Abstract
Assembly of E-cadherin–based adherens junctions (AJ) is obligatory for establishment of polarized epithelia and plays a key role in repressing the invasiveness of many carcinomas. Here we show that type Iγ phosphatidylinositol phosphate kinase (PIPKIγ) directly binds to E-cadherin and modulates E-cadherin trafficking. PIPKIγ also interacts with the μ subunits of clathrin adaptor protein (AP) complexes and acts as a signalling scaffold that links AP complexes to E-cadherin. Depletion of PIPKIγ or disruption of PIPKIγ binding to either E-cadherin or AP complexes results in defects in E-cadherin transport and blocks AJ assembly. An E-cadherin germline mutation that loses PIPKIγ binding and shows disrupted basolateral membrane targeting no longer forms AJs and leads to hereditary gastric cancers. These combined results reveal a novel mechanism where PIPKIγ serves as both a scaffold, which links E-cadherin to AP complexes and the trafficking machinery, and a regulator of trafficking events via the spatial generation of phosphatidylinositol-4,5-bisphosphate.
Introduction
Tight junctions, adherens junctions (AJs), and desmosomes promote adhesion between epithelial cells, initiate the assembly of the mechanical cytoskeleton linkage, and facilitate the formation of a polarized epithelial monolayer (Gumbiner, 1996). AJs initiate these processes and are essential for morphogenesis, wound healing, and the retention of cell polarity and tissue integrity (Perez-Moreno et al., 2003). In epithelia, AJ formation is mediated by the calcium-dependent homophilic binding of E-cadherin molecules on neighboring cells (Gumbiner, 1996). These interactions link adjacent cells and promote the nucleation of a cytoplasmic protein complex consisting of p120-, β-, and α-catenins, which bridges E-cadherin clusters and the actin cytoskeleton. The biological necessity of AJ proteins has been underscored by a high correlation between the malfunctioning of AJ proteins, E-cadherin in particular, and tumor metastasis (Kang and Massague, 2004).
During tumor progression, the E-cadherin gene can be functionally silenced or inactivated by distinct mechanisms (Nelson and Nusse, 2004). In addition to transcriptional repression by SIP-1, δEF-1, Snail/Slug, E12/47, and Twist (Huber et al., 2005), posttranslational regulation of E-cadherin stability modulates its activity. Precisely tuned exocytic and endocytic pathways control the amount of E-cadherin residing on the plasma membrane (PM) and are important for modulation of E-cadherin function and AJ assembly (Bryant and Stow, 2004). Recent evidence suggests that Rab11 (Lock and Stow, 2005), p120-catenin, ARF6, tyrosine phosphorylation, and ubiquitination (D'Souza-Schorey, 2005) all control the trafficking and assembly of E-cadherin in mammalian cells. Additionally, transport of E-cadherin is regulated by the composition of the cadherin–catenin complex as well as the vesicular trafficking machinery (D'Souza-Schorey, 2005), where multiple adaptor and signaling proteins orchestrate trafficking specificity and efficiency.
Clathrin adaptor protein (AP) complexes are important in the sorting of cargoes containing dileucine or tyrosine-based sorting motifs (Bonifacino and Traub, 2003). In epithelial cells, AP1B is the unique isoform that mediates basolateral transport (Folsch et al., 1999; Folsch, 2005). Although AP1B is closely related to the more ubiquitously expressed form of AP1, AP1A, it targets to a distinct membrane compartment defined as the recycling endosome (Folsch et al., 2003; Folsch, 2005). Recently, it has been shown that this compartment is an intermediary in transport from the Golgi to the PM (Ang et al., 2004) and also functions in the recycling of internalized basolateral membrane proteins (Gan et al., 2002; Folsch, 2005).
Phosphoinositides are key mediators of membrane trafficking (Roth, 2004). Membrane assembly and cargo binding of AP2 are both dependent on binding to phosphatidylinositol-4, 5-bisphosphate (PI4,5P2) via its α and μ subunits (Collins et al., 2002; Honing et al., 2005). There is evidence that other AP complexes are also modulated by phosphoinositide lipid messengers (Baust et al., 2006). In addition, PI4,5P2 regulates actin polymerization, focal adhesion assembly, and several components of the vesicular trafficking machinery (Doughman et al., 2003). However, the mechanism by which PI4,5P2 generation is regulated to mediate these trafficking events has not been defined.
Recent studies have unveiled that the spatial targeting and temporal regulation of type I phosphatidylinositol phosphate kinases (PIPKIs) is a critical mechanism for PI4,5P2 generation (Ling et al., 2006). Here we show that in epithelial cells PIPKIγ targets to AJs by a direct interaction with the E-cadherin dimer. PIPKIγ regulates E-cadherin trafficking by acting as a scaffold between E-cadherin and AP complexes. We also demonstrate that localized generation of PI4,5P2 via these complexes is necessary for E-cadherin transport and AJ formation.
Results
PIPKIγ interacts with cadherins
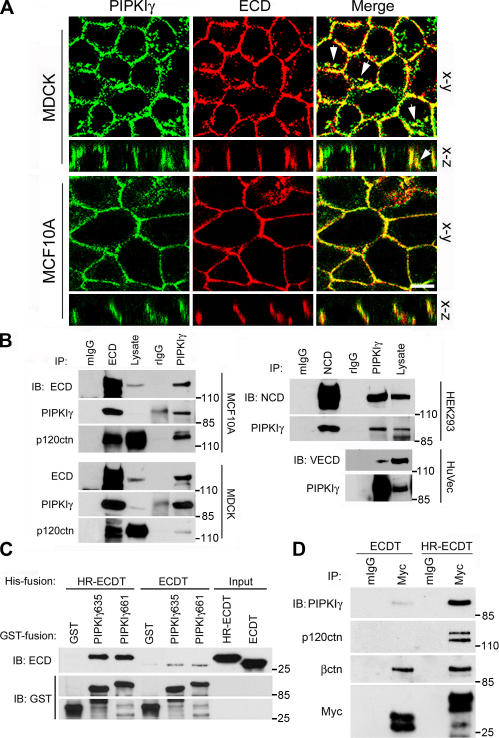
Upon examination of the basolateral membrane in polarized epithelial cells, we found that PIPKIγ colocalized with E-cadherin (Fig. 1 A) but not with occludin (not depicted). PIPKIγ also presented in a cytosolic vesicular compartment and partially colocalized with E-cadherin at this site (Fig. 1 A, arrows). These regions of colocalizion were confirmed by constructing vertical sections of z-series images shown in Fig. 1 A, suggesting an interaction between PIPKIγ and a component of AJs. To examine this possibility, E-cadherin and PIPKIγ were immunoprecipitated. As shown in Fig. 1 B, PIPKIγ and E-cadherin associate in vivo, along with other cadherin-associated proteins, demonstrating that PIPKIγ associates with E-cadherin complexes. N-cadherin and VE-cadherin also associate with PIPKIγ (Fig. 1 B), suggesting that PIPKIγ associates with the classical cadherin complexes.
Figure 1.
PIPKIγ targets to AJs by a direct interaction with E-cadherin. (A) PIPKIγ targets to AJs in both MDCK and MCF10A cells. Horizontal (x-y) and vertical (x-z) sections show colocalization of PIPKγ and E-cadherin (ECD). Arrows show the vesicular compartments where PIPKIγ and E-cadherin colocalize. Bar, 10 μm. (B) PIPKIγ associates with cadherin complexes. Immunoprecipitations (IP) and immunoblots (IB) were performed as indicated. NCD, N-cadherin; VECD, VE-cadherin. Normal mouse (mIgG) or rabbit IgG (rIgG) were used as controls. (C) PIPKIγ directly and preferentially interacts with the dimerized cytoplasmic domain of E-cadherin. A GST pull-down assay was performed using 1 μg of each purified protein. (D) PIPKIγ preferentially binds the E-cadherin dimmer in vivo. Myc-tagged ECDT or HR-ECDT was overexpressed in HEK293 cells and immunoprecipitated.
PIPKIγ is predominantly expressed as two distinct splice variants, PIPKIγ635 and PIPKIγ661, which differ by a 26–amino acid C-terminal extension. HA-tagged PIPKIγ splice variants were expressed in human embryonic kidney 293 (HEK293) cells, and their association with the endogenous N-cadherin complex was analyzed. PIPKIγ635 and PIPKIγ661 both coimmunoprecipitated with N-cadherin indistinguishably (Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200606023/DC1), indicating that this association does not depend on the PIPKIγ661 C-terminal extension. However, the endogenous PIPKIγ associated with E-cadherin was indistinguishable in apparent molecular weight from PIPKIγ661, which is the predominant splice variant expressed in these cells. To ascertain whether this association was direct, in vitro GST pull-down assays were performed using recombinant GST-tagged PIPKIγ and His-tagged E-cadherin cytoplasmic domain (ECDT). ECDT showed specific binding to both GST-PIPKIγ635 and GST-PIPKIγ661, but not to GST alone (Fig. 1 C) or GST-PIPKIα (Fig. S1 B).
E-cadherin molecules form lateral homodimers in vivo, and oligomer formation is critical for AJ assembly and stability (Patel et al., 2003). Consequently, we examined if dimerization was important for association with PIPKIγ. A parallel dimeric ECDT was constructed by inserting a heptad repeat (HR) peptide sequence between the His tag and ECDT (Ling et al., 2003; Fig. S1, C and D). The dimeric construct had a greater binding affinity for PIPKIγ compared with the monomeric protein (Fig. 1 C). This was not caused by the HR tag because an HR-fused integrin cytoplasmic domain did not bind PIPKIγ (Ling et al., 2003; Fig. S1 E). Moreover, when expressed in cells, the Myc-tagged dimeric ECDT (Myc-HR-ECDT) selectively bound PIPKIγ and p120-catenin with ∼10-fold greater affinity compared with the monomer (Fig. 1 D and Fig. S1 F). Consistent with a previous paper (Huber et al., 2005), β-catenin bound the monomeric and dimeric E-cadherin C terminus with the same affinity.
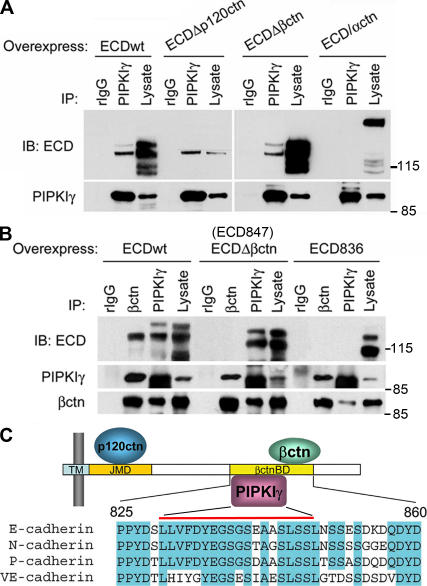
To further determine whether PIPKIγ binding to E-cadherin involves other AJ components, wild-type or mutated E-cadherin was expressed in HEK293 cells and assessed for endogenous PIPKIγ association (Fig. 2 A). Elimination of either the p120-catenin (ECDΔp120; 762EED764 to AAA) or β-catenin (ECDΔβctn; ECD847, deletion of the last 35 amino acids) binding sites had no effect on PIPKIγ association. A chimera of truncated E-cadherin (deletion of the last 70 amino acids) fused to a truncated α-catenin that lacks the β-catenin binding site (Imamura et al., 1999) abrogated both β-catenin (not depicted) and PIPKIγ binding (Fig. 2 A). These results indicate that PIPKIγ binding to E-cadherin is independent of α-, β-, or p120-catenin and narrowed the PIPKIγ interaction region on E-cadherin to residues 837–847. To confirm this putative PIPKIγ binding site, the last 45 amino acids of E-cadherin were truncated (ECD836). This truncation resulted in ablation of both β-catenin and PIPKIγ binding (Fig. 2 B). These combined data demonstrate that PIPKIγ directly interacts within a region including amino acids 837–847 of E-cadherin that is a highly conserved domain in the type I classical cadherins (Fig. 2 C).
Figure 2.
Determination of the PIPKIγ-interacting region on E-cadherin. (A) Endogenous PIPKIγ was immunoprecipitated from HEK293 cells overexpressing wild-type (ECDwt) or the indicated mutant E-cadherin constructs. (B) Endogenous PIPKIγ was immunoprecipitated from HEK293 cells overexpressing wild-type or truncated E-cadherin constructs. The immunoprecipitates were analyzed as indicated. (C) A schematic model representing the predicted minimum region on E-cadherin required for PIPKIγ binding. TM, transmembrane domain; JMD, juxtamembrane domain; βctnBD, β-catenin binding domain. Regions corresponding to βctnBD in E-, N-, P-, and VE-cadherin were aligned and the conserved residues were highlighted.
To examine whether PIPKIγ modulates E-cadherin function through this direct interaction, we introduced wild-type or p120/β-catenin binding site–deleted Myc-HR-ECDT into MDCK cells to compete with endogenous E-cadherin for PIPKIγ binding (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200606023/DC1). Expression of dimeric ECDT that specifically binds PIPKIγ resulted in a loss of AJs identified by E-cadherin staining and a cytosolic accumulation of PIPKIγ in cells, indicating that this phenotype is likely caused by sequestration of PIPKIγ (Fig. S2 B). Overexpression of PIPKIγ661 was sufficient to rescue the loss-of-AJ phenotype induced by wild-type Myc-HR-ECDT expression (Fig. S2 C). These data establish that a specific interaction between PIPKIγ and E-cadherin plays a key role in E-cadherin function and appears to be a limiting factor in AJ formation.
PIPKIγ modulates AJ assembly by facilitating E-cadherin trafficking
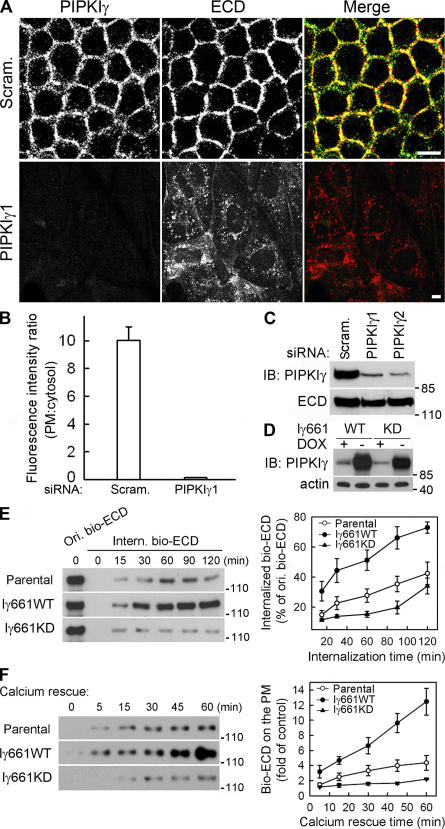
To further determine the functional role of PIPKIγ at AJs, we knocked down endogenous PIPKIγ expression using siRNAs. Although the cellular E-cadherin content was not changed (Fig. 3 C), loss of PIPKIγ caused a striking loss of E-cadherin from the PM with an apparent accumulation in a cytoplasmic compartment (Fig. 3, A and B). Upon loss of PM E-cadherin, the cells spread (Fig. 3 A) and underwent a morphological transition from a polarized epithelial to a more migratory mesenchymal- like phenotype (Fig. S2 D), supporting the requirement for PIPKIγ in E-cadherin–mediated AJs assembly.
Figure 3.
PIPKIγ is required for E-cadherin assembly and trafficking. (A) MDCK cells were treated with scrambled (Scram.) or PIPKIγ-specific siRNA (PIPKIγ1). PIPKIγ (green) and ECD (red) were visualized by indirect immunofluorescence. Bars, 10 μm. (B) PM or cytosolic fluorescence intensity of cells in A was quantified from six randomly picked fields (5 cells/field) representing three independent experiments were randomly counted. The intensity ratio was plotted using SigmaPlot 8.0. Error bars are ± SEM. (C) PIPKIγ and E-cadherin protein levels in cells treated with scrambled (Scram.) or PIPKIγ-specific siRNAs (PIPKIγ1 and PIPKIγ2) were shown by immunoblots. (D) Protein levels of endogenous and inducibly overexpressed PIPKIγ661wild type (Iγ661WT) or knock down (Iγ661KD) in stable MDCK lines were determined. Actin was used as a loading control. (E) After surface biotinylation, cells expressing the indicated proteins were lysed before or after exposure to 0.5 mM EGTA at 18°C to examine the original biotinylated E-cadherin (Ori. bio-ECD) and the internalized biotinylated E-cadherin (Intern. bio-ECD). Data was quantified and plotted from four independent experiments. (F) Calcium restoration subsequent to EGTA treatment induced E-cadherin recycling. The extent of this recycling was quantified in cells expressing the indicated proteins. Data were quantified and plotted from three independent experiments. The values representing surface E-cadherin without calcium rescue were used as control. Error bars are ± SEM.
In addition, MDCK stable cell lines were generated that inducibly express HA-tagged wild-type (PIPKIγ661WT) or kinase-dead PIPKIγ661 (PIPKIγ661KD), the major endogenous PIPKIγ isoform associated with cadherins (Fig. S1 A). Expression of PIPKIγ661WT or PIPKIγ661KD was induced by removing doxycyclin from the growth media (Fig. 3 D). As shown in Fig. S3 A (available at http://www.jcb.org/cgi/content/full/jcb.200606023/DC1), upon PIPKIγ661KD expression, both E-cadherin PM targeting and AJ assembly appeared defective compared with parental or PIPKIγ661WT-expressing cells (Fig. S3 A). These cells formed E-cadherin–mediated cell–cell contacts much more slowly then parental cells when maintained at confluence (Fig. S3 A, 72 vs. 16 h). These observations are consistent with a dominant-negative effect for PIPKIγ661KD and also establish a requirement for PI4,5P2 generation in AJ assembly.
Our combined results demonstrate a highly specific role for PIPKIγ in the assembly of E-cadherin–based AJs, possibly by modulating the trafficking of E-cadherin. It has been shown that depletion of extracellular calcium by EGTA results in a loss of E-cadherin homoligation, internalization of E-cadherin, disassembly of AJs, and cell scattering (Chitaev and Troyanovsky, 1998). To explore possible modulation of E-cadherin trafficking by PIPKIγ, the exocytic and endocytic trafficking of E-cadherin was quantified in parental, PIPKIγ661WT-, and PIPKIγ661KD-expressing MDCK cells.
To quantify internalization, cell surface E-cadherin was biotinylated, followed by extracellular calcium removal to induce E-cadherin internalization. Shown in Fig. 3 E, PIPKIγ661WT expression considerably enhanced, whereas PIPKIγ661KD expression inhibited, E-cadherin internalization when compared with parental cells. These data indicate that E-cadherin endocytosis is dependent on PIPKIγ661 kinase activity and PI4,5P2 generation. E-cadherin internalization can be reversed upon replenishment of calcium, providing a method to assess the role of PIPKIγ in recycling E-cadherin back to the PM. Compared with parental cells, recycling of E-cadherin was accelerated when PIPKIγ661WT was expressed, whereas PIPKIγ661KD blocked PM deposition of E-cadherin (Fig. 3 F). Immunofluorescent staining experiments supported these results, as cells overexpressing PIPKIγ661WT had a dramatically faster rate of E-cadherin internalization and also showed an accumulation of both E-cadherin and PIPKIγ in an intracellular compartment (Fig. S3 B). In contrast, overexpression of PIPKIγ661KD significantly slowed the internalization of E-cadherin (Fig. S3 B).
PI4,5P2 regulates multiple events, including actin reorganization, that could affect E-cadherin assembly and AJ formation. Because changes in PIPKIγ expression levels may alter global PI4,5P2 levels and induce nonspecific responses, cellular PI4,5P2 was quantified by HPLC analysis (Fig. S4, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200606023/DC1). No considerable changes in global cellular PI4,5P2 levels were observed when PIPKIγ expression levels or activity was altered. The overall structure of the actin cytoskeleton also showed no substantial change between PIPKIγ661WT- or KD-overexpressing cells and control cells (unpublished data). Upon depletion of PIPKIγ, cells exhibited an increase in actin stress fibers and prominent membrane ruffles, indicating a morphological transition from a polarized epithelial to migratory phenotype (unpublished data). Further effort was made to observe localized changes in PI4,5P2 levels via a bead-based adhesion assay using latex beads coated with recombinant E-cadherin ectodomain (hE/Fc). As shown in Fig. S4 C, both E-cadherin and PIPKIγ661 assembled on the surface of the hE/Fc-coated beads but not the poly-lysine–coated control beads, demonstrating that PIPKIγ661 was recruited to the nascent AJs. Although our previous data indicate that PI4,5P2 is necessary for E-cadherin assembly, the level of PI4,5P2 detected by the pleckstrin homology domain along the hE/Fc-coated bead surface was similar to the surrounding PM signal (Fig. S4 D), supporting the hypothesis that local PI4,5P2 levels are sufficient to regulate E-cadherin assembly.
PIPKIγ links E-cadherin to AP complexes
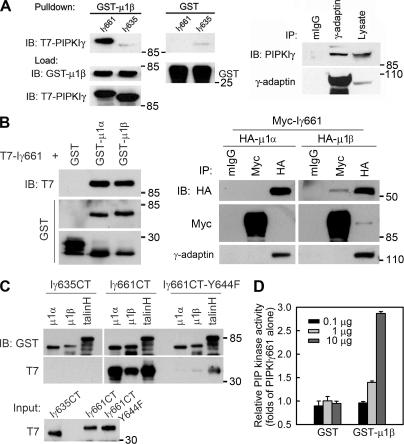
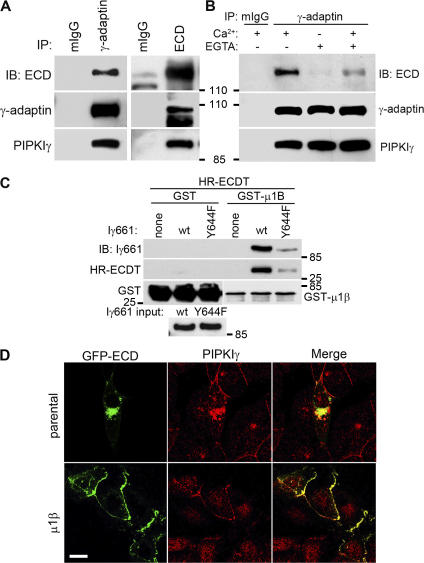
Multiple components of the trafficking machinery, including the AP complexes, bind to and are regulated by PI4,5P2 (Martin, 2001; Roth, 2004; D'Souza-Schorey, 2005). A yeast two-hybrid screen using the C terminus of PIPKIγ661 as bait identified interactions with the μ subunits of both AP1B (μ1β; amino acids 135–423) and AP2 (μ2; full length). This was an exciting observation as the μ subunits are key regulatory subunits of the AP complexes (Bonifacino and Traub, 2003; Folsch, 2005). The direct interaction of PIPKIγ661 with μ1β was confirmed by direct binding of purified components (Fig. 4 A, left), and the in vivo association was established by coimmunoprecipitation (Fig. 4 A, right). PIPKIγ635 did not interact with either μ subunit (Fig. 4 A; Bairstow et al., 2006), indicating that the last 26 residues of PIPKIγ661 are required. In addition, in vitro binding of μ1β-adaptin stimulated the kinase activity of PIPKIγ661 (Fig. 4 D), whereas binding of the soluble ECDT (His-HR-ECDT) had no effect on PIPKIγ661 activity under these conditions (not depicted).
Figure 4.
PIPKIγ661 associates with AP1B. (A, left) A GST pull-down assay was performed using 1 μg of each purified protein, and one fourth of the precipitates were analyzed by immunoblotting as indicated. A stock concentration of T7-tagged PIPKIγ661 and PIPKIγ635 was created for the GST pull-down assay. (right) A coimmunoprecipitation was performed and analyzed as indicated. MDCK cells from confluent 100-mm dishes were used to make 1-ml lysates for immunoprecipitation. All of each immunoprecipitate and 40 μl of each lysate were analyzed. (B, left) GST pull-down assays were performed using 1 μg of His-T7–tagged PIPKIγ or GST-fused proteins. (right) HEK293 cells were transfected with the indicated constructs using calcium phosphate. 48 h after transfection, cells were lysed and specific components were immunoprecipitated. The precipitates were analyzed by Western blot as indicated. The γ-adaptin subunit was coimmunoprecipitated with each of the HA-μ subunits, indicating that the overexpressed μ subunits were incorporated into AP1 complexes. However, when PIPKIγ was overexpressed, γ-adaptin association was weak; whereas in the reverse immunoprecipitation, association of PIPKIγ was readily detected. This could result from the competition of other PIPKIγ binding partners or from the sensitivity of the anti–γ-adaptin antibody. (C) GST pull-down assays were performed using 1 μg of His-T7–tagged indicated PIPKIγ C terminus or GST-fused proteins. The precipitates were analyzed by Western blot as indicated. (D) The indicated amount of purified GST or GST-μ1B was incubated with PIPKIγ at room temperature for 10 min before the kinase assay. Kinase activity was quantified from three independent experiments. Error bars are ± SEM.
Epithelial cells typically express two variants of the AP1 complex, AP1A and AP1B. These AP1 complexes differ only in their μ subunits, μ1α and μ1β, which are almost 80% identical but target to distinct membrane compartments and have distinct functions (Folsch, 2005). To further define the interaction between PIPKIγ and the AP1 complexes, we examined the interaction between PIPKIγ and both μ1α and μ1β in parallel experiments. As shown in Fig. 4 B (left), PIPKIγ661 binds to μ1α and μ1β indistinguishably in vitro. The primary binding site of the μ subunits is in the last 26 amino acids of PIPKIγ661 because the C terminus of PIPKIγ661 but not PIPKIγ635 bound the μ subunits (Fig. 4 C). A weak interaction between full-length PIPKIγ635 and the μ subunits was observed under less rigorous GST pull-down conditions, where detergent and BSA concentrations were decreased. This may be consistent with the observations by Krauss et al. (2006), which indicate that there may be a secondary μ subunit binding site in the kinase domain of the PIPKIs. However, in vivo the interaction is specific for PIPKIγ661 and only the interaction between μ1β subunit (AP1B) and PIPKIγ661 was detected (Fig. 4 B, right). The in vivo specificity of PIPKIγ661 for μ1β may be regulated by targeting to AP1B-positive membrane compartments via an interaction with other trafficking components, or the PIPKIγ661–AP1B interaction maybe specifically regulated by other mechanisms.
Endogenous E-cadherin associates with the PIPKIγ–AP1 complex (Fig. 5 A). This association was disrupted by internalization of E-cadherin triggered by extracellular calcium depletion (Fig. 5 B). When calcium was restored and E-cadherin recycling to the PM was triggered, E-cadherin reassembled into the PIPKIγ–AP1 complex (Fig. 5 B). To further examine the interactions between E-cadherin, PIPKIγ661, and AP1, GST pull-down assays were performed. Although there is no direct interaction between the μ1β subunit of AP1 and HR-ECDT, PIPKIγ661 was sufficient to link HR-ECDT to μ1β in a GST pulldown experiment (Fig. 5 C). PIPKIγ661 contains a Yxxφ sorting motif (644YSPL647; Bonifacino and Traub, 2003; Bairstow et al., 2006). The substitution of the tyrosine with phenylalanine in the sorting motif was reported to reduce binding to the μ subunits (Ohno et al., 1998; Aikawa and Martin, 2003; Bairstow et al., 2006). Concurrent with these results, the Y644F mutation diminished PIPKIγ661 binding to μ1β (Fig. 4 C and Fig. 5 C), and consequently the amount of E-cadherin C terminus pulled down by μ1β was considerably reduced (Fig. 5 C), indicating that the interaction between PIPKIγ661 and μ1β-adaptin is necessary and sufficient to link E-cadherin to the AP1B complex.
Figure 5.
PIPKIγ regulates E-cadherin transport by recruiting E-cadherin to AP1. (A) E-cadherin or AP1 were immunoprecipitated from polarized MDCK cells. (B) E-cadherin was immunoprecipitated from polarized MDCK cells, polarized MDCK cells treated with 2 mM EGTA for 20 min, or polarized MDCK cells treated with EDTA for 20 min followed by regular medium for 10 min. The immunoprecipitates were analyzed as indicated. (C) GST pull-down assays were performed using 1 μg of GST, GST-μ1B, His-PIPKIγ661, and His-HR-ECDT and analyzed by immunoblotting as indicated. (D) GFP-fused E-cadherin (ECD-GFP) was transiently introduced into parental (μ1B deficient) or μ1B-expressing LLC-PK1 cells using Lipofectamine 2000 for 8 h and then visualized using fluorescence microscopy. Bar, 10 μm.
Because AP complexes play an important role in protein transport, our data suggest that PIPKIγ661 regulates E-cadherin trafficking via a direct interaction with and regulation of AP complexes. Such a model would require AP1B for E-cadherin transport to the PM. To address this hypothesis, we used LLC-PK1 cells, which do not express μ1β (i.e., are AP1B deficient). In LLC-PK1 cells, many basolateral proteins are mistargeted and cell polarity is disrupted (Folsch et al., 1999; Folsch, 2005). To assess the role of μ1β in E-cadherin transport, GFP–E-cadherin was expressed (Fig. 5 D). A small fraction of GFP–E-cadherin was able to translocate to the PM; however, the majority was observed in a perinuclear compartment, indicating inefficient transport of E-cadherin to the PM. In cells expressing GFP–E-cadherin, there was a greatly enhanced recruitment of endogenous PIPKIγ to GFP–E-cadherin–containing compartments, which is consistent with the association between PIPKIγ and E-cadherin. Upon expression of μ1B in the LLC-PK1 cells, GFP–E-cadherin was efficiently targeted to sites of cell–cell adhesion, and endogenous PIPKIγ colocalized with E-cadherin at AJs (Fig. 5 D). The expression of μ1α, however, did not rescue E-cadherin trafficking to the PM (unpublished data). These data support a model were PIPKIγ associates with E-cadherin and this interaction is required for functional recruitment of AP1B to PIPKIγ via its interaction with the YSPL motif in the PIPKIγ661 C terminus.
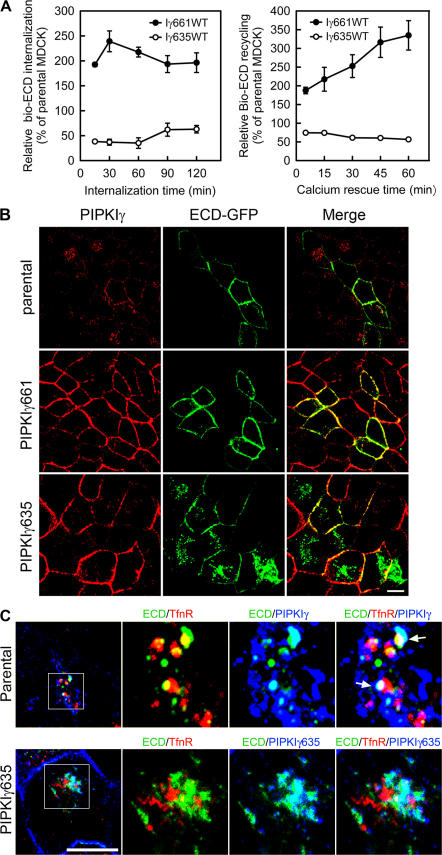
PIPKIγ regulates E-cadherin transport by recruitment to AP1B compartments
The functional relationship between PIPKIγ and AP1B is reinforced by the observation that endogenous PIPKIγ and AP1 colocalized in vesicle compartments (Fig. 6 A). Both E-cadherin and PIPKIγ partially colocalized with γ-adaptin in cytoplasmic compartments after removal of calcium (Fig. 6 B, arrows), suggesting a functional link between E-cadherin, PIPKIγ661, and AP1 in E-cadherin trafficking. Interestingly, when E-cadherin recycling was triggered by replenishing calcium, we observed that overexpression of PIPKIγ661 enhanced the recruitment of AP1B to PM. In parental MDCK cells, AP1 showed typical perinuclear localization with a small fraction targeting to the PM. When PIPKIγ661 was expressed, AP1 targeted to the basolateral membrane where it colocalized with E-cadherin and PIPKIγ661 (Fig. 6 C). However, when PIPKIγ635 was expressed, AP1 organization was strikingly distinct, as it was concentrated in a central perinuclear compartment with no localization near the PM and little colocalization with E-cadherin (Fig. 6 C). In these cells, E-cadherin was largely trapped in the cytosol and was not efficiently targeted to the PM. In PIPKIγ661KD-expressing cells, AP1 weakly localized beneath the PM or showed strong colocalization of both E-cadherin and PIPKIγ661KD in a large perinuclear compartment, but there was little detectable PM E-cadherin (Fig. 6 C). These data again support a model where both the PIPKIγ–AP1 interaction and PIPKIγ kinase activity are necessary for recruitment of AP1 to the PM and the efficient trafficking of E-cadherin to the PM.
Figure 6.
PIPKIγ is functionally related with AP1 and E-cadherin trafficking in vivo. (A) Endogenous PIPKIγ and AP1 colocalize at vesicular compartments. PIPKIγ (FITC, green) and AP1 (Texas red, red) were visualized in MDCK cells by indirect inmmunofluorescence. (B) PIPKIγ and AP1 colocalize with the internalized E-cadherin. Parental MDCK cells were plated on coverslips and allowed to form AJs. They were then treated with 2 mM EGTA for 30 min. Cells were fixed and indirect immunofluorescence staining was performed using anti-PIPKIγ and anti–γ-adaptin, followed by Cy5-conjugated anti–rabbit IgG antibody and Texas red–conjugated anti–mouse IgG antibodies. Cells were then incubated with 0.5 μg/ml of normal mouse IgG at 37°C for 30 min and then with FITC-conjugated anti– E-cadherin antibodies. Arrows indicate the colocalization of AP1, internalized ECD, and PIPKIγ. (C) PIPKIγ661 recruits AP1 to functional sites of E-cadherin recycling. Parental or PIPKIγ isoform–overexpressing MDCK cells were treated with 2 mM EGTA for 30 min followed by complete medium for 10 min at 37°C to induce the recycling of E-cadherin back to the PM. Immunofluorescence staining was then performed to visualize AP1 (γ-adaptin), E-cadherin, and PIPKIγ. Both horizontal and vertical sections were collected to show precise subcellular localizations. Bars, 10 μm.
To explore this hypothesis, the internalization and recycling of E-cadherin in cells ectopically expressing PIPKIγ635 was first determined. As shown in Fig. 7 A, when internalization and recycling of E-cadherin was measured by surface biotinylation, overexpression of PIPKIγ635 had a dominant-negative effect and inhibited E-cadherin trafficking to and from the PM compared with parental cells. Again, these results support a functional role for the PIPKIγ661–AP1B interaction in modulation of E-cadherin trafficking. Consistent with this conclusion, both ectopically expressed GFP–E-cadherin (Fig. 7 B) and endogenous E-cadherin (Fig. 6 C) were sequestered in a cytosolic compartment in PIPKIγ635-overexpressing cells, displaying a phenotype similar to that observed when endogenous PIPKIγ was knocked down.
Figure 7.
The interaction between PIPKIγ and AP1B is necessary for E-cadherin transport. (A) MDCK cells with or without PIPKIγ635 overexpression were used to analyze E-cadherin internalization and recycling by surface biotinylation as described. Immunoblot data from three independent experiments were quantified by ImageJ and plotted with SigmaPlot 8.0. Error bars are ± SEM. (B) Parental, PIPKIγ635-, or PIPKIγ661-expressing MDCK cells were transiently transfected with ECD-GFP. PIPKIγ was visualized by indirect immunofluorescence and ECD-GFP using direct fluorescence microscopy. (C, top) Parental MDCK cells were incubated at 20°C for 2 h, treated with 2 mM EGTA for 30 min, and then fixed and triple- labeled using monoclonal anti-CD71, FITC-conjugated anti–E-cadherin, and rabbit anti-PIPKIγ antibodies. (bottom) MDCK cells with the expression of HA-PIPKIγ635 induced for 72 h were maintained at 20°C for 2 h. The cells were then fixed and stained using monoclonal anti-CD71, FITC-conjugated anti–E-cadherin, and rabbit anti-HA antibodies. Arrows indicate the colocalization of ECD, PIPKIγ, and the recycling endosome. Bars, 10 μm.
To further characterize these E-cadherin–containing vesicles in PIPKIγ635-expressing cells, we induced accumulation of transferrin receptor (TfnR) in the recycling endosome using an established approach (Sheff et al., 1999). As shown in Fig. 7 C (top), internalized E-cadherin in parental MDCK cells showed partial colocalization with both endogenous PIPKIγ and internalized TfnR, representing the recycling endosome, and colocalization among these three proteins was also observed (Fig. 7 C, arrows). Interestingly, overexpression of PIPKIγ635, which interacts with E-cadherin but not AP1B, blocked E-cadherin colocalization with the TfnR compartment, but E-cadherin did colocalize with PIPKIγ635 (Fig. 7 C, bottom). These data suggest that PIPKIγ661 mediates the transport of E-cadherin from the trans-Golgi network to the recycling endosome, which has been argued to serve as an intermediate between the trans-Golgi network and the basolateral PM (Ang et al., 2004; Folsch, 2005). Further, this data establishes that trafficking of E-cadherin to this compartment requires a functional interaction between PIPKIγ661 and AP1B.
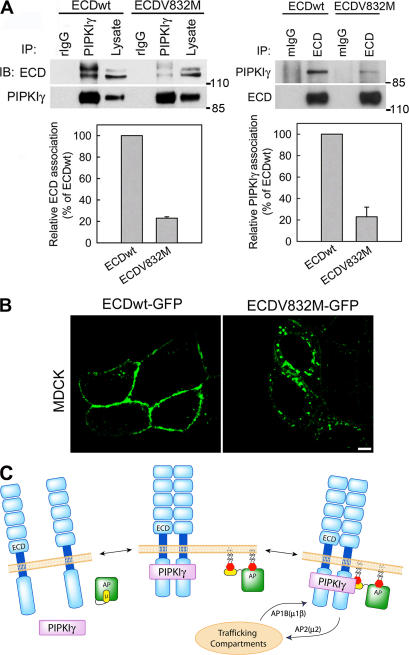
If PIPKIγ serves as an adaptor between E-cadherin and AP complexes, one would expect that an E-cadherin mutant lacking or with diminished PIPKIγ binding would not be transported efficiently to the PM. A V832M germline mutation was identified in hereditary diffuse gastric cancer (Yabuta et al., 2002), which lacks the ability to mediate cell–cell adhesion or suppress invasion (Suriano et al., 2003). In these patients, the wild-type E-cadherin gene is repressed, and only the mutant is expressed in the carcinomas (Yabuta et al., 2002). Interestingly, the V832M mutation lies in the PIPKIγ binding region. To determine whether this mutation impacts PIPKIγ binding, E-cadherin V832M was introduced into HEK293 cells and its association with PIPKIγ was quantified. This mutant showed a substantially reduced ability to bind PIPKIγ (Fig. 8 A). Consistent with published data (Suriano et al., 2003), β-catenin binding was normal (not depicted). The basolateral transport of this V832M mutation was also explored in both LLC-PK1∷μ1β (unpublished data) and MDCK cells using GFP-fused E-cadherinV832M. As shown in Fig. 8 B, although the V832M mutant was visualized on the PM as reported by others (Suriano et al., 2003), a large accumulation of this E-cadherin mutant was observed in a cytosolic compartment. This phenotype was similar to that of wild-type E-cadherin observed in the PIPKIγ635-overexpressing cells (Fig. 7 B) or the LLC-PK1 cells deficient in μ1β (Fig. 5 D). Wild-type E-cadherin in LLC-PK1∷μ1β (Fig. 5 D) and MDCK (Fig. 8 B) cells was transported efficiently to the basolateral membrane and little was visualized in the cytosol. This result is consistent with a requirement for an interaction between E-cadherin and PIPKIγ661 for normal trafficking of E-cadherin.
Figure 8.
The interaction between PIPKIγ and E-cadherin is required for E-cadherin transport. (A) Indicated E-cadherin constructs were overexpressed in HEK293 cells, and their association with PIPKIγ was determined, quantified, and plotted from four independent experiments. Error bars are ± SEM. (B) MDCK cells were transfected with GFP-ECD(V832M). Cells were analyzed by fluorescence microscopy 8–10 h after transfection. Bar, 10 μm. (C) A model representing the mechanism for PIPKIγ661-mediated E-cadherin trafficking. The dual interaction of PIPKIγ661 with the E-cadherin dimer and the AP complex provides a mechanism for the specific regulation of E-cadherin trafficking. This dual interaction mediates the scaffold between AP complexes and E-cadherin to facilitate the assembly of this cargo into the trafficking machinery. In addition, the interaction between PIPKIγ661 and AP complexes ensures the localized generation of PI4,5P2, which regulates the function of AP complexes and other processes in E-cadherin trafficking.
Discussion
Proteins that interact with the ECDT not only mediate the elemental functions of E-cadherin, such as AJ assembly, actin organization, and cell proliferation, but also regulate E-cadherin by modulating its expression and trafficking. Here we have shown that PIPKIγ directly binds to both E-cadherin and AP complexes. This dual interaction supports a mechanism for the highly regulated generation of PI4,5P2 to spatially drive the assembly of the trafficking machinery and to specifically control E-cadherin trafficking. These results reveal a novel mechanism where PIPKIγ661 functions as both scaffolding and signaling molecule during E-cadherin trafficking (Fig. 8 C). In this model, the AP complex interacts indirectly with the E-cadherin cargo via the PIPKIγ661 scaffold, which directly binds to AP complexes via a Yxxφ sorting motif in its C terminus. This represents a novel paradigm in which PIPKIγ661 serves as a cargo adaptor for AP complexes. Although PIPKIγ661 binds to both AP1A and AP1B indistinguishably in vitro, we found that PIPKIγ661 preferentially interacts with AP1B in vivo, and regulation of E-cadherin trafficking to the basolateral PM appears to be specific for AP1B. AP1A and AP1B both use Yxxφ sorting motifs for cargo recognition. However, despite the substantial sequence and structural homology of the μ1 subunits, the AP1 complexes are targeted to distinct compartments by an unknown mechanism (Folsch et al., 2003; Folsch, 2005). PIPKIγ661 might be specifically recruited to AP1B-containing membrane domains in vivo via an interaction with one or more other proteins.
PIPKIγ661 may also have additional lower affinity binding contacts with AP1B, as Krauss et al. (2006) recently reported that multiple PIPKIs bind to the μ2 subunit of AP2 complex via the kinase domain. In our hands, the YSPL motif of PIPKIγ661 was the preferential binding site for the μ subunits of AP1 and AP2 (Bairstow et al., 2006), but PIPKIγ635 did bind μ1- and μ2-adaptin subunits under less rigorous conditions (unpublished data), suggesting additional lower affinity interacting sites between the AP complexes and PIPKIγ. For E-cadherin trafficking, the YSPL motif of PIPKIγ661 is the key interaction with AP complexes and subsequent interactions may regulate kinase activity. As the kinase domains of the PIPKI isoforms are highly homologous, other isoforms of PIPKIs (e.g., PIPKIα) may interact with and regulate some AP complex–dependent trafficking events (Barbieri et al., 2001). These putative interactions could be through the conserved kinase domains or, like PIPKIγ661, could be mediated by specific binding partners via interaction with variable regions of the PIPKI isoforms. Nevertheless, because PI4,5P2 is a key moderator of the recruitment and assembly of trafficking machinery (Simonsen et al., 2001; Roth, 2004), the localized generation of PI4,5P2 at sites where E-cadherin and other cargoes are assembled into the trafficking machinery is an indispensable step in this process. This finding suggests that any association between a PIPK and the trafficking machinery must be spatially and temporally regulated. The interaction between the PIPKIγ661 and the AP1B complex fits this criterion, as this association is detected when E-cadherin is recycled back to the PM but not when E-cadherin is being internalized (Fig. 5 B). This observation supports the concept of cellular signals coordinating the interactions between PIPKIγ661 and the AP complexes.
A dileucine motif in the juxtamembrane region of the ECDT is required for basolateral sorting (Miranda et al., 2001), and this motif was proposed to be a cargo signal recognized by the β subunit of the AP1 complex (Rapoport et al., 1998). There is no solid evidence supporting this interaction at present. However, if this is true, the E-cadherin–PIPKIγ661–AP1B complex could be further stabilized via the interaction of the E-cadherin dileucine motif with the β subunit of AP1B. Alternatively, other trafficking components may recognize this motif and cooperate with PIPKIγ and AP1B to provide specificity.
The E-cadherin–PIPKIγ661–AP1B interaction serves as a foundational signal for exocytic targeting and basolateral sorting of E-cadherin. Indeed, internalized E-cadherin accumulated at the recycling endosome, as indicated by internalized TfnR. This compartment contained, in addition, endogenous PIPKIγ supporting a role for PIPKIγ in trafficking though this compartment. Consistent with this observation, the overexpression of PIPKIγ635, which binds E-cadherin but not μ1β (AP1B), blocked E-cadherin trafficking to the TfnR-positive compartment of the recycling endosome. E-cadherin did colocalize with PIPKIγ635, indicating that PIPKIγ interacts with E-cadherin in this compartment. The combined results demonstrate that the association of PIPKIγ661 with AP1B is require for E-cadherin trafficking through this compartment. The recycling endosome is a major site of AP1B, and this further supports our hypothesis that PIPKIγ functions as an adaptor in E-cadherin trafficking and facilitates E-cadherin transport to and from the recycling endosome via binding to AP1B and generation of PI4,5P2.
E-cadherin endocytosis can occur in a clathrin-dependent (Palacios et al., 2001; Ivanov et al., 2004) or independent manner (Paterson et al., 2003). Calcium removal stimulates E-cadherin endocytosis by the clathrin-dependent pathway (Ivanov et al., 2004). As there is no known Yxxφ sorting motif in the ECDT, the interaction between PIPKIγ661 and E-cadherin may recruit AP2 for clathrin-dependent E-cadherin endocytosis. Additionally, Arf6 promotes E-cadherin internalization (Palacios et al., 2002) and has been shown to associate with and stimulate the activity of PIPKIγ (Aikawa and Martin, 2003, 2005). Arf6, in cooperation with PI4,5P2, was also shown to directly interact with and promote the recruitment of AP2 to the PM (Krauss et al., 2003; Paleotti et al., 2005). These combined results suggest that PIPKIγ661, E-cadherin, AP2, and Arf6 may cooperate to regulate E-cadherin internalization in epithelial cells. This would position PIPKIγ661 as a nexus between AP complexes and E-cadherin in endocytic recycling. Nevertheless, there is no direct evidence demonstrating that AP2 mediates the internalization of E-cadherin, and further investigation is needed to characterize the role of PIPKIγ in E-cadherin endocytosis.
Our data demonstrates that a loss of PIPKIγ in cultured epithelial cells results in the severe mistargeting of E-cadherin, suggesting a strong functional connection between PIPKIγ and E-cadherin. Interestingly, the PIPKIγ knockout mouse does not share the same phenotype as the E-cadherin knockout mouse (Larue et al., 1994; Di Paolo et al., 2004). This is not surprising, as the knockout phenotypes of other modifiers of E-cadherin function, such as p120-catenin, differ from that of the E-cadherin knockout as well (Pettitt et al., 2003; Davis and Reynolds, 2006). Considering the existence of multiple pathways for E-cadherin trafficking, the roles of these E-cadherin modifiers may only become apparent during the development of specific tissues in later stages of animal development.
Dimerization is an essential property of E-cadherin assembly driving AJ formation (Yap et al., 1997). The association of both PIPKIγ and p120 catenin with the E-cadherin dimer may be a mechanism to functionally regulate E-cadherin assembly and could promote AJ formation by stimulating E-cadherin clustering. Because PIPKIγ specifically binds to E-cadherin dimers, the in situ PI4,5P2 generation resulting from this interaction may drive other local complementary cellular events, such as actin reorganization (Janmey and Lindberg, 2004). Actin assembly is important not only in AJ assembly but also for E-cadherin internalization/exocytosis (D'Souza-Schorey, 2005). The association of PIPKIγ with E-cadherin may be crucial for downstream signaling, as Rac and phosphoinositide-3 kinase are activated by E-cadherin and both regulate the stability of AJs by modulating actin assembly (Noren et al., 2001; Yap and Kovacs, 2003; D'Souza-Schorey, 2005). Phosphoinositide-3 kinase requires PI4,5P2 for signaling, and Rho family small G proteins regulate some PIPKI isoforms (Fruman et al., 1998). As a result, PIPKIγ may also regulate AJ assembly through local cooperation with phosphoinositide-3 kinase and small G protein signaling.
The generation of phosphoinositide messengers upon assembly of AJs has implications beyond simple control of E-cadherin trafficking. Because E-cadherin is a major suppressor of invasion of epithelial tumors, the cell biological data suggest that PIPKIγ may play a similar role. In exploring this possibility, we have discovered that a loss of E-cadherin correlates with a loss of PIPKIγ in human breast cancers (unpublished data). This finding supports a physiological role for PIPKIγ in assembly of E-cadherin junctions and potentially a role in progression of epithelial tumors.
Materials and methods
Constructs and antibodies
The C terminus of E-cadherin was amplified by PCR and constructed into normal or modified (Ling et al., 2003) pET28 to generate the His-tagged E-cadherin tail or HR–E-cadherin tail, which were then subcloned into pCMV-Myc vector (CLONTECH Laboratories, Inc.). Wild-type E-cadherin, E-cadherinΔp120ctn, E-cadherinΔβctn, and E-cadherin/αctn were provided by B. Gumbiner (University of Virginia, Charlottesville, VA). E-cadherin836 was amplified by PCR, and E-cadherinV832M was generated using the QuikChange II Site-Directed Mutagenesis kit (Stratagene) according to the manufacturer's instructions. Both μ1α and μ1β constructs were provided by I. Mellman (Yale University, New Haven, CT). cDNAs encoding N-terminal truncated μ1α and μ1β (1–135 aa truncated) were amplified by PCR and subcloned into pET42. All of the PIPKI constructs were created as described previously (Ling et al., 2002, 2003; Bairstow et al., 2006). Duplexes of siRNA oligos (for both human and mouse: aagttctatgggctgtactgc, aaggacctggacttcatgcag; for canine: gaaggctcttgttcacgat) were synthesized by Dharmacon.
Monoclonal antibodies for E-cadherin (recognizing the C terminus), N-cadherin, human VE-cadherin, p120catenin, β-catenin, γ-adaptin, and FITC-conjugated anti–E-cadherin were purchased from Transduction Laboratories. The H68.4 monoclonal anti-CD71 (TfnR) antibody was purchased from BioGenex Inc. Polyclonal PIPKIγ antibody was generated as described previously (Ling et al., 2002). Regular mouse and rabbit IgG and secondary antibodies were obtained from Jackson ImmunoResearch Laboratories. Anti-HA antibody was purchased from Covance. Anti-Myc and FITC-conjugated anti-Myc were obtained from Upstate Biotechnology. HRP-conjugated anti-GST antibodies were purchased from GE Healthcare. Anti–E-cadherin antibodies recognizing the ectodomain were purchased from Zymed Laboratories (monoclonal, for immunoblotting) and Sigma-Aldrich (rat monoclonal, for immunofluorescence).
Cell culture, transfection, immunofluorescence, and confocal microscopy
MDCK-TetOff cells (CLONTECH Laboratories, Inc.) and HEK293 cells were cultured in Dulbecco's modified eagle medium (Mediatech, Inc.) supplemented with 10% FBS (Invitrogen). Lysates of human umbilical vein endothelial cells were obtained from E. Bresnick (University of Wisconsin-Madison, Madison, WI). MDCK cells were transfected using FuGENE 6 (Roche) for 48 h, and then 100 μg/ml hygromycin B was added to the medium to select stable clones and 10 mg/ml doxycycline was used to suppress PIPKIγ expression. To induce expression, doxycycline was removed for 72 h. HEK293 cells were transfected using the calcium phosphate–DNA coprecipitation method for 48 h. For siRNA knockdown, MDCK cells in a 6-well plate were transfected twice at 48-h intervals with 5 pmol/well siRNA using the calcium phosphate–DNA coprecipitation method. Cells were analyzed 48 h after the second transfection. LLC-PK1 cell lines were provided by I. Mellman and cultured as described previously (Folsch et al., 1999).
Indirect immunofluorescence and confocal microscopy were performed as described previously (Ling et al., 2002). For triple labeling, double labeled samples were blocked by 0.5 mg/ml of normal mouse IgG in 3% BSA/PBS at 37°C for 30 min, rinsed in PBS twice, and incubated with FITC-conjugated anti–E-cadherin or anti-Myc antibodies in 3% BSA/PBS at 37°C for 1 h. Confocal images were acquired using photomultiplier tubes through LaserSharp2000 (Carl Zeiss MicroImaging, Inc.) with a PlanApo 100× oil objective (NA 1.4) on an inverted microscope (Eclipse TE2000; Nikon) with Radiance 2100 MP Rainbow (Bio-Rad Laboratories). Z series were created by sequentially scanning FITC, Texas red, or Cy5 channel at 0.3-μm steps. Single sections were exported to Photoshop CS2 (Adobe) for final image processing. Fluorescence intensity was quantified using ImageJ 1.62 (National Institutes of Health) and plotted using SigmaPlot 8.0.
Immunoprecipitation, GST pull-down assay, and immunoblotting
Cells were lysed in lysis buffer (50 mM Tris⋅HCl, pH 7.5, 150 mM NaCl, 0.5% NP-40, 1 mM EDTA, 1 mM PMSF, and 10% glycerol), and then used for immunoprecipitation (Ling et al., 2002). The immunocomplexes were separated by SDS-PAGE and analyzed. Unless stated, immunoprecipitations were performed using 800 μl of cell lysate from one confluent 60-mm dish. One fourth of each precipitate and 20 μl of each lysate were analyzed. GST-tagged PIPKIα, PIPKIγ635, and PIPKIγ661, His-tagged E-cadherin, or HR-E-cadherin tail were purified from Escherichia coli, and GST pull-down assays were performed (Ling et al., 2003). One fourth of the GST beads for each pull down were loaded on the gel and 20 μl of each purified protein was loaded as an input control. Images were scanned and exported to Photoshop CS2 for final processing. Intensity of bands was quantified using ImageJ 1.62 and plotted using SigmaPlot 8.0.
Calcium depletion, surface biotinylation, and trafficking of E-cadherin
Cells were allowed to grow on coverslips for 72 h to reach confluence and were then incubated with 2 mM EGTA for 20 min before performing indirect immunoflurescence. Confluent MDCK cells grown in 24-mm-diam Transwells (Costar) were biotinylated by 1 mg/ml sulfo-NHS-SS-biotin (Pierce Chemical Co.) and analyzed as described previously (Le et al., 1999). Cells were lysed in 500 μl of lysis buffer and one third of the precipitates were analyzed. Internalization of E-cadherin was induced by 0.5 mM EGTA at 18°C. To measure the recycling of E-cadherin, MDCK cells were treated with 2 mM EGTA for 40 min at 37°C and chased in normal medium, and then surface biotinylation was performed.
PIPK activity assay
Activity of 10 μg of purified recombinant PIPKIγ proteins was assayed against 20 μg of Folsch Brain Extract III as previously described (Di Paolo et al., 2002). Kinase activity was quantified using Storm 840 (Molecular Dynamics) and plotted using SigmaPlot 8.0.
Online supplemental material
Fig. S1 shows that PIPKIγ directly binds E-cadherin. Fig. S2 shows that the direct interaction with PIPKIγ is important for E-cadherin assembly. Fig. S3 shows that functional PIPKIγ is required for E-cadherin assembly. Fig. S4 shows that a modification of PIPKIγ activity has no effect on global PI4,5P2 level. Online supplemental material, including Figs. S1−S4 and supplemental Materials and methods, is available at http://www.jcb.org/cgi/content/full/jcb.200606023/DC1.
Acknowledgments
We thank Drs. Barry M. Gumbiner and Alpha Yap for their reagents. We thank Heike Folsch for valuable discussions. We are grateful for the exceptional technical help and scientific discussion of Matt Wagoner, Nick Schill, Jessica Heck, and Xiaojing Su. We thank Drs. Anna Huttenlocher and Patricia Keely for excellent discussions. We thank Dr. Emery Bresnick for reagents.
This work was supported by grants CA104708 and GM057549 to R.A. Anderson and an American Heart Association Fellowship and Scientist Development grants to K. Ling.
The authors declare that they have no competing financial interests.
Abbreviations used in this paper: AJ, adherens junction; AP, clathrin adaptor protein; ECDT, E-cadherin cytoplasmic domain; HEK, human embryonic kidney; HR, heptad repeat; PI4,5P2, phosphatidylinositol-4,5-bisphosphate; PIPKI, type I phosphatidylinositol phosphate kinase; PM, plasma membrane; TfnR, transferrin receptor.
References
- Aikawa, Y., and T.F. Martin. 2003. ARF6 regulates a plasma membrane pool of phosphatidylinositol(4,5)bisphosphate required for regulated exocytosis. J. Cell Biol. 162:647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa, Y., and T.F. Martin. 2005. ADP-ribosylation factor 6 regulation of phosphatidylinositol-4,5-bisphosphate synthesis, endocytosis, and exocytosis. Methods Enzymol. 404:422–431. [DOI] [PubMed] [Google Scholar]
- Ang, A.L., T. Taguchi, S. Francis, H. Folsch, L.J. Murrells, M. Pypaert, G. Warren, and I. Mellman. 2004. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 167:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairstow, S.F., K. Ling, X. Su, A.J. Firestone, C. Carbonara, and R.A. Anderson. 2006. Type Igamma 661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J. Biol. Chem. 281:20632–20642. [DOI] [PubMed] [Google Scholar]
- Barbieri, M.A., C.M. Heath, E.M. Peters, A. Wells, J.N. Davis, and P.D. Stahl. 2001. Phosphatidylinositol-4-phosphate 5-kinase-1beta is essential for epidermal growth factor receptor-mediated endocytosis. J. Biol. Chem. 276:47212–47216. [DOI] [PubMed] [Google Scholar]
- Baust, T., C. Czupalla, E. Krause, L. Bourel-Bonnet, and B. Hoflack. 2006. Proteomic analysis of adaptor protein 1A coats selectively assembled on liposomes. Proc. Natl. Acad. Sci. USA. 103:3159–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino, J.S., and L.M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395–447. [DOI] [PubMed] [Google Scholar]
- Bryant, D.M., and J.L. Stow. 2004. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 14:427–434. [DOI] [PubMed] [Google Scholar]
- Chitaev, N.A., and S.M. Troyanovsky. 1998. Adhesive but not lateral E-cadherin complexes require calcium and catenins for their formation. J. Cell Biol. 142:837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, B.M., A.J. McCoy, H.M. Kent, P.R. Evans, and D.J. Owen. 2002. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 109:523–535. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey, C. 2005. Disassembling adherens junctions: breaking up is hard to do. Trends Cell Biol. 15:19–26. [DOI] [PubMed] [Google Scholar]
- Davis, M.A., and A.B. Reynolds. 2006. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev. Cell. 10:21–31. [DOI] [PubMed] [Google Scholar]
- Di Paolo, G., L. Pellegrini, K. Letinic, G. Cestra, R. Zoncu, S. Voronov, S. Chang, J. Guo, M.R. Wenk, and P. De Camilli. 2002. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature. 420:85–89. [DOI] [PubMed] [Google Scholar]
- Di Paolo, G., H.S. Moskowitz, K. Gipson, M.R. Wenk, S. Voronov, M. Obayashi, R. Flavell, R.M. Fitzsimonds, T.A. Ryan, and P. De Camilli. 2004. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 431:415–422. [DOI] [PubMed] [Google Scholar]
- Doughman, R.L., A.J. Firestone, and R.A. Anderson. 2003. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J. Membr. Biol. 194:77–89. [DOI] [PubMed] [Google Scholar]
- Folsch, H. 2005. The building blocks for basolateral vesicles in polarized epithelial cells. Trends Cell Biol. 15:222–228. [DOI] [PubMed] [Google Scholar]
- Folsch, H., H. Ohno, J.S. Bonifacino, and I. Mellman. 1999. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 99:189–198. [DOI] [PubMed] [Google Scholar]
- Folsch, H., M. Pypaert, S. Maday, L. Pelletier, and I. Mellman. 2003. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 163:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman, D.A., R.E. Meyers, and L.C. Cantley. 1998. Phosphoinositide kinases. Annu. Rev. Biochem. 67:481–507. [DOI] [PubMed] [Google Scholar]
- Gan, Y., T.E. McGraw, and E. Rodriguez-Boulan. 2002. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat. Cell Biol. 4:605–609. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B.M. 1996. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 84:345–357. [DOI] [PubMed] [Google Scholar]
- Honing, S., D. Ricotta, M. Krauss, K. Spate, B. Spolaore, A. Motley, M. Robinson, C. Robinson, V. Haucke, and D.J. Owen. 2005. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol. Cell. 18:519–531. [DOI] [PubMed] [Google Scholar]
- Huber, M.A., N. Kraut, and H. Beug. 2005. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17:548–558. [DOI] [PubMed] [Google Scholar]
- Imamura, Y., M. Itoh, Y. Maeno, S. Tsukita, and A. Nagafuchi. 1999. Functional domains of α-catenin required for the strong state of cadherin-based cell adhesion. J. Cell Biol. 144:1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, A.I., A. Nusrat, and C.A. Parkos. 2004. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell. 15:176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey, P.A., and U. Lindberg. 2004. Cytoskeletal regulation: rich in lipids. Nat. Rev. Mol. Cell Biol. 5:658–666. [DOI] [PubMed] [Google Scholar]
- Kang, Y., and J. Massague. 2004. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 118:277–279. [DOI] [PubMed] [Google Scholar]
- Krauss, M., M. Kinuta, M.R. Wenk, P. De Camilli, K. Takei, and V. Haucke. 2003. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Iγ. J. Cell Biol. 162:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss, M., V. Kukhtina, A. Pechstein, and V. Haucke. 2006. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes. Proc. Natl. Acad. Sci. USA. 103:11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue, L., M. Ohsugi, J. Hirchenhain, and R. Kemler. 1994. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc. Natl. Acad. Sci. USA. 91:8263–8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, T.L., A.S. Yap, and J.L. Stow. 1999. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Ling, K., R.L. Doughman, A.J. Firestone, M.W. Bunce, and R.A. Anderson. 2002. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 420:89–93. [DOI] [PubMed] [Google Scholar]
- Ling, K., R.L. Doughman, V.V. Iyer, A.J. Firestone, S.F. Bairstow, D.F. Mosher, M.D. Schaller, and R.A. Anderson. 2003. Tyrosine phosphorylation of type Iγ phosphatidylinositol phosphate kinase by Src regulates an integrin–talin switch. J. Cell Biol. 163:1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, K., N.J. Schill, M.P. Wagoner, Y. Sun, and R.A. Anderson. 2006. Movin' on up: the role of PtdIns(4,5)P(2) in cell migration. Trends Cell Biol. 16:276–284. [DOI] [PubMed] [Google Scholar]
- Lock, J.G., and J.L. Stow. 2005. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol. Biol. Cell. 16:1744–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, T.F. 2001. PI(4,5)P(2) regulation of surface membrane traffic. Curr. Opin. Cell Biol. 13:493–499. [DOI] [PubMed] [Google Scholar]
- Miranda, K.C., T. Khromykh, P. Christy, T.L. Le, C.J. Gottardi, A.S. Yap, J.L. Stow, and R.D. Teasdale. 2001. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. J. Biol. Chem. 276:22565–22572. [DOI] [PubMed] [Google Scholar]
- Nelson, W.J., and R. Nusse. 2004. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 303:1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren, N.K., C.M. Niessen, B.M. Gumbiner, and K. Burridge. 2001. Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276:33305–33308. [DOI] [PubMed] [Google Scholar]
- Ohno, H., R.C. Aguilar, D. Yeh, D. Taura, T. Saito, and J.S. Bonifacino. 1998. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 273:25915–25921. [DOI] [PubMed] [Google Scholar]
- Palacios, F., L. Price, J. Schweitzer, J.G. Collard, and C. D'Souza-Schorey. 2001. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 20:4973–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios, F., J.K. Schweitzer, R.L. Boshans, and C. D'Souza-Schorey. 2002. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat. Cell Biol. 4:929–936. [DOI] [PubMed] [Google Scholar]
- Paleotti, O., E. Macia, F. Luton, S. Klein, M. Partisani, P. Chardin, T. Kirchhausen, and M. Franco. 2005. The small G-protein Arf6GTP recruits the AP-2 adaptor complex to membranes. J. Biol. Chem. 280:21661–21666. [DOI] [PubMed] [Google Scholar]
- Patel, S.D., C.P. Chen, F. Bahna, B. Honig, and L. Shapiro. 2003. Cadherin-mediated cell–cell adhesion: sticking together as a family. Curr. Opin. Struct. Biol. 13:690–698. [DOI] [PubMed] [Google Scholar]
- Paterson, A.D., R.G. Parton, C. Ferguson, J.L. Stow, and A.S. Yap. 2003. Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: the initial fate of unbound E-cadherin. J. Biol. Chem. 278:21050–21057. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno, M., C. Jamora, and E. Fuchs. 2003. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 112:535–548. [DOI] [PubMed] [Google Scholar]
- Pettitt, J., E.A. Cox, I.D. Broadbent, A. Flett, and J. Hardin. 2003. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin–catenin function during epidermal morphogenesis. J. Cell Biol. 162:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport, I., Y.C. Chen, P. Cupers, S.E. Shoelson, and T. Kirchhausen. 1998. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 17:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, M.G. 2004. Phosphoinositides in constitutive membrane traffic. Physiol. Rev. 84:699–730. [DOI] [PubMed] [Google Scholar]
- Sheff, D.R., E.A. Daro, M. Hull, and I. Mellman. 1999. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145:123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, A., A.E. Wurmser, S.D. Emr, and H. Stenmark. 2001. The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 13:485–492. [DOI] [PubMed] [Google Scholar]
- Suriano, G., D. Mulholland, O. de Wever, P. Ferreira, A.R. Mateus, E. Bruyneel, C.C. Nelson, M.M. Mareel, J. Yokota, D. Huntsman, and R. Seruca. 2003. The intracellular E-cadherin germline mutation V832 M lacks the ability to mediate cell-cell adhesion and to suppress invasion. Oncogene. 22:5716–5719. [DOI] [PubMed] [Google Scholar]
- Yabuta, T., K. Shinmura, M. Tani, S. Yamaguchi, K. Yoshimura, H. Katai, T. Nakajima, E. Mochiki, T. Tsujinaka, M. Takami, et al. 2002. E-cadherin gene variants in gastric cancer families whose probands are diagnosed with diffuse gastric cancer. Int. J. Cancer. 101:434–441. [DOI] [PubMed] [Google Scholar]
- Yap, A.S., and E.M. Kovacs. 2003. Direct cadherin-activated cell signaling: a view from the plasma membrane. J. Cell Biol. 160:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, A.S., W.M. Brieher, and B.M. Gumbiner. 1997. Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13:119–146. [DOI] [PubMed] [Google Scholar]