Abstract
The expression of tissue-specific genes during mammary gland differentiation relies on the coincidence of two distinct signaling events: the continued engagement of β1 integrins with the extracellular matrix (ECM) and a hormonal stimulus from prolactin (Prl). How the integrin and Prl receptor (PrlR) systems integrate to regulate milk protein gene synthesis is unknown. In this study, we identify Rac1 as a key link. Dominant-negative Rac1 prevents Prl-induced synthesis of the milk protein β-casein in primary mammary epithelial cells cultured as three-dimensional acini on basement membrane. Conversely, activated Rac1 rescues the defective β-casein synthesis that occurs under conditions not normally permissive for mammary differentiation, either in β1 integrin–null cells or in wild-type cells cultured on collagen. Rac1 is required downstream of integrins for activation of the PrlR/Stat5 signaling cascade. Cdc42 is also necessary for milk protein synthesis but functions via a distinct mechanism to Rac1. This study identifies the integration of signals provided by ECM and hormones as a novel role for Rho family guanosine triphosphatases.
Introduction
Morphogenesis and function of epithelial tissues involve the coordinated regulation of proliferation, apoptosis, differentiation, and migration. In many cases, these physiological processes are orchestrated by a combination of signals from the ECM through integrins and soluble factors including steroid or peptide hormones and growth factors (Giancotti and Tarone, 2003). One tissue that has been used to understand the molecular basis of epithelial differentiation is the mammary gland. This tissue develops in a temporal and spatially regulated manner so that the epithelial cells only produce their differentiation products, such as milk proteins, at the right time and place (i.e., during lactation and in cells that are spatially restricted to acini). Although endocrine signals such as prolactin (Prl) control differentiation in a temporal fashion, adhesion to basement membrane (BM; a specialized form of the ECM) is also required for lactation. Thus, to respond to the biological requirements of the organism, the epithelial cells need to integrate signals from both soluble factors and the ECM. Our laboratory has used the mammary gland system as a paradigm to dissect the molecular basis of signal integration by soluble factors and ECM, and, in the present study, we demonstrate a novel and key role for Rho family GTPases.
The ECM control of mammary epithelial cell (MEC) differentiation occurs at two distinct levels. First, matrix specificity is critical because the BM protein laminin-1 supports Prl-dependent activation of the Jak2–Stat5 signaling pathway and the transcription of Prl- and Stat5-regulated milk protein genes (e.g., β-casein), whereas adhesion to the stromal protein collagen I does not (Streuli et al., 1995b). Second, β1 integrins are actively required for Prl signaling both in culture and in vivo because function-perturbing anti–β1 integrin antibodies block MEC differentiation (Streuli et al., 1991), a dominant-negative (DN) β1 integrin transgene compromises Stat5 activation and milk production (Faraldo et al., 2002), and Prl cannot activate Stat5 in β1 integrin–null MECs (Naylor et al., 2005). Thus, integrins regulate Stat5 transcription factor activation and expression of tissue-specific genes, but the mechanism underpinning the requirement for adhesion receptors is not yet known.
Rho GTPases are good candidates to relay the adhesion-mediated signals provided by integrins. These enzymes are molecular switches that are turned on by guanine nucleotide exchange factors and have a broad function in cell division, survival, migration, and polarity (Ridley, 2001). They coordinate various cellular responses through specific effector proteins to regulate focal adhesion complexes, cell–cell junctions, actin dynamics, and the generation of reactive oxygen species (Akhtar and Hotchin, 2001; DeMali et al., 2003; Radisky et al., 2005), but their role in differentiation and gene expression has not been studied widely. Because Rho GTPases can affect the activity of receptors within the plasma membrane (e.g., epidermal growth factor receptor; Wu et al., 2003), we reasoned that they might provide a mechanistic link to integrate ECM and Prl signals and, thus, control epithelial cell differentiation.
Rho GTPases have a role in the morphogenesis and differentiation of some cell types; for example, Rac and Cdc42 regulate lumen formation in endothelial capillaries, the establishment of apical-basal polarity and tubulogenesis in kidney epithelia, and keratinocyte terminal differentiation (Rogers et al., 2003; Benitah et al., 2005). In the mammary gland, Rho GTPases have been studied in cancer cells, where it has been shown that Rac1 and Cdc42 mediate motility, whereas Rho is important for the tubulogenesis of T47D cells. Rac1 also influences survival through nuclear factor κB in transformed HMT-3522 cells, and Rac1B contributes to the genomic instability of breast cancer (Keely et al., 1997; Wozniak et al., 2003; Zahir et al., 2003; Radisky et al., 2005).
In this study, we uncover a key role for Rac1 in the differentiation of normal, untransformed MECs. We have demonstrated that laminin and β1 integrins are essential for Prl signaling and milk protein gene expression and now show that Rac1 provides a mechanism for their integration. This study is the first to demonstrate the involvement of Rho family GTPases in the expression of tissue-specific genes during the process of glandular epithelial differentiation.
Results
Rac and Cdc42 signaling is required for milk protein synthesis
Cultured mammary epithelia organize themselves into 3D acini when they are plated onto reconstituted BM matrix (Matrigel) and secrete milk proteins into the inner lumina when stimulated with lactogenic hormones (Barcellos-Hoff et al., 1989). They are morphologically and functionally similar to lactating acini in vivo (Aggeler et al., 1991). To analyze the effects of Rho GTPases on milk protein synthesis and, thus, lactational differentiation, primary MECs were infected with adenoviruses expressing DN forms of RhoA, Rac1, and Cdc42 (Ad-mycN19RhoA, Ad-mycN17Rac1, or Ad-mycN17Cdc42) or a control adenovirus (Ad–β-galactosidase) for 1 h in suspension at an MOI of 50 (which yielded >90 ± 5% infection). Infected cells were plated on BM matrix to assemble into 3D acini for 24 h and stimulated with Prl for a further 24 h. Prl induced the synthesis of β-casein, a mammary-specific differentiation marker, in uninfected cells; however, synthesis was either completely abolished or substantially decreased in cells expressing N17Rac1 or N17Cdc42, respectively (Fig. 1 A). In contrast, β-galactosidase and N19RhoA had no discernable effects on β-casein synthesis even though it did prevent stress fiber formation (unpublished data). The prevention of milk protein synthesis was confirmed by immunofluorescence staining (Fig. 1 B). The effects of N17Rac1 and N17Cdc42 were not caused by apoptosis (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200601059/DC1). These data indicate that DN Rac1 and DN Cdc42 specifically block milk protein synthesis, suggesting that these GTPases have a key role in regulating epithelial cell differentiation.
Figure 1.
Rac and Cdc42 are essential for milk protein expression. (A) MECs were infected with Ad–β-galactosidase (lane 4), Ad-mycN19RhoA (lane 5), Ad-mycN17Rac1 (lane 6), Ad-mycN17Cdc42 (lane 7), or were not infected (lanes 1–3) and plated onto BM matrix for 24 h to assemble into 3D acini. Cultures were stimulated with Prl in differentiation medium for a further 24 h (lanes 2–7) or left untreated (lane 1), lysed, and immunoblotted with β-casein, β-galactosidase, or myc antibodies. Equal loading of protein in each lane was determined by levels of Erk. (B) As in A, but acini were stained for β-casein (red) and nuclei (blue). Micrographs are from sections taken midway through the spherical acinar structure. Bar, 7 μm.
DN Rac and DN Cdc42 inhibit the Prl signaling cascade
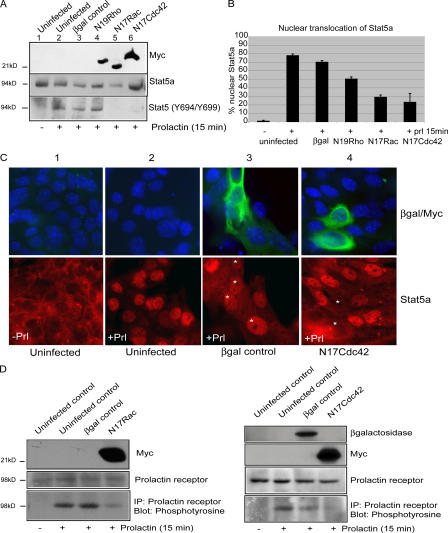
Prl-dependent β-casein expression involves tyrosine phosphorylation and nuclear translocation of the transcription factor Stat5. Therefore, we reasoned that Rac1 and Cdc42 might regulate β-casein expression through modulation of this signaling cascade. The aforementioned virally infected cells were plated onto BM matrix for 24 h to assemble into 3D acini, serum starved for a further 24 h, and stimulated with Prl for 15 min before analysis by immunoblotting with a phospho–Stat5-Y694/699 antibody. In a separate assay to quantify the percentage of cells in which Prl induced Stat5 translocation to the nucleus, monolayer cultured cells were differentiated by adding diluted BM matrix to the culture medium (i.e., 2D culture with BM overlay) before stimulation with Prl for 15 min.
Prl induced Stat5 tyrosine phosphorylation in 3D acini (Fig. 2 A) and nuclear translocation of Stat5a in ∼75% of monolayer cells treated in the overlay assay (Fig. 2, B and C). In virally infected cells, N17Rac1 and N17Cdc42 but not control β-galactosidase abolished Stat5 phosphorylation and reduced its nuclear translocation by 70–80%. N19RhoA did not affect Stat5 phosphorylation, but, interestingly, it slightly inhibited Stat5a nuclear translocation compared with uninfected controls.
Figure 2.
Rac and Cdc42 regulate signal transduction through PrlR and Stat5. (A) MECs were uninfected (lanes 1 and 2) or infected with adenovirus as shown and plated onto BM matrix. 24 h later, acini were serum starved in differentiation medium for 24 h, stimulated with Prl for 15 min (lanes 2–6) or left untreated (lane 1), lysed, and immunoblotted with phospho-Stat5a/b (Y694/Y699) antibody or myc antibody. Total levels of Stat5 were determined by stripping and reprobing with an anti-Stat5a antibody. (B and C) Nuclear translocation of Stat5a was determined in virus-infected monolayer cells, and BM matrix was added to the medium (i.e., 2D culture with BM overlay). Cells were stimulated with Prl for 15 min or left untreated, fixed, and stained with an anti-Stat5a antibody and an anti-myc or anti–β-galactosidase antibody as described. (B) Percentage of cells displaying nuclear Stat5a (mean of six counts from two separate experiments). Error bars represent SD. (C) Representative micrographs. In noninfected cells, Stat5a translocates to the nucleus in response to Prl (compare panels 1 with 2); in Ad–β-galactosidase–infected cells (green, panel 3), Stat5a still translocates. However, the expression of N17Cdc42 inhibits Stat5a translocation (green, panel 4); similar images were obtained with N17Rac1 (not depicted). Asterisks represent infected cells. (D) MECs infected with adenovirus and plated onto BM matrix as in A. Tyrosine phosphorylation of PrlR was analyzed by immunoprecipitation with an anti-PrlR antibody followed by immunoblotting with 4G10. Total levels of PrlR was determined by blotting with anti-PrlR antibody, and viral infection was confirmed with anti– β-galactosidase or anti-myc antibodies.
To determine whether Rac1 or Cdc42 is required for signaling upstream of Stat5, we examined Prl receptor (PrlR) tyrosine phosphorylation. PrlR has no intrinsic kinase activity, and Jak2 phosphorylates its tyrosine residues in response to ligand binding. Although N17Rac1 and N17Cdc42 had no effect on the total levels of PrlR, both substantially diminished PrlR phosphorylation by its ligand, Prl (Fig. 2 D).
Thus, DN Rac1 and DN Cdc42 block the Prl signaling axis, thereby providing an explanation for the prevention of milk protein gene expression. The ability of Prl to signal through its receptor is already known to require both laminin and β1 integrins. Our experiments suggest that small GTPases may provide a functional link to integrate PrlR and integrin signals.
Rac1 activity is regulated by adhesion to BM, whereas Cdc42 is activated by lactogenic hormones
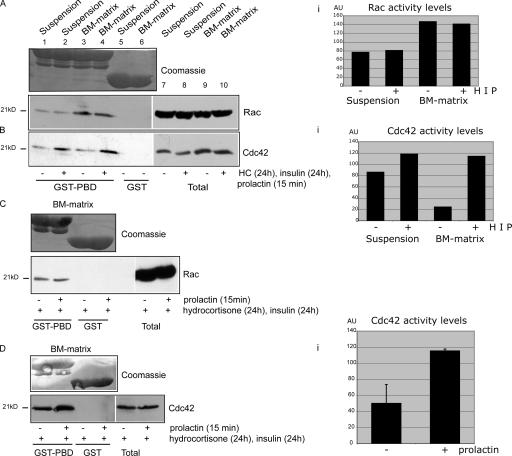
To determine which extracellular factors regulate endogenous GTPase activity during the differentiation process, we analyzed their activity using pull downs with the p21-activated protein kinase (PAK)–binding domain (PBD) either in the absence of integrin-mediated adhesion (i.e., in suspension cultures on polyHEMA) or in 3D acini cultured on BM matrix in the presence or absence of hydrocortisone, insulin, and Prl.
Rac activity was dependent on cell interactions with ECM because it was unable to bind PBD efficiently in suspension (Fig. 3 A), as seen in other cell types (del Pozo et al., 2000).Rac activity was not dependent on the milieu of lactogenic hormones (Fig. 3, A and C). In contrast, Cdc42 did not require adhesion to the BM to be active but instead was sensitive to the hormones, hydrocortisone, insulin, and Prl (Fig. 3 B). To establish whether Prl can regulate Cdc42 independently, cells cultured in the continuous presence of hydrocortisone and insulin were stimulated with Prl for 15 min. A twofold increase in Cdc42 activity was observed in Prl-treated acini (Fig. 3 D).
Figure 3.
Rac activity is regulated by BM matrix, whereas lactogenic hormones regulate Cdc42. (A and B) Rac (A) and Cdc42 (B) activity were analyzed using recombinant GST–Pak-binding domain (PBD) in pull-down assays of lysates from 3D acini plated on growth factor–reduced BM matrix (for 4 d) or cells placed in suspension (for 2 h). Free GST was used in controls to confirm specificity. MECs were cultured in differentiation medium with or without hydrocortisone and insulin continuously and Prl for 15 min. The Coomassie stain demonstrates that equivalent levels of GST-PBD and free GST were used in each sample. Lanes 1–4 were scanned, and the relative levels of Rac and Cdc42 activity were plotted in Ai and Bi. HIP; hydrocortisone, insulin, and Prl. (C and D) Rac (C) and Cdc42 (D) activity was assessed in 3D acini cultured in differentiation medium containing hydrocortisone and insulin and stimulated with Prl (15 min) or left untreated. Prl treatment increased Cdc42 activity but not Rac activity. The relative level and Cdc42 activity is plotted in Di. Error bars represent SD. AU, arbitrary units. White lines indicate that intervening lanes have been spliced out.
Therefore, Rac and Cdc42 are regulated by distinct mechanisms in primary MECs. Rac activation requires adhesion to the BM matrix but is not hormone dependent. On the other hand, Cdc42 is triggered primarily by hydrocortisone and insulin and can be activated further by Prl.
Rac and Cdc42 regulate acinar organization and lumen formation
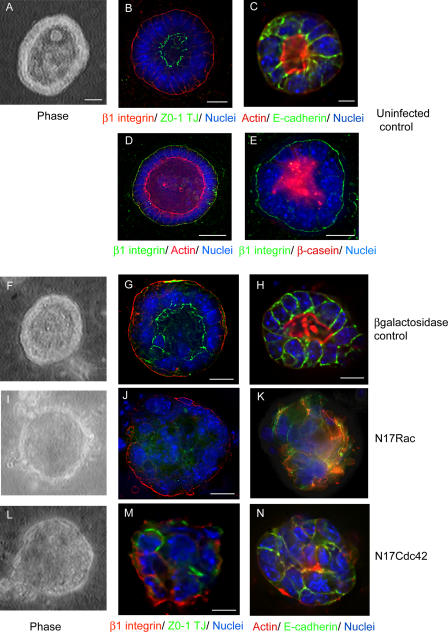
Because Rho GTPases regulate the actin cytoskeleton, cell–cell adhesion, cell polarity, and focal adhesion dynamics, it is possible that they contribute to mammary differentiation through one or more of those mechanisms. To determine their role in 3D acinar configuration, we investigated the effects of N17Rac1 and N17Cdc42 on the distribution of basal (β1 integrin) and lateral (E-cadherin) proteins as well as apical markers (actin and ZO-1, which localizes at the apical region of lateral surfaces). Small acini ranging between 15 and 25 μm in diameter were analyzed because these polarize within 2 d of plating onto BM matrix and lumen formation does not require apoptotic clearance of cells, which is in contrast to those that form larger acini (Debnath et al., 2002). In primary cultures, acini form by reorganization of cells rather than proliferation from a single cell. Stacks of fluorescent images were deconvolved to reveal protein localization within the center of the acini, where lumina are visible, and are shown as individual (Fig. 4) or multiple acini (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200601059/DC1).
Figure 4.
N17Rac and -Cdc42 disrupt the morphogenesis of 3D acini. (A–E) Cells plated on BM matrix form polarized 3D acini. (A) Phase-contrast micrograph of uninfected MECs showing cells at the periphery of an acinus and a central lumen. (B and C) 2-d-old polarized acini stained for ZO-1 (green) and β1 integrin (red; B) or actin (red) and E-cadherin (green; C). (D) 4-d-old acinus with expanded lumen stained for actin (red) and β1 integrin (green). (E) Prl-stimulated acinus showing β-casein (red) secreted into the lumen and β1 integrin (green) at the basal domain. Low-power views of some of these panels to show more acini are presented in Fig. S2 (available at http://www.jcb.org/cgi/content/full/jcb.200601059/DC1). (F–N) MECs were infected with Ad–β-galactosidase (F–H), Ad-mycN17Rac1 (I–K), or Ad-mycN17Cdc42 (L–N) viruses for 1 h in suspension and were plated onto BM matrix–coated coverslips for 48 h. 3D acini were stained for β1 integrin (red) and ZO-1 (green; G, J, and M) or E-cadherin (green) and actin (red; H, K, and N). Phase-contrast micrographs show the absence of lumen in N17Rac1 and N17Cdc42 acini (I and L, respectively) but not in controls (F). Nuclei are stained blue. Virus infection was confirmed by immunostaining with anti– β-galactosidase antibody or anti-myc antibody (not depicted). Micrographs are sections taken midway through acini. Bar (D),10 μM; (A–C, E, G, H, J, and M) 7 μM.
Polarized control acini were contained as a single layer of cells surrounding a hollow lumen (Fig. 4, A and F; and Fig. S2, A and B). β1 integrins were localized to the basal domain of each cell, which faced the exterior and contacted the matrix (Fig. 4, B, D, E, and G; and Fig. S2, E and F). E-cadherin was located at intercellular junctions (Fig. 4, C and H). The apical domain was opposed to the lumen, where ZO-1 tight junctions (Fig. 4, B and G) and a concentration of actin structures assembled (Fig. 4, C, D, and H). Small lumens were visible in 2-d-old acini (Fig. 4, C and G), but these expanded during extended culture of >4 d (Fig. 4 D), and milk proteins were secreted into the lumen when stimulated with Prl (Fig. 4 E).
Acini expressing N17Rac1 and N17Cdc42 lost their polarized structure, and nuclei filled the luminal space. No visible lumina were evident by phase-contrast microscopy in acini expressing N17Rac1 or N17Cdc42 compared with controls (Fig. 4, I and L; and Fig. S2, C and D).
N17Rac1-expressing acini displayed disorganized apical F-actin in 89% of cases (Fig. 4 K) and ZO-1 in 92% of acini (Fig. 4 J); E-cadherin was disrupted at intercellular regions in 68% of acini (Fig. 4 K). However, β1 integrins were still retained at the basal plasma membrane, with perturbation in only 22% of cases (Fig. 4 J and Fig. S2 G).
Similar to N17Rac1, the arrangement of F-actin (Fig. 4 N) and ZO-1 (Fig. 4 M and Fig. S2 H) in N17Cdc42-expressing acini was disrupted in 78 and 72% of cases, respectively; E-cadherin was only partially disrupted at intercellular junctions (42%; Fig. 4 N). However, in contrast to N17Rac1, the basal distribution of integrins was perturbed in 80% of acini (Fig. 4 M and Fig. S2 H).
These results highlight distinct effects of N17Cdc42 and N17Rac1. DN Cdc42 has a dramatic effect on acinar polarization and also disrupts the basal distribution of integrins. In contrast, DN Rac1 partially perturbs polarity, but β1 integrins remain at the basal surface of acini, where cells contact the BM.
Rac and Cdc42 regulate epithelial differentiation through distinct mechanisms
To establish whether altered acinar organization is a mechanism by which these GTPases affect differentiation, we inhibited Cdc42 and Rac1 function in acini after they had formed and become polarized. Under these conditions, F-actin still localized apically and small lumens were present (Fig. 5, B and C; long arrow), although some cells were visible in the lumens (Fig. 5, B and C; short arrows).
Figure 5.
Rac and Cdc42 regulate differentiation through distinct mechanisms. Preassembled polarized 3D acini with lumens that formed after plating on BM matrix for 48 h were left untreated (A and F) or were infected directly with Ad-mycN17Cdc42 (B, D, G, and J) or Ad-mycN17Rac1 (C, E, H, I, K, and L). Acini were stained 24 h later for β1 integrin (red) and actin (green; A–C). Short arrows indicate cells collapsing into the lumen; long arrows indicate lumens. For milk studies, acini were stimulated with Prl for 24 h and stained for β-casein (red; F–I). N17Cdc42 did not inhibit β-casein synthesis, as indicated by immunofluorescence and blotting (G and M, respectively). N17Rac1 blocked β-casein synthesis (H and I). In I and L, only part of the acinus expressed N17Rac1 (dashed lines); only these cells failed to lactate. Viral infection was confirmed with myc antibody (D, E, and J–L). Bar, 7 μM.
In contrast to infection before acinar formation (Fig. 4), N17Cdc42 expression in postpolarized acini did not disrupt the discrete basal distribution of β1 integrins (Fig. 5 B), and, importantly, it did not block β-casein synthesis, as shown by immunofluorescence (Fig. 5 G) and blotting (Fig. 5 M). This indicates that N17Cdc42 blocked mammary differentiation in the previous experiments (Fig. 1) primarily by interfering with the organization of acini. Therefore, we did not further examine the role of Cdc42 in this study.
We reasoned that Rac1 operates through a distinct mechanism from Cdc42 to influence differentiation because β1 integrin was retained at the basal acinar surface in the presence of N17Rac1 (Fig. 4 J and Fig. S2 G). Direct infection of polarized 3D acini with Ad-mycN17Rac1 also prevented β-casein synthesis (Fig. 5, H and I) while leaving acinar structure relatively intact (Fig. 5 C). This indicates that Rac regulates Prl signaling downstream of integrins and that its effects are not coupled to acinar organization.
Rac1 links ECM signals to mammary differentiation
To confirm that Rac1 integrates ECM adhesion signals with the epithelial differentiation process, we asked whether a constitutively active V12Rac1 could rescue milk protein synthesis in two separate culture models in which differentiation is compromised by altered cell–ECM interactions.
Adhesion of MECs to collagen I is integrin mediated, and the cells form 2D monolayers on this ECM. However, this matrix is not permissive for Prl-induced signaling even though the cells retain their differentiation potential if subsequently provided with laminin (Streuli et al., 1995b). Therefore, we tested whether V12Rac1 could rescue this defect in cells cultured in two dimensions on collagen I (with no BM overlay). Control cells failed to differentiate in response to Prl; however, under the same culture conditions, V12Rac1-infected cells synthesized β-casein (Fig. 6 A). Moreover, V12Rac1 restored the ability of Prl to translocate Stat5a to the nucleus (Fig. 6, B and C). Thus, when the ECM signals do not have the specificity to allow Prl signaling, constitutively active Rac1 can bypass the defect. The mechanism for the rescue of differentiation is not caused by the induction of laminin synthesis and assembly, which can occur in MDCK cells (O'Brien et al., 2001), because V12Rac1 did not cause any noticeable laminin deposition on the surface of MECs (unpublished data).
Figure 6.
V12Rac1 stimulates milk protein synthesis in cells cultured on nonpermissive collagen I substratum. (A) MECs were uninfected or infected with Ad-mycV12Rac1 and plated onto collagen I, where they formed 2D monolayers. Cells were stimulated with Prl for 24 h and were analyzed for β-casein expression by blotting. Infection was confirmed with myc or Rac antibody. These cultures were not overlaid with BM matrix, and it is well established that under these conditions, Prl cannot stimulate differentiation (lane 2). V12Rac1 rescued the defect (duplicate lanes 3 and 4). (B and C) Stat5A nuclear translocation was analyzed in uninfected cells or those infected with Ad-mycV12Rac1, plated onto collagen I–coated coverslips (2D culture with no BM matrix overlay), stimulated with Prl for 15 min, and stained with Stat5a and myc antibodies. (B) Percentage of cells displaying nuclear Stat5a; histogram is a mean of six counts from two experiments. Error bars represent SD. (C) Micrographs demonstrating the nuclear translocation of Stat5a (red) in cells expressing V12Rac1 (green) but not in uninfected controls. Note that monolayer-cultured MECs treated with BM overlay respond to Prl (Fig. 2 C), but in the experiment shown here, no exogenous BM is provided, and the cells cannot respond to Prl (ii). V12Rac1 rescues this defect (iv).
This suggests that the effect of V12Rac1 lies downstream of integrins. Thus, we examined the effect of V12Rac1 in β1 integrin–null acini. Such acini can be generated by the Cre-mediated deletion of integrin alleles harboring LoxP sequences, an approach we used previously to demonstrate the importance of β1 integrins for Prl signaling and milk protein synthesis both in culture and in vivo (Naylor et al., 2005). Cells isolated from Itgβ1fx/fx mice were infected in monolayer with Ad-CreM1 virus, leading to a significant reduction in β1 integrin levels within 24 h (Fig. 7 A). The deletion of β1 integrin also abolished Rac1 activity in 3D acini on BM matrix, indicating that Rac1 is regulated by β1 integrins (Fig. 7 C). β1 integrin–null cells or control cells were then reinfected with either Ad–β-galactosidase or Ad-mycV12Rac1 and replated onto BM matrix to assemble into 3D acini for 48 h. The deletion of β1 integrin largely prevented Prl-induced synthesis of β-casein (Fig. 7 B, lane 5). However, V12Rac1 but not β-galactosidase expression in β1-null acini rescued milk protein synthesis (Fig. 7 B, compare lanes 6 with 7).
Figure 7.
V12Rac1 restores the lactation defect observed in β1 integrin–null acini. (A) MECs from Itgβ1fx/fx mice were left untreated or were infected in monolayer with Cre-expressing adenovirus (Ad-CreM1) for 24 h and examined for β1 integrin by immunoblotting. (B) Cells treated as in A were reinfected with Ad–β-galactosidase (lanes 3 and 6) or Ad-mycV12Rac1 (lanes 4 and 7) in suspension for 1 h and were replated onto BM matrix for 24 h. 3D acini were untreated or stimulated with Prl for 24 h and analyzed for β-casein synthesis by immunoblotting. (C) Rac activity in 3D acini on BM matrix either uninfected or infected with Ad–β-galactosidase or Ad-CreM1. Rac activity was almost abolished in β1 integrin–null acini.
Thus, an activated form of Rac1 can bypass the requirement of MECs for both laminin and β1 integrin, thereby enabling Prl signaling and milk protein synthesis in nonpermissive environments. Moreover, Rac1 activity is regulated by BM matrix and β1 integrins, which are both essential for lactation. Together, the data strengthen our argument for Rac1 as a key intermediate in coordinating integrin and cytokine signaling pathways.
The adhesion signals for MEC differentiation may be mediated through the suppression of SHP2
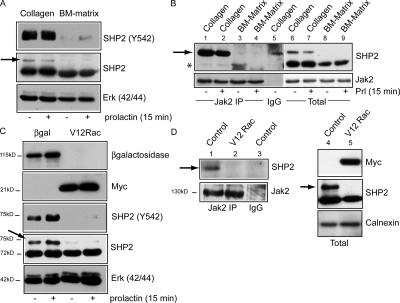
In a previous study, we demonstrated that the inhibition of Prl signaling in cells cultured without BM proteins (i.e., in two dimensions on collagen I) occurs through protein tyrosine phosphatases (PTPs; Edwards et al., 1998). Therefore, we reasoned that the effects of Rac1 on differentiation might correlate with the altered function of phosphatases. SHP2 binds the PrlR–Jak2 complex directly and regulates Jak–Stat signaling both positively and negatively (Yin et al., 1997; Berchtold et al., 1998; Kim and Baumann, 1999; Stofega et al., 2000). Phosphorylation of SHP2 on Y542 triggers its phosphatase activity by binding and displacing the N-SH2 domain from the catalytic cleft (Lu et al., 2001).
In MECs cultured on collagen I, the phosphorylation of SHP2-Y542 was elevated considerably compared with cells cultured with BM, either as acini on BM matrix (Fig. 8 A) or as 2D cultures with BM overlay (not depicted). This form of SHP2 was also detected as a slower migrating band when immunoblotted with a total SHP2 antibody (Fig. 8 A, arrow). In coimmunoprecipitation experiments, SHP2 complexed with Jak2, but the slower migrating phosphorylated form only associated with Jak2 in cells on collagen 1 (Fig. 8 B, lanes 1 and 2; arrow). This suggests that a possible mechanism for the inability of Prl to signal on this substratum is that Jak2-bound SHP2 is phosphorylated on Y542 and, therefore, is active as an inhibitory PTPase.
Figure 8.
Phosphorylation of SHP2 is elevated in cells on collagen I and inhibited by V12Rac1. (A) MECs cultured in two dimensions on collagen I (no BM overlay) or as 3D acini on BM matrix were either untreated or stimulated with Prl for 15 min and immunoblotted for SHP2-pY542. The slower migrating band in the SHP2 blot (arrows in A–D) is a phosphorylated form of SHP2, which disappears after alkaline phosphatase treatment (not depicted) and comigrates with the position of SHP2-pY542. (B) Cell lysates prepared as in A were immunoprecipitated with Jak2 antibody (lanes 1–4) or rabbit IgG (lane 5) and immunoblotted with SHP2 antibody (top). Jak2 immunoblotting confirmed that equivalent levels were immunoprecipitated (bottom). Total levels of SHP2 and Jak2 are shown in lanes 6–9. Low levels of total SHP2 associate with Jak2 (asterisk), and SHP2-pY542 (arrow) only associates in the cells cultured on collagen I. The band in lane 5 is nonspecific and migrates more rapidly than SHP2. (C) MECs infected with Ad–β-galactosidase or Ad-V12Rac1 were plated on collagen I (no BM overlay) for 48 h and immunoblotted for SHP2-pY542 or total SHP2. V12Rac1 inhibits SHP2 phosphorylation. (D) Coimmunoprecipitation experiments show that phosphorylated SHP2 no longer complexes with Jak2 in the presence of V12Rac1. Jak2 immunoprecipitates (lanes 1 and 2) prepared from uninfected or V12Rac1-infected cells on collagen I were immunoblotted with SHP2 or Jak2 antibody. Expression of virus and total levels of SHP2 are shown in lanes 4 and 5.
To confirm a link between SHP2 dephosphorylation and differentiation, we examined the effect of Ad-mycV12Rac1 on SHP2-pY542 in MECs cultured on collagen with no BM overlay. Strikingly, V12Rac1 inhibited the phosphorylation of this residue (Fig. 8 C) and prevented phospho-SHP2 from complexing with Jak2 (Fig. 8 D). Thus, the ability of V12Rac1 to rescue the differentiation defect in cells cultured on collagen I (Fig. 6) correlates with SHP2-pY542 dephosphorylation.
These data suggest that in the absence of appropriate cell–matrix interactions, SHP2 may have a key role in preventing the differentiation response. V12Rac1 suppresses SHP2-pY542 and, by implication, its PTP activity, thereby providing conditions favorable for Prl to stimulate its signaling pathway and permit differentiation.
Discussion
The mammary gland system provides an example of how adhesion and hormones coordinate the timing of epithelial differentiation and tissue function in a spatial and temporal context. It is well established that BM and β1 integrins are necessary for Prl to activate its downstream signaling components in MECs (Streuli et al., 1995a; Edwards et al., 1998). Recently, we confirmed this using a genetic approach by demonstrating that deletion of the β1 integrin gene prevents Prl-activated signaling and differentiation both in primary tissue culture and in vivo (Naylor et al., 2005). However, until now, the mechanism of signal integration between β1 integrins and the PrlR signaling pathway has remained elusive. The key finding in this study is that the small GTPase Rac1 acts as a link between integrin and cytokine receptors in coordinating cellular differentiation.
Rac1 regulates differentiation
The central lines of evidence to support our argument that Rac1 is involved in differentiation are that (1) DN Rac1 inhibited both Prl signaling and milk protein synthesis (Figs. 1, 2, and 5) and (2) activated Rac1 rescued tissue-specific gene expression in cells cultured either on an ECM that is normally nonpermissive for differentiation (i.e., collagen I) or in β1 integrin–null cells (Figs. 6 and 7). Our observations are summarized schematically in Fig. 9.
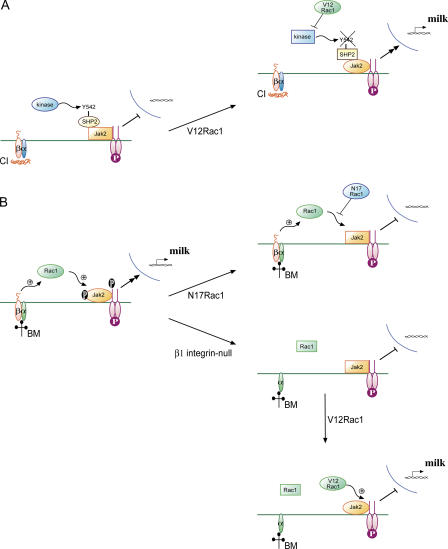
Figure 9.
Models for the involvement of Rac1 in MEC differentiation. (A) In MECs cultured on collagen I (CI), Prl (P) signaling is not active because the SHP2 associated with Jak2 is phosphorylated on Y542 by an unknown kinase. V12Rac1 suppresses this activity, leading to SHP2-Y542 dephosphorylation and Prl signaling. This builds on our previously proposed model for PTP involvement in MEC differentiation (Edwards et al., 1998). Active enzymes are oval, and inactive enzymes are rectangular. (B) In cells cultured on BM, Prl signaling requires Rac1. In either the presence of N17Rac1 or absence of β1 integrin, Rac1 activity is suppressed, leading to the inability of Prl to signal. V12 Rac1 can rescue Prl signaling in integrin-null MECs, but the mechanism may be different than that in cells on collagen I.
A possible mechanism is that Rac1 is required for MEC polarization. Rac1 is known to be involved with the polarization of some epithelia (e.g., MDCK cells; O'Brien et al., 2001), and, in mammary acini, the expression of DN Rac1 perturbed some aspects of polarization both at E-cadherin–containing cell–cell junctions and toward their apical surfaces, where F-actin and ZO-1 became disorganized. However, several observations suggest that it is unlikely that either altered polarization or E-cadherin are involved. First, milk proteins are still synthesized in E-cadherin–null mammary glands and in cultured acini disrupted with function-blocking anti–E-cadherin antibodies (Streuli et al., 1991; Boussadia et al., 2002). Second, 2D monolayer cultures overlaid with diluted BM matrix are not well polarized but still respond to Prl by synthesizing milk proteins (Streuli et al., 1995b). Third, single cells embedded within a BM matrix and separated from each other have no apical polarity; these cells position their basal β1 integrin–containing surfaces to contact the BM and are still able to express milk proteins (Streuli et al., 1991). Furthermore, in occasional control acini, the lumens are disorganized and filled with cells, yet those in contact with matrix still synthesize β-casein (unpublished data).
Although a perturbation of Rac1 affected lateral and apical surfaces of MECs in our study, β1 integrins remained at the basal surface, where they contact ECM (Fig. 4). Therefore, we reasoned that Rac1 is not required for the localization of β1 integrins but rather acts downstream to control their signaling output. Indeed, we demonstrated that this was the case by two complementary experiments. First, DN Rac1 blocked differentiation when it was expressed in acini that were already polarized and had basally localized β1 integrins (Fig. 5). Second, an activated form of Rac1 rescued β-casein expression in β1 integrin–null cells (Fig. 7). Together, our studies argue that Rac1 acts downstream of integrins to regulate differentiation and that it does so by integrating the cell's response to adhesion and cytokine signals.
RhoA and Cdc42 in mammary differentiation
The effects of Rac1 on MEC function are quite distinct to those of RhoA and Cdc42. Rho is involved with cytoskeletal tension and contractility in MECs and is important for tubulogenesis (Wozniak et al., 2003; Paszek et al., 2005). However, inhibiting its function had no effect on differentiation in respect to milk protein expression (Fig. 1) even though DN RhoA did perturb the cytoskeleton (unpublished data). Interestingly, the DN form of RhoA slightly affected Stat5 translocation, but this may be explained by a functional redundancy between different Stat5 isoforms because Rho affects the phosphorylation of Stat5a but not Stat5b in MDCK cells (Benitah et al., 2003).
In some studies, Cdc42 has been suggested to have a similar role to Rac. For example, in mammary cancer cells, both are involved with cell motility and invasiveness (Keely et al., 1997). We identified a separate role for Cdc42 in MEC function, which is to promote the establishment of a fully polarized phenotype (Fig. 4), as seen in other cell types (Etienne-Manneville, 2004). Although our experiments with the DN forms of Cdc42 and Rac1 reveal some overlap in the control of polarity, the correct positioning of β1 integrins at the ECM–cell interface was only disrupted in the presence of DN Cdc42 (Fig. 4 and Fig. S2). Thus, we reasoned that the effect of DN Cdc42 on Prl signaling and differentiation in Figs. 1 and 2 were coupled to integrin localization. To test this possibility, we expressed DN Cdc42 in acini with preexisting interactions with the ECM; in contrast to the expression of DN Rac1, DN Cdc42 did not inhibit milk protein synthesis under these conditions (Fig. 5). Thus, our data suggest a distinct role for Cdc42, which may be necessary to organize acini and establish the BM–integrin axis, thereby activating signaling through adhesion complexes and cross talk with PrlR, although further experiments are necessary to confirm this.
An interesting and novel observation is that Cdc42 activity itself is regulated by Prl (Fig. 3). Although we have not explored the mechanism for this, the guanine exchange factor Vav1 complexes with and can be activated by PrlR when overexpressed in COS cells (Kline et al., 2001). Intriguingly, the requirement of Cdc42 for PrlR phosphorylation and Stat5 nuclear translocation suggests that the activation of PrlR and its downstream signaling cassette may involve a positive feedback loop through Cdc42.
Activation of Rac1
Our observation that Rac1 is required actively and downstream of integrin for the signaling pathways that drive differentiation raises the questions of how Rac becomes activated to achieve this phenotype and what the link to influence Prl signaling might be.
Integrins are located basally in N17Rac1 mammary acini. Interestingly, they are not fully functional because FAK and paxillin phosphorylation are compromised under these conditions (unpublished data). However, we have recently discovered that the genetic deletion of integrin-linked kinase inhibits Stat5 activation and milk production and that V12Rac1 can rescue this (unpublished data). Thus, it is likely that Rac1 acts downstream of the adhesion complex to integrate with Prl signaling, and an interesting possibility is that an integrin-linked kinase-regulated guanine nucleotide exchange factor (e.g., α-Pix) might locally activate Rac1 and may therefore be associated with MEC differentiation (Mishima et al., 2004). Because Rac is widely involved with integrin-mediated signaling, it will be important to determine how those adhesion complexes that form specifically in MEC cultured with BM matrices, as opposed to other ECMs, uniquely instruct Prl signaling.
A possible mechanistic link between Rac1 and Prl signaling
Rac controls several essential cellular processes in epithelia, including organization of the cytoskeleton and polarity, directional cell motility, survival, and stem cell maintenance (O'Brien et al., 2001; Zahir et al., 2003; Benitah et al., 2005; Pankov et al., 2005). Our work adds glandular differentiation to the list, and it is therefore important to consider how Rac1 might drive this phenotype. The cytoskeleton is unlikely to be involved directly because disassembling it with cytochalasin D has no effect on proximal signaling through the Prl pathway (Zoubiane et al., 2004). An alternate possibility is that the Rac effector p21PAK has a role because it phosphorylates Stat5a on S779 (Wang et al., 2003). Although this might provide part of the link to differentiation, other mechanisms are also involved because tyrosine phosphorylation of PrlR itself is compromised in the presence of N17Rac1 (Fig. 2).
We have shown previously that adhesion influences lactational differentiation at two separate levels because Prl signaling and tissue-specific expression of milk protein genes requires both ECM specificity and active integrin signaling. This study indicates that Rac1 provides a link to coordinate signaling inputs from adhesion and cytokines because DN Rac1 blocks differentiation, whereas active Rac1 rescues it. However, the mechanisms preventing accurate integration of adhesion and cytokine signals in wild-type MECs cultured in the absence of a permissive ECM (i.e., without BM) and in cells genetically lacking β1 integrin may be distinct.
In a previous study, we demonstrated a key role for PTPs in the ECM control of MEC differentiation. When cells are cultured without BM (i.e., on collagen I), PTPs dominantly inhibit Prl signaling (Edwards et al., 1998). SHP2 has since been found to regulate Jak2/Stat5 negatively and, therefore, is a potential candidate for preventing Prl signaling (Kim and Baumann, 1999; Stofega et al., 2000). We have now discovered that phosphorylation on Y542, which triggers SHP2 phosphatase activity (Lu et al., 2001), is ECM dependent in mammary cells. This site was phosphorylated strongly in cells on collagen I, correlating with the lack of Prl signaling under these culture conditions (Fig. 8, A and B). Importantly, V12Rac1, which restored Prl-regulated translocation of Stat5a to the nucleus as well as β-casein synthesis (Fig. 6), blocked Y542 phosphorylation (Fig. 8, C and D). Thus, our current model is that in the absence of BM (i.e., when cells are cultured on collagen I), an unidentified kinase phosphorylates SHP2-Y542, thereby activating the PTP and preventing Prl signaling (Fig. 9 A). We suggest that V12Rac1 alters the activity of enzymes controlling SHP2-Y542 phosphorylation, thus permitting Prl signaling.
Although our evidence points to a key role for Rac1 in MEC differentiation, we observed that N17Rac1 did not stimulate an increase in the phosphorylation of SHP2-Y542 in cells cultured with BM (unpublished data). There are several possible explanations for this finding. First, the identity, location, and mechanism of regulation of the intermediate tyrosine kinase or phosphatase involved with Y542 phosphorylation are not known, and inhibiting Rac activation may not have the same effect on a downstream phosphorylation site as overexpressing active Rac. Second, there are context-specific molecular differences between MECs cultured on collagen and BM. The (so far unidentified) SHP2-Y542 kinase may be spatially restricted or even not expressed and, thus, may be unable to phosphorylate SHP2 in the presence of N17Rac1. It is known that SHP2 has multiple roles in Prl signaling, as it is both required for Prl signaling and can inhibit it. In addition, SHP2 is regulated by multiple mechanisms, so although Rac1 may influence SHP2 in MECs cultured on BM, this could occur via a Y542-independent mechanism (Clevenger and Kline, 2001; Neel et al., 2003). For example, we are investigating the possibility that Rac1 elevates cellular reactive oxygen species in MECs (Werner and Werb, 2002), thereby inhibiting PTPs (Knebel et al., 1996), and, interestingly, we have recently discovered that reactive oxygen species are required for Prl signaling in MECs (unpublished data). Resolving these issues will require more insight into the location and regulatory mechanisms of the enzymes controlling SHP2-Y542 phosphorylation.
In summary, our study highlights a novel role for Rac1 as an integrator of the spatial information provided by ECM with the temporal hormonal signals for differentiation. We suggest that Rac1 may act as a nodal point to connect signaling networks, and it will now be important to identify the immediate elements both upstream and downstream of this essential GTPase.
Materials and methods
Cell culture
Primary mouse MECs were isolated from 15.5–17.5-d pregnant ICR mice and cultured in F-12 medium supplemented with 10% FBS, 5 μg/ml insulin, 1 μg/ml hydrocortisone, and 5 ng/ml EGF (growth medium) and were plated on collagen I–coated dishes in growth medium to allow them to dissociate from in vivo acini and form cell monolayers. For some experiments, cells were isolated from Itgβ1fx/fx transgenic mice (Naylor et al., 2005). Differentiation medium consisted of DME–Hams F-12 medium containing insulin and hydrocortisone with or without 3 μg/ml Prl.
Antibodies
Mouse anti–β-casein, rabbit anti–β1 integrin, and rabbit anti-PrlR antibodies have been described previously (Streuli et al., 1991). Commercial primary antibodies were as follows: Cdc42, Stat5a, Erk, and SHP2 (Santa Cruz Biotechnology, Inc.); Rac (23A8), phosphotyrosine (4G10), phospho-Stat5 (Y694/Y699), and Jak2 (Upstate Biotechnology); myc (9E10; Roche); β-galactosidase (Promega); SHP2 (Y542), paxillin, and FAK (Cell Signaling Technology); paxillin (Y31) and FAK (Y397; Biosource International); E-cadherin (ECCD2; Takara Bio Inc.); ZO-1 and Cre (Chemicon); and calnexin (Bioquote). Secondary antibodies were HRP-conjugated anti–mouse and anti–rabbit IgG, Cy2-conjugated anti–mouse and anti–rat IgG, Cy5-conjugated anti–mouse and anti–rabbit IgG, and conjugated rhodamine RX (Jackson ImmunoResearch Laboratories).
Adenovirus purification and infection
Amino-terminal myc-tagged N19RhoA, N17Rac1, N17Cdc42, V12Rac1, and Ad–β-galactosidase adenoviruses were gifts from A. Ridley (Ludwig Institute for Cancer Research, London, United Kingdom; Wojciak-Stothard et al., 2001), and Ad-CreM1 was obtained from Microbix Biosystems. Recombinant adenoviral DNA was amplified in E1-competent 293T human embryonic kidney cells. Infected cells were harvested, and viable adenoviral particles were purified on a caesium chloride gradient as previously described (Watkin and Streuli, 2002). Adenovirus titre was determined by scoring cytopathic effect in 293T cells using the tissue culture infectious dose 50 method.
Primary cells cultured on collagen I dishes for 3 d were trypsinized, and single cells in suspension were infected (for 1 h at 37°C) and plated onto either collagen I (7 × 104 cells/cm−2) or BM matrix dishes (2 × 105 cells/cm−2) saturated with growth medium as described previously (Watkin and Streuli, 2002). Cells were infected with adenoviruses at an MOI of 50, which produced a >90% rate of infection. Adenoviral proteins were expressed for 24 h in cells cultured in growth medium before incubation in differentiation medium for up to 24 h. Single cells attach to the BM matrix and assemble into 3D acini over 2 d; over this time frame, only small acini form.
For rescue experiments, cells were infected for 2 h in monolayer with Ad-CreM1. 24 h later, cells were trypsinized and reinfected in suspension with Ad–β-ga1actosidase or Ad-mycV12Rac1 viruses and replated onto BM matrix. For infection directly in three dimensions, small acini (15–25 μm) were assembled by plating 8 × 105 cells per 35-mm dish onto BM matrix for 48 h. Acini were infected by incubating with 200 MOI viruses for 3 h in serum-free medium. For milk protein analysis, Prl was added for 24 h in differentiation medium.
Immunohistochemistry
Expression and distribution of various proteins were visualized by indirect immunofluorescence. 48 h after plating, cells were fixed for 10 min in PBS/4% (wt/vol) PFA and permeabilized for 7 min using PBS/0.2% (vol/vol) Triton X-100. Nonspecific sites were blocked with PBS/10% goat serum (for 1 h at RT) before incubation with antibodies diluted in PBS/2% goat serum (for 1 h at RT each). F-actin was detected by incubating cells with TRITC-phalloidin (Sigma-Aldrich) or Texas red phalloidin (Invitrogen) for 1 h at RT, whereas nuclei were stained using 4 μg/ml Hoechst 33258 (Sigma-Aldrich) for 2 min at RT. Cells were washed in PBS before mounting in either DAKO (DakoCytomation) for monolayers or prolong antifade (Invitrogen) for 3D acini. Immunostained cells were visualized with a microscope (Axioplan2; Carl Zeiss MicroImaging, Inc.) using plan-Apochromat 100× and 63× NA 1.40 lenses with Immersol 518F oil at RT. 0.2-μm z sections were captured with a camera (Orca ER; Hamamatsu) and analyzed with Volocity 3.5.1 software (Improvision) using iterative deconvolution. Quantification of acini displaying a particular phenotype was scored by analyzing 100 acini for each condition. Where approximately half the cells within an acinus displayed a change in morphology, these acini were scored as positive for that change. Nonbiased cell counts were performed by concealing the identity of each slide.
Immunoprecipitation and immunoblotting
Milk proteins and Stat5 phosphorylation were analyzed as described previously (Edwards et al., 1998). For PrlR and Jak2 immunoprecipitations, cells were scrape lysed in 2× NP-40 lysis buffer for BM matrix or 1× for monolayer (10% [wt/vol] glycerol, 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1% [wt/vol] NP-40, 2 mM MgCl2, and fresh protease/phosphatase inhibitors). Lysates were rotated for 30 min at 4°C, homogenized, and ultracentrifuged at 70,000 g for 15 min at 4°C to remove both detergent-insoluble proteins and the dense part of the BM matrix. Antibodies and protein A–Sepharose beads were incubated with lysates for 2 h each at 4°C.
Stat5 nuclear translocation assay
Primary cells were plated onto collagen I–coated coverslips in growth medium for 48–72 h. Adenoviruses at an MOI of 50 were added to attached cells for 2 h in growth medium. Virus-containing medium was removed, and cells were overlaid with diluted BM matrix (1:50) in differentiation medium for 48 h followed by stimulation with Prl for 15 min. Nuclear translocation of Stat5a was detected by immunostaining, and nonbiased cell counts were performed by concealing the identity of each slide.
Assay for the activity of endogenous Rac and Cdc42
Cells plated on factor-reduced BM matrix or collagen I were rapidly scrape lysed into 2× or 1× NP-40 lysis buffer and ultracentrifuged at 70,000 g for 15 min at 4°C. Cells in suspension were pelleted before lysing in an equivalent volume of NP-40 lysis buffer. 25 μg of recombinant GST alone or GST-PAK PBD purified from the bacterial expression vector system and coupled to glutathione agarose beads was used to precipitate GTP-bound Rac and Cdc42 from cell lysates for 40 min at 4°C (del Pozo et al., 2000). Active Rac and Cdc42 were detected by immunoblotting with anti-Rac or anti-Cdc42 antibodies and quantified using ID Software (Kodak).
Online supplemental material
Fig. S1 shows that the inhibition of differentiation by N17Rac1 and N17Cdc42 is not caused by apoptosis. Fig. S2 presents multiple acini showing the effects of N17Rac1 and N17Cdc42 on acinar morphology. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200601059/DC1.
Supplementary Material
Acknowledgments
We would like to thank Andreas Prokop, Martin Humphries, Christoph Ballestrem, Anne Ridley, and Matthew Naylor for critical reading of the manuscript.
This work was supported by a Wellcome Trust program grant.
Abbreviations used in this paper: BM, basement membrane; DN, dominant negative; MEC, mammary epithelial cell; PAK, p21-activated protein kinase; PBD, PAK-binding domain; Prl, prolactin; PrlR, Prl receptor; PTP, protein tyrosine phosphatase.
References
- Aggeler, J., J. Ward, L.M. Blackie, M.H. Barcellos-Hoff, C.H. Streuli, and M.J. Bissell. 1991. Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo. J. Cell Sci. 99:407–417. [DOI] [PubMed] [Google Scholar]
- Akhtar, N., and N.A. Hotchin. 2001. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol. Biol. Cell. 12:847–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff, M.H., J. Aggeler, T.G. Ram, and M.J. Bissell. 1989. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 105:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitah, S.A., P.F. Valeron, H. Rui, and J.C. Lacal. 2003. STAT5a activation mediates the epithelial to mesenchymal transition induced by oncogenic RhoA. Mol. Biol. Cell. 14:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitah, S.A., M. Frye, M. Glogauer, and F.M. Watt. 2005. Stem cell depletion through epidermal deletion of Rac1. Science. 309:933–935. [DOI] [PubMed] [Google Scholar]
- Berchtold, S., S. Volarevic, R. Moriggl, M. Mercep, and B. Groner. 1998. Dominant negative variants of the SHP-2 tyrosine phosphatase inhibit prolactin activation of Jak2 (janus kinase 2) and induction of Stat5 (signal transducer and activator of transcription 5)-dependent transcription. Mol. Endocrinol. 12:556–567. [DOI] [PubMed] [Google Scholar]
- Boussadia, O., S. Kutsch, A. Hierholzer, V. Delmas, and R. Kemler. 2002. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev. 115:53–62. [DOI] [PubMed] [Google Scholar]
- Clevenger, C.V., and J.B. Kline. 2001. Prolactin receptor signal transduction. Lupus. 10:706–718. [DOI] [PubMed] [Google Scholar]
- Debnath, J., K.R. Mills, N.L. Collins, M.J. Reginato, S.K. Muthuswamy, and J.S. Brugge. 2002. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 111:29–40. [DOI] [PubMed] [Google Scholar]
- del Pozo, M.A., L.S. Price, N.B. Alderson, X.D. Ren, and M.A. Schwartz. 2000. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19:2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali, K.A., K. Wennerberg, and K. Burridge. 2003. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 15:572–582. [DOI] [PubMed] [Google Scholar]
- Edwards, G.M., F.H. Wilford, X. Liu, L. Hennighausen, J. Djiane, and C.H. Streuli. 1998. Regulation of mammary differentiation by extracellular matrix involves protein-tyrosine phosphatases. J. Biol. Chem. 273:9495–9500. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S. 2004. Cdc42–the centre of polarity. J. Cell Sci. 117:1291–1300. [DOI] [PubMed] [Google Scholar]
- Faraldo, M.M., M.A. Deugnier, S. Tlouzeau, J.P. Thiery, and M.A. Glukhova. 2002. Perturbation of beta1-integrin function in involuting mammary gland results in premature dedifferentiation of secretory epithelial cells. Mol. Biol. Cell. 13:3521–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti, F.G., and G. Tarone. 2003. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 19:173–206. [DOI] [PubMed] [Google Scholar]
- Keely, P.J., J.K. Westwick, I.P. Whitehead, C.J. Der, and L.V. Parise. 1997. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 390:632–636. [DOI] [PubMed] [Google Scholar]
- Kim, H., and H. Baumann. 1999. Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells. Mol. Cell. Biol. 19:5326–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline, J.B., D.J. Moore, and C.V. Clevenger. 2001. Activation and association of the Tec tyrosine kinase with the human prolactin receptor: mapping of a Tec/Vav1-receptor binding site. Mol. Endocrinol. 15:832–841. [DOI] [PubMed] [Google Scholar]
- Knebel, A., H.J. Rahmsdorf, A. Ullrich, and P. Herrlich. 1996. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 15:5314–5325. [PMC free article] [PubMed] [Google Scholar]
- Lu, W., D. Gong, D. Bar-Sagi, and P.A. Cole. 2001. Site-specific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol. Cell. 8:759–769. [DOI] [PubMed] [Google Scholar]
- Mishima, W., A. Suzuki, S. Yamaji, R. Yoshimi, A. Ueda, T. Kaneko, J. Tanaka, Y. Miwa, S. Ohno, and Y. Ishigatsubo. 2004. The first CH domain of affixin activates Cdc42 and Rac1 through alphaPIX, a Cdc42/Rac1-specific guanine nucleotide exchanging factor. Genes Cells. 9:193–204. [DOI] [PubMed] [Google Scholar]
- Naylor, M.J., N. Li, J. Cheung, E.T. Lowe, E. Lambert, R. Marlow, P. Wang, F. Schatzmann, T. Wintermantel, G. Schuetz, et al. 2005. Ablation of {β}1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J. Cell Biol. 171:717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel, B.G., H. Gu, and L. Pao. 2003. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28:284–293. [DOI] [PubMed] [Google Scholar]
- O'Brien, L.E., T.S. Jou, A.L. Pollack, Q. Zhang, S.H. Hansen, P. Yurchenco, and K.E. Mostov. 2001. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 3:831–838. [DOI] [PubMed] [Google Scholar]
- Pankov, R., Y. Endo, S. Even-Ram, M. Araki, K. Clark, E. Cukierman, K. Matsumoto, and K.M. Yamada. 2005. A Rac switch regulates random versus directionally persistent cell migration. J. Cell Biol. 170:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek, M.J., N. Zahir, K.R. Johnson, J.N. Lakins, G.I. Rozenberg, A. Gefen, C.A. Reinhart-King, S.S. Margulies, M. Dembo, D. Boettiger, et al. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell. 8:241–254. [DOI] [PubMed] [Google Scholar]
- Radisky, D.C., D.D. Levy, L.E. Littlepage, H. Liu, C.M. Nelson, J.E. Fata, D. Leake, E.L. Godden, D.G. Albertson, M.A. Nieto, et al. 2005. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 436:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J. 2001. Rho family proteins: coordinating cell responses. Trends Cell Biol. 11:471–477. [DOI] [PubMed] [Google Scholar]
- Rogers, K.K., T.S. Jou, W. Guo, and J.H. Lipschutz. 2003. The Rho family of small GTPases is involved in epithelial cystogenesis and tubulogenesis. Kidney Int. 63:1632–1644. [DOI] [PubMed] [Google Scholar]
- Stofega, M.R., J. Herrington, N. Billestrup, and C. Carter-Su. 2000. Mutation of the SHP-2 binding site in growth hormone (GH) receptor prolongs GH-promoted tyrosyl phosphorylation of GH receptor, JAK2, and STAT5B. Mol. Endocrinol. 14:1338–1350. [DOI] [PubMed] [Google Scholar]
- Streuli, C.H., N. Bailey, and M.J. Bissell. 1991. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J. Cell Biol. 115:1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli, C.H., G.M. Edwards, M. Delcommenne, C.B. Whitelaw, T.G. Burdon, C. Schindler, and C.J. Watson. 1995. a. Stat5 as a target for regulation by extracellular matrix. J. Biol. Chem. 270:21639–21644. [DOI] [PubMed] [Google Scholar]
- Streuli, C.H., C. Schmidhauser, N. Bailey, P. Yurchenco, A.P. Skubitz, C. Roskelley, and M.J. Bissell. 1995. b. Laminin mediates tissue-specific gene expression in mammary epithelia. J. Cell Biol. 129:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R.A., R.K. Vadlamudi, R. Bagheri-Yarmand, I. Beuvink, N.E. Hynes, and R. Kumar. 2003. Essential functions of p21-activated kinase 1 in morphogenesis and differentiation of mammary glands. J. Cell Biol. 161:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkin, H., and C.H. Streuli. 2002. Adenoviral-mediated gene transfer in two-dimensional and three-dimensional cultures of mammary epithelial cells. Methods Cell Biol. 69:403–423. [DOI] [PubMed] [Google Scholar]
- Werner, E., and Z. Werb. 2002. Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases. J. Cell Biol. 158:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard, B., S. Potempa, T. Eichholtz, and A.J. Ridley. 2001. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J. Cell Sci. 114:1343–1355. [DOI] [PubMed] [Google Scholar]
- Wozniak, M.A., R. Desai, P.A. Solski, C.J. Der, and P.J. Keely. 2003. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J. Cell Biol. 163:583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, W.J., S. Tu, and R.A. Cerione. 2003. Activated Cdc42 sequesters c-Cbl and prevents EGF receptor degradation. Cell. 114:715–725. [DOI] [PubMed] [Google Scholar]
- Yin, T., R. Shen, G.S. Feng, and Y.C. Yang. 1997. Molecular characterization of specific interactions between SHP-2 phosphatase and JAK tyrosine kinases. J. Biol. Chem. 272:1032–1037. [DOI] [PubMed] [Google Scholar]
- Zahir, N., J.N. Lakins, A. Russell, W. Ming, C. Chatterjee, G.I. Rozenberg, M.P. Marinkovich, and V.M. Weaver. 2003. Autocrine laminin-5 ligates α6β4 integrin and activates RAC and NFκB to mediate anchorage-independent survival of mammary tumors. J. Cell Biol. 163:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubiane, G.S., A. Valentijn, E.T. Lowe, N. Akhtar, S. Bagley, A.P. Gilmore, and C.H. Streuli. 2004. A role for the cytoskeleton in prolactin-dependent mammary epithelial cell differentiation. J. Cell Sci. 117:271–280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.