Abstract
One of the most surprising discoveries in cell biology in the past 5–10 years is the number of diverse human diseases that result from defects in ciliary assembly and/or motility, so-called ciliopathies (Badano, J.L., N. Mitsuma, P.L. Beales, and N. Katsanis. 2006. Annu. Rev. Genomics Hum. Genet. 7:125–148). The results presented by Lechtreck and Witman (see p. 473 of this issue) provide yet another example of how work in the model organism Chlamydomonas reinhardtii can reveal important insights into the underlying mechanisms of ciliary assembly/function and the diseases associated with defects in these organelles. By taking advantage of the wide array of experimental approaches C. reinhardtii offers, Lechtreck and Witman determined the precise axonemal location of hydin, a protein that, when mutated, causes hydrocephalus, and defined a unique role for hydin in ciliary motility.
In mammals, motile cilia/flagella are required for sperm propulsion, removal of debris from the respiratory tract, circulation of cerebrospinal fluid, and even for determination of the left–right body plan during development (Satir and Christensen, 2006). As a consequence, defects in motility may result in impaired fertility, respiratory distress, hydrocephalus, and/or randomization of the left–right body axis. Because these complex organelles are composed of 500 or more polypeptides (Pazour et al., 2005), identifying candidate genes that, when mutated, cause these diseases has been challenging. Fortunately, the genes encoding these proteins are highly conserved. Therefore, work in model organisms such as C. reinhardtii has provided important insights into molecular mechanisms of motility/assembly, as well as a powerful means for identifying diseases that result from defects in these organelles.
Recent studies of mouse models of hydrocephalus identified Hydin as a gene that, when mutated, causes hydrocephalus (Davy and Robinson, 2003). Additional studies in mice, trypanosomes, and C. reinhardtii have all indicated that hydin is a large protein that localizes to cilia/flagella (Davy and Robinson, 2003; Pazour et al., 2005; Broadhead et al., 2006). However, neither the precise localization of hydin within this organelle nor its role in motility was known. Lechtreck and Witman (see p. 473 of this issue) have now demonstrated that hydin localizes to a specific projection on a single microtubule of the central apparatus; that hydin appears to be involved in the assembly or stability of additional central apparatus components, including the kinesin, KLP1; and that hydin is required for motility.
To address questions of function, the authors knocked down expression of hydin in C. reinhardtii using RNA-mediated interference. For the majority of mutant cells, their flagella are paralyzed and randomly arrested in the “hands-up” or “hands-down” position. This unique phenotype combined with the localization of hydin to the central apparatus raises intriguing possibilities for the role of hydin in ciliary/flagellar motility (Fig. 1).
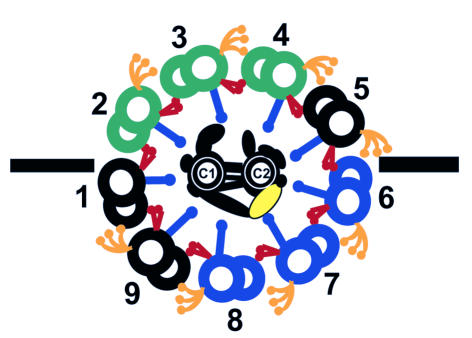
Figure 1.
Transverse section of C. reinhardtii flagellar axoneme. The dark line represents the plane of bending. In the principle bend of the effective stroke, active sliding is generated by dynein arms on doublets 2–4 (green). In the principle bend of the recovery spoke, the generation of active sliding switches to doublets 6–8 (blue). When the bend initiates, the central apparatus is oriented parallel to the plane of bending, with the C1 tubule of the central pair oriented toward doublet 1 (Mitchell, 2003). However, as the bend propagates, the central apparatus rotates with a slight twist. Therefore, in C. reinhardtii, the orientation of the central apparatus changes relative to regions of active sliding. Based on the work of Lechtreck and Witman (2007), hydin (yellow; C2 central tubule) may play a role in regulating the switch between zones of active sliding.
For decades, our understanding of ciliary/flagellar motility has been summarized in a sliding microtubule model (Summers and Gibbons, 1971; Brokaw, 1972). Force generated by the dynein arms causes sliding between adjacent microtubules. However, to generate complex waveforms, dynein activity must be precisely regulated on specific doublet microtubules (Satir, 1985). At any given time, dynein arms on one side of the axoneme must be active, and on the other side inactive (Fig. 1). As the direction of bending changes, the location of active and inactive zones of dynein activity must switch to opposite sides of the axoneme.
A substantial amount of data has indicated that the central apparatus and radial spokes form a scaffold for anchoring proteins involved in signal transduction pathways that ultimately regulate dynein activity on subsets of doublet microtubules (Smith and Yang, 2004). The asymmetric distribution of central pair projections and the polypeptides that comprise them suggests a model in which the central apparatus plays a role in the asymmetric modulation of dynein activity (Mitchell, 2003; Wargo and Smith, 2003). The phenotype of the C. reinhardtii hydin knockdown strain observed by Lechtreck and Witman (2007) provides additional support for this model. The arrest of mutant flagella in either the hands-up or hands-down position reveals that these flagella get stuck in either of two switch points in the beat cycle, the beginning of the effective stroke or the beginning of the recovery stroke, respectively. Therefore, the hydin-associated projection may play an important role in regulating switching of dynein activity. Future studies will most likely include investigations of whether hydin and its associated polypeptides make contact with the radial spokes as well as the functional consequences of these interactions.
An important remaining question is whether hydin performs a similar function in cilia/flagella of other organisms. Knock down of hydin causes paralyzed flagella in trypanosomes, indicating that it also has an essential role there (Broadhead et al., 2006). However, we know that the central apparatus rotates within the nine axonemal doublets of certain cell types, such as C. reinhardtii, but does not rotate in others, including trypanosomes (Omoto et al., 1999). This rotation may indicate functional specialization, and therefore, the role of central apparatus–associated proteins, such as hydin, might also vary between cell types.
The phenotype of hydin mutant mice is hydrocephalus and the eventual loss of cilia from many ventricular ependymal cells (Davy and Robinson, 2003). What is not known is whether the cilia in these mice have any residual motility and the relationship of ciliary defects to the timing and development of hydrocephalus. It is also not known whether mutations in hydin are a leading cause of hydrocephalus in humans. A few studies have provided evidence that hydrocephalus in humans can result from ciliary defects, and in one case a hydrocephalus-causing mutation has been mapped to the vicinity of the HYDIN gene (Callen et al., 1990; Ibanez-Tallon et al., 2004; Kosaki et al., 2004). Therefore, it would not be surprising to find that mutations in hydin in humans result in ciliary defects that lead to hydrocephalus. The current study of Lechtreck and Witman (2007) in C. reinhardtii provides ample data for generating testable hypotheses of hydin function in humans as well as other organisms.
References
- Badano, J.L., N. Mitsuma, P.L. Beales, and N. Katsanis. 2006. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7:125–148. [DOI] [PubMed] [Google Scholar]
- Broadhead, R., H.R. Dawe, H. Farr, S. Griffiths, S.R. Hart, N. Portman, M.K. Shaw, M.L. Ginger, S.J. Gaskell, P.G. McKean, and K. Gull. 2006. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 440:224–227. [DOI] [PubMed] [Google Scholar]
- Brokaw, C.J. 1972. Flagellar movement: a sliding filament model. Science. 178:455–462. [DOI] [PubMed] [Google Scholar]
- Callen, D.F., E.G. Baker, and S.A. Lane. 1990. Re-evaluation of GM2346 from a del(16)(q22) to t(4;16)(q35;q22.1). Clin. Genet. 38:466–468. [DOI] [PubMed] [Google Scholar]
- Davy, B.E., and M.L. Robinson. 2003. Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Hum. Mol. Genet. 12:1163–1170. [DOI] [PubMed] [Google Scholar]
- Ibanez-Tallon, I., A. Pagenstecher, M. Fliegauf, H. Olbrich, A. Kispert, U.P. Ketelsen, A. North, N. Heintz, and H. Omran. 2004. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 13:2133–2141. [DOI] [PubMed] [Google Scholar]
- Kosaki, K., K. Ikeda, K. Miyakoshi, M. Ueno, R. Kosaki, D. Takahashi, M. Tanaka, C. Torikata, Y. Yoshimura, and T. Takahashi. 2004. Absent inner dynein arms in a fetus with familial hydrocephalus-situs abnormality. Am. J. Med. Genet. A. 129:308–311. [DOI] [PubMed] [Google Scholar]
- Lechteck, K.-F., and G.B. Witman. 2007. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J. Cell Biol. 176:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, D.R. 2003. Orientation of the central pair complex during flagellar bend formation in Chlamydomonas. Cell Motil. Cytoskeleton. 56:120–129. [DOI] [PubMed] [Google Scholar]
- Omoto, C.K., I.R. Gibbons, R. Kamiya, C. Shingyoji, K. Takahashi, and G.B. Witman. 1999. Rotation of the central pair microtubules in eukaryotic flagella. Mol. Biol. Cell. 10:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour, G.J., N. Agrin, J. Leszyk, and G.B. Witman. 2005. Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 170:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir, P. 1985. Switching mechanisms in the control of ciliary motility. In Modern Cell Biology. B. Satir, editor. Alan R. Liss, Inc., New York. 1–46.
- Satir, P., and S.T. Christensen. 2006. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 69:14.1–14.24. [DOI] [PubMed] [Google Scholar]
- Smith, E.F., and P. Yang. 2004. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil. Cytoskeleton. 57:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers, K.E., and I.R. Gibbons. 1971. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc. Natl. Acad. Sci. USA. 68:3092–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo, M.J., and E.F. Smith. 2003. Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella. Proc. Natl. Acad. Sci. USA. 100:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]