Abstract
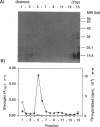
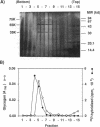
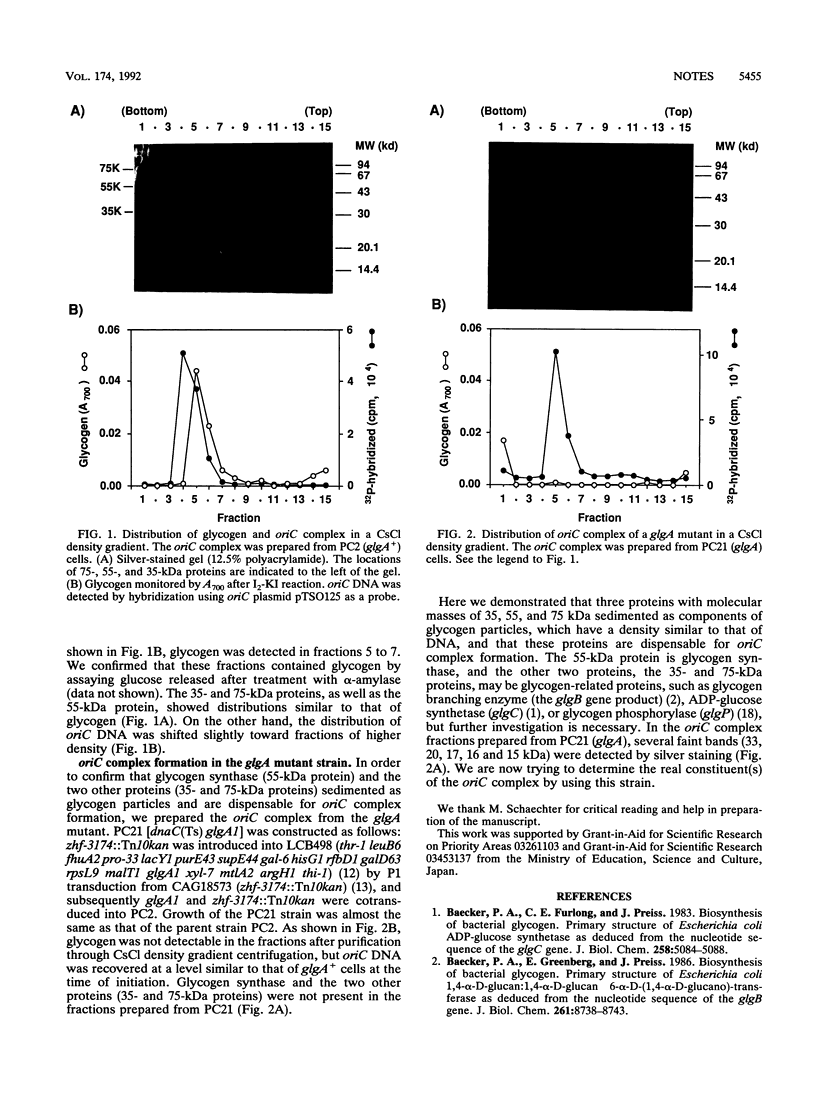
Three proteins with molecular masses of 35, 55, and 75 kDa were found in an oriC complex fraction after purification through CsCl density gradient centrifugation (W. G. Hendrickson, T. Kusano, H. Yamaki, R. Balakrishnan, M. King, J. Murchie, and M. Schaechter, Cell 30:915-923, 1982). Of these three proteins, the 55-kDa protein was determined to be glycogen synthase on the basis of the N-terminal amino acid sequence and the molecular weight. The oriC complex was formed in glgA mutant cells, which produce no detectable glycogen, as well as in wild-type cells. None of the 35-, 55-, and 75-kDa proteins were detected in the fraction from this mutant. The results indicate that these proteins were not constituents of the oriC complex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baecker P. A., Furlong C. E., Preiss J. Biosynthesis of bacterial glycogen. Primary structure of Escherichia coli ADP-glucose synthetase as deduced from the nucleotide sequence of the glg C gene. J Biol Chem. 1983 Apr 25;258(8):5084–5088. [PubMed] [Google Scholar]
- Baecker P. A., Greenberg E., Preiss J. Biosynthesis of bacterial glycogen. Primary structure of Escherichia coli 1,4-alpha-D-glucan:1,4-alpha-D-glucan 6-alpha-D-(1, 4-alpha-D-glucano)-transferase as deduced from the nucleotide sequence of the glg B gene. J Biol Chem. 1986 Jul 5;261(19):8738–8743. [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Gayama S., Kataoka T., Wachi M., Tamura G., Nagai K. Periodic formation of the oriC complex of Escherichia coli. EMBO J. 1990 Nov;9(11):3761–3765. doi: 10.1002/j.1460-2075.1990.tb07589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W. G., Kusano T., Yamaki H., Balakrishnan R., King M., Murchie J., Schaechter M. Binding of the origin of replication of Escherichia coli to the outer membrane. Cell. 1982 Oct;30(3):915–923. doi: 10.1016/0092-8674(82)90296-3. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Gayama S., Takahashi K., Wachi M., Yamasaki M., Nagai K. Only oriC and its flanking region are recovered from the complex formed at the time of initiation of chromosome replication in Escherichia coli. Res Microbiol. 1991 Feb-Apr;142(2-3):155–159. doi: 10.1016/0923-2508(91)90024-5. [DOI] [PubMed] [Google Scholar]
- Kumar A., Larsen C. E., Preiss J. Biosynthesis of bacterial glycogen. Primary structure of Escherichia coli ADP-glucose:alpha-1,4-glucan, 4-glucosyltransferase as deduced from the nucleotide sequence of the glgA gene. J Biol Chem. 1986 Dec 5;261(34):16256–16259. [PubMed] [Google Scholar]
- Kusano T., Steinmetz D., Hendrickson W. G., Murchie J., King M., Benson A., Schaechter M. Direct evidence for specific binding of the replicative origin of the Escherichia coli chromosome to the membrane. J Bacteriol. 1984 Apr;158(1):313–316. doi: 10.1128/jb.158.1.313-316.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffan J., Firshein W. DNA replication by a DNA-membrane complex extracted from Bacillus subtilis: site of initiation in vitro and initiation potential of subcomplexes. J Bacteriol. 1987 Jun;169(6):2819–2827. doi: 10.1128/jb.169.6.2819-2827.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Hendrickson W., Balakrishnan R., Yamaki H., Boyd D., Schaechter M. Isolation of a replication origin complex from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):262–266. doi: 10.1073/pnas.77.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N., Puig J. Etude génétique des mutants du système de la glycogénèse chez Escherichia coli K 12. C R Acad Sci Hebd Seances Acad Sci D. 1968 Oct 7;267(15):1223–1226. [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Oka A., Sugisaki H., Takanami M., Nishimura A., Yasuda Y., Hirota Y. Nucleotide sequence of Escherichia coli K-12 replication origin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):575–579. doi: 10.1073/pnas.76.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto M., Kambe-Honjoh H., Nagai K., Tamura G. Early replicative intermediates of Escherichia coli chromosome isolated from a membrane complex. EMBO J. 1986 Apr;5(4):787–791. doi: 10.1002/j.1460-2075.1986.tb04282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Jen Y., Takeuchi E., Inouye M., Nakayama H., Tagaya M., Fukui T. Alpha-glucan phosphorylase from Escherichia coli. Cloning of the gene, and purification and characterization of the protein. J Biol Chem. 1988 Sep 25;263(27):13706–13711. [PubMed] [Google Scholar]