Abstract
S-palmitoylation is a posttranslational modification that regulates membrane–protein interactions. However, palmitate is more than just a hydrophobic membrane anchor, as many different types of protein are palmitoylated, including transmembrane proteins. Indeed, there is now compelling evidence that palmitoylation plays a key role in regulating various aspects of protein sorting within the cell.
Introduction
Modification of otherwise soluble proteins with hydrophobic moieties, such as myristoyl or isoprenyl groups, is essential for their targeting to cell membranes. These modifications occur in the cytosol and are irreversible. In contrast, thioester linkage of palmitate, a C16 saturated fatty acid, to cysteine residues (S-palmitoylation) is a reversible modification catalyzed by membrane-bound palmitoyl transferases (PATs). Thus, palmitoylation can be viewed as a secondary signal for membrane association, as other primary signals must bring the protein to the membrane to allow access to PAT enzymes. In some cases, palmitoylation provides a stable membrane anchor to proteins that have arrived at a membrane compartment via weak or transient interactions, including protein–protein interactions or prenylation/myristoylation. However, palmitoylation also occurs on proteins that are already tightly associated with membranes, including transmembrane proteins, indicating that palmitate is more than just a membrane tether.
The recent identification of a large family of PATs containing a signature DHHC cysteine-rich domain has brought about a renewed interest in the mechanisms and functions of protein palmitoylation (Fukata et al., 2004). There is now accumulating evidence supporting a role for palmitoylation in regulating many aspects of protein trafficking within the cell. In this mini-review, we focus on specific studies that highlight the diversity of palmitoylation as a signal for protein sorting, before ending with a discussion of the possible mechanisms that underlie palmitoylation-dependent sorting.
Palmitoylation as a cue for traffic or retention
The effects of palmitoylation on protein sorting are not easily predicted, and indeed modification of different cysteines in the same protein can have distinct effects on trafficking. This is the case for the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, a ligand-gated cation channel that mediates the fast component of glutamate-induced excitatory postsynaptic currents. All AMPA receptor subunits, GluR1-GluR4, are palmitoylated; palmitoylation sites are located at the intracellular face of the second transmembrane domain (site 1) and in the C terminus of the protein just downstream of the fourth transmembrane domain (site 2) (Hayashi et al., 2005). Palmitoylation of site 1 was enhanced by coexpression of the PAT DHHC-3 (also called GODZ), promoting accumulation of the receptor in the Golgi and decreasing cell surface expression levels. As DHHC3 is localized to the Golgi (Keller et al., 2004), this implies that palmitate addition to this cysteine residue promotes retention of the receptor at this compartment. Interestingly, several other proteins exhibit a similar intracellular retention upon overexpression of specific PAT enzymes (Huang et al., 2004; Keller et al., 2004); however, the mechanism for this is not clear. In contrast to the effects of palmitoylation of site 1 in GluR subunits, palmitoylation of site 2 did not appear to regulate steady-state cell surface levels of the receptor. However, mutation of this cysteine residue in GluR1/2 inhibited activity- dependent internalization. Thus, regulated palmitoylation/depalmitoylation of both sites in GluR subunits is likely to play a key role in regulating surface expression of the AMPA receptor, albeit by different mechanisms.
Many other transmembrane proteins rely on palmitoylation for correct sorting in mammalian cells. Recent examples of this include the human δ opioid receptor, a G protein–coupled receptor, which required palmitoylation for efficient biosynthetic delivery to the plasma membrane (PM; Petaja-Repo et al., 2006), and the mucin-like MUC1 protein in which palmitoylation of two cysteine residues, although not required for biosynthetic delivery to the cell surface, was linked to efficient trafficking from recycling endosomes to the PM (Kinlough et al., 2006). The role of palmitoylation in regulating the sorting of transmembrane proteins is also apparent in lower eukaryotes, including the yeast Saccharomyces cerevisiae. Chs3 is a chitin synthase involved in cell wall growth that localizes to the tip and neck of the bud and also to an intracellular compartment. The polytopic Chs3 protein is palmitoylated by the ER-localized DHHC protein Pfa4, and preventing this palmitoylation caused Chs3 to be retained in the ER (Lam et al., 2006). Interestingly, unpalmitoylated Chs3 displayed an increased level of aggregation, consistent with the idea that palmitoylation may stabilize membrane interactions of the transmembrane helices of Chs3.
Palmitoylation also plays an important role in the sorting of proteins lacking transmembrane peptide sequences that are tethered to the cytosolic surface of membranes. In many such cases, a key function of palmitate is to serve as a membrane “trap” by increasing relative membrane affinity, and this is an important difference compared with palmitoylation of transmembrane proteins. A well-characterized example of this is palmitoylation-dependent sorting of H- and N-Ras. The primary signal for membrane association of these proteins is C-terminal farnesylation (isoprenylation), which mediates the association of Ras with ER and Golgi membranes (Choy et al., 1999). However, such single lipid modifications provide only a weak membrane affinity (Peitzsch and McLaughlin, 1993; Shahinian and Silvius, 1995), and farnesylated Ras undergoes diffusional exchange between the cytosol and endomembranes (Goodwin et al., 2005; Rocks et al., 2005). In contrast, two tandem lipid modifications provide a relatively stable membrane anchor (Shahinian and Silvius, 1995); thus, subsequent palmitoylation of Ras increases the strength of membrane interaction (Magee et al., 1987). This membrane trapping of Ras facilitates targeting to post-ER compartments, particularly the PM and Golgi. In a manner similar to AMPA receptor trafficking, each of the two palmitoylated cysteines in H-Ras (cys-181 and -184) was suggested to differentially affect protein localization (Roy et al., 2005). Monopalmitoylation of cysteine-184 led to accumulation of H-Ras in the Golgi. As Ras PAT (DHHC9/GCP16) is predominantly localized to the Golgi in mammalian cells (Swarthout et al., 2005), this implies that palmitate addition to cys-184 may act as a membrane trap but without conferring any additional targeting information. Another possibility is that the small amount of DHHC9/GCP16 present on ER membranes mediates palmitoylation of H-Ras at this compartment and, hence, that modification of cys-184 supports transport from the ER to the Golgi. In contrast, monopalmitoylation of cys-181 more effectively directed PM delivery of H-Ras.
Sorting of palmitoylated proteins to specific PM domains
Clustering of AMPA receptors at postsynaptic sites is regulated by postsynaptic density protein-95 (PSD-95). The exclusive targeting of PSD-95 to postsynaptic clusters in neurons is dependent on dual palmitoylation of a specific amino acid sequence, MDCLCIV (Craven et al., 1999). Replacing this sequence with the palmitoylation code from the axonally localized GAP43 protein (MLCCMRR) disrupted the exclusive postsynaptic targeting of PSD-95 and redistributed a fraction of the protein to axons (El-Husseini et al., 2001). Features of the palmitoylation domains of PSD-95 and GAP-43 that were important for postsynaptic and axonal targeting, respectively, included the spacing of palmitoylated cysteines and the presence of basic amino acids downstream of the cysteines. It is not clear how these specific features contribute to sorting, but they may be important for directing the proteins to distinct transport vesicles (El-Husseini et al., 2000). Alternatively, the different palmitoylation motifs may form recognition sites for distinct PAT enzymes, with the localization of specific PATs dictating the final distribution of GAP-43 and PSD-95 (El-Husseini et al., 2001). For example, the GAP-43 PAT may reside in a compartment (or specific microdomain of a compartment) that links to an axonal trafficking pathway, whereas the PSD-95 PAT may link to a postsynaptic pathway. In addition to regulating polarized trafficking in neurons, there is also evidence implying a role for palmitoylation in sorting to the myelin membrane in oligodendrocytes (Schneider et al., 2005) and to tight junctions in epithelial cells (Van Itallie et al., 2005).
The effects of palmitoylation on sorting to PM domains are not restricted to polarized cells and may direct the nanometer-scale microlocalization of proteins within the same membrane. Although this topic is beyond the scope of this mini-review, it is worth noting that palmitoylation of cys-184 of H-Ras (see previous section), although not required for PM delivery, was essential for regulating the correct distribution of GDP- and GTP-bound forms of the protein between cholesterol-rich microdomains and domains that were insensitive to cholesterol extraction (Roy et al., 2005).
Palmitoylation and the ubiquitination pathway
The previous sections highlight the role of palmitoylation in regulating membrane retention or directly influencing protein sorting. In contrast, palmitoylation of the yeast SNARE protein Tlg1 appears to play a more indirect role in membrane targeting of this protein. SNAREs are a family of proteins located on various membrane compartments that regulate intracellular membrane fusion events; Tlg1 regulates membrane traffic between the endosomes and Golgi. Palmitoylation by the DHHC protein Swf1 ensured that Tlg1 is retained on TGN/endosome membranes by protecting the protein from ubiquitination (Valdez-Taubas and Pelham, 2005). Indeed, mutation of the palmitoylation sites in Tlg1 or genetic inactivation of Swf1 led to Tlg1 ubiquitination by the ubiquitin ligase Tul1, causing Tlg1 to be routed to the vacuole and degraded.
However, it is interesting to note that cysteine mutants of Tlg1 displayed a subcellular distribution similar to wild-type Tlg1 when ubiquitin ligases were inactivated. This implies that palmitoylation is not required for membrane targeting of Tlg1 per se, but simply to prevent ubiquitination. Palmitoylation was suggested to fix the position of the transmembrane domain of Tlg1 relative to the bilayer, perhaps ensuring that membrane-proximal acidic residues are not exposed to membrane lipids, a situation that would be predicted to lead to ubiquitination by Tul1 (Valdez-Taubas and Pelham, 2005).
Dynamic palmitoylation and protein sorting
Palmitoylation is a reversible process, and several cellular proteins undergo dynamic palmitoylation (Smotrys and Linder, 2004). The reversibility of palmitoylation can enhance the versatility of this lipid modification as a protein sorting signal. AMPA receptors exhibit stimulation-dependent changes in palmitoylation status as well as regulated internalization (Hayashi et al., 2005). Interestingly, mutation of palmitoylation site 2 (refer to previous sections) in GluR1/2 subunits inhibited activity-dependent internalization. Furthermore, palmitoylation of this site was associated with an inhibition of GluR1/2 binding to the cytoskeleton-associated 4.1N protein. Binding to 4.1N was suggested to trap GluR1/2 at the cell surface, suggesting that palmitoylation of site 2 enhances internalization of GluR1/2 by regulating this interaction (Fig. 1 A).Interestingly, activity-dependent changes in palmitoylation also regulate surface distribution of PSD-95, a protein that modulates synaptic clustering of AMPA receptors (El-Husseini et al., 2002). Indeed, these palmitoylation changes in PSD-95 were also suggested to be required for glutamate-mediated internalization of AMPA receptors. Thus, dynamic palmitoylation plays a key role both directly and indirectly (via PSD-95) in regulating surface distribution of AMPA receptors and hence synaptic activity.
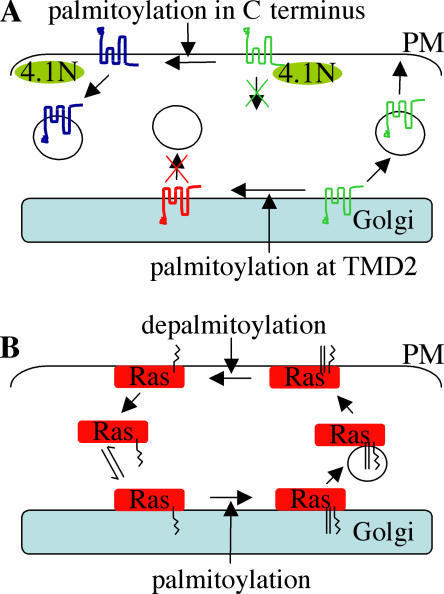
Figure 1.
Palmitoylation-dependent sorting of AMPA receptors and Ras proteins. (A) Palmitoylation of AMPA receptor GluR subunits at transmembrane 2 (TMD2) promotes retention of the receptor in the Golgi, preventing cell surface delivery. When at the cell surface, internalization of AMPA receptors is regulated by interaction with 4.1N. Palmitoylation in the C terminus of GluR subunits inhibits the 4.1N interaction, facilitating internalization of the receptor. The different colors of GluR indicate palmitoylation status: green is unpalmitoylated, red is palmitoylated at TMD2, and blue is palmitoylated at the C terminus. (B) Ras modified by farnesylation (zigzag line) has a weak affinity for Golgi membranes. Subsequent palmitoylation (straight lines) at the Golgi mediates membrane trapping and facilitates Ras trafficking to the PM. At the PM, Ras is depamitoylated, releasing the protein into the cytosol, where it can rebind to Golgi membranes.
Recent work also uncovered a dynamic palmitoylation pathway that regulates Ras trafficking. This pathway operates constitutively and is essential for maintaining correct Ras localization (Goodwin et al., 2005; Rocks et al., 2005). The results of these recent studies also implied that the half-life of palmitate on Ras proteins may be far less than the originally reported values of 20 min for N-Ras (Magee et al., 1987) and 2 h for H-Ras (Lu, 1995; Baker et al., 2003), suggesting an incredibly fast rate of palmitate turnover. This dynamic palmitoylation of Ras proteins results in a constant flux of the proteins between endomembranes and the PM. In this system, palmitoylation at Golgi membranes directs Ras to the PM, whereas depalmitoylation at the PM releases the protein into the cytosol, allowing it to rebind to Golgi membranes, where it is once again palmitoylated and trafficked to the PM (Fig. 1 B). This palmitoylation cycle appears to be essential for maintaining the appropriate subcellular distribution of Ras, as the addition of palmitate-like hexadecylated groups with noncleavable thioether bonds to N-Ras caused a marked missorting of the protein (Rocks et al., 2005). This observation suggests that although palmitoylation of Ras drives PM delivery, it may not be sufficient to maintain Ras at the PM during ongoing membrane remodeling, for example, exocytosis and endocytosis. However, these experiments may be more difficult to interpret, as much of the microinjected hexadecylated protein will presumably associate with intracellular membranes independently of the secretory pathway and thus not experience the same initial trafficking cues as endogenous Ras. Identification of this rapid Ras cycling pathway also introduces an extra dimension to trafficking analyses of monopalmitoylated H-Ras mutants.
Intriguingly, similar palmitoylation cycles were also observed for short N-terminal sequences of other palmitoylated proteins (Rocks et al., 2005), suggesting that dynamic palmitoylation may regulate the intracellular distribution of many proteins. However, this study examined relatively short palmitoylated peptides, and the presence of other domains in the full-length proteins is likely to regulate the intrinsic palmitoylation/depalmitoylation cycle (Rocks et al., 2005); indeed, many proteins, such as SNAP25, appear to be stably palmitoylated (Kang et al., 2004).
Mechanisms for palmitoylation-dependent sorting
Palmitoylation could affect protein trafficking by several distinct mechanisms. It is important to stress that the role palmitoylation serves in the trafficking of integral membrane proteins may be different from that of proteins with a weak membrane affinity, such as H- and N-Ras. In the simplest case, palmitoylation could trap proteins with a weak membrane affinity on an appropriate intracellular membrane by enhancing the strength of membrane interaction (Fig. 2 A). This membrane trapping is well characterized for palmitoylated proteins like Ras, where the addition of a second lipid modification leads to a large increase in membrane residency time (Magee et al., 1987; Shahinian and Silvius, 1995). This enhanced membrane association would allow the protein to associate more efficiently with budding vesicles and ensure that the protein does not dissociate from the membrane during vesicle transport. In this model, sorting is regulated by the strength of membrane interaction and not palmitoylation per se. The specificity of palmitoylation-dependent sorting, in this case, may be dictated by the localization of the appropriate PAT enzyme. For example, although Ras proteins can presumably “sample” a variety of intracellular membranes by virtue of a hydrophobic farnesyl modification, the restricted distribution of Ras PAT to Golgi/ER membranes may ensure the correct sorting of the proteins to the PM and Golgi. In addition, the specific subcellular localization of Ras thioesterases may also contribute to maintaining the appropriate distribution of Ras proteins. By analogy, it may be the intracellular localization of PAT enzymes that modify the specific palmitoylation sequences of PSD-95 and GAP-43 that ensures correct polarized sorting of these proteins in neuronal cells.
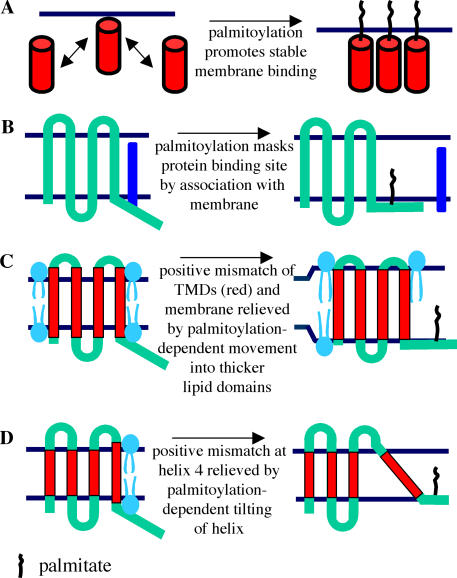
Figure 2.
Regulation of membrane interactions by palmitoylation. (A) Membrane “trapping” by palmitoylation. The figure shows a protein with a relatively weak membrane affinity (such as farnesylated Ras) undergoing dynamic exchange between the cytosol and membrane. Subsequent palmitoylation traps the protein at the membrane by increasing the strength of the hydrophobic anchor. (B) Illustration of a potential mechanism whereby palmitoylation of a cysteine residue masks a protein binding site by pulling it into close proximity to the membrane. One possible outcome would be that the palmitoylated protein is now free to traffic to a distinct membrane compartment. (C) Model depicts palmitoylation modifying the lateral distribution of a protein within the membrane. In this instance, the association with thicker membrane domains relieves a hydrophobic mismatch between the hydrophobic part of transmembrane helices (shown in red) and the original membrane domain. The stabilization of the hydrophobic segments may directly allow the protein to traffic, for example, by preventing aggregation, or, alternatively, the association of the protein with distinct membrane domains might drive subsequent sorting. TMD, transmembrane domain. (D) Hydrophobic mismatch of helix 4 is relieved by palmitoylation, which in this instance changes the tilt of the transmembrane domain. This modified membrane association of the protein may facilitate trafficking. Note that the extent of mismatch shown in C and D has been exaggerated for clarity. The relative positions of polar and nonpolar regions of membrane phospholipids are shown for reference.
In addition to this passive role in intracellular trafficking, palmitoylation may also actively drive the association of proteins with budding vesicles or specific microdomains that facilitate sorting. This model applies both to proteins with a weak membrane affinity (such as Ras) and to integral membrane proteins. There is a steep concentration gradient of cholesterol within cells, with the highest levels in endosomes and the PM. In contrast, the membrane of the ER, where cholesterol is synthesized de novo, contains very little cholesterol. The majority of synthesized cholesterol is transported to the PM directly by nonvesicular traffic; however, a significant fraction is also transported via vesicular traffic through the Golgi (Heino et al., 2000). Cholesterol forms tightly packed domains with saturated phospholipids, and palmitoylated proteins are thought to have a high affinity for these ordered domains. Thus, the association of palmitate groups with cholesterol-rich domains may allow protein movement from the early secretory pathway by vesicular transport. An additional intriguing possibility is that palmitate groups interact directly with cholesterol. Although phospholipids with saturated acyl chains (such as palmitate) exhibit a high affinity for cholesterol in model membranes, the phospholipid head groups are thought to play an important role in this association (Ali et al., 2006). Therefore, it is not clear whether palmitoylated proteins could have an affinity similar to saturated phospholipids for cholesterol, although there is some evidence to support this possibility (Uittenbogaard and Smart, 2000; Roy et al., 2005).
Another important mechanism underlying palmitoylation-dependent protein sorting involves the regulation of protein–protein interactions. These interactions may be with sorting receptors or cargo or with specific proteins such as 4.1N in the case of the AMPA receptor. Palmitoylation could regulate many of these interactions by controlling the conformation of the modified protein. For example, cysteine palmitoylation and subsequent membrane integration may force flanking residues into closer membrane proximity (Fig. 2 B); if these residues form part of a protein binding pocket, then this would inhibit binding. Similarly, the cysteine residue itself may mediate protein interactions that would be inhibited by palmitoylation. Palmitoylation may also bring a protein binding domain into closer proximity to a membrane receptor, enhancing the possibility of productive interactions. In addition to these direct effects on protein interactions, palmitoylation may regulate protein interactions by spatially coupling or segregating proteins within lipid microdomains.
The above discussion has highlighted how association with specific membrane microdomains and the regulation of protein–protein interactions could mediate palmitoylation-dependent protein sorting. These models apply equally to integral membrane proteins and to proteins with weak membrane affinities that have been trapped on membranes by palmitoylation. In addition, palmitoylation may specifically affect sorting of integral membrane proteins by regulating interactions of transmembrane domains with the lipid bilayer. An interesting possibility in this regard is that palmitoylation regulates trafficking of transmembrane proteins by enhancing hydrophobic matching between transmembrane domains and the lipid bilayer (de Planque and Killian, 2003; Kandasamy and Larson, 2006). Hydrophobic mismatch occurs when a discrepancy exists between the thickness of the hydrophobic region of the phospholipid bilayer and the length of the hydrophobic transmembrane helix (Fig. 2 C), and this mismatching is thought to be energetically unfavorable. In relation to intracellular trafficking, the exposure of hydrophobic domains by mismatching could lead to protein aggregation or ER retention (as observed for Chs3) or association with ubiquitin ligases (as seen for Tlg1).
Palmitoylation may affect the extent of hydrophobic mismatching by inducing lateral movement of the protein into distinct membrane microdomains (as discussed previously in this paper). For example, when there is a positive mismatch (hydrophobic domain of protein longer than the thickness of the hydrophobic part of the membrane), palmitoylation may move the protein into cholesterol-rich domains of the membrane (Fig. 2 C). Cholesterol plays an important role in controlling bilayer thickness, and the addition of 30% mol/mol cholesterol to C16:0/C18:1 phosphatidylcholine bilayers increased the thickness of the hydrophobic core of the bilayer by ∼15% (Nezil and Bloom, 1992). Thus, association with cholesterol-rich domains would be predicted to alleviate the positive mismatch. Another idea is that palmitoylation relieves hydrophobic mismatch by altering the tilt of transmembrane helices within the bilayer (Kandasamy and Larson, 2006; Fig. 2 D); indeed, palmitoylation of hydrophobic membrane-spanning peptides was suggested to modify peptide orientation in lipid vesicles (Joseph and Nagaraj, 1995). It is important to note that although palmitate groups may preferentially partition into cholesterol-rich domains, bulky transmembrane helices are generally excluded from these tightly packed domains. An interesting possibility, therefore, is that the affinity of palmitate for ordered lipid domains, coupled with the exclusion of the transmembrane helix from these regions, promotes tilting of the helix as it resists entry into ordered domains occupied by the adjacent palmitate. The effects on transmembrane tilting may be particularly relevant for proteins that have palmitoylation sites in membrane-spanning regions. Finally, palmitoylation may affect the orientation of transmembrane domains by regulating the interfacial localization of aromatic and basic amino acids that typically flank the hydrophobic segments of transmembrane helices.
Concluding remarks and perspective
Recent work has highlighted the diverse nature of palmitate as a protein sorting signal. An important area of investigation now is to identify common mechanisms that regulate palmitoylation-induced protein sorting and to “crack” the palmitoylation codes within proteins to reveal how a palmitoylated peptide sequence relates to the final destination of a protein in the cell. Like the search for palmitoylation consensus sequences, these questions are likely to present a significant challenge.
Acknowledgments
We are very grateful to Christine Salaun (University of Glasgow) and Ian Prior (University of Liverpool) for helpful comments and suggestions.
Work in the authors' laboratory is funded by the Wellcome Trust (grant 070835), Diabetes UK (grant BDA: RD06/0003167), and Tenovus Scotland (grant S06/1).
Abbreviations used in this paper: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; PAT, palmitoyl transferase; PM, plasma membrane.
References
- Ali, M.R., K.H. Cheng, and J. Huang. 2006. Ceramide drives cholesterol out of the ordered lipid bilayer phase into the crystal phase in 1-palmitoyl- 2-oleoyl-glycero-3-phosphocholine/cholesterol/ceramide ternary mixtures. Biochemistry. 45:12629–12638. [DOI] [PubMed] [Google Scholar]
- Baker, T.L., H. Zheng, J. Walker, J.L. Coloff, and J.E. Buss. 2003. Distinct rates of palmitate turnover on membrane-bound cellular and oncogenic H-Ras. J. Biol. Chem. 278:19292–19300. [DOI] [PubMed] [Google Scholar]
- Choy, E., V.K. Chiu, J. Silletti, M. Feoktistov, T. Morimoto, D. Michaelson, I.E. Ivanov, and M.R. Philips. 1999. Endomembrane trafficking of Ras: The CAAX motif targets proteins to the ER and Golgi. Cell. 98:69–80. [DOI] [PubMed] [Google Scholar]
- Craven, S.E., A.E. El-Husseini, and D.S. Bredt. 1999. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 22:497–509. [DOI] [PubMed] [Google Scholar]
- de Planque, M.R.R., and J.A. Killian. 2003. Protein-lipid interactions studied with designed transmembrane peptides: role of hydrophobic matching and interfacial anchoring. Mol. Membr. Biol. 20:271–284. [DOI] [PubMed] [Google Scholar]
- El-Husseini, A.-D., S.E. Craven, S.C. Brock, and D.S. Bredt. 2001. Polarized targeting of peripheral membrane proteins in neurons. J. Biol. Chem. 276:44984–44992. [DOI] [PubMed] [Google Scholar]
- El-Husseini, A.-D., E. Schnell, S. Dakoji, N. Sweeney, Q. Zhou, O. Prange, C. Gauthier-Campbell, A. Aguilera-Moreno, R.A. Nicoll, and D.S. Bredt. 2002. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 108:849–863. [DOI] [PubMed] [Google Scholar]
- El-Husseini, A.E., S.E. Craven, D.M. Chetkovich, B.L. Firestein, E. Schnell, C. Aoki, and D.S. Bredt. 2000. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J. Cell Biol. 148:159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata, M., Y. Fukata, H. Adesnik, R.A. Nicoll, and D.S. Bredt. 2004. Identification of PSD-95 palmitoylating enzymes. Neuron. 44:987–996. [DOI] [PubMed] [Google Scholar]
- Goodwin, J.S., K.R. Drake, C. Rogers, L. Wright, J. Lippincott-Schwartz, M.R. Philips, and A.K. Kenworthy. 2005. Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J. Cell Biol. 170:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, T., G. Rumbaugh, and R.L. Huganir. 2005. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 47:709–723. [DOI] [PubMed] [Google Scholar]
- Heino, S., S. Lusa, P. Somerharju, C. Ehnholm, V.M. Olkkonen, and E. Ikonen. 2000. Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc. Natl. Acad. Sci. USA. 97:8375–8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, K., A. Yanai, R. Kang, P. Arstikaitis, R.R. Singaraja, M. Metzler, A. Mullard, B. Haigh, C. Gauthier-Campbell, and C.-A. Gutekunst. 2004. Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron. 44:977–986. [DOI] [PubMed] [Google Scholar]
- Joseph, M., and R. Nagaraj. 1995. Interaction of peptides corresponding to fatty acylation sites in proteins with model membranes. J. Biol. Chem. 270:16749–16755. [DOI] [PubMed] [Google Scholar]
- Kandasamy, S.K., and R.G. Larson. 2006. Molecular dynamics simulations of model trans-membrane peptides in lipid bilayers: a systematic investigation of hydrophobic mismatch. Biophys. J. 90:2326–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, R., R. Swayze, M.F. Lise, K. Gerrow, A. Mullard, W.G. Honer, and A. El-Husseini. 2004. Presynaptic trafficking of synaptotagmin I is regulated by protein palmitoylation. J. Biol. Chem. 279:50524–50536. [DOI] [PubMed] [Google Scholar]
- Keller, C.A., X. Yuan, P. Panzanelli, M.L. Martin, M. Alldred, M. Sassoe-Pognetto, and B. Luscher. 2004. The γ2 subunit of GABAA receptors is a substrate for palmitoylation by GODZ. J. Neurosci. 24:5881–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlough, C.L., R.J. McMahan, P.A. Poland, J.B. Bruns, K.L. Harkleroad, R.J. Stremple, O.B. Kashlan, K.M. Weixel, O.A. Weisz, and R.P. Hughey. 2006. Recycling of MUC1 is dependent on its palmitoylation. J. Biol. Chem. 281:12112–12122. [DOI] [PubMed] [Google Scholar]
- Lam, K.K.Y., M. Davey, B. Sun, A.F. Roth, N.G. Davis, and E. Conibear. 2006. Palmitoylation by the DHHC protein Pfa4 regulates the ER exit of Chs3. J. Cell Biol. 174:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, J.-Y. 1995. Depalmitoylation of CAAX motif proteins. J. Biol. Chem. 270:7251–7256. [DOI] [PubMed] [Google Scholar]
- Magee, A.I., L. Gutierrez, I.A. McKay, C.J. Marshall, and A. Hall. 1987. Dynamic fatty acylation of p21N-ras. EMBO J. 6:3353–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezil, F.A., and M. Bloom. 1992. Combined influence of cholesterol and synthetic amphiphillic peptides upon bilayer thickness in model membranes. Biophys. J. 61:1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitzsch, R., and S. McLaughlin. 1993. Binding of acylated proteins and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry. 32:10436–10443. [DOI] [PubMed] [Google Scholar]
- Petaja-Repo, U.E., M. Hogue, T.T. Leskela, P.M.H. Markkanen, J.T. Tuusa, and M. Bouvier. 2006. Distinct subcellular localization for constitutive and agonist-modulated palmitoylation of the human δ opioid receptor. J. Biol. Chem. 281:15780–15789. [DOI] [PubMed] [Google Scholar]
- Rocks, O., A. Peyker, M. Kahms, P.J. Verveer, C. Koerner, M. Lumbierres, J. Kuhlmann, H. Waldmann, A. Wittinghofer, and P.I.H. Bastiaens. 2005. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 307:1746–1752. [DOI] [PubMed] [Google Scholar]
- Roy, S., S. Plowman, B. Rotblat, I.A. Prior, C. Muncke, S. Grainger, R.G. Parton, Y.I. Henis, Y. Kloog, and J.F. Hancock. 2005. Individual palmitoyl residues serve distinct roles in H-Ras trafficking, microlocalization, and signaling. Mol. Cell. Biol. 25:6722–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, A., H. Lander, G. Schulz, H. Wolburg, K.-A. Nave, J.B. Schulz, and M. Simons. 2005. Palmitoylation is a sorting determinant for transport to the myelin membrane. J. Cell Sci. 118:2415–2423. [DOI] [PubMed] [Google Scholar]
- Shahinian, S., and J. Silvius. 1995. Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry. 34:3813–3822. [DOI] [PubMed] [Google Scholar]
- Smotrys, J.E., and M.E. Linder. 2004. Palmitoylation of intracellular signaling proteins: regulation and function. Annu. Rev. Biochem. 73:559–587. [DOI] [PubMed] [Google Scholar]
- Swarthout, J.T., S. Lobo, L. Farh, M.R. Croke, W.K. Greentree, R.J. Deschenes, and M.E. Linder. 2005. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J. Biol. Chem. 280:31141–31148. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard, A., and E.J. Smart. 2000. Palmitoylation of caveolin-1 is required for cholesterol binding, chaperone complex formation, and rapid transport of cholesterol to caveolae. J. Biol. Chem. 275:25595–25599. [DOI] [PubMed] [Google Scholar]
- Valdez-Taubas, J., and H. Pelham. 2005. Swf1-dependent palmitoylation of the SNARE Tlg1 prevents its ubiquitination and degradation. EMBO J. 24:2524–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie, C.M., T.M. Gambling, J.L. Carson, and J.M. Anderson. 2005. Palmitoylation of claudins is required for efficient tight-junction localization. J. Cell Sci. 118:1427–1436. [DOI] [PubMed] [Google Scholar]