Abstract
Endoplasmic reticulum (ER) homeostasis requires transfer and subsequent processing of the glycan Glc3Man9GlcNAc2 (G3M9Gn2) from the lipid-linked oligosaccharide (LLO) glucose3mannose9N-acetylglucosamine2-P-P-dolichol (G3M9Gn2-P-P-Dol) to asparaginyl residues of nascent glycoprotein precursor polypeptides. However, it is unclear how the ER is protected against dysfunction from abnormal accumulation of LLO intermediates and aberrant N-glycosylation, as occurs in certain metabolic diseases. In metazoans phosphorylation of eukaryotic initiation factor 2α (eIF2α) on Ser51 by PERK (PKR-like ER kinase), which is activated by ER stress, attenuates translation initiation. We use brief glucose deprivation to simulate LLO biosynthesis disorders, and show that attenuation of polypeptide synthesis by PERK promotes extension of LLO intermediates to G3M9Gn2-P-P-Dol under these substrate-limiting conditions, as well as counteract abnormal N-glycosylation. This simple mechanism requires eIF2α Ser51 phosphorylation by PERK, and is mimicked by agents that stimulate cytoplasmic stress-responsive Ser51 kinase activity. Thus, by sensing ER stress from defective glycosylation, PERK can restore ER homeostasis by balancing polypeptide synthesis with flux through the LLO pathway.
Introduction
As nascent polypeptides enter the ER lumenal space, N-linked glycans modify asparaginyl residues in the context Asn-Xaa-Ser/Thr (Kornfeld and Kornfeld, 1985). This is catalyzed by oligosaccharyltransferase (OT), which transfers a preformed oligosaccharide unit, glucose3mannose9GlcNAc2 (G3M9Gn2), from the lipid-linked oligosaccharide (LLO) glucose3mannose9 N-acetylglucosamine2-P-P-dolichol (G3M9Gn2-P-P-Dol). Synthesis of G3M9Gn2-P-P-Dol starts with dolichol-P (Dol-P), and sequentially requires 2 residues of GlcNAc from UDP-GlcNAc (the first transfer also forming the pyrophosphate linkage), 5 residues of mannose from GDP-mannose, 4 residues of mannose from mannose-P-Dol (synthesized from Dol-P and GDP-mannose), and 3 residues of glucose from glucose-P-Dol (synthesized from Dol-P and UDP-glucose). During biosynthesis, Dol-P and G3M9Gn2-P-P-Dol are oriented at the cytoplasmic and lumenal faces, respectively, of the ER membrane, with flipping of the key intermediate M5Gn2-P-P-Dol (Snider and Rogers, 1984; Helenius et al., 2002). Upon transfer of G3M9Gn2 by OT, Dol-P-P is released, and is recycled to Dol-P for additional rounds of glycosylation (Schenk et al., 2001).
N-linked G3M9Gn2 is sequentially digested by ER glucosidases and mannosidases to generate high-mannose processing intermediates with functions in protein folding, quality control, and degradation (Sayeed and Ng, 2005; van Anken and Braakman, 2005). Inhibition of LLO synthesis by tunicamycin, accumulation of LLO intermediates such as M2-6Gn2-P-P-Dol caused by glucose deprivation, mutations affecting mannosyl precursor synthesis, and interference with ER-processing glycosidases all disturb ER homeostasis (Lehrman, 2006). To minimize damage, mitigate the source of stress, and restore ER homeostasis to normal, the resulting ER stress activates a set of coordinated signals known collectively as the unfolded protein response (UPR). UPR signaling uses resident ER membrane proteins with lumenal stress-sensing domains that control activation of their respective cytoplasmic effector domains (Schröder and Kaufman, 2005). Of particular significance here, the cytoplasmic domain of the stress-sensor PKR-like ER kinase (PERK; Harding et al., 1999), also termed PEK (Sood et al., 2000), is a kinase activated by transautophosphorylation that phosphorylates eukaryotic initiation factor (eIF)-2α. The resultant eIF2α-P interferes with translation initiation, which is sufficient to inhibit protein synthesis by 70–90% after robust ER stress. Importantly, translation attenuation by PERK reduces stress by diminishing the load of ER client protein (Harding et al., 2000b).
Several lines of evidence suggest that metabolic deficiencies affecting G3M9Gn2-P-P-Dol synthesis or N-linked glycosylation might be compensated for by ER stress responses, implying homeostatic adaptation (Lehrman, 2006). The goal of this study was to investigate the potential role of PERK in such adaptation. To do so, we took advantage of the fact that, for many cell types normally maintained in physiological (≥4 mM) glucose, brief incubations with 0.3–0.5 mM glucose hinder conversion of undermannosylated LLO intermediates to G3M9Gn2-P-P-Dol. This is distinguished from the glucose-starvation effect, which requires glucose-free medium and causes a rather discrete shift from G3M9Gn2-P-P-Dol to M5Gn2-P-P-Dol (Chapman and Calhoun, 1988). We focused on our prior finding that dermal fibroblasts incubated 20 min in medium with 0.5 mM glucose accumulated M2-6Gn2-P-P-Dol. Although the improperly glycosylated proteins that resulted were expected to compromise ER function, the treatment by itself was too brief to activate an ER stress response. Significantly, ER stress induced by dithiothreitol (DTT), thapsigargin (TG), castanospermine, azetidine-2-carboxylic acid, or geldanamycin all restored G3M9Gn2-P-P-Dol levels in the fibroblasts to normal (Doerrler and Lehrman, 1999; Shang et al., 2002). The underlying mechanism was not determined, although regulated glycogenolysis was later proposed (Gill et al., 2002). The brief treatments used argued against considerable contributions of UPR transcriptional programs (Schröder and Kaufman, 2005).
In this study, we identify a surprisingly simple protective mechanism by which the ER stress response modulates G3M9Gn2-P-P-Dol synthesis and N-linked glycosylation. It is known that ER stress from aberrant G3M9Gn2-P-P-Dol production activates PERK. PERK is shown to reduce LLO consumption by attenuating synthesis of glycoprotein precursor polypeptides. This facilitates extension of undermannosylated intermediates to G3M9Gn2-P-P-Dol, restoring correct N-linked glycosylation. In this way, PERK balances glycoprotein synthesis with LLO flux.
Results
Evaluation of G3M9Gn2-P-P-Dol synthesis
Most mammalian cells in conventional media containing physiological glucose concentrations synthesize G3M9Gn2-P-P-Dol efficiently, a state we operationally term unrestricted LLO synthesis. However, as we already mentioned, brief incubations with low glucose concentrations can hinder extension of undermannosylated intermediates. We categorize conditions such as these, which impede extension of LLO intermediates, as restricted LLO synthesis (Fig. 1, A). Consequently, the flux of LLO intermediates through the LLO pathway is reduced. We distinguish LLO flux from two other factors, LLO capacity (total LLO that can be synthesized, dependent on availability of Dol-P) and LLO consumption (dependent on transfer of glycan to polypeptide). Deficits in neither capacity nor consumption would be expected to cause LLO intermediates to accumulate. As presented below, restricted conditions were used to determine whether ER stress-activated PERK could compensate for reduced LLO flux. Unrestricted conditions were used to determine whether any such compensation was related to attenuated consumption of LLO, altered LLO capacity, or enhancement of LLO flux. LLOs in cultured cells are conveniently labeled with [2-3H]mannose. Established solvent extraction procedures can then recover the entire [3H] LLO pool (i.e., LLO intermediates plus G3M9Gn2-P-P-Dol), or HPLC can be used to examine glycans of individual [3H] LLOs. Efficient incorporation of [3H]mannose requires low-glucose medium, resulting in restricted or unrestricted LLO synthesis, depending on the cell type and the glucose concentration.
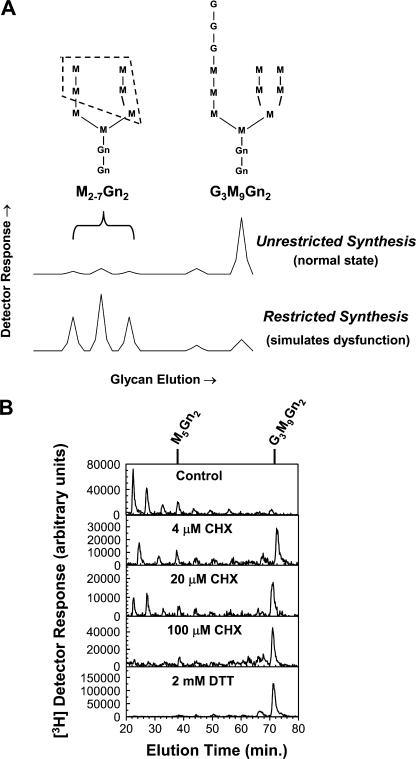
Figure 1.
Restricted and unrestricted LLO synthesis, and the effect of CHX. (A) Hypothetical high-pressure liquid chromatograms of LLO glycans illustrate efficient (unrestricted) synthesis of G3M9Gn2-P-P-Dol versus accumulation of M2-7Gn2-P-P-Dol intermediates under restricted conditions that simulate metabolic dysfunction. The dashed lines indicate the mannosyl residues that vary in the respective intermediates (Helenius and Aebi, 2004). (B) Dermal fibroblasts were treated with the indicated concentrations of CHX for 50 min. Medium with 0.5 mM glucose was used. For the final 20 min, 40 μCi/ml [3H]mannose was added. [3H] LLO glycans were isolated and characterized by HPLC. The positions of standards are shown. Note that addition of CHX converts the pattern of LLO glycans from restricted to unrestricted. For comparison, an experiment is shown with 2 mM DTT added for 30 min instead of CHX.
Treatment with cycloheximide (CHX) suggests that translation attenuation can modulate LLO synthesis
As discussed in the Introduction, LLO synthesis in dermal fibroblasts is restricted with 0.5 mM glucose, and ER stress promotes extension of the accumulated LLO intermediates to G3M9Gn2-P-P-Dol. To simulate the contribution of translation attenuation by PERK during the ER stress response we used CHX. We found that mild treatment (4 μM; inhibiting protein synthesis by 25–30%; unpublished data) reduced accumulation of [3H] LLO intermediates and greatly shifted the LLO profile in favor of [3H]G3M9Gn2-P-P-Dol (Fig. 1 B). Treatment with 100 μM CHX (inhibiting translation by ∼50%) caused even greater enhancement, comparable to the effect of 2 mM DTT. Because CHX slows consumption of LLOs for protein N-glycosylation (Gao and Lehrman, 2002b), we surmise that 4–100 μM CHX may have allowed more time for extension of LLO intermediates, thereby compensating for reduced flux. This also implicated translation attenuation by PERK as a key factor in the ER stress response–mediating extension of undermannosylated intermediates.
Specific activation of PERK's kinase activity drives extension of LLO intermediates
To assess PERK's potential contribution to LLO synthesis, the transfected CHO-K1 line “Fv2E-PERK” was used. This line expresses a cytoplasmic fusion protein with the PERK kinase domain joined to dual FKBP12-derived domains that bind AP20187, a cell-permeant bifunctional “dimerizer.” Such fusion proteins are normally monomers, and are inactive. Addition of dimerizer oligomerizes the Fv2E-PERK fusion proteins, resulting in transautophosphorylation and activation of the kinase domains. The kinase domains then phosphorylate Ser51 of eIF2α, inhibiting translation (Lu et al., 2004).
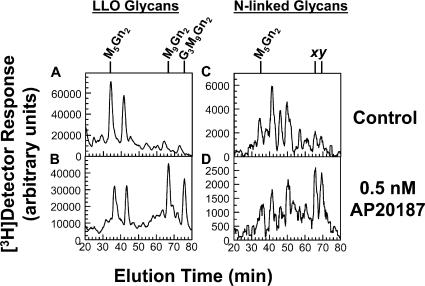
AP20187 caused graded, regulated inhibition of protein synthesis in Fv2E-PERK transfectants, but not untransfected CHO-K1 cells (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200607007/DC1). We used 0.5 nM AP20187 (inhibiting protein synthesis by ∼35%) to replicate translation attenuation expected to occur with moderate ER stress. Fv2E-PERK cells underwent restricted LLO synthesis when incubated in 0.3 mM glucose medium for 20 min (Fig. 2 A), with AP20187 (Fig. 2 B) causing a robust effect on extension of [3H] LLO intermediates to [3H]G3M9Gn2-P-P-Dol. Moreover, N-linked glycans derived from [3H]G3M9Gn2-P-P-Dol (labeled x and y in Fig. 2 [C and D]) were sparse under restricted conditions (C), but were increased greatly by AP20187 (D). Thus, moderate translation attenuation by PERK was sufficient to drive extension of LLO intermediates to G3M9Gn2-P-P-Dol, and reestablish correct N-linked glycosylation.
Figure 2.
PERK's kinase activity is sufficient to rectify LLO biosynthetic defects and aberrant N-linked glycosylation. Fv2E-PERK cells were cultured in the absence (A and C) or presence (B and D) of 0.5 nM AP20187 for 60 min. Incubation with or without AP20187 was then continued for an additional 20 min in medium containing 0.3 mM glucose (causing restricted LLO synthesis), 10% dialyzed FBS, and 40 μCi/ml [3H]mannose. [3H] LLO glycans (A and B) and [3H]N-linked glycans (C and D) were detected by HPLC. The positions of standards are indicated. x and y indicate N-linked glycans assigned the structures M9Gn2 and G1M9Gn2, respectively, and thus derived from G3M9Gn2-P-P-Dol rather than undermannosylated LLO intermediates (Shang and Lehrman, 2004c). Because of low [3H] labeling in N-glycan experiments, interference from spurious electronic noise was minimized by subjecting HPLC data to root-mean-square smoothing with PSI-Plot V.8 (Poly Software International).
ER stress inducers failed to extend LLO intermediates in the absence of phosphorylatable eIF2α
Compared with mouse embryonic fibroblasts (MEFs) expressing normal eIF2α (eIF2αS51/S51), MEFs with alanine substitutions at Ser51 (eIF2αA51/A51) have greatly reduced translation attenuation in response to ER stress because Ser51 is phosphorylated by PERK (Scheuner et al., 2001). Incubation of eIF2αS51/S51 or eIF2αA51/A51 MEFs with 0.3 mM glucose for 20 min resulted in restricted LLO synthesis (Fig. 3, A and E). This provided an opportunity to formally demonstrate the role of eIF2α phosphorylation, and therefore translation attenuation, in stimulation of LLO intermediate extension by ER stress. We considered analogous experiments with PERK−/− MEFs, but reasonable conditions causing restricted LLO synthesis were not identified (unpublished data). Rather, in these cells, glucose deprivation diminished G3M9Gn2-P-P-Dol without accumulation of intermediates, with reversal by CHX (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200607007/DC1).
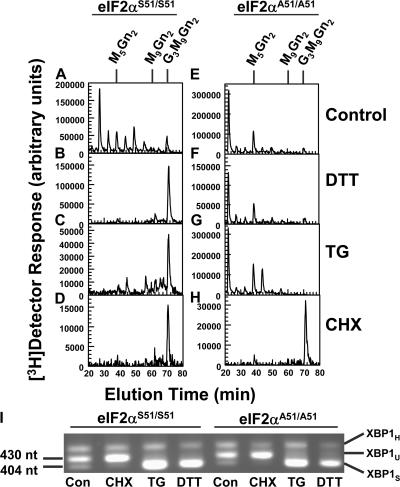
Figure 3.
Acute ER stress does not alter LLO synthesis in eIF2αA51/A51 MEFs. MEFs with two normal eIF2α alleles (eIF2αS51/S51; A–D) or two alleles with Ser51Ala replacements (eIF2αA51/A51; E–H) were cultured in the absence (A and E) or presence of 2 mM DTT for 5 min (B and F), 100 nM TG for 10 min (C and G), or 20 μM CHX for 10 min (D and H). Treatments were then continued for an additional 20 min in medium containing 10% dialyzed FCS, 0.3 mM glucose, and 40 μCi/ml [3H]mannose. [3H] LLO glycans were characterized by HPLC, as described for Fig. 2. The causes of the somewhat different patterns of undermannosylated intermediates in untreated eIF2αS51/S51 and eIF2αA51/A51 samples in this figure (A and E) and in Fig. 9 are unknown. mRNA samples corresponding to experiments in A–H were tested for splicing of XBP1 mRNA (I). Spliced (XBP1S), unspliced (XBP1U), and hybrid (XBP1H) PCR products are indicated, as well as the sizes (nucleotides) of the XBP1S and XBP1U fragments.
Consistent with prior results with dermal fibroblasts, treatments of eIF2αS51/S51 MEFs with DTT (25 min) or TG (30 min) promoted extension of [3H] LLO intermediates (Fig. 3, B and C). However, ER stress did not enhance extension in eIF2αA51/A51 MEFs (Fig. 3, F and G), although treatment with 20 μM CHX (Fig. 3, D and H) demonstrated that the LLO pathway in these cells could respond to translation attenuation. Splicing of XBP1 mRNA is mediated by IRE1, and is a quantitative measure of ER stress (Shang and Lehrman, 2004a). Assays for this reaction verified that TG and DTT caused robust ER stress with both MEF lines (Fig. 3 I).
Hence, within the first 30 min of the ER stress response, translation attenuation via eIF2α phosphorylation appears to be the only factor that significantly stimulates extension of LLO intermediates in MEFs.
PERK's kinase activity during the ER stress response inhibits G3M9Gn2-P-P-Dol consumption
Ostensibly, PERK could aid LLO synthesis if a labile protein inhibited the pathway, or if a stimulatory protein was made upon attenuation of translation initiation (Harding et al., 2000a). However, another explanation comes from work by Hubbard and Robbins (1980), which was extended by us (Gao and Lehrman, 2002b), showing that under unrestricted conditions of LLO synthesis, the translation inhibitors CHX and puromycin prevent synthesis of [3H]mannose-labeled G3M9Gn2-P-P-Dol. This is because G3M9Gn2-P-P-Dol consumption is inhibited in the absence of nascent glycoprotein precursor polypeptides, and the G3M9Gn2-P-P-Dol pool does not turn over. Consequently, Dol-P is not regenerated, so new LLO cannot be made with [3H]mannose. Thus, the results shown in Figs. 1–3 (under restricted conditions) may be explained by slowed LLO consumption, with more time for extension of undermannosylated intermediates to G3M9Gn2-P-P-Dol.
Two types of measurements were required to determine whether ER stress slows G3M9Gn2-P-P-Dol consumption. For inhibition of G3M9Gn2-P-P-Dol synthesis, metabolic labeling with [3H]mannose was used. In parallel, to detect unlabeled G3M9Gn2-P-P-Dol, we used fluorophore-assisted carbohydrate electrophoresis (FACE), by which glycans cleaved from LLOs are tagged at their reducing termini with the anionic fluorophore 7-amino-1,3-naphthalenedisulfonic acid (ANDS; Gao and Lehrman, 2002a). The negatively charged ANDS-glycan conjugates can be separated by electrophoresis with a high-percentage polyacrylamide gel, and detected with ultraviolet light. FACE allows glycans from individual LLO species to be measured quantitatively, and can be used regardless of the medium's glucose concentration.
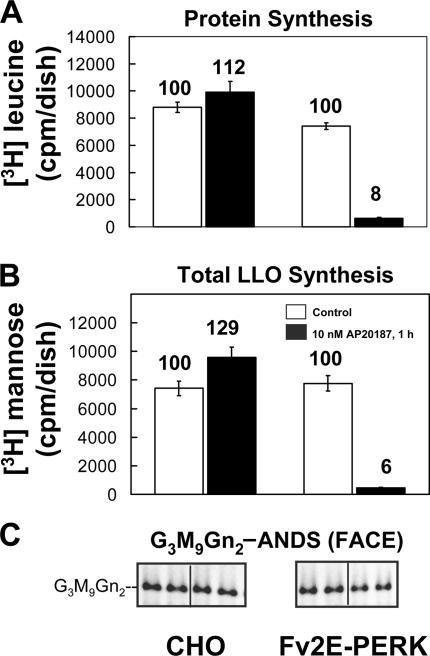
CHO-K1 cells (and CHO-K1–derived Fv2E-PERK cells; see below) underwent unrestricted G3M9Gn2-P-P-Dol synthesis in 0.5 mM glucose medium (Fig. S2 C and not depicted). Synthesis of total [3H] LLO in CHO-K1 cells with 0.5 mM glucose was inhibited by DTT and TG (Fig. 4), but no breakdown products were observed (Fig. S2 C). Importantly, no losses of G3M9Gn2-P-P-Dol were detected by FACE (Fig. 4; increases were apparent, and are explored in Fig. S2 B). PERK's activity was sufficient to block G3M9Gn2-P-P-Dol consumption because 10 nM AP20187 strongly inhibited both protein (Fig. 5, A) and total [3H] LLO (Fig. 5, B) synthesis in Fv2E-PERK transfectants, but not in CHO-K1 cells, whereas G3M9Gn2-P-P-Dol detected by FACE was unaffected in all cases (Fig. 5, C).
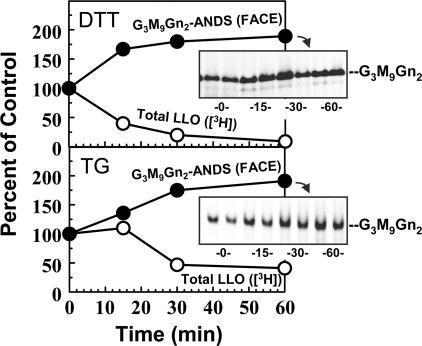
Figure 4.
ER st ress in CHO-K1 cells reduces G3M9Gn2-P-P-Dol consumption. CHO-K1 cells (∼90% confluent; duplicate 100-mm dishes for [3H]mannose labeling and duplicate 150-mm dishes for FACE) were incubated with normal medium (10 mM glucose) in the absence or presence of 2 mM DTT (top) or 150 nM TG (bottom) for the indicated times, and then for 20 min (maintaining absence or presence of DTT or TG) in medium with 0.5 mM glucose (unrestricted LLO synthesis). Open symbols: 10 μCi/ml [3H]mannose was included during the final 20 min, and total [3H] LLO was determined. As shown in Fig. S2 C, under comparable conditions the majority of radioactivity was incorporated into G3M9Gn2-P-P-Dol. Closed symbols: no [3H]mannose was included; instead, G3M9Gn2-ANDS was measured by FACE. Data points are averages of duplicates. (insets) FACE gels displaying G3M9Gn2-ANDS from duplicate dishes obtained at the times (minutes) indicated.
Figure 5.
PERK's kinase activity is sufficient to reduce G3M9Gn2-P-P-Dol consumption. (A–C) Control CHO-K1 (left) or Fv2E-PERK (right) cells were treated without (white bars) or with (black bars) 10 nM AP20187 for 1 h, followed by measurements of [3H]leucine incorporation into protein (means of quadruplicates ± the SEM; A), measurements of [3H]mannose incorporation into total LLO (averages of quadruplicates ± the SEM; B), or detection of G3M9Gn2-ANDS by FACE (duplicates; C) in medium with 0.5 mM glucose (unrestricted LLO synthesis under this condition). For A and B, values above the bars are the percentage of incorporation relative to that in the absence of AP20187.
These results show that PERK's kinase activity is sufficient to inhibit G3M9Gn2-P-P-Dol consumption under unrestricted conditions, just as it is sufficient to drive extension of undermannosylated intermediates under restricted conditions (Fig. 2).
PERK is necessary for inhibition of G3M9Gn2-P-P-Dol consumption by ER stress
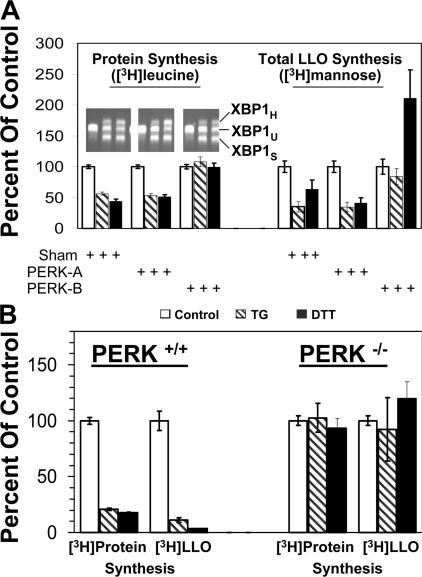
The necessity of PERK was addressed because IRE1 has also been reported to attenuate translation in response to ER stress (Iwawaki et al., 2001). HeLa S3 cells were transfected with siRNA duplexes directed against distinct regions of PERK mRNA. The PERK-A duplex was ineffective for RNA interference, and was therefore used along with sham transfection as a negative control. The PERK-B duplex efficiently knocked down PERK mRNA (losses of 58 ± 3% with 5 h of transfection and 77 ± 1% with 16 h of transfection). Because some effects on cell viability were noticed with the 16-h transfection, subsequent experiments were done with 5-h transfections. DTT and TG each inhibited synthesis of protein and total [3H] LLO under unrestricted conditions by about half in sham and PERK-A–treated cells (Fig. 6, A), whereas the PERK-B duplex fully prevented DTT- and TG-induced translation arrest and [3H] LLO synthesis inhibition. For reasons that are unclear, DTT treatment tended to elevate total [3H] LLO labeling in PERK-B–transfected cells above that in nonstressed cells, but the key point is that LLO synthesis was not inhibited. Splicing of XBP1 mRNA (inset) verified that the PERK-B duplex did not prevent DTT or TG from inducing ER stress.
Figure 6.
Hindering PERK expression by RNA interference or gene disruption prevents inhibition of both protein synthesis and LLO consumption by ER stress inducers. Metabolic labeling of HeLa S3 and MEF lines was done with 0.5 mM glucose (unrestricted LLO synthesis conditions; HPLC data not depicted). (A) HeLa S3 cells were subjected to sham transfection, or transfection with siRNA duplexes PERK-A or -B for 5 h. Cells were left untreated (control; open bars) or treated with either 100 nM TG for 30 min (striped bars) or 2 mM DTT for 20 min (shaded bars). Synthesis of protein (with [3H]leucine; left) and LLO (with [3H]mannose; right) were measured as percentages of the untreated controls. Bars represent means (± the SEM) for 7–8 replicates (protein synthesis) or 8–12 replicates (LLO synthesis) done over 4 independent sets of transfections. (inset) RT-PCR analyses of XBP1 mRNA (representative of 4 independent experiments) from a single gel, cropped for alignment with appropriate bars. Spliced (XBP1S), unspliced (XBP1U), and hybrid (XBP1H) PCR products are indicated, with sizes indicated in Fig. 3. (B) MEFs with normal alleles (PERK+/+; left) or MEFs harboring disrupted PERK alleles (PERK−/−; right) were treated with TG or DTT, followed by protein and LLO synthesis measurements, as for A. Bars represent averages (± the SEM) of 3–5 or 6–9 replicates for TG and DTT, respectively, encompassing at least 2 independent experiments.
Because eIF2α−Ser51 can be phosphorylated by the kinase PKR in response to double-stranded RNA (Scheuner et al., 2001), the potential concern over the use of RNA interference was addressed with MEFs bearing two normal PERK alleles (PERK+/+) or two disrupted alleles (PERK−/−). The results (Fig. 6 B) confirmed those obtained by RNA interference. Synthesis of protein and total [3H] LLO under unrestricted conditions were both strongly inhibited by ER stress inducers in the presence, but not the absence, of PERK. XBP1 mRNA splicing assays (unpublished data) verified that DTT and TG induced similarly robust ER stress in both MEF types.
Pulse-chase analysis of LLO flux
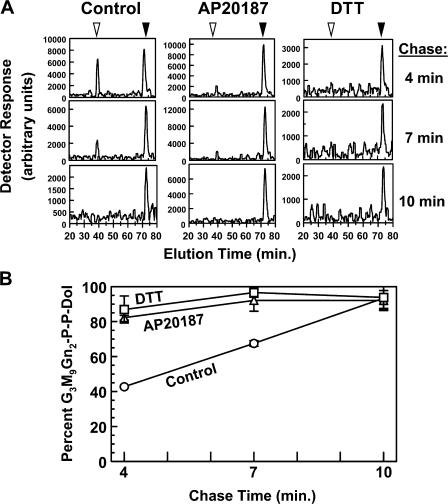
PERK's effects on LLO flux might be explained entirely by a compensatory reduction of LLO consumption because PERK activity was replicated by attenuating translation with either CHX (Fig. 1) or activators of cytoplasmic eIF2α kinases (see the following section). As a more direct test of PERK's effect on LLO flux, we performed pulse-chase experiments with Fv2E-PERK cells. After incubation in the absence or presence of AP20187 or DTT, the cells were labeled for 2 min with [3H]mannose in medium containing 1 mM glucose (which allowed G3M9Gn2-P-P-Dol to be made efficiently, yet permitted sufficient uptake of [3H]mannose), and then chased in medium lacking [3H]mannose for up to 10 min. Although several [3H] species were detected, the only ones confirmed as LLOs (by sensitivity to tunicamycin and comparison with standards) were [3H]M5Gn2-P-P-Dol and [3H]G3M9Gn2-P-P-Dol. Neither LLO was detected during the 2-min pulse (unpublished data). In untreated cells, both [3H] LLOs were detected after 4 min of chase, and [3H]G3M9Gn2-P-P-Dol was the only [3H] LLO significantly detected after 10 min of chase (Fig. 7, A and B). By comparison, after activation of PERK with AP20187 or introduction of ER stress with DTT, [3H]G3M9Gn2-P-P-Dol was the predominant LLO detected after 4 min of chase.
Figure 7.
Pulse-chase analysis of PERK's effect on LLO flux. Fv2E-PERK cells were pulse- labeled with [3H]mannose for 2 min and chased for up to 10 min, as described in Materials and methods. Cells lacked additional treatments (control) or were treated with either 1 nM AP20187 for 1 h or 2 mM DTT for 20 min before pulse labeling, as well as during the pulse (but not during the chase). The 4-min chase was the earliest point at which [3H] LLOs were reliably detected. (A) LLO glycans from cells chased for the indicated times were analyzed by HPLC. The positions of M5Gn2 and G3M9Gn2 are shown by the open and closed arrowheads, respectively. (B) Results from A and a second identically performed experiment were combined (all points are means ± the SEM). HPLC peak heights for M5Gn2 and G3M9Gn2 were normalized to mannose content, and G3M9Gn2 percentages were calculated. Circles, control; triangles, AP20187; squares, DTT.
These results suggest that PERK may have a direct effect on LLO flux, in addition to the aforementioned compensatory effect on consumption. In contrast, there is no evidence that ER stress affects LLO capacity because in CHO-K1 cells in 10 mM glucose undergoing unrestricted G3M9Gn2-P-P-Dol synthesis (FACE analysis; Fig. S2 B), G3M9Gn2-P-P-Dol quantity was not altered by ER stress (we did notice, however, that 0.5 mM glucose incubation caused an unexpected reduction of G3M9Gn2-P-P-Dol that was reversed by ER stress, perhaps because of reduced consumption). Specific activation of PERK's kinase activity in Fv2E-PERK cells with AP20187 also failed to alter G3M9Gn2-P-P-Dol content (Fig. 5).
Phosphorylation of eIF2α–Ser51 explains how cytoplasmic stress inducers counteract abnormalities of LLO biosynthesis and protein glycosylation
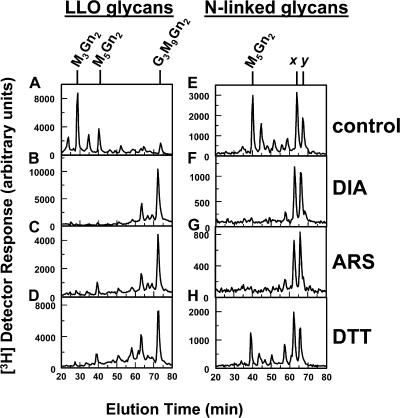
Eukaryotes contain multiple cytoplasmic eIF2α Ser51 kinases distinct from PERK (Scheuner et al., 2001), suggesting an alternative way to modulate LLO biosynthesis. Arsenite (ARS) and diamide (DIA) induce transcription of the cytoplasmic stress marker HSP70 mRNA, but not the ER stress marker GRP78 mRNA (Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200607007/DC1), which is a result opposite to that obtained with ER stress inducers (Shang et al., 2002). By incubating dermal fibroblasts as described for Fig. 1 B, ARS and DIA greatly enhanced the extension of [3H] LLO intermediates to G3M9Gn2-P-P-Dol (Fig. 8, A–C), and diminished N-linked glycoproteins with undermannosylated glycans (Fig. 8, E–G). Their effects rivaled those of DTT (Fig. 8, D and H).
Figure 8.
Treatments with ARS and DIA promote synthesis of G3M9Gn2-P-P-Dol and glycosylation of proteins with G3M9Gn2. Dermal fibroblasts were untreated (controls; A and E), or treated with 0.2 mM DIA for 20 min (B and F), 40 μM ARS for 1 h (C and G), or 2 mM DTT for 20 min (D and H). Cells were incubated with medium containing 10% dialyzed FBS and 40 μCi/ml [3H]mannose for 20 min either during (DIA and DTT) or after (ARS) stress treatments. LLO glycans (A–D) and N-linked glycans (E–H) were analyzed by HPLC. The positions of standards are indicated as in Fig. 2.
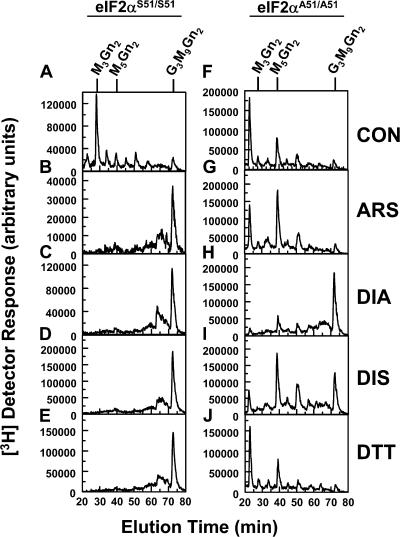
Because the effects of ARS and DIA on LLO synthesis correlated with their abilities to inhibit protein synthesis (Figs. S3 and S4, available at http://www.jcb.org/cgi/content/full/jcb.200607007/DC1), but not with changes in mannose uptake or hexose-phosphate metabolism (not depicted), the requirement for eIF2α-Ser51 phosphorylation was tested with eIF2αS51/S51 and eIF2αA51/A51 MEFs (as described for Fig. 3). Disulfiram (DIS), which is another cytoplasmic stress inducer (Table S1), was included. All three agents inhibited protein synthesis in eIF2αS51/S51 MEFs (Table I) by at least half, and robustly promoted extension of [3H] LLO intermediates (Fig. 9, A–D). However, their responses were quite disparate in eIF2αA51/A51 MEFs. ARS failed to appreciably affect protein (Table I) or [3H] LLO (Fig. 9 G) synthesis in eIF2αA51/A51 MEFs, showing that ARS acted mainly through an eIF2α-Ser51 kinase, with a specificity comparable to that of DTT (Fig. 9, E and J). DIA and DIS both inhibited protein synthesis (Table I) and promoted LLO extension (Fig. 9, H and I) in eIF2αA51/A51 MEFs, but not as well as with eIF2αS51/S51 MEFs, indicating that they acted partly through eIF2α-Ser51 phosphorylation, and partly through a second means of translation attenuation.
Table I.
Effects of replacing eIF2αS51 with eIF2αA51 on protein synthesis
| Genotype | Protein Synthesis | ||||
|---|---|---|---|---|---|
| ARSa
(40 μM, 1 h) |
DIAb
(0.2 mM, 20 min) |
DISc
(10 μM, 2 h) |
|||
| % | |||||
| eIF2αS51/S51 | 46.4 ± 2.7 | 18.6 ± 0.7 | 35.0 ± 0.4 | ||
| eIF2αA51/A51 | 93.2 ± 1.6 | 66.3 ± 3.9 | 83.0 ± 0.9 | ||
eIF2αS51/S51 and eIF2αA51/A51 MEFs were treated with stress inducers. Except for the final 5 min, conventional DMEM plus 10% FBS was used. For the final 5 min, DMEM with 0.3 mM glucose, 10% dialyzed FBS, and 5 μCi/ml [3H]leucine was used. Results are means of triplicates ± the SEM of the percentage of untreated controls. The use of 0.3 mM glucose was not necessary for protein synthesis measurements, but it simplified comparisons with [3H]mannose-labeling experiments.
Figure 9.
Importance of eIF2α-Ser51 for the actions of cytoplasmic stress inducers on LLO synthesis. eIF2αS51/S51 (A–E) or eIF2αA51/A51 (F–J) MEFs (Fig. 3) were left untreated (CON; A and F) or treated with 40 μM ARS for 1 h (B and G), 0.2 mM DIA for 5 min (C and H), 10 μM DIS for 2 h (D and I), or 2 mM DTT for 5 min (E and J). Incubations were continued for 20 min in medium containing the respective agents and 0.3 mM glucose, 10% dialyzed FBS, and 40 μCi/ml [3H]mannose. [3H] LLO glycans were analyzed by HPLC as in Fig. 3.
Collectively, translation attenuation by eIF2α-Ser51 kinase activity explains the effects of cytoplasmic stress inducers on LLO synthesis, and represents a merge point with the mechanisms of ER stress inducers.
Discussion
How is the synthesis of G3M9Gn2-P-P-Dol controlled? Evidence exists for regulation of specific reactions in the LLO pathway (Kean et al., 1999; Banerjee et al., 2005) and for developmental induction of key enzymes (Lehrman, 1991; Crick and Waechter, 1994; Crick et al., 1994). However, mechanisms that might acutely regulate the pathway based on the availability of G3M9Gn2-P-P-Dol have been elusive. Because G3M9Gn2-P-P-Dol is ultimately needed for N-linked glycosylation, and hence ER homeostasis, processes that sense ER stress are candidate regulatory inputs for adjustment of G3M9Gn2-P-P-Dol synthesis.
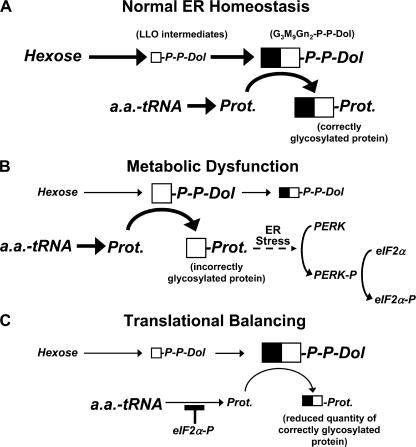
This study shows that decreased synthesis of polypeptide acceptors by activation of PERK reduces LLO consumption and, consequently, enhances extension of LLO intermediates, replenishing G3M9Gn2-P-P-Dol (Fig. 10). Because PERK is activated by extended periods of ER stress resulting from hindered G3M9Gn2-P-P-Dol synthesis, and hence, aberrant N-glycosylation (Harding et al., 2000b), PERK can balance glycoprotein precursor polypeptide synthesis with LLO pathway flux. Thus, in addition to reducing ER stress by lessening the load of client protein, this mechanism allows PERK to ensure proper N-glycosylation of the polypeptides that continue to be made. The importance of this synergy is emphasized by a recent study showing that maintenance of favorable diffusional properties in the ER lumen is much more dependent on efficient functioning of the lectin-chaperone system (which requires proper N-glycosylation) than the total load of polypeptide (Snapp et al., 2006). In addition to decreasing LLO consumption, pulse-chase experiments suggested that PERK stimulates LLO flux itself. The mechanism responsible for this is unclear at this time.
Figure 10.
Translational balancing by PERK restores correct protein glycosylation. Symbol and font sizes reflect relative amounts of LLO, protein, and hexose. Arrow thicknesses represent relative activity at each step. Glycans from LLO intermediates are indicated by white squares, with extension to G3M9Gn2 indicated by attachment of black squares. (A) Under normal conditions, LLO intermediates are efficiently extended to G3M9Gn2-P-P-Dol, with proper N-glycosylation. (B) Metabolic dysfunction (for example, by limited hexose supply) reduces flux through the LLO pathway, leading to accumulation of LLO intermediates, incorrect protein glycosylation, ER stress, PERK activation, and phosphorylation of eIF2α. (C) Translational balancing requires only moderate translation attenuation by eIF2α-P. This reduces LLO consumption, allowing LLO intermediates to be extended to G3M9Gn2-P-P-Dol even with metabolic dysfunction. Note that fewer glycoproteins are produced than normal, but they are correctly glycosylated with G3M9Gn2.
Though used here to accumulate undermannosylated intermediates, glucose deprivation may also be a physiological cause of ER stress (Scheuner et al., 2001). A 30-min reduction of glucose concentration to 2.5 mM (just below the typical fasting level of 4 mM) can cause significant accumulation of LLO intermediates in fibroblasts (Gao et al., 2005). Although the brief (20 min) incubations in low-glucose media used here were insufficient to appreciably inhibit protein synthesis (unpublished data) or cause ER stress (Doerrler and Lehrman, 1999; Shang et al., 2002), seminal studies showed that extended glucose deprivation can trigger an ER stress response, as well as interfere with protein glycosylation (Pouyssegur et al., 1977; Shiu et al., 1977). In our hands, incubation of dermal fibroblasts for 12 h in medium with 0.5 mM glucose (a restricted condition when used for only 20–30 min) triggered an ER stress response, and both G3M9Gn2-P-P-Dol synthesis and proper protein N-glycosylation were restored (Doerrler and Lehrman, 1999). However, at such extended time-points, UPR-dependent transcription of LLO biosynthetic enzymes (Lehrman, 2006) and increased glucose transport (Doerrler and Lehrman, 1999) would be expected, as well as PERK, to participate in enhancement of G3M9Gn2-P-P-Dol synthesis. Other conditions that interfere with LLO synthesis (Elbein, 1987; Datema et al., 1987) include exposures to glucosamine and 2-deoxyglucose, which trigger ER stress responses (Pouyssegur et al., 1977; Peluso et al., 1978; Qiu et al., 2005), and to tunicamycin (Lehrman, 1991), which induces ER stress and causes PERK-dependent translation arrest (Harding et al., 2000b). In all cases, a compensatory role of PERK would be anticipated.
The vigorous effects of only 20–35% translation attenuation on LLO intermediates were surprising, suggesting influence by translational variations within the physiological range (Scheuner et al., 2001). The failure to detect acute ER stress effects on LLO synthesis in eIF2αA51/A51 MEFs suggests that, absent of translational control, there may be no other strongly stimulatory mechanisms in these cells during the first ∼30 min of the response. This also argues against G3M9Gn2-P-P-Dol metabolism being influenced by potential secondary effects of ER stress (such as misfolding of polypeptide acceptors or disruption of OT). LLO extension in ER stressed-dermal fibroblasts correlated temporally with loss of glycogen and elevation of glucosyl phosphates, suggesting that regulated glycogenolysis might elevate sugar precursor pools and drive LLO extension (Gill et al., 2002). However, a direct link was not established. Given our current results, changes in glycogen metabolism do not appear to have major importance for regulation of LLO synthesis by robust ER stress in MEFs.
The mechanism for PERK reported in this study, termed “translational balancing” in Fig. 10, does not involve complex ER stress-signaling pathways and may be analogous to regulation by other eIF2α kinases, notably HRI (Han et al., 2001). Iron deficiency hinders conversion of protoporphyrin IX to heme and releases HRI from its heme-inhibited state to phosphorylate eIF2α-Ser51. This, in turn, reduces α- and β-globin chain synthesis to balance hemoglobin synthesis with heme. In addition to reducing undesired malfolded globin chains (Han et al., 2005), translational balancing should increase heme relative to protoporphyrin. Accordingly, translational balancing may be a simple, general mechanism by which eIF2α-Ser51 kinases adjust metabolic pathways whose end products interact with newly synthesized proteins.
Translational control also has implications for the type I congenital disorders of glycosylation (CDG-I), which involve mutations in genes required for G3M9Gn2-P-P-Dol synthesis (Jaeken and Matthijs, 2001; Freeze and Aebi, 2005), resulting in aberrant glycosylation of serum proteins. Fibroblasts from CDG-I patients exhibit several criteria of chronic ER stress, suggesting that their LLO defects may be partially offset by beneficial effects of the ER stress response (Lehrman, 2006). CDG-I may be amenable to correction, as G3M9Gn2-P-P-Dol production is not completely impaired because of the presence of at least one partially active allele. Because Ib is the only treatable subtype of the 12 CDG-Is (a–l; Niehues et al., 1998), the compensatory effects of DIS (Fig. 9) are particularly interesting. DIS is a clinically approved drug used to discourage alcoholism (Fleming et al., 2006), and is innocuous unless alcohol is consumed. Though toxic, ARS also has a history of therapeutic use (Kosnett, 2004). In preliminary experiments with CDG-Ia fibroblasts, we noted that DIS, ARS, and DIA all had some ability to restore synthesis of G3M9Gn2-P-P-Dol, although the effects were highly variable (unpublished data), advocating further development of such agents.
In conclusion, we find that PERK can balance ER glycoprotein synthesis with flux through the G3M9Gn2-P-P-Dol pathway. Upon accumulation of LLO intermediates, aberrant N-linked glycosylation would create ER stress and activate PERK. PERK's kinase activity would then reduce the load of glycoprotein precursor polypeptides, slow LLO consumption, facilitate extension of LLO intermediates to G3M9Gn2-P-P-Dol, and reestablish correct N-linked glycosylation.
Materials and methods
Reagents and cell cultures
All stress inducers were obtained from Sigma-Aldrich. An ARGENT Regulated Homodimerization kit containing AP20187 was a gift from Ariad Pharmaceuticals (www.ariad.com/regulationkits). [2-3H]mannose (15 Ci/mmol) and [3H]leucine (164 Ci/mmol) were purchased from GE Healthcare. Cell culture media were obtained from Invitrogen, and sera were obtained from Atlanta Biologicals. CHO-K1 (Camp et al., 1993), PERK-Fv2E–expressing CHO-K1 transfectants (Lu et al., 2004), PERK+/+ and PERK−/− MEFs (Harding et al., 2000b), eIF2αS51/S51 and eIF2αA51/A51 MEFs (Scheuner et al., 2001), HeLa S3 (Elbashir et al., 2001), and dermal fibroblasts (Doerrler and Lehrman, 1999; Shang et al., 2002) were obtained and grown in the culture media described. However, to aid adhesion, MEFs were grown on standard 100-mm tissue culture dishes pretreated with 10 ml autoclaved 0.1% type B bovine gelatin (Sigma-Aldrich) for at least 1 h. After removal of the solution, the dishes were dried for at least 30 min.
Analysis of LLO glycans and N-linked glycans by [3H]mannose incorporation
Cell cultures were incubated (see previous section) for 20–30 min (except for Figs. 5 and 6; 5 min) in media with 0.3–0.5 mM glucose containing 10% dialyzed FBS and [2-3H]mannose. [3H] LLOs were extracted with chloroform/methanol/water (10:10:3). Either the total LLO-associated tritium was measured by liquid scintillation spectroscopy or the [3H] LLOs were treated with weak acid to release water-soluble glycans, which were then fractionated and detected by HPLC with in-line liquid scintillation spectroscopy (Doerrler and Lehrman, 1999; Shang and Lehrman, 2004c). The HPLC system resolves glycans on the basis of single-sugar differences, with the largest glycans eluting the latest. The proteinaceous pellets remaining after organic extraction were digested with pronase and N-glycanase (Calbiochem), and the released N-glycans were analyzed by HPLC (Shang and Lehrman, 2004c).
For LLO pulse-chase studies with Fv2E-PERK cells, both [3H]mannose and [3H]glucosamine were evaluated, but only [3H]mannose was deemed suitable. Conditions were optimized by varying the times and [3H]mannose concentrations for pulse labeling. In most [3H]mannose pulses, we detected multiple species eluting from the HPLC column earlier than 20 min, but these were disregarded because they were refractory to inhibition by tunicamycin (an inhibitor of LLO synthesis). For the experiment presented in Fig. 7, Fv2E-PERK cells were incubated in F-12 medium with 10% dialyzed FBS, 1.0 mM glucose, and 250 μCi/ml [3H]mannose for 2 min. Labeling was then terminated by removal of [3H] medium and addition of methanol (no chase), or cells were washed twice with prewarmed phosphate-buffered saline, and the incubation continued in the same medium, but without [3H]mannose before terminating the reactions (chase). LLOs were recovered from methanolic suspensions in sequential chloroform/methanol (2:1) and chloroform/methanol/water (10:10:3) extracts, which were combined for recovery of all LLO species, and processed for HPLC as described in the previous paragraphs.
Analysis of LLO glycans by FACE
LLO glycans from unlabeled cells were recovered by techniques described in the preceding section, coupled to ANDS, and analyzed with FACE oligosaccharide profiling gels. Gel images were acquired with a Fluor-S MultiImager (Bio-Rad Laboratories) using a 530DF60 filter. When necessary, individual ANDS conjugates were quantified with Quantity One software supplied with the scanner (Gao and Lehrman, 2002a). For clarity, some images were adjusted with brightness and contrast tools in PowerPoint 2003 (Microsoft), treating all data from a single gel identically. Cropping and joining of lanes in a single gel is indicated by vertical lines. For critical direct comparisons, only samples loaded on the same FACE gel were considered. This limited most experiments to duplicate determinations. Thus, we present original FACE data for the reader's inspection. Per 107 cells, 50% of the sample was usually loaded per gel lane.
Protein synthesis assays
Incorporation of [3H]leucine into total protein involved incubation in media with 10% dialyzed FBS and 5 μCi/ml [3H]leucine for 5 min, collection of the material insoluble in 5% trichloroacetic acid (Shang and Lehrman, 2004b), and determination of tritium by liquid scintillation spectroscopy. Incorporation of 125 μCi/ml [35S]methionine for 20 min was done exactly as described (Shang et al., 2002) by phosphorimager analysis of polyacrylamide gels. We have not noticed any differences in the validity or reliability of these two assays.
Application of siRNAs
siRNA duplexes targeting human PERK (accession no. NM_004836) were PERK-A (sense 5′-CAAGAGGAAGACAUCCUGCtt-3′, antisense 5′-GCAGGAUGUCUUCCUCUUGtt-3′) and PERK-B (sense 5′-UGGACCAUGAGGACAUCAGtt-3′, antisense 5′-CUGAUGUCCUCAUGGUCCAtt-3′), corre- sponding to coding region nucleotides 691–709 and 2,237–2,255, respectively (synthesized by the RNA Oligonucleotide Synthesis Core of University of Texas Southwestern Medical Center). Individual oligonucleotides were resuspended in 500 μl DEPC-treated H2O (concentrations determined by OD260), mixed, and diluted to 20 μM each in annealing buffer (100 mM potassium acetate, 30 mM Hepes-KOH, pH 7.4, and 2 mM magnesium acetate), heated for 3 min at 90°C, and incubated for 1 h at 37°C to form duplexes. HeLa S3 cells were plated in DME with 10% FBS without antibiotics in 60-mm dishes, and used at ∼30–50% confluence. Transfection of siRNAs was done with Oligofectamine (Invitrogen) according to the manufacturer's instructions. Cells were incubated with buffer only (“sham”) or with duplexes for 5 or 16 h. Cells were passaged once into the desired numbers of 100-mm dishes. 3 d after the initiation of transfection, cells were treated with the stress inducers indicated or used to harvest RNA. PERK mRNA was quantified by northern analysis and normalized to actin mRNA (Shang et al., 2002).
Splicing of XBP1 mRNA
Total mRNA was isolated with a RNeasy Mini kit (QIAGEN). Splicing of XBP1 mRNA was assessed by RT-PCR (Shang and Lehrman, 2004a). PCR products representing spliced XBP1 (XBP1S), unspliced XBP1 (XBP1U), and a hybrid (formed during the chain reaction, composed of one strand each of XBP1U and XBP1S, [XBP1H]) were resolved by agarose gel electrophoresis.
Online supplemental material
Fig. S1 shows the inhibition of protein synthesis in Fv2E-PERK cells by AP20187. Fig. S2 shows ancillary LLO analyses. Fig. S3 shows the inhibition of protein synthesis by cytoplasmic stress inducers. Fig. S4 shows the recovery from DIA treatment. Table S1 shows mRNA responses in dermal fibroblasts for cytoplasmic stress inducers. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200607007/DC1.
Acknowledgments
We thank Dr. Joel Goodman for valuable comments on the manuscript, and Mr. Biswanath Pramanik for assistance with cell culture.
We acknowledge generous support from the National Institutes of Health (GM38545 to M.A. Lehrman; DK42394 to R.J. Kaufman; DK47119 and ES08681 to D. Ron) and the Robert Welch Foundation (I-1168 to MAL).
Abbreviations used in this paper: ANDS, 7-amino-1,3-naphthalenedisulfonic acid; ARS, arsenite; CDG, congenital disorders of glycosylation; CHX, cycloheximide; DIA, diamide; DIS, disulfiram; Dol, dolichol; DTT, dithiothreitol; eIF, eukaryotic initiation factor; FACE, fluorophore-assisted carbohydrate electrophoresis; G3M9Gn2-P-P-Dol, glucose3mannose9N-acetylglucosamine2-P-P-dolichol; LLO, lipid-linked oligosaccharide; MEF, mouse embryonic fibroblast; OT, oligosaccharyltransferase; PERK, PKR-like ER kinase; TG, thapsigargin; UPR, unfolded protein response.
References
- van Anken, E., and I. Braakman. 2005. Versatility of the endoplasmic reticulum protein folding factory. Crit. Rev. Biochem. Mol. Biol. 40:191–228. [DOI] [PubMed] [Google Scholar]
- Banerjee, D.K., E.A. Carrasquillo, P. Hughey, J.S. Schutzbach, J.A. Martinez, and K. Baksi. 2005. In vitro phosphorylation by cAMP-dependent protein kinase up-regulates recombinant Saccharomyces cerevisiae mannosylphosphodolichol synthase. J. Biol. Chem. 280:4174–4181. [DOI] [PubMed] [Google Scholar]
- Camp, L.A., P. Chauhan, J.D. Farrar, and M.A. Lehrman. 1993. Defective mannosylation of glycosylphosphatidylinositol in Lec35 Chinese hamster ovary cells. J. Biol. Chem. 268:6721–6728. [PubMed] [Google Scholar]
- Chapman, A.E., and J.C. Calhoun. 1988. Effects of glucose starvation and puromycin treatment on lipid-linked oligosaccharide precursors and biosynthetic enzymes in Chinese hamster ovary cells in vivo and in vitro. Arch. Biochem. Biophys. 260:320–333. [DOI] [PubMed] [Google Scholar]
- Crick, D.C., J.R. Scocca, J.S. Rush, D.W. Frank, S.S. Krag, and C.J. Waechter. 1994. Induction of dolichyl-saccharide intermediate biosynthesis corresponds to increased long chain cis-isoprenyltransferase activity during the mitogenic response in mouse B cells. J. Biol. Chem. 269:10559–10565. [PubMed] [Google Scholar]
- Crick, D.C., and C.J. Waechter. 1994. Long-chain cis-isoprenyltransferase activity is induced early in the developmental program for protein N-glycosylation in embryonic rat brain cells. J. Neurochem. 62:247–256. [DOI] [PubMed] [Google Scholar]
- Datema, R., S. Olofsson, and P.A. Romero. 1987. Inhibitors of protein glycosylation and glycoprotein processing in viral systems. Pharmacol. Ther. 33:221–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerrler, W.T., and M.A. Lehrman. 1999. Regulation of the dolichol pathway in human fibroblasts by the endoplasmic reticulum unfolded protein response. Proc. Natl. Acad. Sci. USA. 96:13050–13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir, S.M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 411:494–498. [DOI] [PubMed] [Google Scholar]
- Elbein, A.D. 1987. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu. Rev. Biochem. 56:497–534. [DOI] [PubMed] [Google Scholar]
- Fleming, M., S.J. Mihic, and R.A. Harris. 2006. Ethanol. In Goodman and Gilman's The Pharmacological Basis of Therapeutics Digital Edition. L.L. Brunton, K. Parker, J. Lazo, I. Buxton, and D. Blumenthal, editors. McGraw-Hill. On-line edition.
- Freeze, H.H., and M. Aebi. 2005. Altered glycan structures: the molecular basis of congenital disorders of glycosylation. Curr. Opin. Struct. Biol. 15:490–498. [DOI] [PubMed] [Google Scholar]
- Gao, N., and M.A. Lehrman. 2002. a. Analyses of dolichol pyrophosphate-linked oligosaccharides in cell cultures and tissues by fluorophore-assisted carbohydrate electrophoresis. Glycobiology. 12:353–360. [DOI] [PubMed] [Google Scholar]
- Gao, N., and M.A. Lehrman. 2002. b. Coupling of the dolichol-P-P-oligosaccharide pathway to translation by perturbation-sensitive regulation of the initiating enzyme, GlcNAc-1-P transferase. J. Biol. Chem. 277:39425–39435. [DOI] [PubMed] [Google Scholar]
- Gao, N., J. Shang, and M.A. Lehrman. 2005. Analysis of glycosylation in CDG-Ia fibroblasts by fluorophore-assisted carbohydrate electrophoresis: Implications or extracellular glucose and intracellular mannose-6-phosphate. J. Biol. Chem. 280:17901–17909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, A., N. Gao, and M.A. Lehrman. 2002. Rapid activation of glycogen phosphorylase by the endoplasmic reticulum unfolded protein response. J. Biol. Chem. 277:44747–44753. [DOI] [PubMed] [Google Scholar]
- Han, A.-P., C. Yu, L. Lu, Y. Fujiwara, C. Browne, G. Chin, M. Fleming, P. Leboulch, S.H. Orkin, and J.-J. Chen. 2001. Heme-regulated eIF2α kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 20:6909–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, A.-P., M.D. Fleming, and J.-J. Chen. 2005. Heme-regulated eIF2α kinase modifies the phenotypic severity of murine models of erythropoietic protoporphyria and β-thalassemia. J. Clin. Invest. 115:1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, H.P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic reticulum resident kinase. Nature. 397:271–274. [DOI] [PubMed] [Google Scholar]
- Harding, H.P., I. Novoa, Y. Zhang, H. Zeng, R. Wek, M. Schapira, and D. Ron. 2000. a. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 6:1099–1108. [DOI] [PubMed] [Google Scholar]
- Harding, H.P., Y. Zhang, A. Bertolotti, H. Zeng, and D. Ron. 2000. b. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 5:897–904. [DOI] [PubMed] [Google Scholar]
- Helenius, A., and M. Aebi. 2004. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73:1019–1049. [DOI] [PubMed] [Google Scholar]
- Helenius, J., D.T.W. Ng, C.L. Marolda, P. Walter, M.A. Valvano, and M. Aebi. 2002. Translocation of lipid-linked oligosaccharides across the ER membrane requires Rft1 protein. Nature. 415:447–450. [DOI] [PubMed] [Google Scholar]
- Hubbard, S.C., and P.W. Robbins. 1980. Synthesis of the N-linked oligosaccharides of glycoproteins. J. Biol. Chem. 255:11782–11789. [PubMed] [Google Scholar]
- Iwawaki, T., A. Hosoda, T. Okuda, Y. Kamigori, C. Nomura-Furuwatari, Y. Kimata, A. Tsuru, and K. Kohno. 2001. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat. Cell Biol. 3:158–164. [DOI] [PubMed] [Google Scholar]
- Jaeken, J., and G. Matthijs. 2001. Congenital disorders of glycosylation. Annu. Rev. Genomics Hum. Genet. 2:129–151. [DOI] [PubMed] [Google Scholar]
- Kean, E.L., Z. Wei, V.E. Anderson, N. Zhang, and L. Sayre. 1999. Regulation of the biosynthesis of N-acetylglucosaminylpyrophosphoryldolichol, feedback and product inhibition. J. Biol. Chem. 274:34072–34082. [DOI] [PubMed] [Google Scholar]
- Kornfeld, R., and S. Kornfeld. 1985. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 54:631–664. [DOI] [PubMed] [Google Scholar]
- Kosnett, M.J. 2004. Heavy Metals & Chelators. In Basic & Clinical Pharmacology. B.G.Katzung, editor. McGraw-Hill, On-line edition.
- Kosower, N.S., E.M. Kosower, B. Wertheim, and W.S. Correa. 1969. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem. Biophys. Res. Commun. 37:593–596. [DOI] [PubMed] [Google Scholar]
- Lehrman, M.A. 1991. Biosynthesis of N-Acetylglucosamine-P-P-Dolichol, the committed step of asparagine-linked oligosaccharide assembly. Glycobiology. 1:553–562. [DOI] [PubMed] [Google Scholar]
- Lehrman, M.A. 2006. Stimulation of N-linked glycosylation and lipid-linked oligosaccharide synthesis by stress responses in metazoan cells. Crit. Rev. Biochem. Mol. Biol. 41:51–75. [DOI] [PubMed] [Google Scholar]
- Lu, P.D., H.P. Harding, and D. Ron. 2004. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehues, R., M. Hasilik, G. Alton, C. Körner, M. Schiebe-Sukumar, H.G. Koch, K.P. Zimmer, R. Wu, E. Harms, K. Reiter, et al. 1998. Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J. Clin. Invest. 101:1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso, R.W., R.A. Lamb, and P.W. Choppin. 1978. Infection with paramyxo viruses stimulates synthesis of cellular polypeptides that are also stimulated in cells transformed by Rous sarcoma virus or deprived of glucose. Proc. Natl. Acad. Sci. USA. 75:6120–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur, J., R.P. Shiu, and I. Pastan. 1977. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 11:941–947. [DOI] [PubMed] [Google Scholar]
- Qiu, W., R. Kohen-Avramoglu, S. Mhapsekar, J. Tsai, R.C. Austin, and K. Adeli. 2005. Glucosamine-induced endoplasmic reticulum stress promotes ApoB100 degradation: evidence for Grp78-mediated targeting to proteasomal degradation. Arterioscler. Thromb. Vasc. Biol. 25:571–577. [DOI] [PubMed] [Google Scholar]
- Sayeed, A., and D.T.W. Ng. 2005. Search and destroy: ER quality control and ER-associated protein degradation. Crit. Rev. Biochem. Mol. Biol. 40:75–91. [DOI] [PubMed] [Google Scholar]
- Schenk, B., F. Fernandez, and C.J. Waechter. 2001. The ins(ide) and outs(ide) of dolichyl phosphate biosynthesis and recycling in the endoplasmic reticulum. Glycobiology. 11:61R–70R. [DOI] [PubMed] [Google Scholar]
- Scheuner, D., B. Song, E. McEwen, C. Liu, R. Laybutt, P. Gillespie, T. Saunders, S. Bonner-Weir, and R.J. Kaufman. 2001. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell. 7:1165–1176. [DOI] [PubMed] [Google Scholar]
- Schröder, M., and R.J. Kaufman. 2005. The mammalian unfolded protein response. Annu. Rev. Biochem. 74:739–789. [DOI] [PubMed] [Google Scholar]
- Shang, J., C. Körner, H. Freeze, and M.A. Lehrman. 2002. Extension of lipid-linked oligosaccharides is a high-priority aspect of the unfolded protein response: endoplasmic reticulum stress in Type I congenital disorder of glycosylation fibroblasts. Glycobiology. 12:307–317. [DOI] [PubMed] [Google Scholar]
- Shang, J., and M.A. Lehrman. 2004. a. Discordance Of UPR Signaling By ATF6 and Ire1p-XBP1 With Levels Of Target Transcripts. Biochem. Biophys. Res. Commun. 317:390–396. [DOI] [PubMed] [Google Scholar]
- Shang, J., and M.A. Lehrman. 2004. b. Inhibition of mammalian RNA synthesis by the cytoplasmic Ca2+ buffer BAPTA. Analyses of [3H]Uridine incorporation and stress-dependent transcription. Biochemistry. 43:9576–9582. [DOI] [PubMed] [Google Scholar]
- Shang, J., and M.A. Lehrman. 2004. c. Metformin-stimulated mannose transport in dermal fibroblasts. J. Biol. Chem. 279:9703–9712. [DOI] [PubMed] [Google Scholar]
- Shiu, R.P., J. Pouyssegur, and I. Pastan. 1977. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteins in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc. Natl. Acad. Sci. USA. 74:3840–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp, E.L., A. Sharma, J. Lippincott-Schwartz, and R.S. Hegde. 2006. Monitoring chaperone engagement of substrates in the endoplasmic reticulum of live cells. Proc. Natl. Acad. Sci. USA. 103:6536–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider, M.D., and O.C. Rogers. 1984. Transmembrane movement of oligosaccharide-lipids during glycoprotein synthesis. Cell. 36:753–761. [DOI] [PubMed] [Google Scholar]
- Sood, R., A.C. Porter, K. Ma, L.A. Quilliam, and R.C. Wek. 2000. Pancreatic eukaryotic initiation factor-2α kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem. J. 346:281–293. [PMC free article] [PubMed] [Google Scholar]
- Voet, D., and J.G. Voet. 1995. Enzymatic Catalysis. In Biochemistry. John Wiley & Sons, New York. pp. 371–410.