Abstract
Elevated permeability of the endothelium is thought to be crucial in atherogenesis because it allows circulating lipoproteins to access subendothelial monocytes. Both local hemodynamics and cytokines may govern endothelial permeability in atherosclerotic plaque. We recently found that p21-activated kinase (PAK) regulates endothelial permeability. We now report that onset of fluid flow, atherogenic flow profiles, oxidized LDL, and proatherosclerotic cytokines all stimulate PAK phosphorylation and recruitment to cell–cell junctions. Activation of PAK is higher in cells plated on fibronectin (FN) compared to basement membrane proteins in all cases. In vivo, PAK is activated in atherosclerosis-prone regions of arteries and correlates with FN in the subendothelium. Inhibiting PAK in vivo reduces permeability in atherosclerosis-prone regions. Matrix-specific PAK activation therefore mediates elevated vascular permeability in atherogenesis.
Introduction
Atherosclerosis involves the progressive accumulation of lipids, immune cells, and ECM in the vessel wall, which can decrease blood flow or rupture to cause acute thrombosis. Endothelial cell dysfunction is the key initiating event in atherogenesis, resulting in decreased flow-induced dilation and inflammatory gene expression (Ross, 1999). Activated endothelium recruits monocytes, which differentiate into macrophages. Elevated permeability of the endothelium is believed to allow entry of lipoproteins into the vessel wall, which become oxidized and propagate endothelial dysfunction (Steinberg, 1997). Macrophages engulf low-density lipoprotein (LDL) and other lipoproteins and become foam cells, which can be visualized as fatty streaks in the vessel wall. In the continued presence of high LDL cholesterol and oxidant stress, fatty streaks progress to advanced atherosclerotic plaques (Ross, 1999).
Despite the systemic nature of most atherogenic stimuli, atherosclerosis is a focal disease affecting discrete regions of the vasculature, such as vessel curvatures and bifurcations. These regions are characterized by complex flow patterns. including flow reversal, flow gradients, secondary flows with rapid changes in flow direction, and, in some regions, turbulence (VanderLaan et al., 2004). We group all of these flow patterns under the rubric of disturbed flow. Endothelial cells sense the force of flowing blood, termed shear stress, and different blood flow patterns regulate endothelial behavior. Regions of blood vessels exposed to undisturbed, unidirectional laminar flow (henceforth termed laminar flow) are protected from atherosclerosis, and in vitro prolonged laminar flow stimulates expression of athero-protective genes (Traub and Berk, 1998; Brooks et al., 2004). By contrast, disturbed flow patterns stimulate proatherosclerotic events, including increased monolayer permeability; decreased antioxidant capacity; and enhanced expression of proinflammatory genes, such as ICAM-1, VCAM-1, and monocyte chemotactic protein-1 (MCP-1; Jo et al., 1991; De Keulenaer et al., 1998; Phelps and DePaola, 2000; Brooks et al., 2004). The correlation between flow patterns and endothelial monolayer permeability has recently been demonstrated in vivo, where vascular permeability is inversely proportional to time-average shear stress and correlated with increased flow oscillation and flow gradients (Himburg et al., 2004; LaMack et al., 2005). Interestingly, onset of laminar shear stimulates many of the same responses as disturbed shear; however, in laminar shear, these events are down-regulated as cells adapt, whereas in disturbed shear, they are sustained. Thus, failure to adapt is thought to be critical for responses to disturbed shear (Orr et al., 2006).
The molecular mechanisms involved in flow-induced endothelial permeability are unknown. Although vesicular transport and transcellular channels may contribute to endothelial permeability, paracellular pore formation is most likely the major pathway for macromolecule transport across arterial endothelium (Ogunrinade et al., 2002). Paracellular permeability is limited by cell–cell interactions, especially those in tight junctions (TJs). Multiple molecular mechanisms implicated in regulation of endothelial paracellular permeability include changes in gene expression, phosphorylation of junctional components, myosin-dependent contractility, and stability of cortical actin (Ogunrinade et al., 2002). Many signaling pathways regulate permeability, most of which affect cortical actin or myosin (Yuan, 2002). Actin remodeling is regulated by the Rho family of small GTPases, including Rho, Rac, and Cdc42 (Jaffe and Hall, 2005). The p21-activated kinase (PAK) family of Ser/Thr kinases is important for Rac and Cdc42-induced cytoskeletal remodeling, affecting both actomyosin contractility and the stability of actin filaments (Bokoch, 2003). Recently, PAK was shown to stimulate paracellular pore formation and increased endothelial cell permeability in response to a wide range of cellular stimuli (Stockton et al., 2004). PAK-mediated permeability responses require the localization of active PAK to cell–cell junctions, where PAK stimulates the phosphorylation of myosin light chain to induce contractility (Stockton et al., 2004). In addition, PAK can also promote paracellular pore formation by phosphorylating VE-cadherin, which results in its arrestin-dependent internalization (Gavard and Gutkind, 2006). PAK contains multiple domains that bind scaffolding proteins, such as Nck and Grb2, capable of regulating PAK localization (Lu et al., 1997; Puto et al., 2003). Interestingly, both PAK localization to cell–cell junctions and PAK-mediated permeability were inhibited with a cell-permeable peptide corresponding to the Nck-binding sequence of PAK (Stockton et al., 2004).
Shear stress activates the integrin family of ECM receptors, and new integrin ligation mediates effects of flow on Rac, Cdc42, and Rho activity (Jalali et al., 2001; Tzima et al., 2001, 2002, 2003). Flow-induced GTPase regulation mediates cell alignment in the direction of flow and stimulates the transcription factor NF-κB, which is important for expression of inflammatory genes in the endothelium (Tzima et al., 2002). The idea that integrin ligation mediates these effects suggested that alterations in the subendothelial matrix composition would affect which integrins become ligated, resulting in differential signaling in response to flow. Indeed, shear stress activates NF-κB when endothelial cells are plated on a fibronectin (FN) or fibrinogen matrix, but not when cells are plated on collagen or laminin. Furthermore, FN and fibrinogen were deposited at sites of disturbed flow in vivo, which correlated with expression of NF-κB target genes (Orr et al., 2005). These results suggest that matrix remodeling plays a causal role in atherogenesis. In this work, we investigate the role of flow and ECM in endothelial permeability in atherogenesis.
Results
Flow stimulates matrix-specific PAK activation and localization to cell–cell junctions
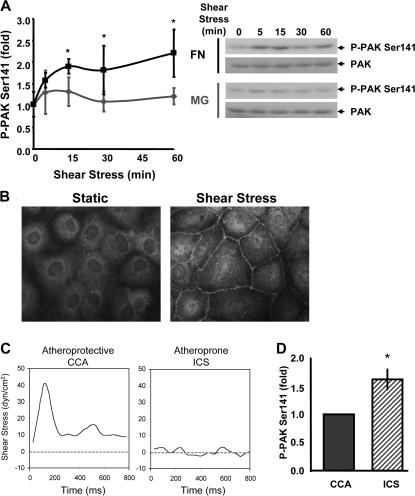
The N terminus of PAK contains a Rac/Cdc42 binding domain that overlaps an autoinhibitory domain (AID) such that binding of active GTPases alleviates an inhibitory interaction between the AID and the C-terminal kinase domain (Bokoch, 2003). PAK activation results in autophosphorylation at multiple sites (Gatti et al., 1999; Chong et al., 2001), including Ser141 at the end of the AID. Phosphorylation of this residue prevents the interaction of the AID with the kinase domain to maintain the active conformation. Flow-induced integrin signaling activates both Rac and Cdc42 (Tzima et al., 2002, 2003), suggesting that PAK might be activated. Using PAK Ser141 phosphorylation as a marker, bovine aortic endothelial (BAE) cells were examined. Cells were plated for 4 h on coverslips coated with either FN or diluted matrigel (MG), which, under these conditions, adsorbs to the glass as a thin layer similar to FN. MG was used as a model for normal basement membrane proteins. We found that flow stimulated biphasic PAK activation on FN; however, no significant activation occurred in cells on MG (Fig. 1 A). Collagen also failed to support PAK activation under these conditions (unpublished data). Immunofluorescence staining showed that activated PAK localized to cell–cell borders (Fig. 1 B). No major changes in cell–cell junctions themselves were noted on this time scale (see Fig. 4). Consistent with previous results (Stockton et al., 2004), this localization was abrogated by the addition of a cell-permeant peptide that blocks the binding of PAK to Nck (unpublished data).
Figure 1.
Flow stimulates matrix-specific PAK phosphorylation. (A) BAE cells plated on MG or FN for 4 h were sheared for the indicated times. Phosphorylation of PAK on Ser141 was assessed by immunoblotting total cell lysates with a phosphorylation site–specific antibody. Values are means ± SD normalized for total PAK (n = 3–4). Representative blots are shown. (B) Endothelial cells plated on FN were sheared for 15 min or kept under static conditions, and PAK phospho-Ser141 localization was assessed by immunocytochemistry. Representative images are shown. (C) Shear stress flow profiles for the CCA and the ICS were determined using MRI-generated near-wall velocity gradient profiles of normal carotid arteries. (D) Endothelial cells on FN were stimulated for 24 h with either CCA or ICS flow. Phosphorylation of PAK on Ser141 normalized to total PAK was assessed as described in A. Values are means ± SD after normalization for total protein (n = 3). *, P < 0.05.
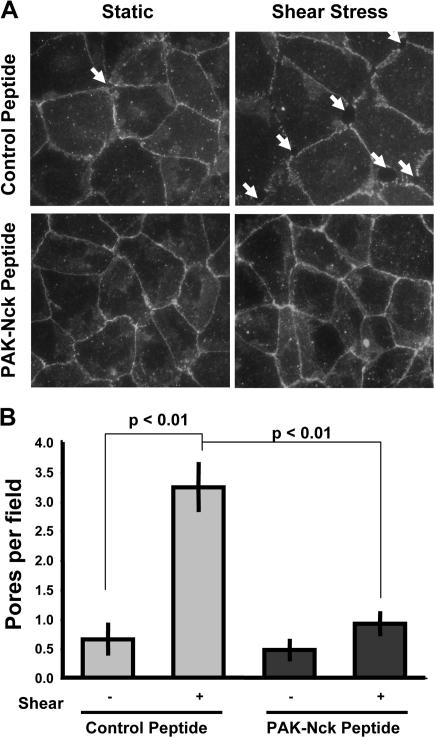
Figure 4.
Flow stimulates PAK-dependent formation of paracellular pores. BAE cells plated on FN were treated with either control or PAK-Nck inhibitory peptide (20 μg/ml for 1 h) and stimulated with flow for 30 min, and cell–cell junctions were visualized by staining for β-catenin. (A) Representative β-catenin stains are shown. Arrows indicate the presence of a paracellular pore. (B) The total number of paracellular pores per high power field was determined. 20 fields were counted per experiment (n = 3).
Responses to the onset of laminar shear are transient but otherwise resemble events triggered by disturbed flow (Orr et al., 2006). We therefore determined PAK activity in endothelial cells exposed to different flow patterns for longer times. BAE cells plated on FN were stimulated for 24 h with flow profiles derived from the athero-protective common carotid artery (CCA) or the athero-prone internal carotid sinus (ICS; Fig. 1 C; Gelfand et al., 2006). Matrix specificity was not determined in this assay because cell-derived matrices deposited over this extended time course could affect signaling responses. Consistent with the adaptation to flow hypothesis, cells stimulated with ICS flow show elevated PAK phosphorylation compared with cells stimulated with CCA flow (Fig. 1 D).
Matrix-dependent PAK signaling regulates flow-induced endothelial permeability
Because PAK regulates permeability of endothelial monolayers (Stockton et al., 2004; Gavard and Gutkind, 2006), we tested whether matrix-specific PAK activation correlates with permeability. To assay flow-mediated endothelial cell permeability, we developed a novel transwell assay that used a modified cone and plate device adapted to 75-cm transwell chambers (Fig. 2 A). Using this system, we applied shear to endothelial cell monolayers and assessed the movement of a tracer across the filter. Membranes were then fixed and stained to ensure that no cell loss occurred during the assay. Consistent with previous results, we found that laminar flow transiently increased endothelial cell permeability, which returned to baseline by 4 h (Fig. 2 B).
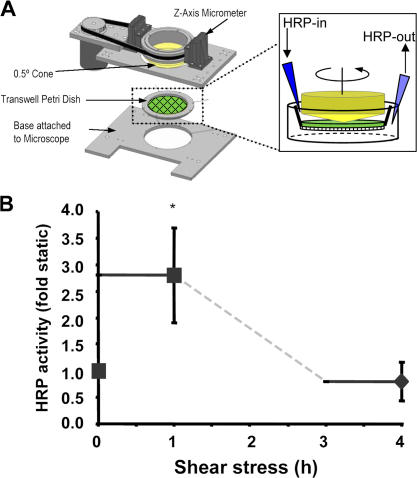
Figure 2.
Onset of flow stimulates endothelial leak. (A) To monitor flow-induced permeability, we used large transwell membranes mounted into a modified cone and plate viscometer and subjected to shear stress. HRP was infused into the top well for 1 h, and permeability was assessed by measuring final HRP activity in the bottom well media. (B) BAE cell monolayers were stimulated with acute onset of flow, and the permeability response was measured as described in A during both the first and fourth hour after application of flow. Values are means ± SD (n = 3). *, P < 0.05.
To determine whether these effects are matrix specific, BAE cells were plated on either FN or diluted MG for 4 h. Endothelial cells formed a complete monolayer with both adherens junctions and TJs as assessed by β-catenin and ZO-1 staining but deposited very little endogenous matrix (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200609008/DC1). Onset of flow triggered a greater increase in permeability in cells on FN compared with MG or collagen IV (Fig. 3 A). In addition, the low level of permeability in cells on MG was enhanced in a dose-dependent manner when overlaid with FN (Fig. 3 B). Matrix proteins alone without cells did not differentially affect permeability (Fig. S2).
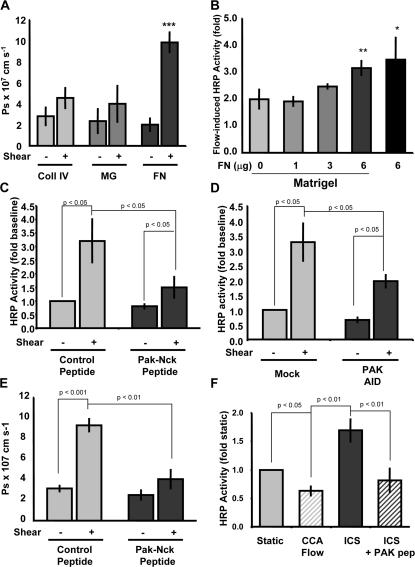
Figure 3.
Flow-induced endothelial permeability is matrix and PAK dependent. (A) BAE cells plated on transwell membranes coated with collagen IV, MG, or FN were stimulated with laminar flow at 12 dynes/cm2. HRP leak across the membrane was assessed during the first hour of flow; data are presented as absolute solute permeability as described in Materials and methods. Values are means ± SD (n = 3). (B) Endothelial cells were plated on transwells coated with MG and increasing concentrations of FN, stimulated with 12 dynes/cm2 laminar flow for 1 h, and HRP leak across the membrane was assessed. Values are means ± SD (n = 3). (C) Endothelial cells plated on FN-coated transwell membranes were treated with either control peptide or PAK-Nck inhibitory peptide (20 μg/ml for 1 h). HRP leak across the membrane was assessed during the first hour of steady laminar flow at 12 dynes/cm2. Values are means ± SD (n = 4). (D) Endothelial cells plated on FN-coated transwell membranes were transfected with HA-tagged PAK AID. At 24 h after transfection, monolayers were exposed to flow, and HRP leak across the membrane was assessed during the first hour. Values are means ± SD (n = 3). Transfection efficiency ranged from ∼35 to ∼50% as determined by immunocytochemistry. (E) Endothelial cells plated on FN-coated transwell membranes were treated with either control or PAK-Nck inhibitory peptide (20 μg/ml for 1 h). Alexa 488–conjugated BSA leak across the membrane was assessed during the first hour of steady laminar flow at 12 dynes/cm2. Data (means ± SD; n = 3) are shown as absolute solute permeability. (F) Endothelial cells plated on FN-coated transwell membranes were stimulated for 4 h with CCA or ICS flow. HRP leak across the membrane was assessed during the last 1-h period. Some cells were pretreated with the PAK-Nck inhibitory peptide (20 μg/ml for 1 h) and stimulated with ICS flow in the continued presence of the peptide. Values are means ± SD (n = 3).
To test whether PAK is involved in flow-induced permeability, cells were either transfected with a construct encoding the PAK AID or treated with a cell-permeant peptide that contains the Nck-binding sequence from PAK. This peptide was previously shown to mimic the dominant-negative effects of kinase-dead PAK, including inhibition of endothelial permeability (Kiosses et al., 2002; Stockton et al., 2004). The peptide blocked the flow-induced increase in permeability by ∼80%, whereas an inactive control peptide containing mutations in key proline residues involved in Nck binding (Kiosses et al., 1999) had no effect (Fig. 3 C). Though transfection efficiency with the PAK AID was ∼50%, the decrease in flow-induced permeability approached 50%, indicating that it is also highly effective (Fig. 3 D). In addition to HRP, Alexa 488–labeled BSA was also used to determine flow-induced permeability. Absolute permeability to both BSA and HRP were similar (Fig. 3, A and E), and both showed sensitivity to PAK inhibition (Fig. 3 E).
Disturbed flow is known to increase permeability compared with steady or arterial flow patterns (Phelps and DePaola, 2000). To confirm these results in our system, BAE cells on FN were exposed to CCA or ICS flow for 4 h, and permeability was assessed. ICS flow increased monolayer permeability nearly twofold compared with CCA flow (Fig. 3 F). Immunofluorescence revealed that active PAK was localized to cell– cell junctions after 4 h of ICS flow but not after CCA flow (unpublished data). The blocking peptide also inhibited permeability induced by ICS flow (Fig. 3 F) as well as junctional phospho-PAK staining (not depicted). Taken together, these data show that matrix-specific PAK activation triggered by onset of flow or prolonged disturbed flow mediates enhanced endothelial monolayer permeability.
Flow stimulates PAK-dependent paracellular pore formation
Multiple growth factors and other bioactive substances use a pathway in which PAK regulates phosphorylation of MLCK to increase cellular contractility, thereby inducing endothelial cell permeability through formation of paracellular pores (Stockton et al., 2004). To test whether flow induces PAK-dependent paracellular pores, BAE cells were treated with either the control or PAK-Nck inhibitory peptide, sheared for 30 min, and assayed for the presence of paracellular pores by staining for the adherens junction protein β-catenin. Flow induced the formation of paracellular pores, which was strongly reduced by the pretreatment with the PAK-Nck inhibitory peptide (Fig. 4).
Effects of cytokines and oxidized LDL (oxLDL)
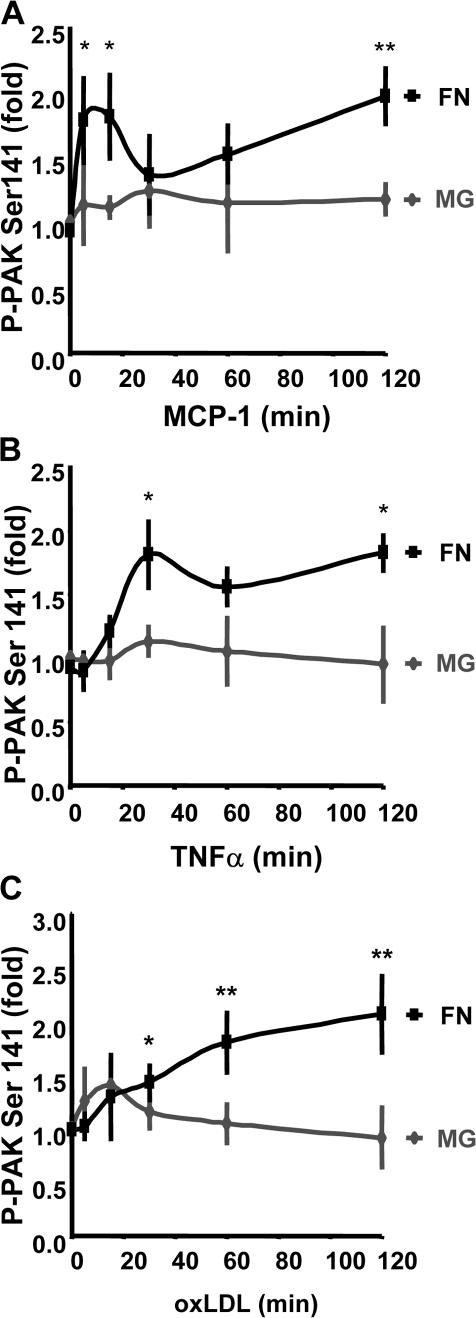
Although flow patterns regulate susceptibility to atherosclerosis, a number of soluble factors also promote atherosclerotic plaque development and likely contribute to endothelial permeability in atherosclerosis. OxLDL stimulates endothelial cell permeability through a Rho-dependent pathway (Essler et al., 1999; Siess et al., 1999). In early atherogenesis, activated endothelial cells and macrophages produce MCP-1, which also stimulates endothelial permeability (Stamatovic et al., 2003), as do the macrophage-derived cytokines TNFα and IL-1β (Martin et al., 1988; Brett et al., 1989). Furthermore, mice deficient in either MCP-1 or TNFα show reduced atherosclerosis (Gu et al., 1998; Ohta et al., 2005). We previously showed that TNFα-induced endothelial permeability was reduced by the PAK-Nck inhibitory peptide (Stockton et al., 2004). To analyze the matrix dependence of these factors, PAK phosphorylation was assessed in endothelial cells plated on FN or MG. Though the time courses were distinct, MCP-1, TNFα, and oxLDL stimulated PAK phosphorylation in cells on FN but not on MG (Fig. 5). In all cases, phosphorylated PAK localized to cell–cell junctions, and this localization was inhibited by the Pak-Nck peptide (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200609008/DC1).
Figure 5.
PAK activation by multiple atherogenic stimuli is matrix dependent. BAE cells plated on MG or FN for 4 h were treated with MCP-1 at 50 ng/ml (A), TNFα at 10 ng/ml (B), or oxLDL at 100 μg/ml (C) for the indicated times. Phosphorylation of PAK on Ser141 was assessed by immunoblotting total cell lysates with a phosphorylation site–specific antibody. Values are means ± SD after normalization for total PAK (n = 3–4). *, P < 0.05; **, P < 0.01.
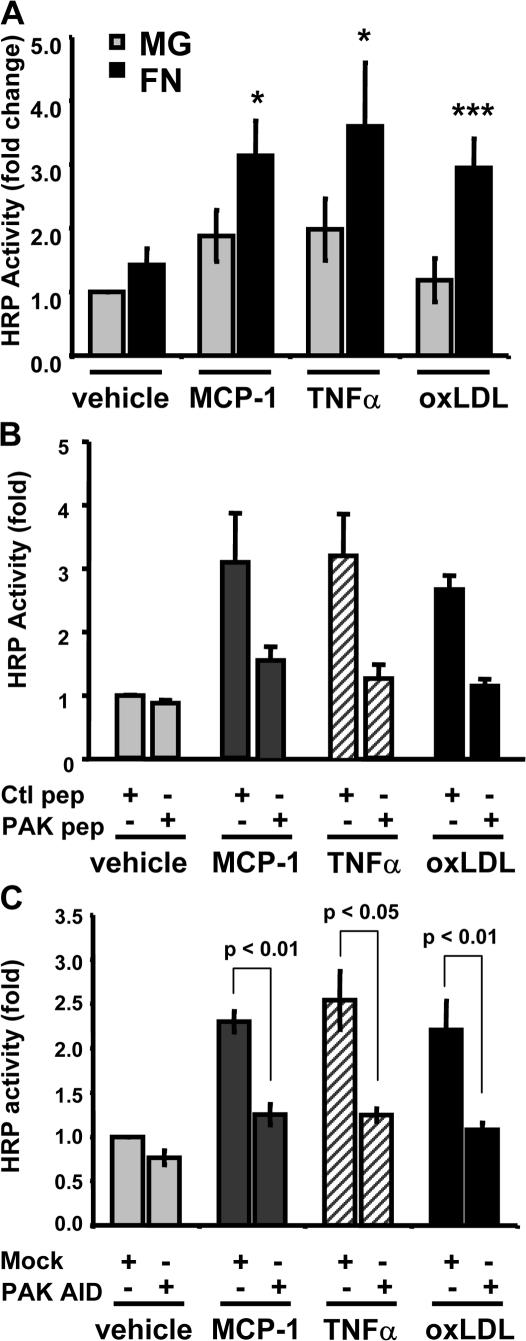
We next examined monolayer permeability. All of these factors triggered matrix-dependent increases in permeability (Fig. 6 A) that were inhibited by the PAK-Nck blocking peptide (Fig. 6 B) and by expression of the PAK AID (Fig. 6 C). Thus, effects of a number of atherogenic soluble factors on PAK- dependent permeability are strongly modulated by the ECM.
Figure 6.
Matrix-specific PAK activation stimulates permeability in response to multiple atherogenic stimuli. (A) Endothelial cells plated on transwell membranes coated with either MG or FN were stimulated with 50 ng/ml MCP-1, 10 ng/ml TNFα, or 100 μg/ml oxLDL for 2 h, and the HRP leak across the membrane was assessed. Values are means ± SD (n = 3–4). (B) BAE cells plated on FN-coated transwell membranes were treated with either a control or a PAK-Nck inhibitory peptide (20 μg/ml for 1 h). Monolayers were stimulated with MCP-1, TNFα, or oxLDL for 2 h, and the HRP leak across the membrane was assessed. Values are means ± SD (n = 3). (C) Endothelial cells plated on FN-coated transwell membranes were transfected with HA-tagged PAK AID. After 24 h, monolayers were stimulated with MCP-1, TNFα, or oxLDL for 2 h, and the HRP leak across the membrane was assessed. Values are means ± SD (n = 3). Transfection efficiency ranged from ∼35 to ∼50% as determined by immunocytochemistry.
PAK is phosphorylated in vivo
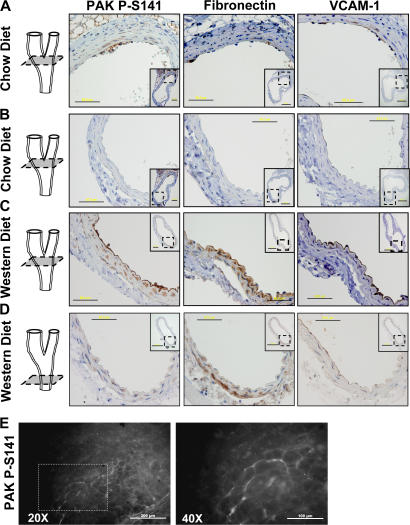
Areas of disturbed flow in vivo show elevated endothelial cell permeability (Himburg et al., 2004; LaMack et al., 2005). These regions also show deposition of FN in the subendothelial ECM and expression of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule (VCAM-1; Orr et al., 2005). We therefore tested whether permeability, PAK activity, and FN correlate in vivo. The carotid arteries from young (20-wk-old) ApoE−/− mice fed either a chow or Western diet were isolated and processed for immunohistochemistry. In mice on a chow diet, the carotid sinus displayed some monocyte infiltration but no foam cell formation. PAK phosphorylation was observed specifically in the atherosclerosis-prone region of these vessels but not nearby athero-resistant regions (Fig. 7 A). Nearby sections showed FN in the subendothelial matrix in the same regions of the artery (Fig. 7 A). Furthermore, enhanced expression of VCAM-1 was detected in these regions, indicating endothelial activation. The opposite side of the carotid sinus can develop atherosclerosis in some cases but in this mouse shows no FN, VCAM-1, or phospho-PAK staining (Fig. 7 B). PAK phosphorylation, VCAM-1, and FN were all enhanced by the Western diet within the carotid sinus (Fig. 7 C) but not in athero-resistant regions of the CCA (Fig. 7 D). Monocytes recruited to athero-prone regions of arteries from these mice also stained positively for phospho-PAK. Thus, PAK activation correlates with subendothelial FN and inflammatory markers.
Figure 7.
PAK activation and FN deposition in vivo. Male ApoE−/− mice fed a chow diet (A and B) or a Western diet (C and D) for 10 wk were killed, and the carotid arteries were removed and embedded in paraffin. Nearby sections were stained for PAK phospho-Ser141, FN, and VCAM-1, and shown at high magnification (40×) with lower magnification (10×) views of the entire vessels shown as insets. A and B show different areas of the same carotid sinus, and C and D show the carotid sinus and CCA, respectively, from the same animal. A 3D representation of the artery indicates the location of the sections. Bars: 50 μm; (insets) 200 μm. (E) Male ApoE−/− mice fed a chow diet for 10 wk were killed, the aortic arch was excised, and en face staining was performed for PAK phospho-Ser141. Representative images are shown at both 20× and 40× magnifications. Bars: (left) 200 μm; (right) 100 μm.
To determine whether active PAK localizes to cell–cell junctions in atherosclerosis in vivo, aortas from ApoE−/− mice on a chow diet were fixed, excised, and examined en face. Staining for platelet-endothelial cell adhesion molecule 1 (PECAM-1) confirmed the ability to visualize endothelial cell junctions in vivo and illustrated endothelial cell alignment in an athero-protected region of the ascending aortic arch (unpublished data). Although athero-resistant regions of the aorta showed no staining, athero-prone regions of the lesser curvature of the arch showed focal areas of high phospho-PAK staining at cell–cell junctions (Fig. 7 E).
PAK inhibition reduces permeability in atherosclerosis in vivo
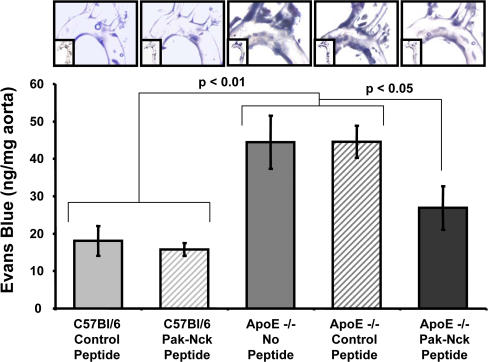
To determine whether PAK is responsible for the increased permeability during development of atherosclerosis, 32-wk-old ApoE−/− mice (chow diet) were given intraperitoneal injections of the PAK-Nck blocking peptide or a control peptide. Mice under these conditions are reported to develop moderate atherosclerotic lesions, though plaque development is slower than in animals on a high-fat Western diet (Reddick et al., 1994). Vascular permeability within the aorta was then assessed by measuring leakage of Evans blue dye into the vascular wall. Aortas from C57Bl/6 mice were used as a source for healthy, atherosclerosis-free vessels. Each mouse received 1 mg of peptide at 24 h and 1 h before Evans blue injection via the tail vein. After 30 min, leakage of dye into the aorta was assessed. Although little Evans blue accumulated in the aorta of C57Bl/6 mice, in ApoE−/− mice, dye was apparent at the lesser curvature of the arch and at branch points for major arteries in both the nontreated and control peptide–treated animals (Fig. 8), consistent with known athero-prone regions. The Pak-Nck peptide inhibited 67% of the increase in permeability, relative to healthy vessels. These data suggest that PAK makes an important contribution to permeability in atherogenesis.
Figure 8.
PAK inhibition reduces permeability in vivo. C57Bl/6 and ApoE−/− mice fed a chow diet were treated with PAK-Nck inhibitory or control peptides, and leakage of Evans blue dye into the aortas was assessed as described in Materials and methods. Images were recorded by bright field microscopy. Results were quantified by extracting the dye and measuring absorbance at 620 nm. Values were normalized to the dry weight of the aorta (n = 3). *, P < 0.05. Representative images are shown.
Discussion
These data support the concept that remodeling of the subendothelial ECM plays a crucial role in atherogenesis. Previous work demonstrated a correlation between enhanced vascular permeability and atherosclerosis (Ogunrinade et al., 2002). In this work, we present evidence for ECM-specific activation of PAK by atherogenic stimuli, leading to increased permeability. PAK activation may be initiated by disturbed flow, though as atherosclerosis develops, soluble factors such as oxLDL and cytokines produced by immune cells and activated endothelium most likely make major contributions. Importantly, PAK activation at athero-prone sites in vivo correlates with areas of FN deposition. Finally, inhibiting PAK function in vivo reduced permeability in athero-prone regions.
The mechanisms regulating the matrix specificity of PAK activation are presently unclear. Flow-induced Rac activation is equivalent on all matrices (unpublished data), suggesting that there may be matrix-specific signals that inhibit PAK activation. Known mechanisms limiting PAK activation include binding of PAK to Nischarin or hPIP1 and dephosphorylation by the phosphatases PP2A and POPX1/2 (Xia et al., 2001; Koh et al., 2002; Kumar and Vadlamudi, 2002; Alahari et al., 2004). Phosphorylation of PAK by protein kinase A also inhibits PAK activation (Howe and Juliano, 2000). Further examination of matrix-specific PAK activation will be an interesting avenue for future work.
The current data suggest that reducing either PAK activation or localization to cell–cell junctions should reduce the permeability of the endothelial cell layer. Recently, the Ser/Thr kinases Akt and protein kinase G (PKG) were found to phosphorylate PAK at Ser21 within the Nck-binding sequence, inhibiting the interaction between PAK and Nck (Zhou et al., 2003; Fryer et al., 2006). Because blocking the PAK–Nck interaction inhibits localization of PAK to cell–cell borders and decreases endothelial permeability, these kinases might decrease permeability in a similar manner. Indeed, both Akt and cyclic GMP/PKG can decrease vascular permeability (Pearse et al., 2003; Chen et al., 2005; Moldobaeva et al., 2006). Whether PAK is the relevant target for these effects remains to be explored.
The mechanisms by which permeability is elevated in the plaque endothelium are not well understood. Dissolution of intercellular interactions during endothelial cell division and apoptosis, both of which are elevated at athero-prone sites in vivo (Weinbaum et al., 1985; Lin et al., 1988), has been suggested as a possible mechanism. However, the correlation between endothelial cell turnover and enhanced permeability in vivo is weak (Penn and Chisolm, 1991; Malinauskas et al., 1995). A more likely mechanism involves TJs in athero-prone regions, which are discontinuous compared with athero-resistant regions (Okano and Yoshida, 1994). Changes in TJ protein expression, phosphorylation, and reorganization could all contribute to decreased barrier function (Ogunrinade et al., 2002). Both flow and cytokines induce permeability too rapidly for changes in gene expression to be an attractive mechanism. Shear stress stimulates occludin phosphorylation on Ser/Thr residues, which could alter occludin localization to TJs or function (Sakakibara et al., 1997; DeMaio et al., 2001). VEGF stimulates PAK-dependent VE-cadherin phosphorylation, resulting in its arrestin- dependent internalization and the formation of paracellular pores (Gavard and Gutkind, 2006). Myosin light chain phosphorylation triggers cell contraction and the formation of paracellular pores (Stockton et al., 2004), and contractility appears to be a common pathway for endothelial cell permeability by multiple atherogenic stimuli (Takeya et al., 1993; Essler et al., 1999; Siess et al., 1999; Ogunrinade et al., 2002; Stamatovic et al., 2003). PAK inhibition decreases myosin phosphorylation and contractility in endothelial cells (Kiosses et al., 1999; Stockton et al., 2004). Thus, effects of PAK on the cytoskeleton appear to be involved in regulation of permeability, though other events, such as VE-cadherin and occludin phosphorylation, are likely to contribute.
PAK regulates cytoskeletal organization, proliferation, and movement in many cell types, making PAK activity by itself an unlikely target for long term therapy. For example, PAK inhibition in mice with a cell-permeable peptide was recently shown to mimic Alzheimer's disease (Zhao et al., 2006). However, specific interactions, such as Nck, may offer more attractive therapeutic targets. The ECM dependence of PAK activity may provide an especially attractive means for therapeutic intervention that would be less perturbing than global inhibition of kinase activity.
Materials and methods
Cell culture, transfection, and shear stress
BAE cells (a gift from H. Sage, Hope Heart Institute, Seattle, WA) were maintained in low-glucose DME containing 10% calf serum (CS), 10 U/ml penicillin, and 10 μg/ml streptomycin (Invitrogen). Cells were plated for 4–24 h on 38- × 75-mm2 glass slides (Corning) precoated with collagen IV (20 μg/ml in PBS; Sigma-Aldrich), MG (1:100 dilution in serum-free media; Calbiochem), or FN (10 μg/ml in PBS). After 4 h, cells were fully attached and spread and formed a confluent monolayer. Slides were then loaded onto a parallel plate flow chamber in 0.5% CS, and 12 dynes/cm2 shear stress was applied for varying times as previously described (Orr et al., 2005). To stimulate BAE cells with athero-prone (ICS) or athero-protective (CCA) shear stress profiles, BAE cells were plated as described except in a custom Petri dish and stimulated as previously described (Blackman et al., 2002). Human hemodynamic shear stress profiles were developed from MRI-generated near-wall velocity profiles of normal carotid arteries (Gelfand et al., 2006). Transient transfection of HA-tagged PAK AID was accomplished by Effectene (QIAGEN) using the manufacturer's protocols.
Immunoblotting and immunocytochemistry
Cell lysis and immunoblotting were performed as previously described (Orr et al., 2002). Antibodies used include rabbit anti–phospho-PAK (Ser141; 1:5,000; Biosource International) and rabbit anti-PAK (1:1,000; Cell Signaling Technologies). For immunocytochemistry, cells were fixed with PBS containing 2% formaldehyde, permeabilized with 0.2% Triton X-100, and blocked for 1 h in PBS containing 1% BSA and 10% goat serum. Primary antibodies were incubated with cells in blocking buffer as follows: rabbit anti–phospho-PAK (Ser141; 1:500 overnight), rabbit anti–β-catenin (1:200 overnight; Santa Cruz Biotechnology, Inc.), and mouse anti–ZO-1 (1:500 overnight). Cells were then incubated in 1 μg/ml Alexa 488– conjugated goat anti–rabbit IgG or goat anti–mouse IgG (Invitrogen). Slides were mounted with Fluoromount G, and images were taken using the 60× oil-immersion objective on a microscope (DiaPhot; Nikon) equipped with a video camera (CoolSnap; Photometrics) using the Inovision ISEE software program.
Permeability assays
A novel transwell well-flow device was developed to assay macromolecule permeability across an intact endothelial monolayer using previously established methods (Stockton et al., 2004). In brief, a previously developed cone-and-plate flow device was adapted to accept a 75-mm chamber transwell insert (Blackman et al., 2002). Custom flanges mounted on the lip of the Petri dish hold inlet and outlet tubing for the top and lower chambers, respectively, to inject and remove HRP without interrupting flow. Transwell chambers (3.0-μm pore size; Costar) were coated with either MG or FN, and BAE cells were allowed to attach for 4–24 h. Some transwells were coated with a fixed concentration of MG followed by increasing concentrations of FN. For flow experiments, cells on 75-mm chambers were serum deprived for 4 h in phenol red-free DME containing 0.5% CS and 2% dextran (wt/vol) and loaded onto the flow device stage, and shear stress was applied using the modified cone-and-plate device. At desired times, the medium was replaced with fresh medium containing 60 μg/ml HRP (Sigma-Aldrich) or Alexa 488–conjugated BSA (Invitrogen). After 1 h, medium was removed from the lower chamber, and cells were fixed in 2% formaldehyde and stained with Coomassie blue to detect cell loss or examined by immunocytochemistry for Ser141 phosphorylated PAK. For cytokine and LDL-induced permeability assays, cells grown on 6.5-mm filters were serum deprived for 4 h in phenol red–free DME containing 0.5% CS and transferred to fresh medium containing soluble factors for 90 min. HRP was then added to the top well to give a final concentration of 60 μg/ml. After 30 min, medium from the bottom well was removed, incubated with 0.5 mM guaiacol, 50 mM Na2HPO4, and 0.6 mM H2O2, and formation of O-phenylenediamine was determined by measure of absorbance at 470 nm. Alexa 488–conjugated BSA was measured using a spectrofluorometer (FluoroLog; Jobin Yvon). Results are shown as a fold increase in HRP activity or in absolute solute permeability. Solute permeability coefficients for the endothelial monolayer were calculated as Ps = ΔCaVa/ΔCΔtS, where ΔCa is the final concentration in the lower well, Va is the volume of the bottom well (ml), ΔC is the concentration in the top well, Δt is the sampling interval (s), and S is the surface area of the transwell (cm2; Kajimura et al., 1997).
Animals and vessel harvest
Nine male ApoE-deficient mice on a C57Bl/6 background from The Jackson Laboratory, 8–12 wk of age and weighing 18–20 g, were used in these experiments. Four mice were fed a Western-type atherogenic diet (TD 88137 [Harlan-Teklad]; containing 21% fat by weight, 0.15% by weight cholesterol, and 19.5% by weight casein without sodium cholate) for 10 wk before sacrifice. Control mice were fed a chow diet during this time. At 20 wk of age (10 wk on diet), mice were perfused with 4% paraformaldehyde, and the aortic arch, left carotid sinus, and right carotid sinus were processed for paraffin embedding. For Evans blue assays, six male C57Bl/6 and nine male ApoE-deficient mice (The Jackson Laboratory) were maintained on chow diets for 8 or 32 wk, respectively.
Immunohistochemistry
5-μm paraffin sections were obtained for immunohistochemistry. Immunohistochemistry for adhesion molecules VCAM-1 (Santa Cruz Biotechnology, Inc.) was performed as previously described (McPherson et al., 2001). After microwave antigen retrieval with antigen unmasking solution (Vector Laboratories), rabbit anti-FN (1:400; Sigma-Aldrich) and rabbit anti-Ser141 phosphorylated PAK (1:250) were applied. Detection of antibodies was with Vetastain Elite kit (Vector Laboratories). Visualization was with diaminobenzidine (DakoCytomation). For en face staining, the aortic arch was cut into rings and stained for either PECAM-1 or Ser141 phosphorylated PAK using Alexa 488–conjugated goat anti–rabbit secondary antibodies to detect localization. Rings were then cut, opened, and mounted between two coverslips for en face viewing by fluorescence microscopy. Images were acquired using the 10× or 40× objective on a microscope (BX51; Olympus) equipped with a digital camera (DP70; Olympus) using ImagePro Plus software (Media Cybernetics).
Permeability to Evans blue in vivo
Mice were injected intraperitoneally with 0.1 ml of either control peptide or the PAK-Nck inhibitory peptide (10 mg/ml) at 24 h and at 1 h before Evans blue injection. Evans blue (0.1 ml of 1% dye in PBS) was injected into the tail vein. After 30 min, mice were killed with ketamine/xylazine and perfused through the left ventricle with 10 ml of 4% formaldehyde in PBS, and the aorta was excised from the cusp to the renal artery branches. Bright field microscopy of excised aortas was performed using the 0.5 and 1.2× objectives on a microscope (SZX12; Olympus) equipped with a DP70 digital camera using ImagePro Plus. Aortas were dried and weighed, Evans blue was extracted by incubation in formamide for 24 h at 60°C, and absorbance at 620 nm was determined. Concentration curves for pure Evans blue were used to calculate the total amount of dye extracted, and this value was normalized to the weight of the isolated aortas.
Online supplemental material
Independent of matrix composition, the 4-h plating time is sufficient to allow both adherens and TJ formation, as assessed by staining cells for β-catenin and ZO-1, respectively (Fig. S1). Matrix-specific effects on monolayer permeability are not due to differences in matrix permeability, which shows no difference between MG and FN (Fig. S2). Localization to cell–cell junctions is required for PAK-dependent permeability (Stockton et al., 2004), and TNFα, MCP-1, and oxLDL all stimulate active PAK localization to cell–cell junctions, which was abrogated by the addition of the PAK-Nck inhibitory peptide (Fig. S3). Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200609008/DC1.
Acknowledgments
The authors acknowledge Bradley Gelfand for assistance with the flow profiles, Melissa Bevard for her assistance in preparing the immunohistochemical stains, and Yinling Yi and Lukas Tamm for assistance with the fluorimeter measurements. The authors would also like to acknowledge Daniel Bennet and Elizabeth Thao Phan for their assistance in vessel isolation and Gina Wimer for assistance with tail vein injections.
This work was supported by U.S. Public Health Service grant RO1 HL75092 to M.A. Schwartz, American Heart Association Mid-Atlantic Affiliate fellowship 0525589U to A.W. Orr, National Institutes of Health grant 1RO1HL66264 to I.J. Sarembock, and The Whitaker Foundation Biomedical Research grant RG-02-0853 to B.R. Blackman.
Abbreviations used in this paper: AID, autoinhibitory domain; BAE, bovine aortic endothelial; CCA, common carotid artery; CS, calf serum; FN, fibronectin; ICS, internal carotid sinus; LDL, low-density lipoprotein; MCP-1, monocyte chemotactic protein-1; MG, matrigel; oxLDL, oxidized LDL; PAK, p21-activated kinase; TJ, tight junction; VCAM-1, vascular cell adhesion molecule 1.
References
- Alahari, S.K., P.J. Reddig, and R.L. Juliano. 2004. The integrin-binding protein Nischarin regulates cell migration by inhibiting PAK. EMBO J. 23:2777–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman, B.R., G. Garcia-Cardena, and M.A. Gimbrone Jr. 2002. A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J. Biomech. Eng. 124:397–407. [DOI] [PubMed] [Google Scholar]
- Bokoch, G.M. 2003. Biology of the p21-activated kinases. Annu. Rev. Biochem. 72:743–781. [DOI] [PubMed] [Google Scholar]
- Brett, J., H. Gerlach, P. Nawroth, S. Steinberg, G. Godman, and D. Stern. 1989. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J. Exp. Med. 169:1977–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, A.R., P.I. Lelkes, and G.M. Rubanyi. 2004. Gene expression profiling of vascular endothelial cells exposed to fluid mechanical forces: relevance for focal susceptibility to atherosclerosis. Endothelium. 11:45–57. [DOI] [PubMed] [Google Scholar]
- Chen, J., P.R. Somanath, O. Razorenova, W.S. Chen, N. Hay, P. Bornstein, and T.V. Byzova. 2005. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat. Med. 11:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, C., L. Tan, L. Lim, and E. Manser. 2001. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J. Biol. Chem. 276:17347–17353. [DOI] [PubMed] [Google Scholar]
- De Keulenaer, G.W., D.C. Chappell, N. Ishizaka, R.M. Nerem, R.W. Alexander, and K.K. Griendling. 1998. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ. Res. 82:1094–1101. [DOI] [PubMed] [Google Scholar]
- DeMaio, L., Y.S. Chang, T.W. Gardner, J.M. Tarbell, and D.A. Antonetti. 2001. Shear stress regulates occludin content and phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 281:H105–H113. [DOI] [PubMed] [Google Scholar]
- Essler, M., M. Retzer, M. Bauer, J.W. Heemskerk, M. Aepfelbacher, and W. Siess. 1999. Mildly oxidized low density lipoprotein induces contraction of human endothelial cells through activation of Rho/Rho kinase and inhibition of myosin light chain phosphatase. J. Biol. Chem. 274:30361–30364. [DOI] [PubMed] [Google Scholar]
- Fryer, B.H., C. Wang, S. Vedantam, G.L. Zhou, S. Jin, L. Fletcher, M.C. Simon, and J. Field. 2006. cGMP-dependent protein kinase phosphorylates p21-activated kinase (Pak) 1, inhibiting Pak/Nck binding and stimulating Pak/vasodilator-stimulated phosphoprotein association. J. Biol. Chem. 281:11487–11495. [DOI] [PubMed] [Google Scholar]
- Gatti, A., Z. Huang, P.T. Tuazon, and J.A. Traugh. 1999. Multisite autophosphorylation of p21-activated protein kinase gamma-PAK as a function of activation. J. Biol. Chem. 274:8022–8028. [DOI] [PubMed] [Google Scholar]
- Gavard, J., and J.S. Gutkind. 2006. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8:1223–1234. [DOI] [PubMed] [Google Scholar]
- Gelfand, B., F. Epstein, and B.R. Blackman. 2006. Spatial and spectral heterogeneity of time-varying shear stress profiles in the carotid bifurcation by phase-contrast MRI. J. Magn. Reson. Imaging. 24:1386–1392. [DOI] [PubMed] [Google Scholar]
- Gu, L., Y. Okada, S.K. Clinton, C. Gerard, G.K. Sukhova, P. Libby, and B.J. Rollins. 1998. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell. 2:275–281. [DOI] [PubMed] [Google Scholar]
- Himburg, H.A., D.M. Grzybowski, A.L. Hazel, J.A. LaMack, X.M. Li, and M.H. Friedman. 2004. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am. J. Physiol. Heart Circ. Physiol. 286:H1916–H1922. [DOI] [PubMed] [Google Scholar]
- Howe, A.K., and R.L. Juliano. 2000. Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat. Cell Biol. 2:593–600. [DOI] [PubMed] [Google Scholar]
- Jaffe, A.B., and A. Hall. 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21:247–269. [DOI] [PubMed] [Google Scholar]
- Jalali, S., M.A. del Pozo, K. Chen, H. Miao, Y. Li, M.A. Schwartz, J.Y. Shyy, and S. Chien. 2001. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc. Natl. Acad. Sci. USA. 98:1042–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, H., R.O. Dull, T.M. Hollis, and J.M. Tarbell. 1991. Endothelial albumin permeability is shear dependent, time dependent, and reversible. Am. J. Physiol. 260:H1992–H1996. [DOI] [PubMed] [Google Scholar]
- Kajimura, M., M.E. O'Donnell, and F.E. Curry. 1997. Effect of cell shrinkage on permeability of cultured bovine aortic endothelia and frog mesenteric capillaries. J. Physiol. 503:413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiosses, W.B., R.H. Daniels, C. Otey, G.M. Bokoch, and M.A. Schwartz. 1999. A role for p21-activated kinase in endothelial cell migration. J. Cell Biol. 147:831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiosses, W.B., J. Hood, S. Yang, M.E. Gerritsen, D.A. Cheresh, N. Alderson, and M.A. Schwartz. 2002. A dominant-negative p65 PAK peptide inhibits angiogenesis. Circ. Res. 90:697–702. [DOI] [PubMed] [Google Scholar]
- Koh, C.G., E.J. Tan, E. Manser, and L. Lim. 2002. The p21-activated kinase PAK is negatively regulated by POPX1 and POPX2, a pair of serine/threonine phosphatases of the PP2C family. Curr. Biol. 12:317–321. [DOI] [PubMed] [Google Scholar]
- Kumar, R., and R.K. Vadlamudi. 2002. Emerging functions of p21-activated kinases in human cancer cells. J. Cell. Physiol. 193:133–144. [DOI] [PubMed] [Google Scholar]
- LaMack, J.A., H.A. Himburg, X.M. Li, and M.H. Friedman. 2005. Interaction of wall shear stress magnitude and gradient in the prediction of arterial macromolecular permeability. Ann. Biomed. Eng. 33:457–464. [DOI] [PubMed] [Google Scholar]
- Lin, S.J., K.M. Jan, G. Schuessler, S. Weinbaum, and S. Chien. 1988. Enhanced macromolecular permeability of aortic endothelial cells in association with mitosis. Atherosclerosis. 73:223–232. [DOI] [PubMed] [Google Scholar]
- Lu, W., S. Katz, R. Gupta, and B.J. Mayer. 1997. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr. Biol. 7:85–94. [DOI] [PubMed] [Google Scholar]
- Malinauskas, R.A., R.A. Herrmann, and G.A. Truskey. 1995. The distribution of intimal white blood cells in the normal rabbit aorta. Atherosclerosis. 115:147–163. [DOI] [PubMed] [Google Scholar]
- Martin, S., K. Maruta, V. Burkart, S. Gillis, and H. Kolb. 1988. IL-1 and IFN-gamma increase vascular permeability. Immunology. 64:301–305. [PMC free article] [PubMed] [Google Scholar]
- McPherson, J.A., K.G. Barringhaus, G.G. Bishop, J.M. Sanders, J.M. Rieger, S.E. Hesselbacher, L.W. Gimple, E.R. Powers, T. Macdonald, G. Sullivan, et al. 2001. Adenosine A(2A) receptor stimulation reduces inflammation and neointimal growth in a murine carotid ligation model. Arterioscler. Thromb. Vasc. Biol. 21:791–796. [DOI] [PubMed] [Google Scholar]
- Moldobaeva, A., L.E. Welsh-Servinsky, L.A. Shimoda, R.S. Stephens, A.D. Verin, R.M. Tuder, and D.B. Pearse. 2006. Role of protein kinase G in barrier-protective effects of cGMP in human pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L919–L930. [DOI] [PubMed] [Google Scholar]
- Ogunrinade, O., G.T. Kameya, and G.A. Truskey. 2002. Effect of fluid shear stress on the permeability of the arterial endothelium. Ann. Biomed. Eng. 30:430–446. [DOI] [PubMed] [Google Scholar]
- Ohta, H., H. Wada, T. Niwa, H. Kirii, N. Iwamoto, H. Fujii, K. Saito, K. Sekikawa, and M. Seishima. 2005. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 180:11–17. [DOI] [PubMed] [Google Scholar]
- Okano, M., and Y. Yoshida. 1994. Junction complexes of endothelial cells in atherosclerosis-prone and atherosclerosis-resistant regions on flow dividers of brachiocephalic bifurcations in the rabbit aorta. Biorheology. 31:155–161. [DOI] [PubMed] [Google Scholar]
- Orr, A.W., M.A. Pallero, and J.E. Murphy-Ullrich. 2002. Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J. Biol. Chem. 277:20453–20460. [DOI] [PubMed] [Google Scholar]
- Orr, A.W., J.M. Sanders, M. Bevard, E. Coleman, I.J. Sarembock, and M.A. Schwartz. 2005. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J. Cell Biol. 169:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, A.W., B.P. Helmke, B.R. Blackman, and M.A. Schwartz. 2006. Mechanisms of mechanotransduction. Dev. Cell. 10:11–20. [DOI] [PubMed] [Google Scholar]
- Pearse, D.B., L.A. Shimoda, A.D. Verin, N. Bogatcheva, C. Moon, G.V. Ronnett, L.E. Welsh, and P.M. Becker. 2003. Effect of cGMP on lung microvascular endothelial barrier dysfunction following hydrogen peroxide. Endothelium. 10:309–317. [DOI] [PubMed] [Google Scholar]
- Penn, M.S., and G.M. Chisolm. 1991. Relation between lipopolysaccharide-induced endothelial cell injury and entry of macromolecules into the rat aorta in vivo. Circ. Res. 68:1259–1269. [DOI] [PubMed] [Google Scholar]
- Phelps, J.E., and N. DePaola. 2000. Spatial variations in endothelial barrier function in disturbed flows in vitro. Am. J. Physiol. Heart Circ. Physiol. 278:H469–H476. [DOI] [PubMed] [Google Scholar]
- Puto, L.A., K. Pestonjamasp, C.C. King, and G.M. Bokoch. 2003. p21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. J. Biol. Chem. 278:9388–9393. [DOI] [PubMed] [Google Scholar]
- Reddick, R.L., S.H. Zhang, and N. Maeda. 1994. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler. Thromb. 14:141–147. [DOI] [PubMed] [Google Scholar]
- Ross, R. 1999. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 340:115–126. [DOI] [PubMed] [Google Scholar]
- Sakakibara, A., M. Furuse, M. Saitou, Y. Ando-Akatsuka, and S. Tsukita. 1997. Possible involvement of phosphorylation of occludin in tight junction formation. J. Cell Biol. 137:1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess, W., K.J. Zangl, M. Essler, M. Bauer, R. Brandl, C. Corrinth, R. Bittman, G. Tigyi, and M. Aepfelbacher. 1999. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. USA. 96:6931–6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic, S.M., R.F. Keep, S.L. Kunkel, and A.V. Andjelkovic. 2003. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J. Cell Sci. 116:4615–4628. [DOI] [PubMed] [Google Scholar]
- Steinberg, D. 1997. A critical look at the evidence for the oxidation of LDL in atherogenesis. Atherosclerosis. 131(Suppl.):S5–S7. [DOI] [PubMed] [Google Scholar]
- Stockton, R.A., E. Schaefer, and M.A. Schwartz. 2004. p21-activated kinase regulates endothelial permeability through modulation of contractility. J. Biol. Chem. 279:46621–46630. [DOI] [PubMed] [Google Scholar]
- Takeya, M., T. Yoshimura, E.J. Leonard, and K. Takahashi. 1993. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum. Pathol. 24:534–539. [DOI] [PubMed] [Google Scholar]
- Traub, O., and B.C. Berk. 1998. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler. Thromb. Vasc. Biol. 18:677–685. [DOI] [PubMed] [Google Scholar]
- Tzima, E., M.A. del Pozo, S.J. Shattil, S. Chien, and M.A. Schwartz. 2001. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 20:4639–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima, E., M.A. del Pozo, W.B. Kiosses, S.A. Mohamed, S. Li, S. Chien, and M.A. Schwartz. 2002. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 21:6791–6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima, E., W.B. Kiosses, M.A. del Pozo, and M.A. Schwartz. 2003. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J. Biol. Chem. 278:31020–31023. [DOI] [PubMed] [Google Scholar]
- VanderLaan, P.A., C.A. Reardon, and G.S. Getz. 2004. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 24:12–22. [DOI] [PubMed] [Google Scholar]
- Weinbaum, S., G. Tzeghai, P. Ganatos, R. Pfeffer, and S. Chien. 1985. Effect of cell turnover and leaky junctions on arterial macromolecular transport. Am. J. Physiol. 248:H945–H960. [DOI] [PubMed] [Google Scholar]
- Xia, C., W. Ma, L.J. Stafford, S. Marcus, W.C. Xiong, and M. Liu. 2001. Regulation of the p21-activated kinase (PAK) by a human Gbeta -like WD-repeat protein, hPIP1. Proc. Natl. Acad. Sci. USA. 98:6174–6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, S.Y. 2002. Protein kinase signaling in the modulation of microvascular permeability. Vascul. Pharmacol. 39:213–223. [DOI] [PubMed] [Google Scholar]
- Zhao, L., Q.L. Ma, F. Calon, M.E. Harris-White, F. Yang, G.P. Lim, T. Morihara, O.J. Ubeda, S. Ambegaokar, J.E. Hansen, et al. 2006. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat. Neurosci. 9:234–242. [DOI] [PubMed] [Google Scholar]
- Zhou, G.L., Y. Zhuo, C.C. King, B.H. Fryer, G.M. Bokoch, and J. Field. 2003. Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol. Cell. Biol. 23:8058–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]