437-440.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheletti P. A., Sment K. A., Konisky J. Isolation of a coenzyme M-auxotrophic mutant and transformation by electroporation in Methanococcus voltae. J Bacteriol. 1991 Jun;173(11):3414–3418. doi: 10.1128/jb.173.11.3414-3418.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. A., Skowronek K., Kauc L., Goodgal S. H. Electroporation of Haemophilus influenzae is effective for transformation of plasmid but not chromosomal DNA. Nucleic Acids Res. 1991 Jul 11;19(13):3625–3628. doi: 10.1093/nar/19.13.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989 Apr;2(2):137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D., Charbit A. High efficiency transformation of Salmonella typhimurium and Salmonella typhi by electroporation. Mol Gen Genet. 1990 Aug;223(1):156–158. doi: 10.1007/BF00315809. [DOI] [PubMed] [Google Scholar]
- Odumeru J. A., Alfa M. J., Martin C. F., Ronald A. R., Jay F. T. Production of monoclonal antibodies specific for Haemophilus ducreyi: a screening method to discriminate specific and cross-reacting antibodies. Hybridoma. 1989 Jun;8(3):337–351. doi: 10.1089/hyb.1989.8.337. [DOI] [PubMed] [Google Scholar]
- Patrick C. C., Kimura A., Jackson M. A., Hermanstorfer L., Hood A., McCracken G. H., Jr, Hansen E. J. Antigenic characterization of the oligosaccharide portion of the lipooligosaccharide of nontypable Haemophilus influenzae. Infect Immun. 1987 Dec;55(12):2902–2911. doi: 10.1128/iai.55.12.2902-2911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell B. K., Richardson J. A., Radolf J. D., Hansen E. J. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J Infect Dis. 1991 Aug;164(2):359–367. doi: 10.1093/infdis/164.2.359. [DOI] [PubMed] [Google Scholar]
- Schalla W. O., Sanders L. L., Schmid G. P., Tam M. R., Morse S. A. Use of dot-immunobinding and immunofluorescence assays to investigate clinically suspected cases of chancroid. J Infect Dis. 1986 May;153(5):879–887. doi: 10.1093/infdis/153.5.879. [DOI] [PubMed] [Google Scholar]
- Sreenivasan P. K., LeBlanc D. J., Lee L. N., Fives-Taylor P. Transformation of Actinobacillus actinomycetemcomitans by electroporation, utilizing constructed shuttle plasmids. Infect Immun. 1991 Dec;59(12):4621–4627. doi: 10.1128/iai.59.12.4621-4627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomb J. F., Barcak G. J., Chandler M. S., Redfield R. J., Smith H. O. Transposon mutagenesis, characterization, and cloning of transformation genes of Haemophilus influenzae Rd. J Bacteriol. 1989 Jul;171(7):3796–3802. doi: 10.1128/jb.171.7.3796-3802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Willson P. J., Albritton W. L., Slaney L., Setlow J. K. Characterization of a multiple antibiotic resistance plasmid from Haemophilus ducreyi. Antimicrob Agents Chemother. 1989 Sep;33(9):1627–1630. doi: 10.1128/aac.33.9.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabarovsky E. R., Winberg G. High efficiency electroporation of ligated DNA into bacteria. Nucleic Acids Res. 1990 Oct 11;18(19):5912–5912. doi: 10.1093/nar/18.19.5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zealey G. R., Loosmore S. M., Yacoob R. K., Cockle S. A., Boux L. J., Miller L. D., Klein M. H. Gene replacement in Bordetella pertussis by transformation with linear DNA. Biotechnology (N Y) 1990 Nov;8(11):1025–1029. doi: 10.1038/nbt1190-1025. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]