Abstract
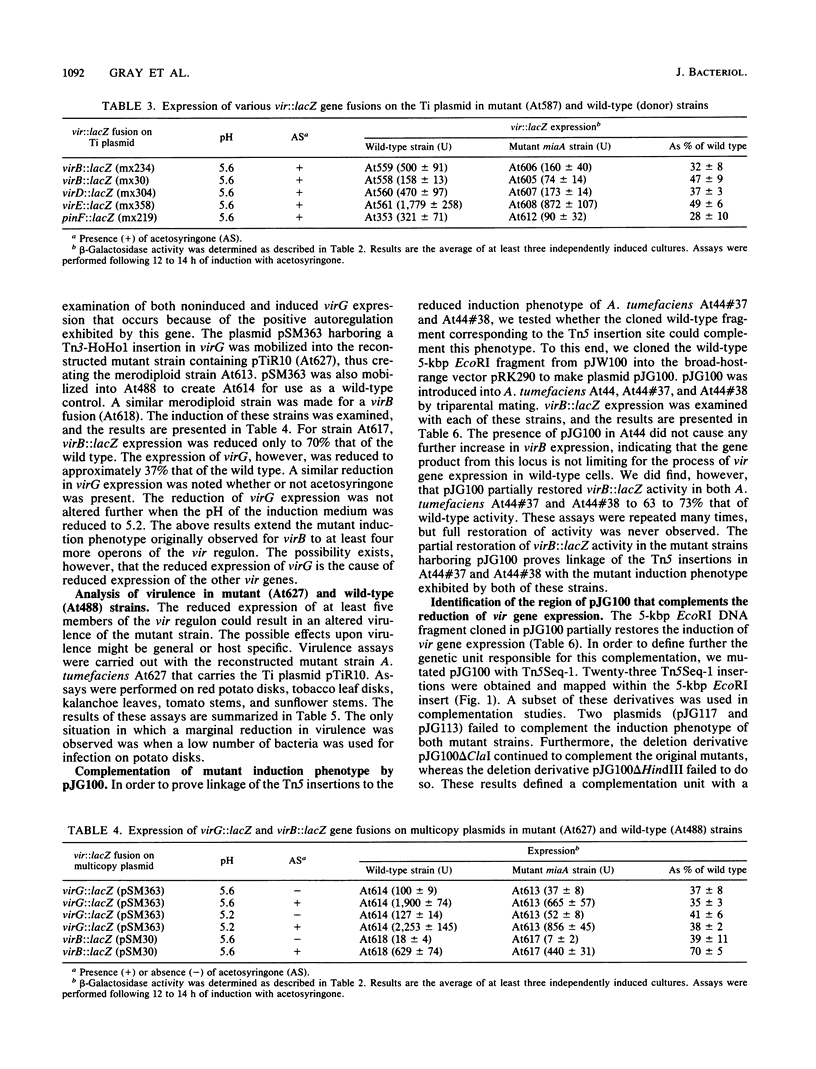
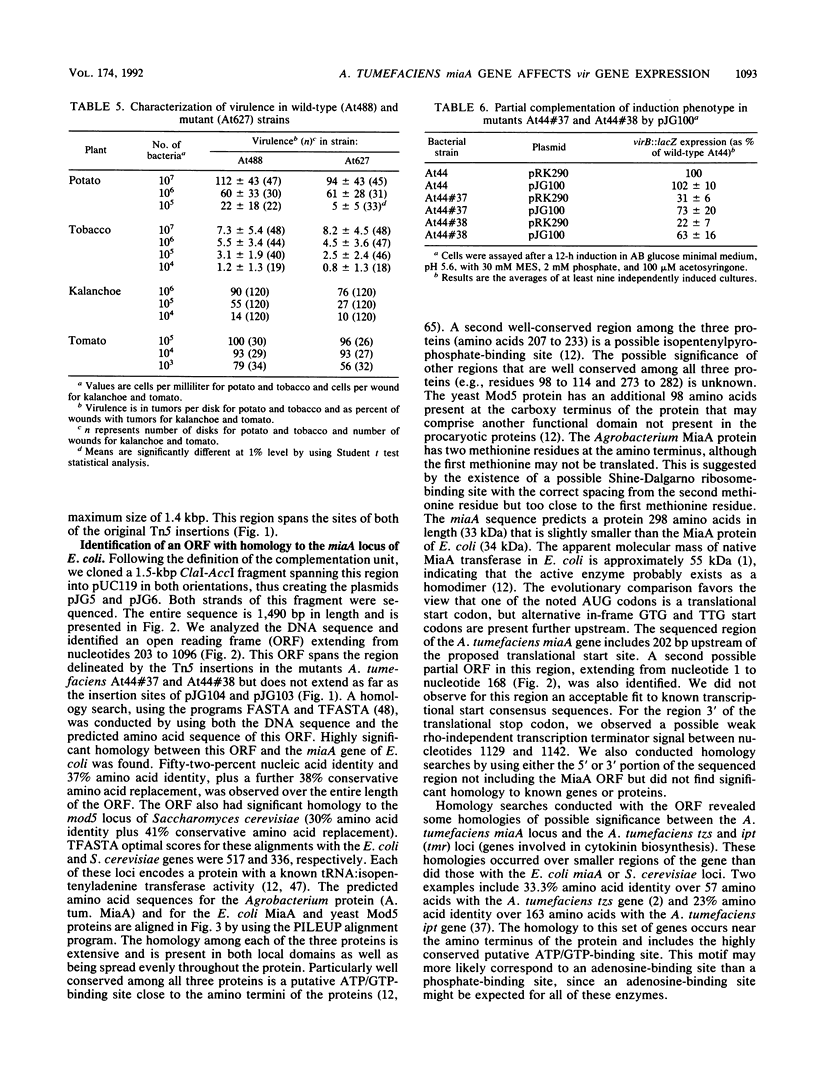
vir regulon expression in Agrobacterium tumefaciens involves both chromosome- and Ti-plasmid-encoded gene products. We have isolated and characterized a new chromosomal gene that when mutated results in a 2- to 10-fold reduction in the induced expression of vir genes by acetosyringone. This reduced expression occurs in AB minimal medium (pH 5.5) containing either sucrose or glucose and containing phosphate at high or low concentrations. The locus was cloned and used to complement A. tumefaciens strains harboring Tn5 insertions in the gene. Sequence analysis of this locus revealed an open reading frame with strong homology to the miaA locus of Escherichia coli and the mod5 locus of Saccharomyces cerevisiae. These genes encode tRNA: isopentenyltransferase enzymes responsible for the specific modification of the A-37 residue in UNN codon tRNA species. The function of the homologous gene in A. tumefaciens was proven by genetic complementation of E. coli miaA mutant strains. tRNA undermodification in A. tumefaciens miaA mutant strains may reduce vir gene expression by causing a reduced translation efficiency. A slight reduction in the virulence of these mutant Agrobacterium strains on red potato plants, but not on tobacco, tomato, kalanchoe, or sunflower plants, was observed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartz J. K., Kline L. K., Söll D. N6-(Delta 2-isopentenyl)adenosine: biosynthesis in vitro in transfer RNA by an enzyme purified from Escherichia coli. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1481–1487. doi: 10.1016/0006-291x(70)90035-5. [DOI] [PubMed] [Google Scholar]
- Blum P. H. Reduced leu operon expression in a miaA mutant of Salmonella typhimurium. J Bacteriol. 1988 Nov;170(11):5125–5133. doi: 10.1128/jb.170.11.5125-5133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Buchmann I., Marner F. J., Schröder G., Waffenschmidt S., Schröder J. Tumour genes in plants: T-DNA encoded cytokinin biosynthesis. EMBO J. 1985 Apr;4(4):853–859. doi: 10.1002/j.1460-2075.1985.tb03710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi G. A., Ankenbauer R. G., Nester E. W. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6708–6712. doi: 10.1073/pnas.87.17.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi G. A., Hung L., Puvanesarajah V., Stacey G., Ozga D. A., Leigh J. A., Nester E. W. Common loci for Agrobacterium tumefaciens and Rhizobium meliloti exopolysaccharide synthesis and their roles in plant interactions. J Bacteriol. 1987 May;169(5):2086–2091. doi: 10.1128/jb.169.5.2086-2091.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Winans S. C. Controlled expression of the transcriptional activator gene virG in Agrobacterium tumefaciens by using the Escherichia coli lac promoter. J Bacteriol. 1991 Feb;173(3):1139–1144. doi: 10.1128/jb.173.3.1139-1144.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherayil J. D., Lipsett M. N. Zeatin ribonucleosides in the transfer ribonucleic acid of Rhizobium leguminosarum, Agrobacterium tumefaciens, Corynebacterium fascians, and Erwinia amylovora. J Bacteriol. 1977 Sep;131(3):741–744. doi: 10.1128/jb.131.3.741-744.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D. M., Winkler M. E. Genetic and physiological relationships among the miaA gene, 2-methylthio-N6-(delta 2-isopentenyl)-adenosine tRNA modification, and spontaneous mutagenesis in Escherichia coli K-12. J Bacteriol. 1989 Jun;171(6):3233–3246. doi: 10.1128/jb.171.6.3233-3246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D. M., Winkler M. E. Structure of Escherichia coli K-12 miaA and characterization of the mutator phenotype caused by miaA insertion mutations. J Bacteriol. 1991 Mar;173(5):1711–1721. doi: 10.1128/jb.173.5.1711-1721.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley M. B., D'Souza M. R., Kado C. I. The virC and virD operons of the Agrobacterium Ti plasmid are regulated by the ros chromosomal gene: analysis of the cloned ros gene. J Bacteriol. 1991 Apr;173(8):2608–2616. doi: 10.1128/jb.173.8.2608-2616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaux Y., Guyon P., Farrand S. K., Petit A., Tempé J. Agrobacterium Ti and Ri plasmids specify enzymic lactonization of mannopine to agropine. J Gen Microbiol. 1986 Sep;132(9):2549–2559. doi: 10.1099/00221287-132-9-2549. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Halperin W., Nester E. W. Agrobacterium tumefaciens mutants affected in attachment to plant cells. J Bacteriol. 1982 Dec;152(3):1265–1275. doi: 10.1128/jb.152.3.1265-1275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S. P., Yarus M., Soll L. The effect of an Escherichia coli regulatory mutation on transfer RNA structure. J Mol Biol. 1979 Nov 25;135(1):111–126. doi: 10.1016/0022-2836(79)90343-7. [DOI] [PubMed] [Google Scholar]
- Engström P., Zambryski P., Van Montagu M., Stachel S. Characterization of Agrobacterium tumefaciens virulence proteins induced by the plant factor acetosyringone. J Mol Biol. 1987 Oct 20;197(4):635–645. doi: 10.1016/0022-2836(87)90470-0. [DOI] [PubMed] [Google Scholar]
- Ericson J. U., Björk G. R. tRNA anticodons with the modified nucleoside 2-methylthio-N6-(4-hydroxyisopentenyl)adenosine distinguish between bases 3' of the codon. J Mol Biol. 1991 Apr 5;218(3):509–516. doi: 10.1016/0022-2836(91)90697-5. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvin S. B., Habeck L. L. vir genes influence conjugal transfer of the Ti plasmid of Agrobacterium tumefaciens. J Bacteriol. 1990 Mar;172(3):1600–1608. doi: 10.1128/jb.172.3.1600-1608.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvin S. B., Karcher S. J., DiRita V. J. Methylation of the T-DNA in Agrobacterium tumefaciens and in several crown gall tumors. Nucleic Acids Res. 1983 Jan 11;11(1):159–174. doi: 10.1093/nar/11.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar J., Pittard J. Regulation of phenylalanine biosynthesis in Escherichia coli K-12: control of transcription of the pheA operon. J Bacteriol. 1982 Jun;150(3):1130–1137. doi: 10.1128/jb.150.3.1130-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heffron F., Bedinger P., Champoux J. J., Falkow S. Deletions affecting the transposition of an antibiotic resistance gene. Proc Natl Acad Sci U S A. 1977 Feb;74(2):702–706. doi: 10.1073/pnas.74.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hirsch P. R., Beringer J. E. A physical map of pPH1JI and pJB4JI. Plasmid. 1984 Sep;12(2):139–141. doi: 10.1016/0147-619x(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Huang M. L., Cangelosi G. A., Halperin W., Nester E. W. A chromosomal Agrobacterium tumefaciens gene required for effective plant signal transduction. J Bacteriol. 1990 Apr;172(4):1814–1822. doi: 10.1128/jb.172.4.1814-1822.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Morel P., Powell B., Kado C. I. VirA, a coregulator of Ti-specified virulence genes, is phosphorylated in vitro. J Bacteriol. 1990 Feb;172(2):1142–1144. doi: 10.1128/jb.172.2.1142-1144.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. F., Simon R., Pühler A. The development of plasmid-free strains of Agrobacterium tumefaciens by using incompatibility with a Rhizobium meliloti plasmid to eliminate pAtC58. Plasmid. 1985 Mar;13(2):99–105. doi: 10.1016/0147-619x(85)90062-9. [DOI] [PubMed] [Google Scholar]
- Jin S., Roitsch T., Ankenbauer R. G., Gordon M. P., Nester E. W. The VirA protein of Agrobacterium tumefaciens is autophosphorylated and is essential for vir gene regulation. J Bacteriol. 1990 Feb;172(2):525–530. doi: 10.1128/jb.172.2.525-530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiss-Chapman R. W., Morris R. O. Trans-zeatin in culture filtrates of Agrobacterium tumefaciens. Biochem Biophys Res Commun. 1976 May 23;76(2):453–459. doi: 10.1016/0006-291x(77)90746-x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein C., Klee H., Montoya A., Garfinkel D., Fuller S., Flores C., Nester E., Gordon M. Nucleotide sequence and transcript mapping of the tmr gene of the pTiA6NC octopine Ti-plasmid: a bacterial gene involved in plant tumorigenesis. J Mol Appl Genet. 1984;2(4):354–362. [PubMed] [Google Scholar]
- Matthysse A. G. Characterization of nonattaching mutants of Agrobacterium tumefaciens. J Bacteriol. 1987 Jan;169(1):313–323. doi: 10.1128/jb.169.1.313-323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers L. S., Regensburg-Tuïnk T. J., Bourret R. B., Sedee N. J., Schilperoort R. A., Hooykaas P. J. Membrane topology and functional analysis of the sensory protein VirA of Agrobacterium tumefaciens. EMBO J. 1989 Jul;8(7):1919–1925. doi: 10.1002/j.1460-2075.1989.tb03595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metts J., West J., Doares S. H., Matthysse A. G. Characterization of three Agrobacterium tumefaciens avirulent mutants with chromosomal mutations that affect induction of vir genes. J Bacteriol. 1991 Feb;173(3):1080–1087. doi: 10.1128/jb.173.3.1080-1087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A. L., Moore L. W., Gordon M. P., Nester E. W. Multiple genes coding for octopine-degrading enzymes in Agrobacterium. J Bacteriol. 1978 Dec;136(3):909–915. doi: 10.1128/jb.136.3.909-915.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. O., Regier D. A., Olson R. M., Jr, Struxness L. A., Armstrong D. J. Distribution of cytokinin-active nucleosides in isoaccepting transfer ribonucleic acids from Agrobacterium tumefaciens. Biochemistry. 1981 Oct 13;20(21):6012–6017. doi: 10.1021/bi00524a014. [DOI] [PubMed] [Google Scholar]
- Nag D. K., Huang H. V., Berg D. E. Bidirectional chain-termination nucleotide sequencing: transposon Tn5seq1 as a mobile source of primer sites. Gene. 1988 Apr 15;64(1):135–145. doi: 10.1016/0378-1119(88)90487-8. [DOI] [PubMed] [Google Scholar]
- Najarian D., Dihanich M. E., Martin N. C., Hopper A. K. DNA sequence and transcript mapping of MOD5: features of the 5' region which suggest two translational starts. Mol Cell Biol. 1987 Jan;7(1):185–191. doi: 10.1128/mcb.7.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Petrullo L. A., Elseviers D. Effect of a 2-methylthio-N6-isopentenyladenosine deficiency on peptidyl-tRNA release in Escherichia coli. J Bacteriol. 1986 Feb;165(2):608–611. doi: 10.1128/jb.165.2.608-611.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell G. K., Hommes N. G., Kuo J., Castle L. A., Morris R. O. Inducible expression of cytokinin biosynthesis in Agrobacterium tumefaciens by plant phenolics. Mol Plant Microbe Interact. 1988 Jul-Aug;1(6):235–242. doi: 10.1094/mpmi-1-235. [DOI] [PubMed] [Google Scholar]
- Regier D. A., Morris R. O. Secretion of trans-zeatin by Agrobacterium tumefaciens: a function determined by the nopaline Ti plasmid. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1560–1566. doi: 10.1016/0006-291x(82)91429-2. [DOI] [PubMed] [Google Scholar]
- Rogowsky P. M., Powell B. S., Shirasu K., Lin T. S., Morel P., Zyprian E. M., Steck T. R., Kado C. I. Molecular characterization of the vir regulon of Agrobacterium tumefaciens: complete nucleotide sequence and gene organization of the 28.63-kbp regulon cloned as a single unit. Plasmid. 1990 Mar;23(2):85–106. doi: 10.1016/0147-619x(90)90028-b. [DOI] [PubMed] [Google Scholar]
- Rong L. J., Karcher S. J., Gelvin S. B. Genetic and molecular analyses of picA, a plant-inducible locus on the Agrobacterium tumefaciens chromosome. J Bacteriol. 1991 Aug;173(16):5110–5120. doi: 10.1128/jb.173.16.5110-5120.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong L., Karcher S. J., O'Neal K., Hawes M. C., Yerkes C. D., Jayaswal R. K., Hallberg C. A., Gelvin S. B. picA, a novel plant-inducible locus on the Agrobacterium tumefaciens chromosome. J Bacteriol. 1990 Oct;172(10):5828–5836. doi: 10.1128/jb.172.10.5828-5836.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. doi: 10.1128/jb.156.3.1292-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda N., Toyoda-Yamamoto A., Nagamine J., Usami S., Katayama M., Sakagami Y., Machida Y. Control of expression of Agrobacterium vir genes by synergistic actions of phenolic signal molecules and monosaccharides. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6684–6688. doi: 10.1073/pnas.87.17.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986 Jul;5(7):1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell. 1986 Aug 1;46(3):325–333. doi: 10.1016/0092-8674(86)90653-7. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Karlinsey J. E., Marks J. R., Hurlbert R. E. Identification of a new virulence locus in Agrobacterium tumefaciens that affects polysaccharide composition and plant cell attachment. J Bacteriol. 1987 Jul;169(7):3209–3216. doi: 10.1128/jb.169.7.3209-3216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Jayaswal R. K., Gelvin S. B. Virulence genes A, G, and D mediate the double-stranded border cleavage of T-DNA from the Agrobacterium Ti plasmid. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1881–1885. doi: 10.1073/pnas.84.7.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Krishnan M., Gould J. H., Smith R. H., Gelvin S. B. Opines stimulate induction of the vir genes of the Agrobacterium tumefaciens Ti plasmid. J Bacteriol. 1989 Jul;171(7):3696–3703. doi: 10.1128/jb.171.7.3696-3703.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Kerstetter R. A., Nester E. W. Transcriptional regulation of the virA and virG genes of Agrobacterium tumefaciens. J Bacteriol. 1988 Sep;170(9):4047–4054. doi: 10.1128/jb.170.9.4047-4054.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C. Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media. J Bacteriol. 1990 May;172(5):2433–2438. doi: 10.1128/jb.172.5.2433-2438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Mutations affecting tRNATrp and its charging and their effect on regulation of transcription termination at the attenuator of the tryptophan operon. J Mol Biol. 1977 Jul 15;113(4):663–677. doi: 10.1016/0022-2836(77)90229-7. [DOI] [PubMed] [Google Scholar]
- Zambryski P. Basic processes underlying Agrobacterium-mediated DNA transfer to plant cells. Annu Rev Genet. 1988;22:1–30. doi: 10.1146/annurev.ge.22.120188.000245. [DOI] [PubMed] [Google Scholar]