Abstract
During mitosis, the chromosomal passenger complex (CPC) orchestrates highly different processes, such as chromosome alignment, histone modification, and cytokinesis. Proper and timely localization of this complex is the key to precise control over the enzymatic core of the CPC, the Aurora-B kinase. We discuss the molecular mechanisms by which the CPC members direct the dynamic localization of the complex throughout cell division. Also, we summarize posttranslational modifications that occur on the CPC and discuss their roles in regulating localization and function of this mitotic complex.
The chromosomal passenger complex (CPC) has recently received much attention as an important mitotic regulatory complex. In early mitosis, this complex promotes chromosome alignment by correcting misattachments between chromosomes and microtubules of the mitotic spindle (Carmena and Earnshaw, 2003). Additionally, the CPC is responsible for the displacement of heterochromatin protein-1 (HP-1) from mitotic chromosomes by modifying Histone H3 (Fischle et al., 2005; Hirota et al., 2005). At the end of mitosis, the CPC regulates the proper execution of cytokinesis (Carmena and Earnshaw, 2003). All these functions can be attributed to the action of the enzymatic heart of the CPC, the Aurora-B serine/threonine protein kinase, as chemical inhibition of this kinase impairs all CPC functions described above (Ditchfield et al., 2003; Hauf et al., 2003; Fischle et al., 2005; Hirota et al., 2005). Here, instead of focusing on the specific functions of the CPC during mitosis, we primarily discuss how the nonenzymatic components of the CPC enable Aurora-B to function properly during mitosis.
The CPC
The CPC can be regarded as a complex similar to the cyclin/CDK kinase complexes, in which the binding of a nonenzymatic protein to its enzymatic partner is essential for functioning of the kinase. Instead of one nonenzymatic/regulatory subunit in a cyclin/CDK complex, the CPC contains three nonenzymatic subunits, all of which are essential for the function of Aurora-B. These subunits determine activity, localization, stability, and possibly also substrate specificity of Aurora-B. In human cells, these subunits are Survivin, the inner centromere protein (INCENP), and Borealin/Dasra-B (hereafter referred to as Borealin; Fig. 1). These proteins are conserved among species, as in all investigated model organisms similar proteins have been identified. Borealin forms an exception, because orthologues have not been identified so far in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Also, the putative functional orthologue of Borealin in Caenorhabditis elegans, CSC-1, is only distantly related to other Borealin proteins (Gassmann et al., 2004). Interestingly, Bir1p, the yeast homologue of Survivin, is much larger than its mammalian orthologues, raising the intriguing possibility that Survivin and Borealin are combined in a single protein in yeast and that Bir1p has diverged into different polypeptides during evolution. In all studied organisms, the CPC proteins function in multiprotein complexes, and in mammalian cells, complex formation between the CPC proteins is needed for protein stability (Honda et al., 2003; Vader et al., 2006). Recent evidence suggests that two distinct passenger complexes exist during mitosis: one containing all four CPC members and another consisting of INCENP and Aurora-B (Gassmann et al., 2004). Of these two complexes, the quaternary CPC functions during chromosome alignment and cytokinesis, whereas the INCENP–Aurora-B complex might be responsible for modifying Histone H3 (Gassmann et al., 2004).
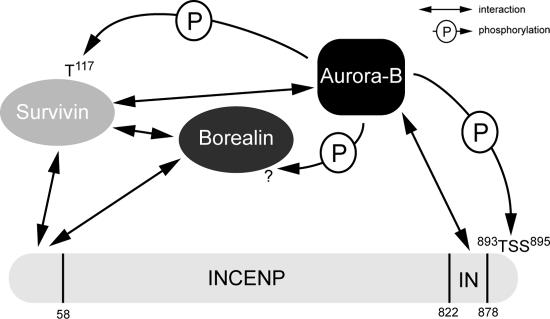
Figure 1.
Interactions within the CPC. Schematic representation of direct interactions between CPC proteins and phosphorylations of Aurora-B within the CPC. Survivin and Borealin interact with the NH2 terminus of INCENP, whereas Aurora-B binds the COOH-terminal IN-box in INCENP. Mapped Aurora-B phosphorylation sites are indicated.
How do these proteins dictate Aurora-B function? The CPC proteins show a very dynamic localization during mitosis that gave them their name (Earnshaw and Bernat, 1991): they initially paint the entire chromatin during the onset of mitosis, move from the chromosome arms toward the inner centromeric chromatin (in between the kinetochores, the sites of microtubule attachment) during prometaphase, relocalize to the microtubules of the central spindle at the metaphase–anaphase transition, and finally concentrate at the midbody during telophase/cytokinesis. This localization correlates with the diverse functions of the CPC during mitosis: modifying histones at the chromatin, correcting misattachments while at the centromere, and regulating cytokinesis at the central spindle (and later midbody). Given the correlation between localization and function, it is apparent that timely and proper localization of the CPC is the key to allowing Aurora-B to exert its diverse functions during mitosis.
Regulation of Aurora-B localization by the CPC proteins
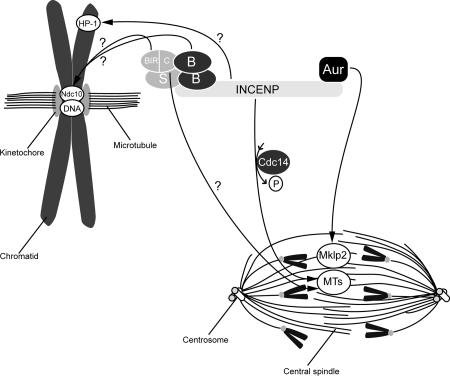
To allow timely CPC localization, one or multiple CPC subunits should recognize a docking site (i.e., receptor) on chromosome arms, centromere, or central spindle. Because of the molecular differences between these structures (e.g., centromeric chromatin versus microtubules on the central spindle), it is likely that different receptors exist on these structures and that different CPC members are involved in the specific targeting of Aurora-B. The mechanism by which the CPC is targeted to the chromosome arms in unclear, but a plausible possibility would be via interaction with HP-1, as INCENP has been described to interact with this chromatin-associated protein (Ainsztein et al., 1998). HP-1 displacement from chromosome arms is mediated by Aurora-B (Fischle et al., 2005; Hirota et al., 2005), which could explain the transient localization of the CPC at chromosome arms during prometaphase. Also, the CPC receptors at the centromeres are unknown, but there are clues to the mechanisms by which the CPC interacts with its centromeric receptors (Fig. 2). INCENP-deletion studies identified an NH2-terminal domain needed for centromere localization of the CPC (Ainsztein et al., 1998). Survivin interacts with INCENP via this domain, and replacement of this domain with Survivin suffices for targeting a functional CPC to the centromeres (Vader et al., 2006). When Survivin is linked covalently to INCENP, a functional CPC can be targeted, albeit less efficiently, to the centromeres and central spindle in the absence of Borealin (Vader et al., 2006). Thus, Borealin appears to play only a minor role in centromere targeting when Survivin and INCENP are forced into a complex. However, Borealin is essential for centromere localization of the endogenous proteins, suggesting it plays a major role in promoting interaction between Survivin and INCENP. Indeed, depletion of Borealin disrupts the interaction between exogenously expressed Survivin and INCENP (Vader et al., 2006). Similarly, efficient in vitro interaction between C. elegans Survivin (BIR [baculovirus IAP repeat] 1) and INCENP (ICP-1) depends on the Borealin orthologue CSC-1 (Romano et al., 2003). Interestingly, recent data showed that Borealin also interacts with the NH2 terminus of INCENP and that Borealin can interact with double-stranded DNA in vitro (Klein et al., 2006), suggesting that, in addition to facilitating the Survivin–INCENP interaction, the contribution of Borealin to centromere targeting is mediated via direct interaction with chromatin (Klein et al., 2006). Collectively, this allows for a model in which Survivin and Borealin cooperatively mediate centromere targeting of the CPC through multiple docking sites, including the chromatin itself. By interacting with the NH2 terminus of INCENP, these proteins can then recruit INCENP and Aurora-B to centromeres. Because Survivin and Borealin can oligomerize in vitro (Chantalat et al., 2000; Muchmore et al., 2000; Verdecia et al., 2000; Gassmann et al., 2004), it is possible that a heterooligomer of Borealin and Survivin assembled on the NH2 terminus of INCENP forms the centromere binding interface of the CPC. Within Survivin, the BIR domain is the most likely domain to interact with putative CPC receptors at the centromere, as disruption of this domain impaired CPC centromere function (but not Borealin interaction; Lens et al., 2006). Interestingly, Bir1p, the S. cerevisiae Survivin orthologue, interacts with Ndc10, a subunit of the centromere binding factor-3 complex (Yoon and Carbon, 1999), making this protein a good candidate for a CPC centromere receptor. However, a mammalian orthologue of Ndc10 has not been identified. Clearly, identifying Survivin (BIR domain) and Borealin interactors is necessary to further elucidate the mechanisms of CPC centromere targeting.
Figure 2.
Targeting mechanisms of the CPC. For chromatin localization during prometaphase, interaction between HP-1 and INCENP might be required. A multimeric complex of Survivin and Borealin might cooperatively serve as binding interface at the centromere. Within Survivin, the BIR domain is important for centromere targeting, whereas the COOH terminus (C) plays a role in central spindle localization. Putative centromere receptors are Ndc10 and centromeric DNA. At the central spindle during anaphase, Aurora-B interacts with Mklp2 and INCENP has an affinity for microtubules (MTs) regulated by Cdc14. Survivin can also interact with microtubules, but it is unknown whether this contributes to central spindle targeting.
Most of our knowledge regarding CPC centromere targeting is based on immunofluorescence data in fixed cells. However, it is clear that association of the CPC to centromeres is highly dynamic. For example, Survivin localizes dynamically to the centromere (Beardmore et al., 2004). Inhibition of Aurora-B or depolymerization of microtubules greatly reduces Survivin turnover at centromeres (Beardmore et al., 2004), suggesting that CPC localization and microtubule attachment are linked during prometaphase/metaphase. Indeed, recent evidence showed that proper dynamics of Survivin (and presumably the entire CPC) at the centromeres is essential for proper chromosome alignment. Vong et al. (2005) identified ubiquitination as a posttranslational modification required for proper targeting and dynamics of Survivin at centromeres. Interference with this process, by removing either a deubiquitinating enzyme (hFAM) or a ubiquitin binding protein (Ufd1) impaired localization and turnover of Survivin at the centromeres and, as a consequence, disturbed chromosome alignment (Vong et al., 2005). Moreover, Survivin is also an Aurora-B substrate, and mimicking constitutive phosphorylation impairs centromere localization (Wheatley et al., 2004). It will be interesting to see whether these modifications are interdependent and/or influence each other and how posttranslational modifications on Survivin can influence the function of the entire CPC.
From centromere to central spindle
To function during cytokinesis, Aurora-B needs to translocate from the centromeres to the central spindle at the metaphase–anaphase transition. In S. cerevisiae, this translocation is negatively regulated by cyclin B/Cdk1–dependent phosphorylation of INCENP. Dephosphorylation of residues within the coiled-coil domain of INCENP by Cdc14 triggers translocation of the CPC to the central spindle (Pereira and Schiebel, 2003). However, a recent phosphoproteomics analysis failed to identify phoshosites in the coiled-coil domain of human INCENP. Yet, multiple putative cyclin B/Cdk1 phosphosites were identified in a region in INCENP previously shown to interact with HP-1 (Ainsztein et al., 1998; Nousiainen et al., 2006). Together with the observation that expression of a nondegradable cyclin B mutant prevented spindle transfer of Aurora-B during anaphase in human cells (Murata-Hori et al., 2002), this suggests that phospho-dependent regulation of CPC spindle transfer is conserved but that different domains in INCENP might be involved. Besides the phosphosites found within the HP-1 binding domain, several additional residues in INCENP were found to be phosphorylated during mitosis, suggesting complex phospho-dependent regulation of the human CPC (Nousiainen et al., 2006). Relocalization of the CPC from centromeres to the central spindle at the metaphase–anaphase transition also requires dynamic microtubules, as treatment of anaphase cells with the microtubule-stabilizing drug taxol impaired central spindle targeting (Wheatley et al., 2001). INCENP interacts directly with polymerized microtubules via its coiled-coil domain (Mackay et al., 1993). Additionally, a small domain in the NH2 terminus of INCENP interacts with β-tubulin and is essential for efficient spindle localization of the CPC (Ainsztein et al., 1998; Wheatley et al., 2001). Survivin can also interact in vitro with polymerized microtubules, and mutation of the coiled-coil domain in Survivin impaired this interaction (Li et al., 1998), suggesting a dual interaction of the CPC with microtubules (i.e., via INCENP and Survivin). However, it is unclear whether this interaction is crucial for proper CPC localization during anaphase. Besides microtubules, central spindle localization of the CPC also depends on Mklp2, a mitotic kinesin. In human cells depleted of Mklp2, the CPC fails to relocalize to the central spindle during anaphase (Gruneberg et al., 2004). Aurora-B directly interacts with Mklp2 in human cells (Gruneberg et al., 2004), and similarly, C. elegans Aurora-B functionally interacts with the related kinesin Zen-4 (Severson et al., 2000). In this case, Aurora-B itself targets the CPC to the central spindle by interacting with Mklp2. Additionally, Mklp2 interacts with and is required for central spindle localization of human Cdc14a, a homologue of S. cerevisiae Cdc14. Altogether, relocalization of the CPC to the central spindle depends on the orchestrated actions of at least spindle microtubules, Mklp2, and Cdc14 (Fig. 2).
Is centromere localization a prerequisite for central spindle localization? Initial experiments in which disruption of centromeric localization also impaired anaphase spindle transfer indicated that it might be (Ainsztein et al., 1998). However, Drosophila melanogaster mutants undergoing meiosis without chromosomes can execute cytokinesis and concomitantly localize Aurora-B to the central spindle (Bucciarelli et al., 2003). Additionally, recent experiments revealed that the COOH-terminal region of Survivin (the region containing the coiled-coil domain that binds microtubules in vitro [Li et al., 1998]) is sufficient to direct a functional CPC to the central spindle without prior centromere concentration (Lens et al., 2006). It will be interesting to investigate whether this domain in Survivin interacts in vivo with one of the known central spindle CPC receptors or with as-yet-unidentified central spindle receptors. Taken as a whole, it seems that, although in normal cells centromeric and central spindle localization are tightly linked, they can be uncoupled and involve different targeting mechanisms.
Activation of Aurora-B and CPC phosphorylation
In vitro experiments have demonstrated that INCENP is critically needed to activate Aurora-B. INCENP interacts with Aurora-B via its conserved COOH-terminal IN-box, and incubation of this domain with Aurora-B causes an increase in kinase activity (Kang et al., 2001; Bolton et al., 2002). Addition of Borealin does not activate Aurora-B in vitro (Gassmann et al., 2004), whereas conflicting data exist regarding the ability of Survivin to activate Aurora-B. In Xenopus laevis extracts, Survivin is needed for full Aurora-B activity (Bolton et al., 2002), but in vitro experiments with human proteins did not reveal a role for Survivin in activating Aurora-B, whereas INCENP could activate Aurora-B in this in vitro setup, suggesting that INCENP is the major Aurora-B activator in human cells (Honda et al., 2003). Alternatively, in vivo regulatory mechanisms might exist (e.g., additional proteins and/or posttranslational modifications) that are needed for additional Survivin-dependent activation of Aurora-B.
INCENP, Survivin, and Borealin are subject to phosphorylation by Aurora-B (Fig. 1). INCENP phosphorylation at a TSS motif close to the IN-box induces a conformational change in Aurora-B, causing full activation of Aurora-B (Sessa et al., 2005). This phosphorylation is essential for in vitro (Honda et al., 2003; Sessa et al., 2005) and in vivo (unpublished data) functionality of Aurora-B. Survivin is phosphorylated on threonine-117 by Aurora-B in vitro (Wheatley et al., 2004), and this phosphorylation is involved in regulating localization (see Regulation of Aurora-B localization by the CPC proteins). The COOH terminus of Borealin is phosphorylated by Aurora-B, but the functionality of this phosphorylation is unknown (Gassmann et al., 2004). Further research regarding these phosphorylations in CPC function (e.g., on localization and dynamics) will be necessary to deepen our understanding of CPC regulation. Additionally, it will also be interesting to explore whether INCENP, Survivin, and Borealin also influence substrate specificity and recognition of Aurora-B.
Concluding remarks
Aurora-B kinase activity is essential for faithful chromosome segregation and execution of cytokinesis. To fulfill these critical functions, the kinase needs to be in its active conformation at the right place at the right time. It is clear that activity and localization of Aurora-B is tightly controlled by its interaction partners INCENP, Survivin, and Borealin. Because Aurora-B is targeted to different structures during prometaphase/metaphase and anaphase (i.e., centromeric chromatin and microtubules/tubulin, respectively), it will be a challenge to build a complete picture of the CPC-specific receptors on these structures and the composition of CPC proteins that serve as ligands for these receptors. Because Aurora-B is a promising anti-cancer drug target (Keen and Taylor, 2004), interference with these receptor–ligand interactions by small molecules could be an alternative therapeutic strategy for disturbing Aurora-B function.
Acknowledgments
We thank Dr. M. van Vugt for comments on the manuscript.
Our work is supported by the Dutch Cancer Society (NKI 2002-2764).
Abbreviations used in this paper: BIR, baculovirus IAP repeat; CPC, chromosomal passenger complex; INCENP, inner centromere protein.
References
- Ainsztein, A.M., S.E. Kandels-Lewis, A.M. Mackay, and W.C. Earnshaw. 1998. INCENP centromere and spindle targeting: identification of essential conserved motifs and involvement of heterochromatin protein HP1. J. Cell Biol. 143:1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardmore, V.A., L.J. Ahonen, G.J. Gorbsky, and M.J. Kallio. 2004. Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J. Cell Sci. 117:4033–4042. [DOI] [PubMed] [Google Scholar]
- Bolton, M.A., W. Lan, S.E. Powers, M.L. McCleland, J. Kuang, and P.T. Stukenberg. 2002. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol. Biol. Cell. 13:3064–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucciarelli, E., M.G. Giansanti, S. Bonaccorsi, and M. Gatti. 2003. Spindle assembly and cytokinesis in the absence of chromosomes during Drosophila male meiosis. J. Cell Biol. 160:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena, M., and W.C. Earnshaw. 2003. The cellular geography of aurora kinases. Nat. Rev. Mol. Cell Biol. 4:842–854. [DOI] [PubMed] [Google Scholar]
- Chantalat, L., D.A. Skoufias, J.P. Kleman, B. Jung, O. Dideberg, and R.L. Margolis. 2000. Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual alpha-helical extensions. Mol. Cell. 6:183–189. [PubMed] [Google Scholar]
- Ditchfield, C., V.L. Johnson, A. Tighe, R. Ellston, C. Haworth, T. Johnson, A. Mortlock, N. Keen, and S.S. Taylor. 2003. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw, W.C., and R.L. Bernat. 1991. Chromosomal passengers: toward an integrated view of mitosis. Chromosoma. 100:139–146. [DOI] [PubMed] [Google Scholar]
- Fischle, W., B.S. Tseng, H.L. Dormann, B.M. Ueberheide, B.A. Garcia, J. Shabanowitz, D.F. Hunt, H. Funabiki, and C.D. Allis. 2005. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 438:1116–1122. [DOI] [PubMed] [Google Scholar]
- Gassmann, R., A. Carvalho, A.J. Henzing, S. Ruchaud, D.F. Hudson, R. Honda, E.A. Nigg, D.L. Gerloff, and W.C. Earnshaw. 2004. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg, U., R. Neef, R. Honda, E.A. Nigg, and F.A. Barr. 2004. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 166:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf, S., R.W. Cole, S. LaTerra, C. Zimmer, G. Schnapp, R. Walter, A. Heckel, J. van Meel, C.L. Rieder, and J.M. Peters. 2003. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore- microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota, T., J.J. Lipp, B.H. Toh, and J.M. Peters. 2005. Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature. 438:1176–1180. [DOI] [PubMed] [Google Scholar]
- Honda, R., R. Korner, and E.A. Nigg. 2003. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell. 14:3325–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J., I.M. Cheeseman, G. Kallstrom, S. Velmurugan, G. Barnes, and C.S. Chan. 2001. Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J. Cell Biol. 155:763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen, N., and S. Taylor. 2004. Aurora-kinase inhibitors as anticancer agents. Nat. Rev. Cancer. 4:927–936. [DOI] [PubMed] [Google Scholar]
- Klein, U.R., E.A. Nigg, and U. Gruneberg. 2006. Centromere targeting of the chromosomal passenger complex requires a ternary sub-complex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol Biol Cell. 10.1091/mbc.E05-12-1133. [DOI] [PMC free article] [PubMed]
- Lens, S.M., J.A. Rodriguez, G. Vader, S.W. Span, G. Giaccone, and R.H. Medema. 2006. Uncoupling the central spindle-associated function of the chromosomal passenger complex from its role at centromeres. Mol. Biol. Cell. 17:1897–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., G. Ambrosini, E.Y. Chu, J. Plescia, S. Tognin, P.C. Marchisio, and D.C. Altieri. 1998. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 396:580–584. [DOI] [PubMed] [Google Scholar]
- Mackay, A.M., D.M. Eckley, C. Chue, and W.C. Earnshaw. 1993. Molecular analysis of the INCENPs (inner centromere proteins): separate domains are required for association with microtubules during interphase and with the central spindle during anaphase. J. Cell Biol. 123:373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore, S.W., J. Chen, C. Jakob, D. Zakula, E.D. Matayoshi, W. Wu, H. Zhang, F. Li, S.C. Ng, and D.C. Altieri. 2000. Crystal structure and mutagenic analysis of the inhibitor-of-apoptosis protein survivin. Mol. Cell. 6:173–182. [PubMed] [Google Scholar]
- Murata-Hori, M., M. Tatsuka, and Y.L. Wang. 2002. Probing the dynamics and functions of aurora B kinase in living cells during mitosis and cytokinesis. Mol. Biol. Cell. 13:1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nousiainen, M., H.H. Sillje, G. Sauer, E.A. Nigg, and R. Korner. 2006. Phosphoproteome analysis of the human mitotic spindle. Proc. Natl. Acad. Sci. USA. 103:5391–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, G., and E. Schiebel. 2003. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 302:2120–2124. [DOI] [PubMed] [Google Scholar]
- Romano, A., A. Guse, I. Krascenicova, H. Schnabel, R. Schnabel, and M. Glotzer. 2003. CSC-1: a subunit of the Aurora B kinase complex that binds to the Survivin-like protein BIR-1 and the Incenp-like protein ICP-1. J. Cell Biol. 161:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa, F., M. Mapelli, C. Ciferri, C. Tarricone, L.B. Areces, T.R. Schneider, P.T. Stukenberg, and A. Musacchio. 2005. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol. Cell. 18:379–391. [DOI] [PubMed] [Google Scholar]
- Severson, A.F., D.R. Hamill, J.C. Carter, J. Schumacher, and B. Bowerman. 2000. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr. Biol. 10:1162–1171. [DOI] [PubMed] [Google Scholar]
- Vader, G., J.J. Kauw, R.H. Medema, and S.M. Lens. 2006. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep. 7:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecia, M.A., H. Huang, E. Dutil, D.A. Kaiser, T. Hunter, and J.P. Noel. 2000. Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat. Struct. Biol. 7:602–608. [DOI] [PubMed] [Google Scholar]
- Vong, Q.P., K. Cao, H.Y. Li, P.A. Iglesias, and Y. Zheng. 2005. Chromosome alignment and segregation regulated by ubiquitination of survivin. Science. 310:1499–1504. [DOI] [PubMed] [Google Scholar]
- Wheatley, S.P., S.E. Kandels-Lewis, R.R. Adams, A.M. Ainsztein, and W.C. Earnshaw. 2001. INCENP binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow. Exp. Cell Res. 262:122–127. [DOI] [PubMed] [Google Scholar]
- Wheatley, S.P., A.J. Henzing, H. Dodson, W. Khaled, and W.C. Earnshaw. 2004. Aurora-B phosphorylation in vitro identifies a residue of survivin that is essential for its localization and binding to inner centromere protein (INCENP) in vivo. J. Biol. Chem. 279:5655–5660. [DOI] [PubMed] [Google Scholar]
- Yoon, H.J., and J. Carbon. 1999. Participation of Bir1p, a member of the inhibitor of apoptosis family, in yeast chromosome segregation events. Proc. Natl. Acad. Sci. USA. 96:13208–13213. [DOI] [PMC free article] [PubMed] [Google Scholar]