Abstract
Certain mutations in gyrA and gyrB, the genes encoding the two subunits of DNA gyrase, are known to influence expression of the his operon (K. E. Rudd and R. Menzel, Proc. Natl. Acad. Sci. USA 84:517-521, 1987). Such mutations lead to a decrease in tRNA(His) levels and consequently to an attenuator-dependent increase in his operon expression. This effect presumably is due to the dependence of the hisR promoter (hisR encodes tRNA(His) on supercoiling for maximal activity. We used a relaxed (Rel-) strain of Escherichia coli to isolate gyrB mutants by selecting for resistance to the histidine antimetabolite 3-amino-1,2,4-triazole and then screening for temperature-sensitive growth on rich medium. Rel- mutants, which generally have lower basal levels of ppGpp (a positive regulator of his operon transcription), are more sensitive than wild-type E. coli to aminotriazole. The chance of isolating spoT mutants, which can be selected with a similar procedure, was decreased by selecting in the presence of a multicopy plasmid that carries the wild-type spoT gene. Under these conditions, gyrB mutants were isolated preferentially. This scheme selects for loss of function of DNA gyrase, rather than for its alteration due to resistance to specific gyrase inhibitors, and thus a greater variety of gyrase mutations might be obtainable.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Justesen J., Watson R. J., Friesen J. D. Cloning the spoT gene of Escherichia coli: identification of the spoT gene product. J Bacteriol. 1979 Mar;137(3):1100–1110. doi: 10.1128/jb.137.3.1100-1110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune M., Schumann R., Wittinghofer F. Cloning and sequencing of the adenylate kinase gene (adk) of Escherichia coli. Nucleic Acids Res. 1985 Oct 11;13(19):7139–7151. doi: 10.1093/nar/13.19.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K. Bacterial topoisomerases and the control of DNA supercoiling. Trends Genet. 1990 Dec;6(12):433–437. doi: 10.1016/0168-9525(90)90306-q. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa N., Wills N., Bossi L. Common sequence determinants of the response of a prokaryotic promoter to DNA bending and supercoiling. EMBO J. 1991 Apr;10(4):941–949. doi: 10.1002/j.1460-2075.1991.tb08028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I., Plantefaber L. C., Olson E. J., Carver D., O'Dea M. H., Gellert M. Mutations in the DNA gyrB gene that are temperature sensitive for lambda site-specific recombination, Mu growth, and plasmid maintenance. J Bacteriol. 1984 Feb;157(2):490–497. doi: 10.1128/jb.157.2.490-497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
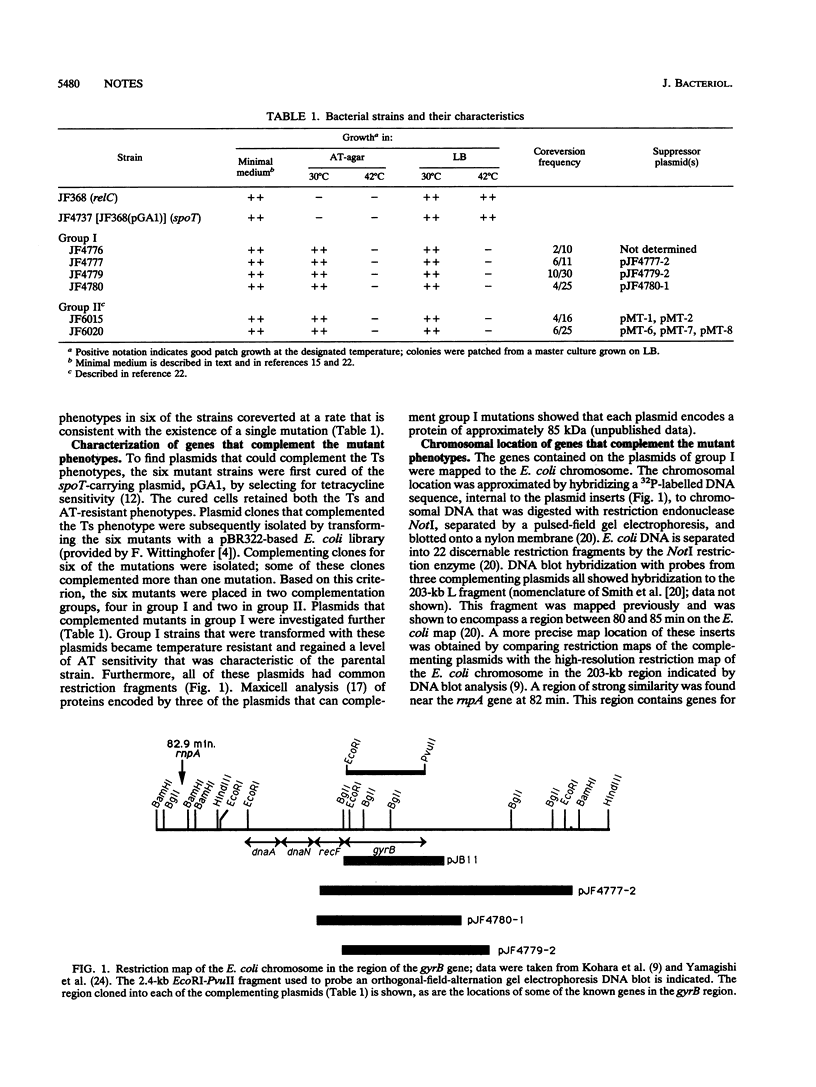
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Watson R. J., Friesen J. D. A relaxed mutant with an altered ribosomal protein L11. Mol Gen Genet. 1976 Feb 27;144(1):111–114. doi: 10.1007/BF00277313. [DOI] [PubMed] [Google Scholar]
- Rudd K. E., Bochner B. R., Cashel M., Roth J. R. Mutations in the spoT gene of Salmonella typhimurium: effects on his operon expression. J Bacteriol. 1985 Aug;163(2):534–542. doi: 10.1128/jb.163.2.534-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd K. E., Menzel R. his operons of Escherichia coli and Salmonella typhimurium are regulated by DNA supercoiling. Proc Natl Acad Sci U S A. 1987 Jan;84(2):517–521. doi: 10.1073/pnas.84.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. Physical map of the recA gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3144–3148. doi: 10.1073/pnas.76.7.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Stephens J. C., Artz S. W., Ames B. N. Guanosine 5'-diphosphate 3'-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4389–4393. doi: 10.1073/pnas.72.11.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toone W. M., Rudd K. E., Friesen J. D. deaD, a new Escherichia coli gene encoding a presumed ATP-dependent RNA helicase, can suppress a mutation in rpsB, the gene encoding ribosomal protein S2. J Bacteriol. 1991 Jun;173(11):3291–3302. doi: 10.1128/jb.173.11.3291-3302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]



