Abstract
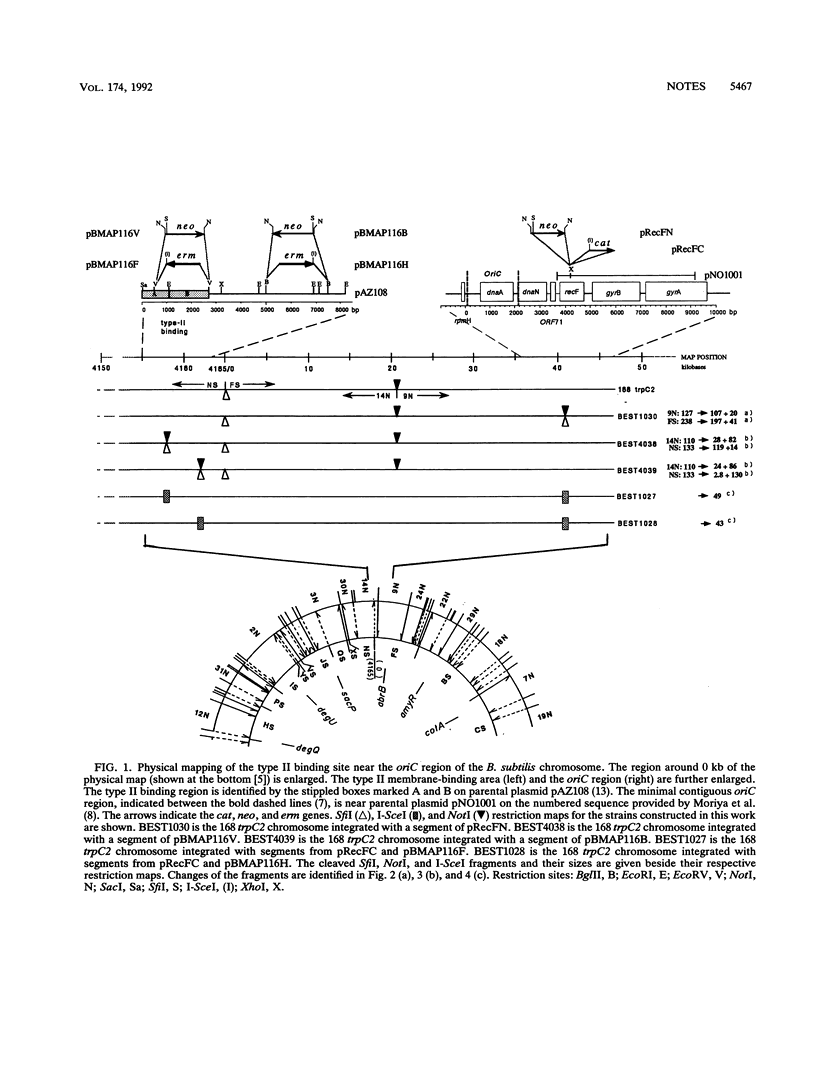
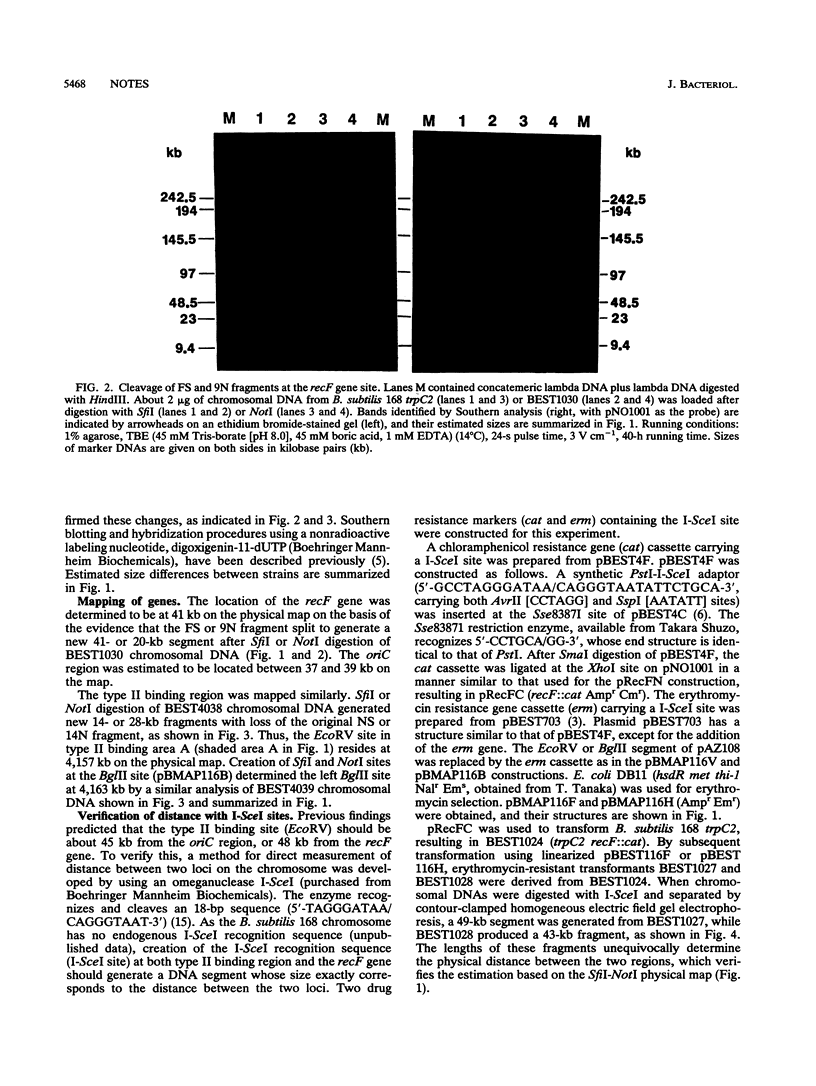
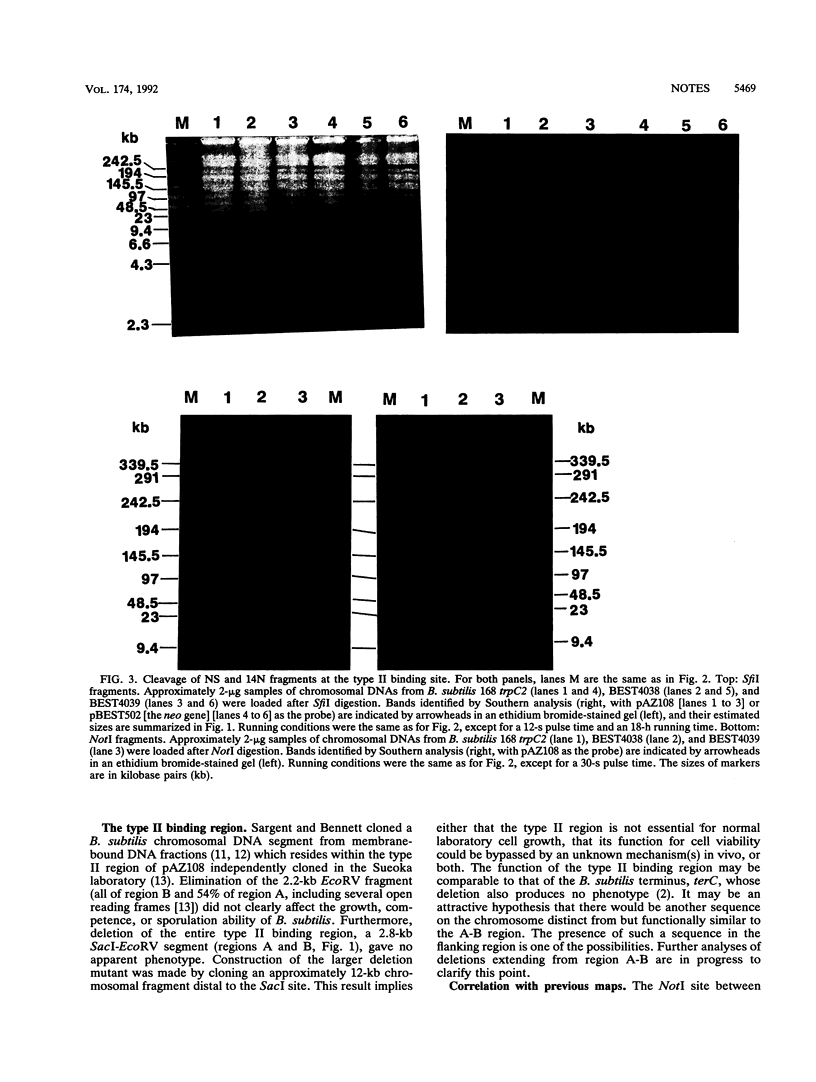
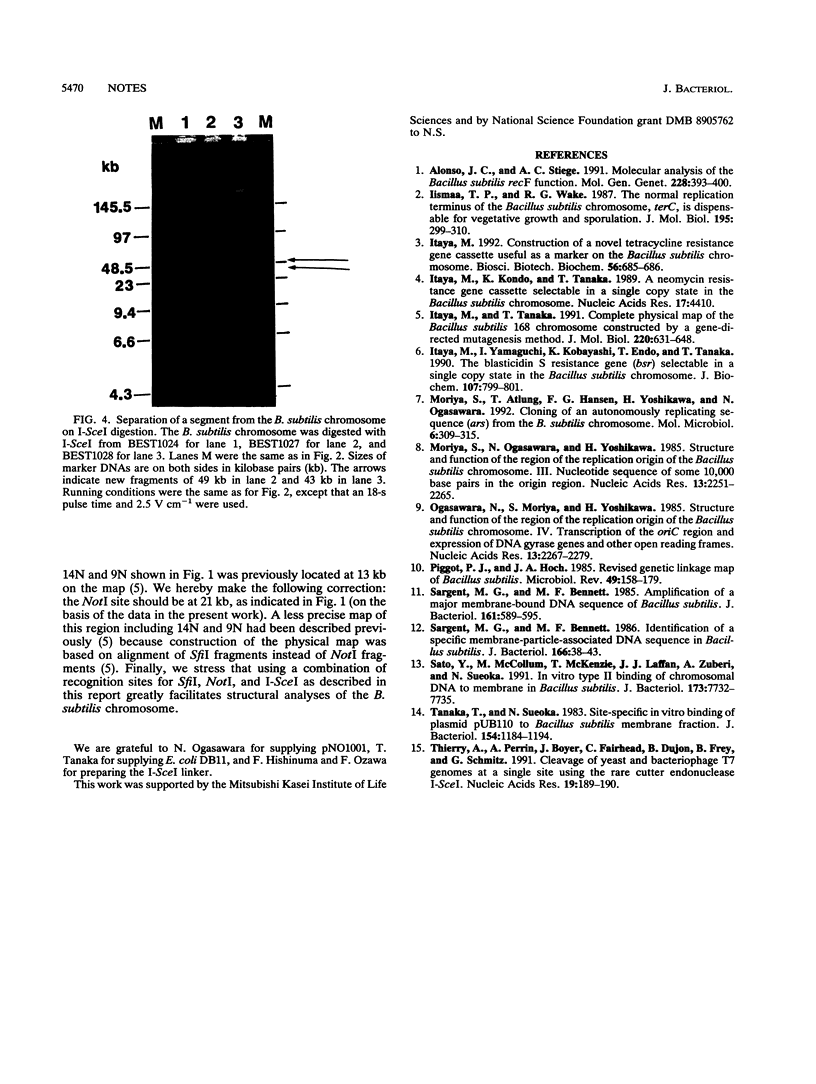
The precise physical locations of the oriC region and the region for type II DNA binding to the membrane on the Bacillus subtilis 168 chromosome were determined. The DNA regions were physically mapped by creating new restriction sites (NotI and SfiI) within these regions. The physical distance between oriC and the type II DNA-binding region was verified with the creation of a novel sequence cleaved by endonuclease I-SceI in each of the above regions. Complete removal of the defined type II membrane-binding region produced no noticeable phenotype.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso J. C., Stiege A. C. Molecular analysis of the Bacillus subtilis recF function. Mol Gen Genet. 1991 Sep;228(3):393–400. doi: 10.1007/BF00260632. [DOI] [PubMed] [Google Scholar]
- Iismaa T. P., Wake R. G. The normal replication terminus of the Bacillus subtilis chromosome, terC, is dispensable for vegetative growth and sporulation. J Mol Biol. 1987 May 20;195(2):299–310. doi: 10.1016/0022-2836(87)90651-6. [DOI] [PubMed] [Google Scholar]
- Itaya M. Construction of a novel tetracycline resistance gene cassette useful as a marker on the Bacillus subtilis chromosome. Biosci Biotechnol Biochem. 1992 Apr;56(4):685–686. doi: 10.1271/bbb.56.685. [DOI] [PubMed] [Google Scholar]
- Itaya M., Kondo K., Tanaka T. A neomycin resistance gene cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acids Res. 1989 Jun 12;17(11):4410–4410. doi: 10.1093/nar/17.11.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya M., Tanaka T. Complete physical map of the Bacillus subtilis 168 chromosome constructed by a gene-directed mutagenesis method. J Mol Biol. 1991 Aug 5;220(3):631–648. doi: 10.1016/0022-2836(91)90106-g. [DOI] [PubMed] [Google Scholar]
- Itaya M., Yamaguchi I., Kobayashi K., Endo T., Tanaka T. The blasticidin S resistance gene (bsr) selectable in a single copy state in the Bacillus subtilis chromosome. J Biochem. 1990 Jun;107(6):799–801. doi: 10.1093/oxfordjournals.jbchem.a123128. [DOI] [PubMed] [Google Scholar]
- Moriya S., Atlung T., Hansen F. G., Yoshikawa H., Ogasawara N. Cloning of an autonomously replicating sequence (ars) from the Bacillus subtilis chromosome. Mol Microbiol. 1992 Feb;6(3):309–315. doi: 10.1111/j.1365-2958.1992.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Moriya S., Ogasawara N., Yoshikawa H. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. III. Nucleotide sequence of some 10,000 base pairs in the origin region. Nucleic Acids Res. 1985 Apr 11;13(7):2251–2265. doi: 10.1093/nar/13.7.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N., Moriya S., Yoshikawa H. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. IV. Transcription of the oriC region and expression of DNA gyrase genes and other open reading frames. Nucleic Acids Res. 1985 Apr 11;13(7):2267–2279. doi: 10.1093/nar/13.7.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Hoch J. A. Revised genetic linkage map of Bacillus subtilis. Microbiol Rev. 1985 Jun;49(2):158–179. doi: 10.1128/mr.49.2.158-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Bennett M. F. Amplification of a major membrane-bound DNA sequence of Bacillus subtilis. J Bacteriol. 1985 Feb;161(2):589–595. doi: 10.1128/jb.161.2.589-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Bennett M. F. Identification of a specific membrane-particle-associated DNA sequence in Bacillus subtilis. J Bacteriol. 1986 Apr;166(1):38–43. doi: 10.1128/jb.166.1.38-43.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., McCollum M., McKenzie T., Laffan J., Zuberi A., Sueoka N. In vitro type II binding of chromosomal DNA to membrane in Bacillus subtilis. J Bacteriol. 1991 Dec;173(23):7732–7735. doi: 10.1128/jb.173.23.7732-7735.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Sueoka N. Site-specific in vitro binding of plasmid pUB110 to Bacillus subtilis membrane fraction. J Bacteriol. 1983 Jun;154(3):1184–1194. doi: 10.1128/jb.154.3.1184-1194.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry A., Perrin A., Boyer J., Fairhead C., Dujon B., Frey B., Schmitz G. Cleavage of yeast and bacteriophage T7 genomes at a single site using the rare cutter endonuclease I-Sce I. Nucleic Acids Res. 1991 Jan 11;19(1):189–190. doi: 10.1093/nar/19.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]