Abstract
Members of the Rab guanosine triphosphatase (GTPase) family are key regulators of membrane traffic. Here we examined the association of 48 Rabs with model phagosomes containing a non-invasive mutant of Salmonella enterica serovar Typhimurium (S. Typhimurium). This mutant traffics to lysosomes and allowed us to determine which Rabs localize to a maturing phagosome. In total, 18 Rabs associated with maturing phagosomes, each with its own kinetics of association. Dominant-negative mutants of Rab23 and 35 inhibited phagosome–lysosome fusion. A large number of Rab GTPases localized to wild-type Salmonella-containing vacuoles (SCVs), which do not fuse with lysosomes. However, some Rabs (8B, 13, 23, 32, and 35) were excluded from wild-type SCVs whereas others (5A, 5B, 5C, 7A, 11A, and 11B) were enriched on this compartment. Our studies demonstrate that a complex network of Rab GTPases controls endocytic progression to lysosomes and that this is modulated by S. Typhimurium to allow its intracellular growth.
Introduction
Movement through the endosomal system involves the progression from an early endosome to a late endosome to a degradative lysosome. This progression is regulated, at least in part, by Rab family GTPases localized to each compartment (Zerial and McBride, 2001). For example, early endosomes are characterized by their rapid recruitment of Rab5 (Gorvel et al., 1991). Progression from an early to a late endosome (with concomitant Rab7 recruitment) occurs through the action of Rab5 effectors (Rink et al., 2005). Phagocytosis is a specialized form of endocytosis that allows the internalization of large particles into the cell and plays an essential role in development and immunity. After their formation, phagosomes undergo a maturation process by interacting with the endosomal pathway (Vieira et al., 2002). During phagosome maturation, Rab5 is necessary for the recruitment of Rab7 and the formation of a late phagosome and a subsequent phagolysosome. Therefore, Rabs are critical regulators of endosome and phagosome maturation and many parallels are likely to exist between these events. However, as there are over 60 known Rabs and multiple Rabs can be present in distinct microdomains on a single endosome (Sonnichsen et al., 2000), it is unclear if and how most Rabs function during endosome/phagosome maturation. The small size of endosomes makes Rab interactions with the endosomal system difficult to study. Therefore, we decided to use a model phagosome to screen a large network of Rabs to determine which Rabs are involved in phagosome maturation.
Results and discussion
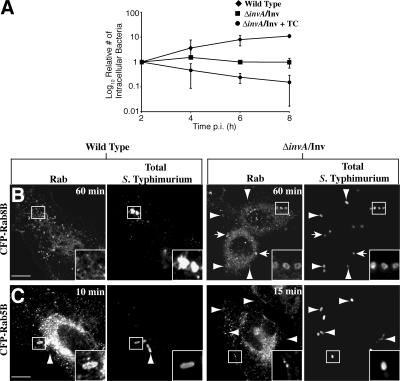
We chose the ΔinvA/Inv strain of Salmonella enterica serovar Typhimurium (S. Typhimurium) as our model to generate phagosomes. The InvA protein is a structural component of the Salmonella pathogenicity island (SPI)–1–encoded type III secretion system (TTSS; Galan and Curtiss, 1989). The TTSS is a needle-like device that S. Typhimurium uses to translocate bacterial effector proteins into the host cell to manipulate the actin cytoskeleton and drive invasion through a ruffling mechanism (Brumell and Grinstein, 2004). The ΔinvA strain is unable to secrete effector proteins into the host cell and is therefore noninvasive. Expression of the invasin (inv) gene of Yersinia pseudotuberculosis allows the bacteria to enter the host cell via an alternative mechanism. The Inv protein induces uptake of the bacteria through binding to β1-integrin (Isberg and Leong, 1990). Inv-mediated uptake of Y. pseudotuberculosis leads to degradation of these bacteria in lysosomes (Mills and Finlay, 1998). Similarly, the ΔinvA/Inv strain of S. Typhimurium is efficiently internalized and traffics to late endocytic compartments where it fails to replicate (Steele-Mortimer et al., 2002). To ensure that the phagosomal maturation of ΔinvA/Inv S. Typhimurium is not manipulated by virulence factors apart from the SPI-1 TTSS, such as the PhoP/PhoQ regulon or the SPI-2 encoded TTSS (Brumell and Grinstein, 2004), we inhibited bacterial protein synthesis through addition of tetracycline 15 min after internalization. This resulted in the trafficking of the bacteria to a lysosome, leading to their degradation (Fig. 1 A).
Figure 1.
Differential trafficking of wild-type and ΔinvA/Inv S. Typhimurium. (A) Cells were infected with wild type (♦), ΔinvA/Inv (▪), or ΔinvA/Inv + tetracycline (•). Semi-log plot of the relative number of intracellular bacteria over an 8-h infection. Results are the means (± SD) of three independent experiments. HeLa cells were transfected with CFP-Rab8B (B) or CFP-Rab5B (C), infected with wild-type or ΔinvA/Inv S. Typhimurium, and fixed at the indicated time. Arrows indicate colocalization between bacterial vacuole and Rab. Arrowheads indicate extracellular bacteria. Bar, 10 μm.
To follow the trafficking of the model phagosome we separately transfected HeLa cells with 48 distinct GFP- or CFP-tagged Rab GTPases. The Rabs chosen for our study constitute a broad representation of the Rab family. We then introduced ΔinvA/Inv S. Typhimurium and, after phagocytosis, tracked Rab association with the model phagosome over 3 h. At each time point investigated, we determined Rab association for at least 100 internalized bacteria, ensuring we only counted cells where expression of the Rab was relatively low. To ensure we counted only internalized bacteria, we immunostained for S. Typhimurium before and after permeabilization of the cellular membrane. In this way we were able to determine which bacteria had invaded and which remained extracellular. We counted a positive association of a Rab with the model phagosome as a distinct ring around the bacteria and in the same focal plane as the bacteria (Fig. 1, B and C).
In parallel, we infected cells with wild-type S. Typhimurium and monitored its association with the same 48 Rab GTPases. Wild-type S. Typhimurium use the SPI-1–encoded TTSS to invade epithelial cells in a manner distinct from receptor-mediated phagocytosis (Brumell and Grinstein, 2004). After invasion, the bacteria reside in a Salmonella-containing vacuole (SCV) that, like a phagosome, interacts with the early endosomal pathway and acquires Rab5. The SCV then undergoes a maturation process that is characterized by the recruitment of lysosomal-associated membrane protein-1 (LAMP-1) and other lysosomal glycoproteins but does not fuse with lysosomes. The manner in which wild-type S. Typhimurium is able to manipulate SCV trafficking is unknown; however, there is evidence it can directly manipulate Rab function (Harrison et al., 2004).
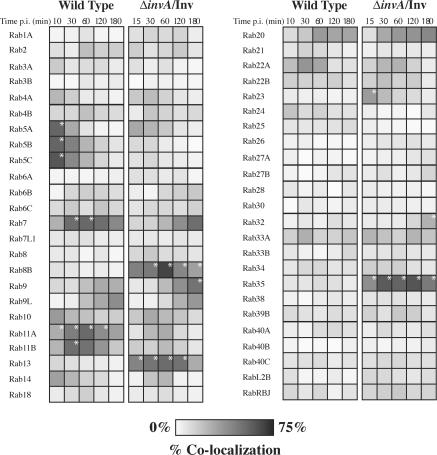
The results of our screen on the model phagosome correlated well with previously characterized Rabs found on phagosomes (Fig. 2). Rab3, 4, 5, 7, 9, 10, 11, and 14 have all been previously identified on purified latex bead phagosomes (Garin et al., 2001) and were all present, with the exception of Rab3, at a level of 20% or higher on model phagosomes at some time during the first 3 h post infection (p.i.; Fig. 2 and Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200611056/DC1). In addition, the association of Rab5A early (15 min p.i.) and Rab7 later (2–3 h p.i.) matched previous results (Vieira et al., 2002), signifying that our model system is an effective tool to study phagosome maturation. Importantly, our studies determined several novel Rab associations (>20% association) with our model phagosome (Fig. 2).
Figure 2.
Rab GTPase localization on wild-type and ΔinvA/Inv S. Typhimurium vacuoles. Wild-type SCVs and ΔinvA/Inv S. Typhimurium model phagosomes were enumerated for their association with 48 Rab GTPases at the indicated times. The scale represents 0 to 75% of vacuoles colocalizing with the Rab GTPase as indicated by degree of shading. Results represent at least two independent experiments. *, P < 0.05, comparing differences between wild-type and ΔinvA/Inv vacuoles at each time point. Significance was tested when n ≥ 3.
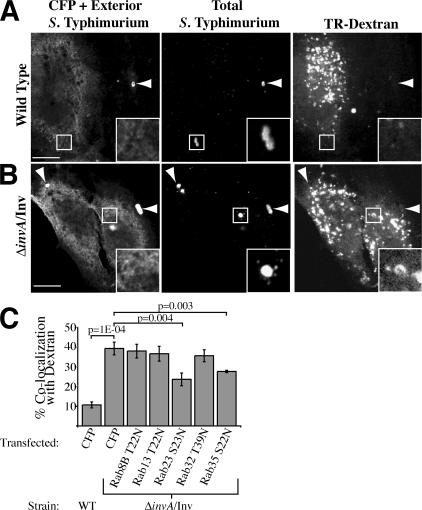
Some phagosome-associated Rabs interacted only with the model phagosome and not with the SCV of wild-type bacteria (Fig. 1 B and Fig. 2). These included Rab8B, 13, 23, 32, and 35. These Rabs also associated with the model phagosome in a macrophage cell line with similar kinetics and with phagosomes containing IgG-coated ΔinvA mutant bacteria (lacking Inv expression) after Fcγ receptor–mediated phagocytosis (Table S2, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200611056/DC1). To test whether these Rabs have a role in phagosome maturation, Texas red–labeled dextran was preloaded into HeLa cells, followed by transfection with CFP-tagged dominant-negative (DN) Rab constructs or a CFP control vector. Before infection, noninternalized dextran was removed, allowing internalized dextran to label lysosomes. At 3 h p.i., cells were fixed and the number of bacteria in lysosomes was enumerated. For wild-type S. Typhimurium, ∼10% of bacteria fused with lysosomes compared with ∼40% of ΔinvA/Inv bacteria (Fig. 3, A–C). Expression of DN mutants of Rab8B, 13, and 32 had no effect on phagosomal fusion with lysosomes compared with the control. However, DN mutants of Rab23 and 35 reduced fusion by >25% compared with the control, demonstrating these Rabs play a role in phagosome–lysosome fusion (Fig. 3 C). Both Rabs are expressed in many cell types, including HeLa cells, and are thought to play a role in endosomal recycling (Evans et al., 2003; Kouranti et al., 2006). Thus, it is possible that Rab23 and Rab35 promote phagosome maturation by mediating recycling from this compartment.
Figure 3.
Rab requirement for phagosome–lysosome fusion. Cells were preloaded with Texas red (TR)–dextran and transfected with CFP. Cells were fixed 3 h after infection with wild-type S. Typhimurium (A), or ΔinvA/Inv S. Typhimurium (B). Arrowheads indicate extracellular bacteria. Bar, 10 μm. (C) Co-localization between the SCV and TR-Dextran was enumerated in cells preloaded with TR-dextran, transfected with CFP or CFP-tagged DN Rab constructs, and fixed 3 h after addition of bacteria to HeLa cells. Results are the means (± SD) of three independent experiments. Levels of significance are indicated by p-values compared with CFP-transfected ΔinvA/Inv-infected control.
Interestingly, we observed some Rabs that, although associated with the model phagosome, had greater association with the wild-type SCV (Fig. 1 C and Fig. 2). These are Rab5A, 5B, 5C, 7, 11A, and 11B. It is possible the bacteria specifically mediate recruitment of these Rabs to the SCV. Indeed, our recent experiments indicate that a bacterial factor promotes recruitment of Rab5 to early SCVs by promoting their fusion with early endosomes (unpublished data). Conversely, the lack of association of some Rabs with the SCV suggests that S. Typhimurium can block their recruitment. This is consistent with the finding that S. Typhimurium can modulate the activity of host GTPases, including members of the Rab family (Harrison et al., 2004). Additionally, S. Typhimurium may mimic the function of host GTPases, obviating a need for their recruitment. Such a scenario was recently suggested for TTSS effectors present in other bacterial pathogens (Alto et al., 2006).
Approximately 3 h p.i., S. Typhimurium activates a second TTSS encoded in SPI-2 and mediates translocation of a distinct set of effector proteins across the SCV and into the host cell. Deletion of ΔssaR, an essential component of the SPI-2 TTSS apparatus, had no effect on the recruitment of Rabs to the SCV during the first 3 h p.i., which is consistent with the kinetics of SPI-2 TTSS expression (Table S1). We also observed that the ΔssaR mutant did not associate with Rab8B, 13, 23, 32, and 35 up to 10 h p.i., indicating this mutant did not traffic to lysosomes (unpublished data). These observations are consistent with the findings of Garvis et al. (2001), who demonstrated that the PhoP/Q regulon mediates avoidance of lysosomes and that the SPI-2 TTSS plays no role in this aspect of SCV trafficking.
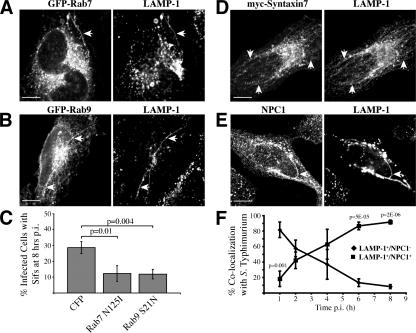
One of the cellular phenotypes attributed to the SPI-2 TTSS is the formation of Salmonella-induced filaments (Sifs). Sifs are long tubular structures extending from the SCV that are characterized by their enrichment with LAMP-1 (Garcia-del Portillo et al., 1993). The purpose of Sif formation is unclear; however, mutants defective for Sif formation are also defective for intracellular growth and for virulence in animal models of infection. The source of membrane from which Sifs are formed is also unclear; hence, we screened the 48 Rabs to determine which are present on Sif membranes. We found both Rab7 (Fig. 4 A) and Rab9 (Fig. 4 B) on the membrane but none of the other Rabs tested (not depicted). Rab7 controls the acquisition of LAMP-1 by the SCV (Meresse et al., 1999) and has been previously localized to Sifs and shown to be required for Sif formation (Brumell et al., 2001).
Figure 4.
A delayed interaction of the wild-type SCV with late endocytic compartments. HeLa cells transfected with GFP-Rab7 (A) or GFP-Rab9 (B) show colocalization between the tagged protein and LAMP-1 8 h p.i. on Sifs. Arrows indicate Sifs. (C) The number of cells with Sifs was counted after an 8-h infection with wild type S. Typhimurium in cells transfected with CFP or DN Rab constructs. Results are the means (± SD) of three independent experiments. Levels of significance are indicated by p-values compared with CFP transfected. Hela cells transfected with myc-Syntaxin 7 (D) or immunostained for NPC1 (E) show colocalization with LAMP-1 8 h p.i. on Sifs. Arrows indicate Sifs. Bars, 10 μm. (F) HeLa cells were infected with wild-type S. Typhimurium, fixed, and immunostained for LAMP-1 and NPC1. SCVs that colocalized with LAMP-1 only (♦) or LAMP-1 and NPC1 (▪) were quantified. Results are the means (± SD) of three independent experiments. Levels of significance are indicated by p-values comparing LAMP-1+/NPC1+ to LAMP-1+/NPC1−.
Rab9 regulates late endosome to Golgi traffic (Lombardi et al., 1993) and its localization to Sifs is a novel observation. Therefore, we determined if Rab9 activity is required for Sif formation by transfection of DN Rab9 in wild-type S. Typhimurium–infected HeLa cells (Fig. 4 C). For these experiments, we transfected cells with DN constructs 2 h after infection with wild-type S. Typhimurium. In this way, we could selectively modulate Rab activity after bacterial establishment of a favorable intracellular niche and focus our analysis on Sif formation, which occurs between 6–8 h p.i. (Birmingham et al., 2005). In CFP control-transfected cells, ∼30% of infected cells had Sifs after an 8-h infection. When transfected with DN Rab7, 12% of infected cells had Sifs after 8 h, confirming the requirement of this GTPase in Sif formation. Transfection of DN Rab5 had no effect on Sif formation, suggesting this effect was not caused by a general inhibition of the endosomal system (unpublished data). In cells transfected with DN Rab9, 11% of infected cells had Sifs after 8 h (Fig. 4 C). Under similar infection conditions, expression of DN Rab5, 7, or 9 did not affect intracellular bacterial growth up to 10 h p.i. (unpublished data). Thus, inhibition of Sifs by DN Rab expression was not caused by an effect on bacterial growth. Together, these studies reveal a novel role for Rab9 in Sif formation.
The presence of Rab9 on Sifs suggested that fusion of late endosomes with the SCV can occur at late times p.i. and is consistent with the presence of cathepsin D and lysobisphosphatidic acid on Sifs (Brumell et al., 2001). To further test this possibility, we examined other late endocytic markers to determine their presence on Sifs. We found that the late endosome-localized syntaxin7 (Nakamura et al., 2000), as well as the late endosome/lysosome-localized transporter Niemann-Pick C1 (NPC1; Higgins et al., 1999), were both present on Sifs (Fig. 4, D and E). However, we did not detect Sif fusion with lysosomes when examined by addition of fluid phase markers (unpublished data), confirming previous results (Garcia-del Portillo et al., 1993). Thus, our data suggests that the SCV fuses with late endosomes and that this compartment could provide a source of membrane for Sifs.
We determined when fusion of the SCV with late endosomes occurs during infection. HeLa cells were infected with wild-type S. Typhimurium and fixed at various times up to 8 h p.i. Bacteria positive for LAMP-1 (a marker for SCVs) were scored for colocalization with NPC1 (Fig. 4 F). At 1 h p.i., 80% of LAMP-1+ SCVs did not associate with NPC1, indicating that fusion with late endosomes was minimal at this time. However, by 4 h p.i. the majority of LAMP-1+ SCVs had accumulated NPC1, indicating late endosome fusion had taken place. Our data demonstrates a two-step maturation of the SCV occurs where there is a delay in fusion with late endosomes. This is reminiscent of the “pregnant pause” mechanism used by Legionella pneumophila and other intracellular bacterial pathogens (Swanson and Fernandez-Moreira, 2002), which temporarily halt maturation of their vacuole so they can form a replicative niche before the vacuole matures to the next state.
Our experimental approach permitted us to study the interactions of a large number of Rabs with both a model phagosome and the SCV of virulent S. Typhimurium. The results we obtained correlated well with previous data describing the association of Rab GTPases with both compartments. Importantly, we were able to localize several uncharacterized Rabs to each compartment. A network of 18 different Rabs was present on model phagosomes and 16 were found on the SCV. The finding that up to 12 different Rabs were present on each compartment at a given time is remarkable and consistent with the hypothesis that endosomes are composed of membrane domains (Sonnichsen et al., 2000). Our data indicate that the process of phagosome maturation is far more complex than a single Rab5 to 7 transition and provide many insights and new avenues of study. Indeed, we found a novel role for both Rab23 and 35 in phagosome maturation. Furthermore, we were able to monitor the trafficking of a pathogenic bacterium and characterize the divergence of its vacuolar compartment from the normal degradative pathway. Our observations reveal the ability of S. Typhimurium to undergo a unique two-step maturation process that involves a delayed interaction of the SCV with late endosomes, but not lysosomes (Fig. 5). Future studies are required to define the molecular mechanisms by which Rab GTPases control phagosome maturation and how intracellular pathogens like S. Typhimurium modulate their function.
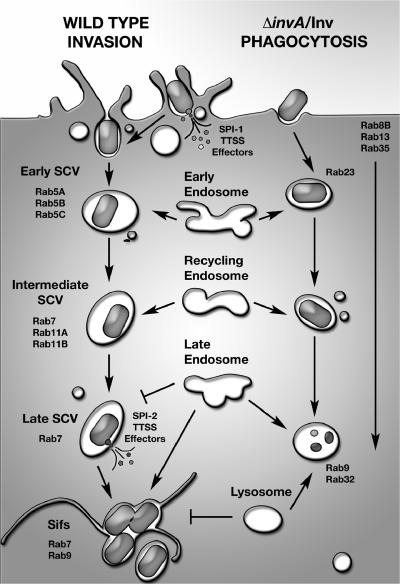
Figure 5.
Model of Rab association during Salmonella invasion. Model showing Rabs enriched on either the wild-type SCV or ΔinvA/Inv model phagosome from invasion through to Sif formation or lysosomal fusion, respectively.
Materials and methods
Cell culture and bacterial strains
HeLa and RAW 264.7 cells were obtained from American Type Culture Collection. Cells were maintained in DME (HyClone) supplemented with 10% FBS (Wisent) at 37°C in 5% CO2 without antibiotics. Cultures were used between passage numbers 3–25. Wild-type SL1344 (Hoiseth and Stocker, 1981), ΔssaR SL1344 (Brumell et al., 2002), ΔinvA (Steele-Mortimer et al., 2002), and ΔinvA/pRI203 (ΔinvA/Inv) 14028S (Steele-Mortimer et al., 2002) S. Typhimurium strains were used in this study.
Plasmids and transfection
Plasmids used in this study are described in the Online supplemental material. GeneJuice transfection reagent (Oncogene Research Products) was used for transient transfection of cells.
Antibodies
Rabbit polyclonal antibodies to S. Typhimurium O antiserum group B were obtained from Difco. Murine monoclonal and rabbit polyclonal anti-GFP antibodies were obtained from Invitrogen. Rabbit polyclonal antibodies to c-myc were purchased from Santa Cruz Biotechnology, Inc. Murine monoclonal anti–human LAMP-1 antibodies (clone H4A3) developed by T. August (University of Iowa, Iowa City, IA) were obtained from the Developmental Studies Hybridoma Bank. Rabbit polyclonal antibodies to NPC1 were obtained from D. Manhuran and R. Bagshaw (Hospital for Sick Children, Toronto, Canada).
Bacterial infection of cell cultures
For wild-type and ΔssaR mutant infections, late-log bacterial cultures were used and prepared using a method optimized for bacterial invasion (Steele-Mortimer et al., 1999). After infection, extracellular bacteria were removed by extensive washing with PBS and addition of growth medium containing 100 μg/ml gentamicin. After 90 min of bacterial infection, the gentamicin concentration was decreased to 10 μg/ml. For Sif studies, cells were transfected 2 h p.i. with wild-type S. Typhimurium (see Plasmids and transfection). Cells were fixed 8 h after addition of bacteria. Intracellular growth assays were performed as previously described (Steele-Mortimer et al., 2002).
For ΔinvA/Inv infections, bacteria were grown for ∼16 h at 37°C with shaking, subcultured (1:33) in Luria-Bertani broth for 3 h, and diluted to an OD600 of 1.0 in Luria-Bertani. Bacterial inocula were prepared by pelleting at 10,000 g for 2 min, directly resuspended and diluted in PBS, pH 7.2, and added to cells at a dilution of 1:20. Cells were spun at 1,000 rpm for 1 min followed by incubation at 37°C for 15 min. After infection, extracellular bacteria were removed by extensive washing with PBS and addition of growth medium containing 200 μg/ml tetracycline. For ΔinvA infections, bacteria were incubated in 2 ml PBS in the presence of 2 mg/ml of human IgG for 1 h at 37°C with shaking before addition to cells, as mentioned for the ΔinvA/Inv strain.
For dextran loading, HeLa cells were incubated with 1 mg/ml Texas red–dextran (Invitrogen) for 24 h, and then chased for 1 h before use. Cells were transfected 16–20 h before use and cells were infected with wild-type and ΔinvA/Inv bacteria as above.
Immunofluorescence
Fixed cells were immunostained as previously described (Brumell et al., 2001). Immunostaining before permeabilization was used when determining the presence of intracellular bacteria. A fluorescence microscope (DMIRE2; Leica) equipped with a 100×/NA 1.4 oil objective (Plan Apochromat; Leica) was used to enumerate association of different proteins with the SCV. Colocalization was determined visually, with distinct signal surrounding the bacteria considered positive, for at least 100 bacteria. The mean ± the range (n = 2) or the SD (n ≥ 3) is presented. Images of fixed cells were obtained using an inverted microscope (Axiovert 200M; Carl Zeiss MicroImaging, Inc.) equipped with a laser scanning confocal imaging system (LSM510; Carl Zeiss MicroImaging, Inc.) and a 63×/NA 1.4 oil immersion objective lens (Plan Apochromat; Carl Zeiss MicroImaging, Inc.). Images were imported into Photoshop (Adobe) and assembled in Illustrator (Adobe).
Statistical analysis
All experiments were repeated two to four times. Statistical analyses were performed using a two-tailed unpaired t test. P-values <0.05 were considered statistically significant.
Online supplemental material
Provided as online supplemental material are descriptions of the plasmids used in this study. Table S1 provides raw data for Fig. 2. Table S2 provides data for colocalization of Rab8B, 13, 23, 32, and 35 with phagosomes containing ΔinvA/Inv bacteria (A) or IgG-coated ΔinvA bacteria (B) in RAW 264.7 macrophages. Table S2 C provides the primers used to generate plasmids in this study. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200611056/DC1.
Supplementary Material
Acknowledgments
We are grateful to those colleagues who generously provided reagents used in this study. We thank members of the Brumell laboratory for critical reading of the manuscript, Malina Bakowski for her generous donation of artwork, Mike Woodside for help with confocal microscopy, and Judith Cirulis for technical assistance.
A.C. Smith is the recipient of a University of Toronto open scholarship, a studentship from the Natural Sciences and Engineering Research Council of Canada, and the Toronto Star student bursary through the Hospital for Sick Children Research Training Centre. C. Macrae was supported by the Samuel Lunenfeld Research Summer Student Program. This work was supported with funding from the Canadian Institutes of Health Research. Infrastructure for the Brumell laboratory was provided by a New Opportunities Fund from the Canadian Foundation for Innovation and the Ontario Innovation Trust. J.H. Brumell holds an Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Abbreviations used in this paper: DN, dominant-negative; Inv, invasin; LAMP-1, lysosomal-associated membrane protein-1; NPC1, Niemann-Pick C1; p.i., post infection; SCV, Salmonella-containing vacuole; Sif, Salmonella-induced filament; SPI, Salmonella pathogenicity island; S. Typhimurium, Salmonella enterica serovar Typhimurium; TTSS, type III secretion system.
References
- Alto, N.M., F. Shao, C.S. Lazar, R.L. Brost, G. Chua, S. Mattoo, S.A. McMahon, P. Ghosh, T.R. Hughes, C. Boone, and J.E. Dixon. 2006. Identification of a bacterial type III effector family with G protein mimicry functions. Cell. 124:133–145. [DOI] [PubMed] [Google Scholar]
- Birmingham, C.L., X. Jiang, M.B. Ohlson, S.I. Miller, and J.H. Brumell. 2005. Salmonella-induced filament formation is a dynamic phenotype induced by rapidly replicating Salmonella enterica serovar typhimurium in epithelial cells. Infect. Immun. 73:1204–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumell, J.H., and S. Grinstein. 2004. Salmonella redirects phagosomal maturation. Curr. Opin. Microbiol. 7:78–84. [DOI] [PubMed] [Google Scholar]
- Brumell, J.H., P. Tang, S.D. Mills, and B.B. Finlay. 2001. Characterization of Salmonella-induced filaments (Sifs) reveals a delayed interaction between salmonella-containing vacuoles and late endocytic compartments. Traffic. 2:643–653. [DOI] [PubMed] [Google Scholar]
- Brumell, J.H., D.L. Goosney, and B.B. Finlay. 2002. SifA, a type III secreted effector of Salmonella typhimurium, directs Salmonella-induced filament (Sif) formation along microtubules. Traffic. 3:407–415. [DOI] [PubMed] [Google Scholar]
- Evans, T.M., C. Ferguson, B.J. Wainwright, R.G. Parton, and C. Wicking. 2003. Rab23, a negative regulator of hedgehog signaling, localizes to the plasma membrane and the endocytic pathway. Traffic. 4:869–884. [DOI] [PubMed] [Google Scholar]
- Galan, J.E., and R. Curtiss III. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. USA. 86:6383–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-del Portillo, F., M.B. Zwick, K.Y. Leung, and B.B. Finlay. 1993. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc. Natl. Acad. Sci. USA. 90:10544–10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin, J., R. Diez, S. Kieffer, J.F. Dermine, S. Duclos, E. Gagnon, R. Sadoul, C. Rondeau, and M. Desjardins. 2001. The phagosome proteome: insight into phagosome functions. J. Cell Biol. 152:165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvis, S.G., C.R. Beuzon, and D.W. Holden. 2001. A role for the PhoP/Q regulon in inhibition of fusion between lysosomes and Salmonella-containing vacuoles in macrophages. Cell. Microbiol. 3:731–744. [DOI] [PubMed] [Google Scholar]
- Gorvel, J.P., P. Chavrier, M. Zerial, and J. Gruenberg. 1991. rab5 controls early endosome fusion in vitro. Cell. 64:915–925. [DOI] [PubMed] [Google Scholar]
- Harrison, R.E., J.H. Brumell, A. Khandani, C. Bucci, C.C. Scott, X. Jiang, B.B. Finlay, and S. Grinstein. 2004. Salmonella impairs RILP recruitment to Rab7 during maturation of invasion vacuoles. Mol. Biol. Cell. 15:3146–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, M.E., J.P. Davies, F.W. Chen, and Y.A. Ioannou. 1999. Niemann-Pick C1 is a late endosome-resident protein that transiently associates with lysosomes and the trans-Golgi network. Mol. Genet. Metab. 68:1–13. [DOI] [PubMed] [Google Scholar]
- Hoiseth, S.K., and B.A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 291:238–239. [DOI] [PubMed] [Google Scholar]
- Isberg, R.R., and J.M. Leong. 1990. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 60:861–871. [DOI] [PubMed] [Google Scholar]
- Kouranti, I., M. Sachse, N. Arouche, B. Goud, and A. Echard. 2006. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr. Biol. 16:1719–1725. [DOI] [PubMed] [Google Scholar]
- Lombardi, D., T. Soldati, M.A. Riederer, Y. Goda, M. Zerial, and S.R. Pfeffer. 1993. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 12:677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meresse, S., O. Steele-Mortimer, B.B. Finlay, and J.P. Gorvel. 1999. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 18:4394–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, S.D., and B.B. Finlay. 1998. Isolation and characterization of Salmonella typhimurium and Yersinia pseudotuberculosis-containing phagosomes from infected mouse macrophages: Y. pseudotuberculosis traffics to terminal lysosomes where they are degraded. Eur. J. Cell Biol. 77:35–47. [DOI] [PubMed] [Google Scholar]
- Nakamura, N., A. Yamamoto, Y. Wada, and M. Futai. 2000. Syntaxin 7 mediates endocytic trafficking to late endosomes. J. Biol. Chem. 275:6523–6529. [DOI] [PubMed] [Google Scholar]
- Rink, J., E. Ghigo, Y. Kalaidzidis, and M. Zerial. 2005. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 122:735–749. [DOI] [PubMed] [Google Scholar]
- Sonnichsen, B., S. De Renzis, E. Nielsen, J. Rietdorf, and M. Zerial. 2000. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149:901–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Mortimer, O., S. Méresse, J.-P. Gorvel, B.-H. Toh, and B.B. Finlay. 1999. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell. Microbiol. 1:33–51. [DOI] [PubMed] [Google Scholar]
- Steele-Mortimer, O., J.H. Brumell, L.A. Knodler, S. Meresse, A. Lopez, and B.B. Finlay. 2002. The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell. Microbiol. 4:43–54. [DOI] [PubMed] [Google Scholar]
- Swanson, M.S., and E. Fernandez-Moreira. 2002. A microbial strategy to multiply in macrophages: the pregnant pause. Traffic. 3:170–177. [DOI] [PubMed] [Google Scholar]
- Vieira, O.V., R.J. Botelho, and S. Grinstein. 2002. Phagosome maturation: aging gracefully. Biochem. J. 366:689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.