Abstract
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) is a threat to human health worldwide. Although progress has been made, mechanisms of CA-MRSA pathogenesis are poorly understood and a comprehensive analysis of CA-MRSA exoproteins has not been conducted. To address that deficiency, we used proteomics to identify exoproteins made by MW2 (USA400) and LAC (USA300) during growth in vitro. Two hundred and fifty unique exoproteins were identified by 2-dimensional gel electrophoresis coupled with automated direct infusion-tandem mass spectrometry (ADI-MS/MS) analysis. Eleven known virulence-related exoproteins differed in abundance between the strains, including alpha-haemolysin (Hla), collagen adhesin (Cna), staphylokinase (Sak), coagulase (Coa), lipase (Lip), enterotoxin C3 (Sec3), enterotoxin Q (Seq), V8 protease (SspA) and cysteine protease (SspB). Mice infected with MW2 or LAC produced antibodies specific for known or putative virulence factors, such as autolysin (Atl), Cna, Ear, ferritin (Ftn), Lip, 1-phosphatidylinositol phosphodiesterase (Plc), Sak, Sec3 and SspB, indicating the exoproteins are made during infection in vivo. We used confocal microscopy to demonstrate aureolysin (Aur), Hla, SspA and SspB are produced following phagocytosis by human neutrophils, thereby linking exoprotein production in vitro with that during host–pathogen interaction. We conclude that the exoproteins identified herein likely account in part for the success of CA-MRSA as a human pathogen.
Introduction
Staphylococcus aureus causes a wide range of human diseases, including impetigo, cellulitis, food poisoning, toxic–shock syndrome, necrotizing pneumonia, endocarditis and sepsis (Lowy, 1998; Diekema et al., 2001). Decades of selective pressure with β-lactam antibiotics and close proximity of susceptible hosts have resulted in a high prevalence of methicillin-resistant S. aureus (MRSA) in hospitals worldwide (Chambers, 2001; Diekema et al., 2001). Although these factors logically explain the high incidence of hospital-associated MRSA infections, the molecular basis for the increased incidence and severity of community-acquired (or associated) MRSA (CA-MRSA) infections among healthy individuals remains incompletely defined (Chambers, 2001; 2005; McDougal et al., 2003; Fridkin et al., 2005; Miller et al., 2005; Zetola et al., 2005). Recent studies indicate strains that are the leading causes of CA-MRSA disease in the United States, represented by pulsed-field gel electrophoresis (PFGE) types USA300-0114 (McDougal et al., 2003; Fridkin et al., 2005; Kazakova et al., 2005; Diep et al., 2006) and USA400 (Centers for Disease Control and Prevention, 1999; Baba et al., 2002; McDougal et al., 2003; Adem et al., 2005), have enhanced virulence compared with leading causes of hospital infections (e.g. USA200) (Voyich et al., 2005). In addition to their distinct PFGE profiles (McDougal et al., 2003), these two CA-MRSA strains can be distinguished from one another and from other S. aureus strains on the basis of multilocus sequence typing (MLST or ST) and sequencing of the variable number tandem repeats in the staphylococcal protein A gene (spa); USA300-0114 is spa-type 1 (Shopsin et al., 1999) and multilocus sequence type ST8, while USA400 is ST1 (Enright et al., 2000). Both strains also have the type IV staphylococcal chromosomal cassette mec element, which is common among CA-MRSA but not typically found in hospital adapted nosocomial MRSA (Baba et al., 2002; Voyich et al., 2005; Diep et al., 2006). In addition, each strain has one or more common names, such as Los Angeles County clone (LAC) or FPR3757 (sequenced strain) used for USA300-0114 (Voyich et al., 2005; Diep et al., 2006), and MW2 for the prototype USA400 strain (Centers for Disease Control and Prevention, 1999; Baba et al., 2002). Enhanced virulence of USA300-0114 (referred to herein as either LAC or USA300) and USA400 (referred to herein as MW2) is linked in part to their ability to circumvent killing by neutrophils and cause host cell lysis (Voyich et al., 2005). It is likely that exoproteins (cell surface-associated and freely secreted proteins) produced by these strains are an important component of this enhanced virulence (Foster, 2005; Voyich et al., 2005; Diep et al., 2006).
Secreted virulence proteins of S. aureus can be categorized based on proven or putative function. Cytolytic toxins, such as haemolysins (Hla, Hlb, HlgABC) and leukocidins (LukD/E and Panton–Valentine leukocidin, PVL), oligomerize to form pores on the cell surface (Walev et al., 1993; Bhakdi et al., 1998). Destruction of leucocytes (especially neutrophils), which can be mediated by these toxins, is likely a key component of CA-MRSA pathogenesis. For example, PVL has high specificity for granulocytes and is linked by epidemiology to CA-MRSA disease (Lina et al., 1999), although our recent studies indicate the toxin has a limited role in virulence (Voyich et al., 2006). Staphylococcal enterotoxins are secreted superantigens (SAg) that bind to major histocompatibility complex (MHC) class II proteins, resulting in CD4+ T-cell activation and immune modulation (Malchiodi et al., 1995; McCormick et al., 2001; Orwin et al., 2002; Llewelyn et al., 2004). S. aureus also secretes numerous proteases and lipases that degrade host components, and proteins that sequester antibody or inactivate antibiotics (Foster, 2005). As a step towards understanding the role played by S. aureus exoproteins in virulence, previous proteomics-based studies identified immunogenic proteins produced by strain COL (Vytvytska et al., 2002), evaluated the role played by S. aureus agr and sigB on secretion of virulence factors (Ziebandt et al., 2001), and tested the effects of linezolid on production of virulence factors (Bernardo et al., 2004). However, a comprehensive analysis of the exoproteins produced by CA-MRSA has not been conducted.
To that end, we used a proteomic approach to identify exoproteins of LAC and MW2 during growth in vitro and evaluated immunogenicity of the proteins using sera from mice infected with each strain. In addition, we used confocal microscopy to determine that selected exoproteins were produced within phagocytic vacuoles of human neutrophils following uptake, a phenomenon accompanied by host cell lysis.
Results
Resolution and identification of culture supernatant proteins produced by CA-MRSA strains
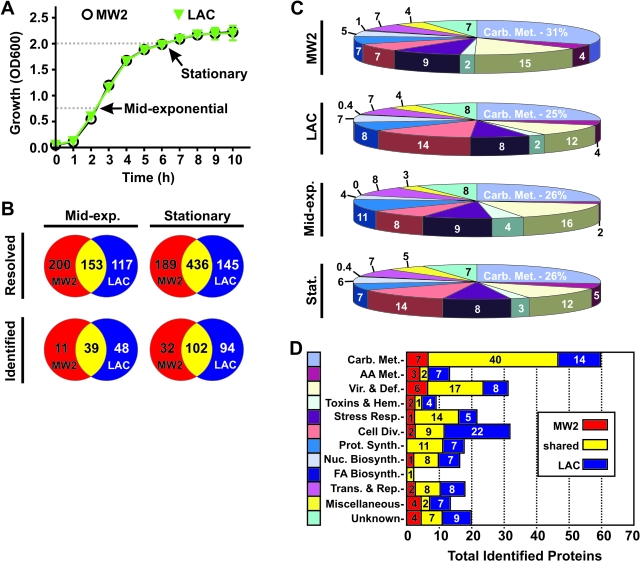
As an initial step towards gaining a comprehensive understanding of exoproteins made by the most prominent CA-MRSA strains, we resolved/identified proteins in MW2 (USA400) and LAC (USA300) culture supernatants using 2-dimensional gel electrophoresis (2-DGE) coupled with automated direct infusion-tandem mass spectrometry (ADI-MS/MS). Three hundred and fifty-three and 270 protein spots were resolved from MW2 and LAC culture media, respectively, at mid-exponential phase of growth (Fig. 1A–D). By comparison, 625 MW2 and 581 LAC proteins were resolved from culture supernatants at stationary phase of growth (Fig. 1B). Of the resolved exoproteins, 153 (60.2 ± 18%) from cultures at mid-exponential growth and 436 (67.9 ± 6.6%) from cultures at stationary growth matched between strains, indicating MW2 and LAC produce numerous proteins that co-migrate during 2-DGE (Fig. 2). We note that this analysis fails to account for variations of same or similar proteins with slightly different migration by 2-DGE. Therefore, our estimate of the degree of similarity between the two strains at the level of exoprotein (60% to 68%) is relatively conservative.
Fig. 1.
Distribution and function of proteins found in MW2 and LAC culture supernatants. A. MW2 and LAC were cultured to mid-exponential (Mid-exp.) or stationary phases of growth as indicated, at which point proteins were identified/resolved using proteomics as described in Experimental procedures. B. Numbers in yellow-shaded regions represent proteins identified in both MW2 and LAC supernatants at the indicated growth phase. Numbers in red- or blue-shaded regions indicate proteins identified in MW2 or LAC supernatants respectively. Results are derived from three separate experiments. C. Proteins identified by ADI-MS/MS were categorized based upon functional annotation. Numbers are the per cent of total proteins identified. D. Proteins identified at mid-exponential and stationary phases of growth were enumerated and categorized by functional annotation. Carb. Met., carbohydrate transport and metabolism; AA Met., amino acid transport and metabolism; Vir. & Def., virulence and defence mechanisms; Toxins & Hem., toxins and haemolysins; Stress Resp., stress response; Cell Div., cell division and maintenance; Prot. Synth., protein synthesis; Nuc. Biosynth., nucleotide biosynthesis; FA Biosynth., fatty acid biosynthesis; Trans. & Rep., transcription and replication.
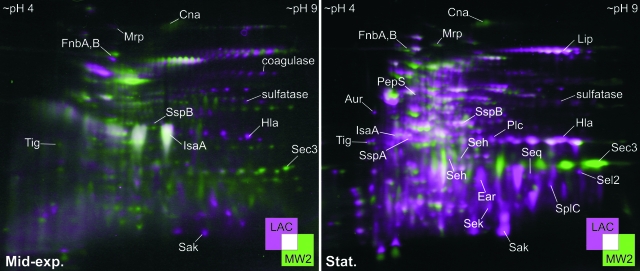
Fig. 2.
2-DGE analyses of culture supernatant proteins produced by MW2 and LAC. Proteins from MW2 (green) and LAC (magenta) culture supernatants were analysed by proteomics. Left panel, proteins from cultures at mid-exponential (Mid-exp.) phase of growth. Right panel, proteins from cultures at stationary (Stat.) phase of growth. White areas are regions of overlap. Selected proteins are indicated. Images are representative of three separate experiments.
Using ADI-MS/MS and excluding protein isoforms and identifications from multiple gels, we identified 250 unique proteins from MW2 and LAC culture supernatants at the two phases of growth combined (98 at mid-exponential phase of growth and 228 at stationary phase) (Fig. 2 and Table 1). Proteins were separated into categories based on functional annotation to facilitate subsequent analyses (Fig. 1B and C, and Table 1).
Table 1.
MW2 and LAC culture supernatant proteins identified by ADI-MS/MS.
| Protein name (Entrez protein name)a | Entrez proteina | MWE | pIE | MP | SC% | MS/MS MOWSE score |
|---|---|---|---|---|---|---|
| Carbohydrate transport and metabolism (61) | ||||||
| Acetate kinase (AckA)C1,D1 | 13701506 | 44029 | 5.7 | 10 | 31 | 226 |
| Acetoin reductase (ButA)D2 | 49482369 | 27199 | 5.0 | 3 | 15 | 104 |
| Aconitate hydratase (AcnA)B1,C1,D1 | 49241672 | 98850 | 4.9 | 24 | 34 | 878 |
| Adenylate kinase (Adk)B1,D2 | 49484445 | 23959 | 4.8 | 6 | 29 | 245 |
| Adenylosuccinate synthetase, putative (PurA)C1 | 49482270 | 47522 | 5.1 | 4 | 12 | 146 |
| Alcohol dehydrogenase (Adh)B5,D6 | 14246373 | 36039 | 5.5 | 13 | 45 | 558 |
| Alcohol dehydrogenase, zinc-containing (AdhE)D1 | 87162223 | 36244 | 5.3 | 4 | 13 | 93 |
| Aldehyde dehydrogenase (AldA1)B1,D1 | 49242475 | 51936 | 5.1 | 7 | 19 | 179 |
| ATP synthase beta chain (AtpD)A1,C2,D1 | 49484327 | 51368 | 4.7 | 4 | 11 | 101 |
| ATP synthase alpha chain (AtpA)D1 | 49484329 | 54550 | 4.9 | 4 | 9 | 82 |
| Citrate synthase II (GltA)B2,C1,D2 | 49483937 | 42566 | 5.4 | 14 | 50 | 507 |
| CoA synthetase protein, putative (FadE)B1,C1,D1 | 49483408 | 42060 | 4.9 | 9 | 23 | 256 |
| Deoxyribose-phosphate aldolase (Dra)B1 | 14247910 | 23327 | 4.7 | 3 | 17 | 56 |
| Dihydrolipoamide acetyltransferase: subunit E2 (PdhC)A2,C2,D2 | 581570 | 46411 | 4.9 | 16 | 46 | 817 |
| Dihydrolipoamide dehydrogenase: subunit E3 (PdhC)A2,B2,C1,D2 | 48874 | 49421 | 5.0 | 16 | 46 | 803 |
| Phosphoglycerate mutase (Gpm)B2 | 14246544 | 56419 | 4.7 | 12 | 29 | 590 |
| Enolase (Eno)A2,B3,C2,D4 | 6015099 | 47088 | 4.5 | 18 | 54 | 1257 |
| Methylenetetrahydrofolate dehydrogenase (FolD)B2,D1 | 49244345 | 30824 | 5.4 | 13 | 68 | 859 |
| Formate tetrahydrofolate ligase (Fhs)B1,C1,D1 | 83288210 | 59876 | 5.8 | 16 | 44 | 759 |
| Formate acetyltransferase (PflB)D2 | 49482458 | 84808 | 5.3 | 6 | 11 | 193 |
| Fructose bisphosphate aldolase class I (Fba)A1,B4,C2,D6 | 88196553 | 33034 | 4.9 | 14 | 57 | 683 |
| D-Fructose 6-phosphate amidotransferase (GlmS)D1 | 49484376 | 65839 | 4.9 | 6 | 15 | 151 |
| 6-Phospho-beta-galactosidase (LacG)A1 | 644835 | 116131 | 5.3 | 1 | 1 | 42 |
| Glucose 6-phosphate isomerase A (GpiA)A1,B1,C1,D1 | 49244181 | 49777 | 4.8 | 20 | 53 | 1008 |
| Glucose 6-phosphate 1-dehydrogenase (Zwf)D1 | 49483755 | 56929 | 5.3 | 7 | 15 | 227 |
| Glyceraldehyde 3-phosphate dehydrogenase 1 (Gap)A2,B5,C3,D5 | 49244087 | 36258 | 4.9 | 13 | 47 | 770 |
| Glyceraldehyde 3-phosphate dehydrogenase subunit B (GapB)D1 | 38195941 | 36899 | 6.0 | 4 | 14 | 106 |
| Glyceraldehyde 3-phosphate dehydrogenase subunit C (GapC)C1,D1 | 38195943 | 36227 | 4.9 | 3 | 10 | 65 |
| Glycerate dehydrogenase (MW2224)B1,D2 | 14248079 | 34681 | 5.1 | 10 | 41 | 377 |
| Glycine cleavage system H protein (GcvH)A1,C1,D2 | 49241194 | 14072 | 4.0 | 2 | 30 | 194 |
| Glycine C-acetyltransferase, similar to (MW0505)D1 | 14246318 | 41426 | 5.3 | 2 | 5 | 58 |
| Hexulose 6-phosphate synthase, putative (SgaH)B1,D1 | 87160968 | 22404 | 4.6 | 12 | 81 | 656 |
| Hydrolase (HAD superfamily) (MW0575)B1,D1 | 49241218 | 27962 | 4.5 | 4 | 16 | 67 |
| Indole-3-pyruvate decarboxylase, putative (IpdC)D1 | 14245955 | 60490 | 5.1 | 1 | 2 | 59 |
| Isocitrate dehydrogenase (Idh)B1,D1 | 49244963 | 46408 | 4.9 | 4 | 10 | 129 |
| L-Lactate dehydrogenase 1 (Ddh)B3,D4 | 57286685 | 34562 | 5.0 | 10 | 34 | 368 |
| Malate:quinone oxidoreductase (Mqo2)A1,B1,C1,D1 | 21205698 | 55892 | 6.2 | 7 | 17 | 301 |
| Mannitol-1-phosphate 5-dehydrogenase (MtlD)B3,D1 | 49242510 | 40801 | 5.0 | 10 | 36 | 357 |
| 2-C-Methyl-D-erythritol 4-phosphate cytidylyltransferase (IspDF)B1 | 49482491 | 26640 | 5.4 | 3 | 12 | 77 |
| NAD-dependent dehydrogenase, putative (MW2068)B2 | 49245380 | 24057 | 5.0 | 15 | 81 | 643 |
| Oxoglutarate dehydrogenase (Ogdh)B1,D1 | 21204471 | 105289 | 5.4 | 14 | 22 | 401 |
| Phosphate acetyltransferase, putative (Pta)A1,B4,C1,D5 | 22212856 | 20383 | 4.8 | 8 | 44 | 523 |
| Phosphoenolpyruvate carboxykinase (PckA)B1,D1 | 49484033 | 59370 | 5.7 | 20 | 44 | 678 |
| Phosphogluconate dehydrogenase (Pgd)A1,C1,D1 | 14247282 | 51751 | 5.1 | 5 | 15 | 122 |
| Phosphopentomutase, putative (Drm)A1,B1,C2,D2 | 14246544 | 56419 | 4.7 | 14 | 36 | 672 |
| Phosphoenolpyruvate-protein phosphatase (PtsI)B1,D1 | 1070386 | 63179 | 4.7 | 12 | 22 | 490 |
| 6-Phosphofructokinase (PfkA)B2,D1 | 49244967 | 34818 | 5.6 | 11 | 34 | 327 |
| 6-Phosphogluconate dehydrogenase, decarboxylating (Gnd)B1,D2 | 49483761 | 51770 | 5.0 | 12 | 30 | 564 |
| Phosphoglycerate kinase (Pgk)A1,B2,C3,D2 | 49483031 | 42575 | 5.2 | 14 | 47 | 615 |
| 1-Pyrroline-5-carboxylate dehydrogenase (RocA)B1,D1 | 21205647 | 56833 | 5.0 | 16 | 39 | 687 |
| Pyruvate carboxylase, putative (PycA)B1,D1 | 49483277 | 128451 | 5.2 | 14 | 14 | 353 |
| Pyruvate dehydrogenase E1 component, alpha subunit, putative (PdhA)A1,C2 | 49244374 | 41357 | 4.9 | 7 | 32 | 239 |
| Pyruvate dehydrogenase E1 component, beta subunit, putative (PdhB)A2,B4,C1,D3 | 57285889 | 35194 | 4.7 | 10 | 43 | 525 |
| Pyruvate kinase (Pyk)A1,B2,C3,D1 | 49242068 | 63103 | 5.2 | 18 | 38 | 610 |
| Short chain dehydrogenase MW2249 (MW2249)D3 | 21205420 | 31769 | 4.6 | 4 | 12 | 179 |
| Succinyl-CoA synthetase (SucD)B1 | 49244528 | 31506 | 5.5 | 5 | 25 | 154 |
| Succinyl-CoA synthetase (SucB)D3 | 49483408 | 42060 | 4.9 | 7 | 18 | 246 |
| Tagatose-bisphosphate aldolase, putative (LacD1)A1,B6 | 49245361 | 30817 | 5.0 | 8 | 40 | 371 |
| Transaldolase, putative (MW1721)B4,D2 | 14247553 | 25742 | 4.8 | 7 | 37 | 273 |
| Transketolase (Tkt)B1,D1 | 57284532 | 72206 | 5.0 | 19 | 35 | 703 |
| Triosephosphate isomerase (Tpi)A1,B3,C2,D3 | 49483032 | 27245 | 4.8 | 8 | 39 | 477 |
| Amino acid transport and metabolism (12) | ||||||
| Alanine dehydrogenase 2 (Ald2)D1 | 49244722 | 40209 | 5.2 | 6 | 18 | 173 |
| Amidophosphoribosyltransferase precursor, putative (PurF)B1 | 49244352 | 54363 | 6.1 | 12 | 23 | 426 |
| Cysteine synthase (CysK)C2 | 82750220 | 32969 | 5.4 | 8 | 52 | 273 |
| 3-Deoxy-7-phosphoheptulonate synthase (MW1680)B1,D1 | 49483977 | 40609 | 5.8 | 7 | 24 | 229 |
| Glucosamine-fructose 6-phosphate aminotransferase (GlmS)D1 | 14247927 | 65795 | 4.9 | 5 | 11 | 110 |
| Glutamine synthetase (GlnA)B1 | 1134886 | 50808 | 5.1 | 11 | 34 | 400 |
| Imidazolonepropionase (HutI)D1 | 87162411 | 45011 | 5.2 | 5 | 15 | 101 |
| Phosphoribosylformylglycinamidine synthase I (PurQ)B1 | 14246838 | 24541 | 5.0 | 3 | 21 | 108 |
| Phosphotransferase system enzyme IIA-like protein (SH1484)D1 | 88195154 | 17949 | 4.5 | 4 | 33 | 144 |
| SNO glutamine amidotransferase family protein (MW0475)D1 | 49482749 | 20617 | 5.7 | 6 | 43 | 211 |
| Thiamine pyrophosphate enzyme, putative (MW0162)B1,D1 | 49240559 | 60503 | 5.0 | 6 | 13 | 181 |
| Urocanate hydratase (HutU)C1 | 14248105 | 60626 | 5.2 | 4 | 12 | 55 |
| Virulence/defence mechanisms (31) | ||||||
| Aminopeptidase PepS (PepS)D1,S | 87161826 | 46805 | 4.8 | 8 | 19 | 226 |
| Aureolysin (Aur)C1,D1,S | 6119705 | 56281 | 5.1 | 3 | 8 | 102 |
| Chitinase (MW0945)D1,S | 49483226 | 11338 | 6.6 | 2 | 32 | 75 |
| Coagulase (Coa)A4,C7,D1,S | 46540 | 71675 | 8.4 | 20 | 37 | 920 |
| Collagen adhesin precursor (Cna)A2,B1,L,M,S | 21205785 | 132921 | 5.9 | 7 | 7 | 199 |
| Ear (Ear)B1,D1,M,S | 21203924 | 20322 | 8.6 | 4 | 19 | 115 |
| Esterase\lipase (MW2501)B1,D2,S | 14248355 | 30986 | 4.7 | 10 | 52 | 415 |
| Fibrinogen-binding protein-related (MW1037)A1,C1,S | 49244435 | 12171 | 10.4 | 3 | 27 | 115 |
| FmtB protein (FmtB)A1,D1 | 14247939 | 263611 | 4.6 | 9 | 4 | 229 |
| Fibronectin-binding protein A (FnbA)C3,L,M,S | 87161146 | 111642 | 4.6 | 9 | 13 | 254 |
| Fibronectin-binding protein B (FnbB)A1,B2,C2,L,M,S | 87162339 | 103492 | 4.7 | 9 | 12 | 265 |
| IgG-binding protein (Sbi)A2,S | 49245643 | 50099 | 9.4 | 11 | 26 | 330 |
| IgG-binding protein A precursor (Spa)A9,C7,D4,L,M,S | 83682325 | 49338 | 5.7 | 16 | 45 | 666 |
| Lipase (Lip)C3,D8,S | 1095875 | 76845 | 7.1 | 19 | 30 | 947 |
| Lysophospholipase, putative (MW1732)D1,S | 87162009 | 31019 | 5.1 | 9 | 53 | 306 |
| Metallo-beta-lactamase superfamily protein (YycJ)B1,S | 49483948 | 25306 | 5.0 | 4 | 24 | 101 |
| Mrp protein (Mrp)C1 | 5834649 | 262876 | 4.6 | 9 | 4 | 250 |
| 1-Phosphatidylinositol phosphodiesterase (Plc)B1,D3,S | 1172527 | 35213 | 6.5 | 13 | 52 | 452 |
| Putative sulfatase (MW0681)B1,C1,D2,M,S | 49244034 | 74353 | 9.0 | 8 | 17 | 349 |
| SspA, V8 protease (SspA)B2,D4,S | 12025238 | 36304 | 5.0 | 6 | 27 | 337 |
| SspB, cysteine protease precursor (SspB)A1,B3,C2,D9,S | 12025239 | 44491 | 5.7 | 15 | 44 | 738 |
| Serine protease SplC (SplC)B1,D2,S | 88195634 | 26082 | 6.3 | 7 | 27 | 212 |
| Staphylokinase precursor (Sak)B4,C1,D6,S | 21205055 | 18483 | 6.8 | 7 | 67 | 411 |
| Succinyl-diaminopimelate desuccinylase (MW1943)B1 | 13701801 | 45109 | 4.6 | 6 | 16 | 158 |
| Tetracycline resistance protein (TetP)B1,M,S | 6094458 | 72677 | 5.3 | 1 | 2 | 60 |
| ThiJ/PfpI family protein, protease 1 (MW1815)B3,D2 | 49240910 | 32171 | 5.0 | 6 | 67 | 323 |
| Trigger factor (prolyl isomerase) (Tig)A2,B3,C2,D2 | 49483918 | 48565 | 4.3 | 8 | 24 | 439 |
| Tripeptidase, similar to (MW1465)B1 | 14247283 | 40172 | 5.0 | 4 | 12 | 88 |
| Truncated MHC class II analogue protein (SAOUHSC 02466)D4 | 88196118 | 15438 | 8.7 | 7 | 55 | 300 |
| Xaa-His dipeptidase (MW1694)B2,C1,D1,S | 49245019 | 52775 | 4.6 | 13 | 34 | 535 |
| Xaa-Pro dipeptidase (MW1482)B1,D3,S | 49483779 | 39357 | 5.2 | 9 | 32 | 314 |
| Toxins and haemolysins (7) | ||||||
| Alpha-haemolysin, chain G (Hla)C3,D4,S | 2914575 | 33227 | 7.9 | 14 | 51 | 666 |
| Enterotoxin C3 (Sec3)B4,S | 295149 | 27634 | 7.2 | 17 | 58 | 799 |
| Enterotoxin H (Seh)B4,S | 9955226 | 25128 | 5.2 | 7 | 38 | 282 |
| Enterotoxin K (Sek)C1,D2,S | 87161791 | 27733 | 8.3 | 6 | 25 | 252 |
| Enterotoxin L, extracellular (Sel2)D2,S | 14247781 | 27479 | 9.0 | 2 | 4 | 83 |
| Enterotoxin Q (Seq)B2,C2,D2,S | 87161054 | 28129 | 7.7 | 8 | 43 | 358 |
| Exotoxin (SAUSA300_0407)C1,S | 88194194 | 25350 | 8.5 | 2 | 15 | 96 |
| Stress response proteins (20) | ||||||
| Alkaline shock protein 23 (Asp23)A1,B7,C5,D11 | 49484402 | 19180 | 5.1 | 8 | 60 | 301 |
| Alkyl hydroperoxide reductase subunit C (AhpC)A1,B2,C2,D4 | 49482631 | 20963 | 4.9 | 7 | 53 | 514 |
| Alkyl hydroperoxide reductase subunit F (AhpF)B1,D1 | 14246148 | 54674 | 4.7 | 5 | 11 | 164 |
| ATP-dependent Clp proteinase chain (ClpP)B2,D3,M | 14248322 | 77810 | 4.8 | 25 | 49 | 1416 |
| Catalase (KatA)B1,C1,D2 | 7161887 | 58287 | 5.3 | 15 | 31 | 680 |
| Chaperone protein DnaK, HSP70 (DnaK)A2,B1,C2,D,M | 1169381 | 66307 | 4.6 | 15 | 34 | 875 |
| Chaperone protein HchA, Hsp31 (HchA)B1 | 49240910 | 32171 | 5.0 | 4 | 14 | 147 |
| Cold shock protein (CspA)B2,C2,D2 | 49483592 | 7317 | 4.5 | 3 | 78 | 188 |
| General stress protein 26 (MW2302)B1,D1 | 49245607 | 15807 | 5.1 | 4 | 40 | 199 |
| GroES (GroES)D1 | 18028156 | 10453 | 5.1 | 2 | 26 | 68 |
| NAD(P)H-flavin oxidoreductase, similar to (Frp)D1 | 14248297 | 25359 | 5.5 | 4 | 17 | 56 |
| Nitric oxide dioxygenase (MW0216)D1 | 14246007 | 42932 | 5.2 | 4 | 12 | 104 |
| OsmC-like protein (MW0781)B3,D1 | 49244117 | 15320 | 4.8 | 3 | 25 | 113 |
| Peroxiredoxin reductase (AhpF)D1 | 49243746 | 11325 | 6.4 | 3 | 42 | 67 |
| SrrA (SrrA)D1 | 37781574 | 28143 | 5.2 | 6 | 30 | 288 |
| Superoxide dismutase (SodA)A1,B2,C1,D2 | 49483802 | 22697 | 5.1 | 6 | 45 | 303 |
| Thioredoxin (TrxA)B1,D2 | 49483308 | 11433 | 4.4 | 5 | 56 | 223 |
| Thioredoxin (MW1870)B1,D1 | 49484170 | 21902 | 5.2 | 7 | 37 | 259 |
| Thioredoxin reductase (TrxB)B1,C1,D2 | 32468851 | 33595 | 5.2 | 9 | 30 | 456 |
| Universal stress protein, putative (MW1653)B1,D5 | 49483951 | 18463 | 5.6 | 8 | 63 | 497 |
| Cell division and maintenance (33) | ||||||
| Aminoglycoside phosphotransferase (AphA)C1 | 11991167 | 30624 | 4.5 | 3 | 16 | 64 |
| Autoinducer-2 production protein (LuxS)D1 | 49484358 | 17502 | 5.4 | 4 | 23 | 140 |
| Autolysin (Atl)A2,B1,C3,D4,S | 14248419 | 69186 | 6.0 | 20 | 43 | 867 |
| Cyclophilin type peptidyl-prolyl cis-trans isomerase, putative (MW0836)D1 | 49483114 | 21605 | 4.6 | 4 | 18 | 108 |
| Cell division initiation protein DivIVA (MW1335)B1,D1 | 49483601 | 29963 | 4.4 | 3 | 11 | 114 |
| Cell division protein FtsZ (FtsZ)A1,B1,D2 | 38604824 | 41413 | 5.0 | 5 | 15 | 167 |
| Cytosol aminopeptidase family protein (PepA)D1 | 49483102 | 54140 | 5.7 | 9 | 23 | 215 |
| GAF domain protein (MW1661)D1 | 88195528 | 17100 | 4.9 | 3 | 22 | 93 |
| HMG-CoA synthase (MvaS)B3,D1 | 9937361 | 43191 | 5.0 | 10 | 31 | 414 |
| Histidine-containing phosphocarrier protein (PtsH)D2 | 46908 | 9505 | 4.4 | 2 | 27 | 109 |
| Ferritin (Ftn)B3,D7 | 49242263 | 19590 | 4.7 | 6 | 31 | 450 |
| Putative non-haem iron-containing ferritin (MW2063)D2 | 49484363 | 16681 | 4.6 | 2 | 19 | 120 |
| Formylmethionine deformylase homologue (Def)D1 | 14246861 | 20546 | 5.8 | 6 | 38 | 111 |
| Fumarylacetoacetate (FAA) hydrolase family protein (Faa)B1 | 49244187 | 33093 | 4.8 | 3 | 13 | 72 |
| Methionine aminopeptidase (Map)D1 | 49484129 | 27485 | 5.2 | 3 | 14 | 105 |
| Malonyl CoA-acyl carrier protein transacylase (FabD)D1 | 14247000 | 33628 | 4.9 | 5 | 23 | 109 |
| Manganese-dependent inorganic pyrophosphatase (PpaC)A1,C1,D1 | 492245182 | 34279 | 4.7 | 6 | 23 | 199 |
| Monofunctional biosynthetic peptidoglycan transglycosylate (MW1814)D1 | 49483860 | 11941 | 3.9 | 2 | 10 | 84 |
| Naphthoate synthase (MenB)D1 | 14246815 | 30392 | 5.4 | 6 | 18 | 162 |
| Peptidoglycan hydrolase (LytM)C2,S | 2239274 | 35147 | 6.1 | 4 | 20 | 207 |
| Putative 3-methyl-2-oxobutanoate hydroxymethyltransferase (PanB)D1 | 49245819 | 29237 | 5.6 | 8 | 30 | 348 |
| Secretory antigen precursor SsaA (SsaA)D1 | 87159889 | 17388 | 5.8 | 1 | 9 | 73 |
| Stage V sporulation protein (SpoVG)B1 | 13700388 | 12312 | 4.9 | 3 | 33 | 62 |
| tRNA, Arginyl-tRNA synthetase (ArgS)B1,D1 | 49240966 | 62312 | 5.1 | 7 | 16 | 220 |
| tRNA, Aspartyl-tRNA synthetase (AspS)D1 | 49483875 | 66527 | 5.0 | 10 | 17 | 163 |
| tRNA, Cysteinyl-tRNA synthetase (CysS)D1 | 46395518 | 53651 | 5.3 | 9 | 23 | 280 |
| tRNA, Glutamyl-tRNA amidotransferase subunit B (GatB)C1,D3 | 82751553 | 53607 | 5.1 | 9 | 24 | 229 |
| tRNA, Glycyl-tRNA synthetase (GlyS)D1 | 49483813 | 53586 | 5.0 | 4 | 8 | 107 |
| tRNA, Isoleucyl-tRNA synthetase (IleS)D1 | 49244476 | 104825 | 5.3 | 8 | 10 | 179 |
| tRNA, Leucyl-tRNA synthetase (LeuS)B1,D1 | 21204871 | 91926 | 5.0 | 9 | 17 | 343 |
| tRNA, Phenylalanyl-tRNA synthetase beta subunit (PheT)C1,D1 | 14246909 | 88885 | 4.7 | 9 | 13 | 317 |
| tRNA, Seryl-trna synthetase (SerS)B1,D1 | 14245776 | 48609 | 5.0 | 10 | 27 | 327 |
| tRNA, Threonyl-tRNA synthetase 1 (ThrS)C1,D1 | 14247455 | 74341 | 5.3 | 11 | 20 | 281 |
| Protein synthesis (18) | ||||||
| Acetyltransferase (GNAT) family protein (MW2324)D2 | 49483339 | 16991 | 4.9 | 5 | 32 | 174 |
| Aminotransferase, putative (RocD)B1,C1 | 492241363 | 54376 | 6.1 | 7 | 19 | 229 |
| Branched-chain amino acid aminotransferase (IlvE)D1 | 82750262 | 40061 | 5.0 | 5 | 20 | 220 |
| Deblocking aminopeptidase, similar to (MW1253)D1 | 49483560 | 37848 | 5.3 | 1 | 3 | 58 |
| Formiminoglutamase (HutG)D1 | 87160628 | 34491 | 5.4 | 6 | 29 | 223 |
| Glutamine ammonia ligase (GlnA)A1,C1,D1 | 14247080 | 50822 | 5.1 | 9 | 34 | 314 |
| 30S Ribosomal protein S1 (RpsA)A1,B1,C1,D2 | 14247247 | 43283 | 4.6 | 15 | 51 | 740 |
| 30S Ribosomal protein S2 (RspB)B2,C1,D1 | 57286011 | 29377 | 5.3 | 7 | 26 | 306 |
| 30S Ribosomal protein S6 (RpsF)A1,B2,C2,D3 | 49482595 | 11588 | 5.1 | 6 | 58 | 268 |
| S30EA Family ribosomal protein, putative (MW0714)B1,D2 | 49244067 | 22199 | 5.2 | 5 | 31 | 211 |
| 50S Ribosomal protein L7/L12 (RplL)A2,B2,C2,D4 | 88194302 | 12704 | 4.6 | 7 | 72 | 352 |
| 50S Ribosomal protein L10 (RplJ)B1,C1,D1 | 49243847 | 17672 | 4.8 | 5 | 51 | 242 |
| 50S Ribosomal protein L25 (RplY)C1 | 49243808 | 23773 | 4.4 | 4 | 23 | 74 |
| O-acetylserine (thiol)-lyase, putative, cysteine synthase (CysK)B2,C1,D2 | 49243820 | 32955 | 5.4 | 11 | 58 | 500 |
| Ornithine aminotransferase (RocD1)B1,D3 | 49483117 | 43444 | 5.4 | 11 | 31 | 543 |
| Secretory antigen precursor (SsaA)C3 | 49242648 | 16997 | 5.8 | 1 | 9 | 86 |
| Secretory antigen precursor SsaA (MW0627)C1 | 49243980 | 28155 | 6.1 | 3 | 14 | 69 |
| Serine hydroxymethyltransferase (GlyA)B1,D1 | 49245349 | 45144 | 5.8 | 10 | 29 | 422 |
| Nucleotide biosynthesis (16) | ||||||
| Adenylosuccinate lyase (PurB)D1 | 49484149 | 49572 | 5.6 | 7 | 18 | 154 |
| Adenylosuccinate synthase (PurA)B1,D2 | 49482270 | 47522 | 5.1 | 5 | 14 | 217 |
| Amidophosphoribosyltransferase precursor (PurF)B1,D2 | 49244352 | 54363 | 6.1 | 12 | 23 | 426 |
| Carbamoyl-phosphate synthase large chain (CarB)C1 | 14246973 | 117098 | 4.9 | 3 | 3 | 54 |
| GMP synthase (GuaA)D2 | 38372353 | 58149 | 4.9 | 6 | 13 | 200 |
| Inositol-monophosphate dehydrogenase (GuaB)A1,B1,C1,D1 | 21203531 | 52790 | 5.6 | 10 | 35 | 338 |
| Phosphoribosylamine-glycine ligase (PurD)D1 | 14246844 | 41946 | 5.0 | 2 | 6 | 50 |
| Phosphoribosylaminoimidazole-succinocarboxamide synthase (PurC)B1,D1 | 88194764 | 26676 | 5.3 | 12 | 62 | 494 |
| Phosphoribosylformylglycinamidine cyclo-ligase (PurM)D2 | 14246841 | 36966 | 4.8 | 7 | 26 | 252 |
| Phosphoribosylformylglycinamidine synthetase (PurL)B1,D1 | 14246839 | 79513 | 4.8 | 10 | 16 | 245 |
| Phosphoribosylformylglycinamidine synthase (PurS)D2 | 14246838 | 24541 | 5.0 | 7 | 43 | 215 |
| Phosphoribosylformylglycinamidine synthase, PurS component (MW0950)B2 | 49241360 | 9929 | 4.7 | 4 | 68 | 225 |
| Polyribonucleotide nucleotidyltransferase (PnpA)B1,D1 | 49244556 | 77342 | 4.9 | 14 | 23 | 530 |
| Purine nucleoside phosphorylase (DeoD1)A1,B3,D2 | 49484362 | 25892 | 4.9 | 6 | 35 | 329 |
| Pyridoxine biosynthesis protein (MW0474)A1,C1,D1 | 49482748 | 31972 | 5.1 | 8 | 31 | 267 |
| Uracil phosphoribosyltransferase (Upp)D1 | 49484336 | 23035 | 6.1 | 9 | 58 | 496 |
| Fatty Acid Biosynthesis (1) | ||||||
| Trans-2-enoyl-ACP reductase (FabI)B1,D1 | 56001093 | 24601 | 5.2 | 7 | 53 | 226 |
| Transcription and Replication (18) | ||||||
| 2′−5′ RNA ligase (MW0896)B1,D1 | 49244233 | 19315 | 4.9 | 6 | 40 | 201 |
| Accessory gene regulator A (AgrA)D1 | 14247812 | 27903 | 5.9 | 5 | 18 | 92 |
| DNA-directed RNA polymerase alpha chain (RpoA)C2 | 49484440 | 34990 | 4.7 | 9 | 40 | 292 |
| DNA polymerase III, beta chain (DnaN)C1,D1 | 49482255 | 41888 | 4.7 | 8 | 25 | 194 |
| DNA-directed RNA polymerase delta subunit (RpoE)C1 | 49245364 | 20868 | 3.6 | 2 | 14 | 84 |
| Translation elongation factor G (Fus)A2,B2,C3,D2 | 49243855 | 76564 | 4.8 | 19 | 40 | 915 |
| Elongation factor TS (Tsf)A2,B3,C3,D5 | 14247027 | 32473 | 5.2 | 15 | 57 | 683 |
| Elongation factor P, putative (Efp)D1 | 49483778 | 20541 | 4.8 | 2 | 11 | 82 |
| RsbW (RsbW)B1 | 37781578 | 17896 | 4.7 | 3 | 20 | 70 |
| TatD related Dnase, putative (MW0446)D1 | 49482718 | 29263 | 5.1 | 4 | 17 | 126 |
| Transcription pleiotropic repressor (CodY)B1,D2 | 49483418 | 28737 | 5.9 | 8 | 35 | 354 |
| Transcription termination-anti-termination factor (NusA)C1,D1 | 14247036 | 43729 | 4.6 | 9 | 29 | 242 |
| Transcriptional regulator (MW0363)B2,D3 | 14246155 | 15127 | 5.0 | 7 | 65 | 301 |
| Transcriptional regulator, LytR family (SAUSA300_0958)D1 | 1723223 | 23880 | 5.7 | 5 | 33 | 170 |
| Transcriptional regulator, LytR family (MW0939)C1,B2 | 57284435 | 45657 | 6.0 | 6 | 20 | 144 |
| Translation initiation factor IF-1 (InfA)B1 | 49484444 | 8274 | 6.7 | 2 | 38 | 64 |
| Translation elongation factor Tu (Tuf)A2,B1,C2,D3 | 49243856 | 43077 | 4.7 | 16 | 59 | 871 |
| Transposase (MW2398)B2,D2 | 49484688 | 16446 | 5.6 | 4 | 33 | 191 |
| Miscellaneous (13) | ||||||
| IIIG9 protein, similar to- (LOC576703)B1 | 72179405 | 52711 | 9.4 | 1 | 2 | 51 |
| 6,7-Dimethyl-8-ribityllumazine synthase (RibH)B1,D1 | 49242141 | 16412 | 5.7 | 7 | 73 | 342 |
| ABC transporter-associated protein, SufB (MW0799)D1 | 49483078 | 52512 | 5.1 | 7 | 18 | 139 |
| Amylase (MalA)D1 | 18145251 | 77435 | 5.9 | 1 | 1 | 54 |
| Aldo/keto reductase family protein (MW2127)D1 | 49482959 | 32339 | 5.2 | 4 | 12 | 114 |
| Arsenate reductase family protein (MW0785)D1 | 49483064 | 13591 | 6.7 | 3 | 49 | 133 |
| Lipoate synthase (LipA)*A1 | 27807337 | 16468 | 6.3 | 2 | 17 | 54 |
| Cell wall surface anchor family protein (MW2416)C1,D1 | 57285190 | 136262 | 5.7 | 16 | 16 | 804 |
| Short chain dehydrogenase (MW2249)B1 | 14248102 | 31777 | 4.7 | 2 | 5 | 72 |
| Immunodominant antigen A (IsaA)A9,B5,C13,D2 | 14248343 | 24189 | 5.9 | 4 | 23 | 259 |
| N-Acetylglucosamine 6-phosphate deacetylase (NagA)D1 | 87161324 | 43089 | 5.4 | 7 | 20 | 123 |
| 4-Nitrophenylphosphatase-probable (MW0811)B1 | 82750544 | 27962 | 4.5 | 4 | 16 | 61 |
| SufD (SufD)D1 | 82750525 | 48518 | 5.4 | 5 | 18 | 69 |
| Unknown (20) | ||||||
| Conserved hypothetical protein (SAUSA300_0871)D1 | 88194663 | 33093 | 4.8 | 9 | 42 | 262 |
| Conserved hypothetical protein (SAUSA300_0916)D1 | 88194708 | 19314 | 5.0 | 7 | 47 | 249 |
| Conserved hypothetical protein (SAUSA300_1856)D2 | 88195657 | 19536 | 6.1 | 7 | 46 | 247 |
| Hypothetical exported protein (MW0347)B6,D7 | 49243694 | 21261 | 5.7 | 3 | 14 | 115 |
| Hypothetical exported protein (MW2606)B1,D1 | 82752265 | 18700 | 4.7 | 3 | 25 | 147 |
| Hypothetical cytosolic protein (MW0395)C1,S | 14246202 | 55465 | 5.1 | 14 | 32 | 487 |
| Hypothetical protein (MW0542)B1 | 14246355 | 29371 | 5.1 | 3 | 14 | 97 |
| Hypothetical protein (MW0819)B1,D1,M | 49244219 | 69762 | 5.1 | 14 | 30 | 604 |
| Hypothetical protein (MW2068)D1,S | 4126674 | 22954 | 5.2 | 10 | 62 | 369 |
| Hypothetical protein (SAUSA300_0408)A1,C5,S | 57285506 | 56443 | 4.8 | 19 | 40 | 899 |
| Hypothetical protein (SAUSA300_0279)**D1,M,S | 77383233 | 24390 | 6.9 | 1 | 5 | 51 |
| Hypothetical protein (MW1884)A1,M,S | 30043928 | 13044 | 9.3 | 2 | 26 | 99 |
| Hypothetical protein (MW0577)B1,S | 49482843 | 18554 | 9.2 | 4 | 25 | 124 |
| Hypothetical cytosolic protein (MW1786)B1,C1,D1 | 49484087 | 13302 | 4.4 | 9 | 85 | 406 |
| Hypothetical protein (MW1795)A2,B1,D2 | 49484096 | 22344 | 5.3 | 10 | 58 | 394 |
| Hypothetical protein (MW2099)B1,D1 | 49484393 | 10000 | 6.1 | 3 | 45 | 95 |
| Hypothetical protein (SAUSA300 2327)D1 | 87161861 | 15876 | 4.9 | 7 | 58 | 336 |
| Hypothetical protein (SAUSA300 pUSA010004)C1,M,S | 87159841 | 21257 | 9.3 | 2 | 11 | 55 |
| Putative exported protein (MW0355)B8,M | 49243745 | 56170 | 5.0 | 17 | 45 | 620 |
| Putative exported protein (MW1757)D2,S | 49245076 | 20371 | 6.8 | 3 | 21 | 100 |
The Mascot search results displayed above (Entrez Protein number, MW, pI, MP, SC%, and MS/MS MOWSE score) are from the best protein match to published S. aureus genomes. In many instances, database searches were performed before the genome sequence of USA300 was published and the best match is to a protein from another published S. aureus strain. However, the Entrez Protein name indicates the name of the likely MW2 or USA300 protein. SC, sequence coverage. MP, matched peptides.
AMW2 supernatants from mid-exponential phase of growth.
BMW2 supernatants from stationary phase of growth.
CLAC supernatants from mid-exponential phase of growth.
DLAC supernatants from stationary phase of growth.
ETheoretical or predicted.
LContains an LPXTG cell wall anchoring signal sequence.
MContains probable transmembrane regions.
SContains a probable N-terminal signal peptide sequence.
1–8Number after the letter A,B,C or D indicates number of times a protein was identified.
Best Mascot search homology was to Cathelicidin 4 [indolicidin] [Bos taurus].
Best Mascot search homology was to Pfl_3008 [Pseudomonas fluorescens PfO-1] (ABA74746).
Identification of CA-MRSA exoproteins associated with virulence
Twenty exoproteins (20%) identified at mid-exponential growth and 33 (15%) of those at early stationary phase of growth are known to be associated with virulence (Fig. 1 and Table 1). There were essentially three subcategories of virulence determinants found in culture media. Proteases or enzymes, including aminopeptidase (PepS), aureolysin (Aur), staphylokinase (Sak), V8 protease (SspA), cysteine protease (SspB), serine protease (SplC), lipase (Lip) and Xaa-Pro dipeptidase, were produced by each strain at either phase of growth (Fig. 1 and Table 1). These enzymes degrade and/or modify proteins and lipids present in the growth environment (McGavin et al., 1997; Karlsson et al., 2001; Massimi et al., 2002; Imamura et al., 2005). For example, cysteine protease/staphopain B (SspB), which directly cleaves kininogen, also works in concert with staphopain A (ScpA) to promote vascular leakage and lower blood pressure, thereby facilitating septic shock (Massimi et al., 2002; Imamura et al., 2005). SspA causes release of cell surface fibronectin-binding protein (FnbB) and immunoglobulin G (IgG)-binding protein A (protein A, Spa), modifying capacity for host interaction and increasing free FnbB and Spa (McGavin et al., 1997; Karlsson et al., 2001).
A second group of molecules identified in CA-MRSA culture media were those involved in bacteria–host interaction or adhesion, such as coagulase (Coa), collagen adhesin (Cna), enolase (Eno), fibrinogen-binding protein, FnbA, FnbB, 1-phosphatidylinositol phosphodiesterase (Plc), Spa and IgG-binding protein (Sbi) (Figs 1 and 2, and Table 1). These molecules can activate the clotting cascade (Coa) (Panizzi et al., 2006), mediate binding to host tissues (Cna, Eno, FnbA and FnbB) (Patti et al., 1992; Greene et al., 1995; Carneiro et al., 2004), and sequester host antibody (Spa and Sbi) (Forsgren and Sjoquist, 1966; Zhang et al., 1998). Although the function of S. aureus Plc is uncharacterized, that of Listeria monocytogenes promotes adhesion to epithelial cells and mediates escape of the pathogen from phagosomes (Krawczyk-Balska and Bielecki, 2005; Wei et al., 2005).
The toxins and haemolysins, namely alpha-haemolysin (Hla), enterotoxin C3 (Sec3), enterotoxin H (Seh), enterotoxin K (Sek), enterotoxin L (Sel2) and enterotoxin Q (Seq), comprised at most 4% of the exoproteins produced by MW2 and/or LAC in mid-exponential or early stationary phases of growth (4/98 at mid-exponential phase and 6/229 at early stationary phase of growth respectively) (Fig. 1 and Table 1). Unexpectedly, we failed to detect gamma-haemolysin subunits (HlgA, HlgB and HlgC), LukD/E, LukM, or either component of PVL (LukS-PV and LukF-PV) in culture supernatants under the two growth conditions tested.
Proteins involved in metabolism, biosynthesis, transcription and replication are present in MW2 and LAC culture supernatants
CA-MRSA culture supernatants contained 73 proteins known to be involved in the transport and utilization of carbohydrates or amino acids for energy (Table 1). Thirty-five proteins known to participate in biosynthesis of nucleotides, proteins and fatty acids, and 51 proteins involved in cell division, transcription and replication, were also identified in culture media (Fig. 1 and Table 1). The observation that cytoplasmic proteins were found in culture supernatant is not unexpected, as numerous cycles of autolysis would have occurred thereby releasing proteins into culture medium (Lei et al., 2000; Chaussee et al., 2001; Trost et al., 2005).
Stress response proteins are present in culture supernatants
Twenty proteins associated with the response to environmental stress were identified in MW2 and LAC culture supernatants (Fig. 1 and Table 1). For example, alkyl hydroperoxide reductase (AhpC and AhpF), catalase (KatA), superoxide dismutase (SodA), thioredoxin (TrxA) and thioredoxin reductase (TrxB), proteins that function to inactivate reactive oxygen species, and heat shock proteins, GroES and DnaK, were identified in culture supernatants at both phases of growth (Fig. 1 and Table 1). Consistent with this observation, genes encoding these proteins are induced in MW2 and LAC during phagocytosis by human neutrophils (Voyich et al., 2005). Further, heat shock proteins such as DnaK and GroEL have been shown to be immunogenic in patients with S. aureus endocarditis (Qoronfleh et al., 1993; 1998), suggesting they are exoproteins in vivo.
MW2 and LAC produced numerous exoproteins of unknown function
We identified 20 culture supernatant proteins with no characterized function, including four putative exported proteins (Fig. 1, Table 1 and Table S1) (Baba et al., 2002; Diep et al., 2006). Fourteen of these proteins were conserved across 10 sequenced strains of S. aureus (Table S1). Genes encoding MW0395 and MW1757 reside within Type II genomic islands of MW2 known as νSaα and νSaβ respectively, and each is located near or among putative virulence determinants (Baba et al., 2002). MW1884 is encoded by MW2 prophage ΦSa3 and is juxtaposed to the gene encoding staphylokinase (sak), a known virulence factor in S. aureus (Baba et al., 2002). Several other exoproteins with no characterized function, such as MW0542, MW1795, MW2068, SAUSA300_0871, SAUSA300_0916 and SAUSA300_2327, have homology to enzymes that participate in metabolism or replication, or respond to stress (Table S1). It will be important to determine whether some of these proteins have a role in virulence and/or if they are potential vaccine targets.
Selected MW2 and LAC exoproteins differ in abundance
Although MW2 and LAC produced many common exoproteins, 11 exoproteins detected at either phase of growth differed in abundance between the strains (Figs 3 and 4). Hla and a putative surface protein (SAUSA300_0408) were in greater abundance in LAC culture supernatants at mid-exponential growth phase, and multiple repeat polypeptide (Mrp), Sak, and Coa were found exclusively in the same supernatants (Fig. 3A). By comparison, Cna, Sec3 and a putative exported protein (MW0355) were identified as exoproteins only in MW2 culture supernatants at mid-exponential growth (Fig. 3B).
Fig. 3.
Quantitative analysis of CA-MRSA culture supernatant proteins produced during growth in vitro. Differential analysis of MW2 and LAC culture supernatant proteins was performed as described under Experimental procedures. Proteins more abundant in/found only in LAC (A and D) or MW2 (B and E) supernatants. Panels C and F represent proteins equally abundant in MW2 and LAC. The phase of growth at which the analysis was performed is indicted to the left of the panels. Results are the mean ± SD of three separate experiments at each phase of growth.
Fig. 4.
Exoproteins produced by MW2 and LAC during infection. Culture supernatant proteins made by MW2 or LAC at the indicated phase of growth were separated by 2-DGE, transferred to nitrocellulose membranes, and probed with convalescent sera from mice infected with each strain or non-immune sera (not shown). Proteins immunoreactive only with sera from infected mice are indicated (boxed). Unidentified immunoreactive proteins are annotated with Arabic numbers. Immunoblots are representative of three separate experiments.
At stationary phase of growth, Hla, Sak, Lip, Seq, SplC, SspA, SspB and Sek were either increased ≥ 2-fold in LAC supernatants or were found only in those supernatants (Fig. 3D). There was far more Cna (39.5-fold) in MW2 supernatants at this phase of growth, and Sec3 and MW0577, a protein of unknown function, were detected only in MW2 culture media (Fig. 3E). Differences in exoproteins between these strains may underlie in part the noted variances in disease phenotypes (Baba et al., 2002; Kazakova et al., 2005; Miller et al., 2005; Voyich et al., 2005; Diep et al., 2006).
Exoproteins made during infection in vivo
To reconcile exoproteins produced by MW2 and LAC in vitro and those made during infection in vivo, we used a mouse abscess model to generate immune/convalescent sera from mice infected with MW2 and LAC (Voyich et al., 2006). Following 2-DGE and transfer to nitrocellulose, MW2 and LAC exoproteins were probed with convalescent serum from mice infected with either MW2 or LAC. Several proteins from these CA-MRSA strains were commonly immunogenic in mice (Fig. 4). For example, AhpC, Atl, formate tetrahydrofolate ligase (Fhs), glyceraldehyde 3-phosphate dehydrogenase (Gap), Lip and SspB were immunogenic in mice infected with either strain (Fig. 4 and Table 2). Cna, Sec3 and Sak are known virulence factors that were immunogenic in mice infected with either MW2 (Cna and Sec3) or LAC (Sak) (Fig. 4 and Table 2), findings consistent with the differential analysis of exoproteins produced in vitro (compare Figs 3 and 4). Although many other immunogenic proteins were detected by our analysis, many were cross-reactive with non-immune sera (unboxed, unmarked protein spots, Fig. 4) or could not be identified with absolute certainty (indicated by numbers, Fig. 4). Taken together, these data provide strong support to the idea that proven or putative virulence factors, such as Atl, Cna, Lip, Sak, Sec3 and SspB, are made during CA-MRSA infection in vivo.
Table 2.
Immunogenic (in vivo expressed) exoproteins of MW2 and LAC.
| Proteina | MW2 | LAC | Immunoreactivity |
|---|---|---|---|
| AhpC, alkyl hydroperoxide reductase | ME, S | ME, S | NI, I |
| AroA, chorismate mutase | – | S | I |
| Asp23, alkaline shock protein 23 | – | S | NI, I |
| Atl, autolysin | ME, S | ME, S | I |
| ClpP, ATP-dependent Clp protease | S | S | NI, I |
| Cna, collagen adhesin precursor | ME, S | – | I |
| Coa, coagulase | ME | – | NI, I |
| DeoD, purine nucleoside phosphorylase | ME | ME | NI, I |
| Ear | S | S | I |
| Eno, enolase | S | S | NI, I |
| Fba, fructose bisphosphate aldolase | S | – | NI, I |
| Fhs, formate tetrahydrofolate ligase | ME, S | ME, S | I |
| Ftn, ferritin | – | S | I |
| Gap, glyceraldehyde 3-phosphate dehydrogenase | ME, S | ME, S | I |
| GlyA, serine hydroxymethyltransferase | – | S | I |
| Gpi, glucose 6-phosphate isomerase | ME | ME | NI, I |
| GuaB, inositol-monophosphate dehydrogenase | ME | – | NI, I |
| Idh1, isocitrate dehydrogenase | – | S | NI, I |
| Lip, lipase | S | S | I |
| MW0525, hexulose 6-phosphate synthase | – | S | I |
| MW0896, 2′−5′ RNA ligase | – | S | NI, I |
| MW1795, hypothetical protein | – | S | NI, I |
| MW1870, thioredoxin | – | S | NI, I |
| PdhA, pyruvate dehydrogenase subunit A | ME | ME | NI, I |
| PdhB, pyruvate dehydrogenase subunit B | S | S | NI, I |
| Pgd, phosphogluconate dehydrogenase | ME | ME | NI, I |
| Pgi, phosphoglucose isomerase | S | S | NI, I |
| Plc, 1-phosphatidylinositol phosphodiesterase | S | S | I |
| Pta, phosphate acetyltransferase | – | S | I |
| RocD, ornithine aminotransferase | S | – | NI, I |
| Sak, staphylokinase | ME | ME, S | I |
| Sec3, staphylococcal enterotoxin C3 | S | – | I |
| Seq, staphylococcal enterotoxin Q | ME | ME | I |
| SodA, superoxide dismutase | S | S | I |
| Spa, immunoglobulin-binding protein A | ME, S | ME, S | NI, I |
| SspB, cysteine protease precursor | ME, S | ME, S | I |
| Sulfatase | S | – | NI, I |
| Tkt, transketolase | S | – | I |
| TpiA, triosephosphate isomerase | – | ME | I |
| Tsf, translation elongation factor Ts | ME | S | I |
| Tuf, translation elongation factor Tu | ME, S | ME, S | NI, I |
| Unknown 1 | ME | – | I |
| Unknown 2 | ME | – | I |
| Unknown 3 | – | ME | I |
| Unknown 4 | – | ME | I |
| Unknown 5 | – | ME | I |
| Unknown 6 | – | ME | I |
Protein identities were obtained by overlay analysis as described under Experimental procedures. Results are representative of three experiments using serum pooled from 10 to 15 mice.
ME, mid-exponential phase of growth; S, stationary phase of growth; NI, non-immune sera; I, immune sera.
Phagocytosis of CA-MRSA by human neutrophils triggers production/secretion of virulence factors
To determine if production of selected exoproteins is triggered by interaction of S. aureus with host cells and/or if the proteins are made within phagosomes, we used confocal laser-scanning microscopy to evaluate production of Aur, Hla, SspA and SspB after phagocytosis by human polymorphonuclear leucocytes (PMNs) (Figs 5 and 6). Notably, there was a time-dependent increase in Aur, Hla, SspA and SspB produced by MW2 and/or LAC within neutrophil phagocytic vacuoles (Figs 5 and 6, yellow arrowheads). There was also redistribution of each molecule over time; at 15 or 60 min proteins were typically localized only to S. aureus, whereas at 3 or 4 h after phagocytosis each molecule was diffused within larger, more mature phagosomes or distributed throughout the cell (Figs 5 and 6, yellow arrowheads). Accumulation of these molecules late during phagocytosis correlates well with the noted PMN lysis caused by MW2 and LAC (Figs 5 and 6) (Voyich et al., 2005). Production of virulence factors within phagosomes is consistent with the notion that these proteins are made during infection in vivo.
Fig. 5.
Production and distribution of selected MW2 (USA400) virulence factors during phagocytosis by human PMNs. Following phagocytosis of MW2, aureolysin (Aur), SspA and SspB were visualized by confocal laser-scanning microscopy. White arrowheads indicate bacteria. Yellow arrowheads indicate areas enriched with the S. aureus protein of interest. The image labelled ‘Merge’ illustrates distribution of neutrophil actin-related protein (ARP, green) and nuclei (blue). DIC, differential interference contrast.
Fig. 6.
Production and distribution of selected LAC (USA300) virulence factors during phagocytosis by human PMNs. Following phagocytosis of LAC, Hla, SspA and SspB were visualized by confocal laser-scanning microscopy. Labelling for this figure is otherwise identical to the legend for Fig. 5.
Discussion
The striking increase in CA-MRSA infections over the past few years has prompted an intense search for the underlying molecular determinants. To date, few virulence factors are associated specifically with CA-MRSA disease and no single determinant appears to account for the increased incidence and severity of CA-MRSA infections (Lina et al., 1999; Baba et al., 2002; de Bentzmann et al., 2004; Diep et al., 2004; 2006; Fridkin et al., 2005; Voyich et al., 2006). It is almost certain that a combination of virulence determinants, including S. aureus exoproteins, and host susceptibility promote disease in otherwise healthy subjects. Inasmuch as exoproteins produced by CA-MRSA probably facilitate evasion of innate host defence (Voyich et al., 2005) and thereby contribute to disease, we performed a comprehensive analysis of exoproteins produced by MW2 and LAC in vitro and during infection.
A limited number of proteomics-based studies have investigated exoproteins of S. aureus, typically using strain COL or laboratory-derived strains (Ziebandt et al., 2001; 2004; Nakano et al., 2002). For example, Ziebrandt et al. recently compared S. aureus strains RN6390 and RN6911 and identified 43 exoproteins produced in vitro, including many controlled by accessory gene regulator (agr) and/or alternative sigma factor óB (sigB) (Ziebandt et al., 2004). Nakano et al. identified 29 exoproteins produced by MRSA strains using 2-DGE coupled with N-terminal peptide sequencing (Nakano et al., 2002). By comparison, numerous studies have reported individual S. aureus exoproteins that promote pathogenesis, including proteases (McGavin et al., 1997; Karlsson et al., 2001; Imamura et al., 2005), enterotoxins and exotoxins (Dinges et al., 2000; McCormick et al., 2001), and leukotoxins and haemolysins (Kaneko and Kamio, 2004). Although progress has been made towards identification and characterization of many important S. aureus exoproteins, there is a noted paucity of information regarding exoproteins produced by CA-MRSA strains.
Our analysis of MW2 (USA400) and LAC (USA300) culture supernatants identified 250 exoproteins (out of 600+ resolved protein spots) between two phases of growth in vitro, at present the single most comprehensive view of S. aureus exoproteins. Differential analysis of MW2 and LAC exoproteins revealed key differences between the strains (Fig. 3). These differences were not due to differences in rate of growth between the strains, because MW2 and LAC have essentially identical growth curves in vitro (Fig. 1A). Although many of the differentially abundant exoproteins, including Atl, Coa, Hla, Lip, Mrp, Sak, Sek, Seq, Sec3, SspA, SspB and SplC, are relatively ubiquitous among S. aureus, it is possible that the observed variances in protein levels relate to distinct strain pathologies. For example, Cna is linked to necrotizing pneumonia (de Bentzmann et al., 2004) and there were higher levels of this exoprotein in MW2 culture supernatants (Fig. 3). MW2 is a strain known to cause lethal pneumonia (Centers for Disease Control and Prevention, 1999). Compared with MW2, more Hla was present in LAC culture supernatants (7.5 ± 1.8- and 9.2 ± 1.8-fold more Hla at mid-exponential and stationary phases of growth respectively). Consistent with that observation, Hla appeared to accumulate more rapidly in LAC-containing neutrophil phagosomes or was more highly diffused in and around deteriorating PMNs after phagocytosis of LAC compared with MW2 containing cells (accumulation of Hla typically occurred by 180 min in LAC-containing PMNs versus 240 min in those with MW2). We recently found dramatic differences in pathophysiology between MW2 and LAC in a mouse skin infection/abscess model (Voyich et al., 2006). LAC produced rapid and pronounced dermonecrosis in infected animals, whereas mice infected with MW2 developed intact abscesses (Voyich et al., 2006). Thus, differences in exoprotein abundance, such as that for Hla, may underlie the differences in strain pathology. Additional studies are needed to test this hypothesis.
We used sera from mice infected with MW2 or LAC to identify exoproteins made during infection in vivo (Fig. 4). Previous serological proteome studies using strain COL identified 15 immunogenic proteins made during human infections, although only four of these proteins (alkaline shock protein, hexose 6-phosphate synthase, PdhB and Tuf) are common with our analysis (Table 2) (Vytvytska et al., 2002). In more recent work, Clarke et al. used bacteriophage expression libraries to identify S. aureus antigens produced during human infections (Clarke et al., 2006). Several of those immunogenic proteins, i.e. chorismate mutase (AroA), autolysin (Atl), Coa, Fhs, Gap, transketolase (Tkt) and triosephosphate isomerase (Tpi), were also identified as in vivo expressed S. aureus antigens by our studies (Table 2). Our work revealed many additional exoproteins produced during infection, such as AhpC, Cna, Ear, ferritin (Ftn), Lip, Plc, phosphate acetyltransferase (Pta), Sak, Sec3, Seq and SspB (Fig. 4). Importantly, these proteins were immunoreactive only with sera from MW2- or LAC-infected animals (as opposed to sera from uninfected animals). Variances in antigenic exoproteins between MW2 and LAC are likely explained in part by differences in gene content or phase of growth used to test antigenicity (Fig. 4). The relative importance of these in vivo expressed proteins remains to be determined.
At least four of the exoproteins identified by our proteomic analysis (Aur, Hla, SspA and SspB) were produced within phagosomes of human neutrophils following uptake (phagocytosis) (Figs 5 and 6). These findings are notable because MW2 and LAC are known to cause rapid lysis of PMNs (Voyich et al., 2005) and the factors responsible for the dramatic host cell lysis remain elusive (Voyich et al., 2006). Consistent with these data, we determined previously that the gene encoding Hla was upregulated during phagocytosis (Voyich et al., 2005). Although our studies do not demonstrate that Aur, Hla, SspA and SspB are directly related to the observed PMN lysis, increased accumulation of these virulence determinants accompanied initial stages of neutrophil destruction (Figs 5 and 6).
The S. aureus genome consists of ∼2600 proteins of which more than 40% have no similarity to proteins of known function (Kuroda et al., 2001). Moreover, 33% of identified proteins are unique to S. aureus (Kuroda et al., 2001). Therefore, it is not surprising that 8.5% of the proteins identified in our study have no known function (Fig. 1). Some of these exoproteins are of significant interest based upon homology to known S. aureus proteins (e.g. SAUSA300_0407 as an exotoxin homologue) or given their location within the genome (Table 1 and Table S1). Several exoproteins identified by our analysis are putative exported or surface proteins, e.g. SAUSA300_0408 and MW0355 (rather than metabolism enzymes, etc.) and also require characterization in the context of CA-MRSA pathogenesis (Fig. 3).
We used a proteomics-based approach to generate a comprehensive view of exoproteins made by prominent CA-MRSA strains, including identification of proteins that are immunogenic and thus produced during infection in vivo. Identification of these exoproteins is an important first step towards development of vaccines, prophylactics, and enhanced therapeutics designed to control CA-MRSA infections.
Experimental procedures
Growth of S. aureus and generation of culture supernatants
Staphylococcus aureus strains MW2 (USA400) (Baba et al., 2002) and LAC (USA300-0114) (Kazakova et al., 2005; Miller et al., 2005; Voyich et al., 2005; Diep et al., 2006) were cultured in tryptic soy broth containing 0.25% dextrose (TSB, Becton, Dickenson, and Company, Franklin Lakes, NJ) filtered sequentially through 10 kDa cut-off and 0.22 μm filters. Cultures were inoculated with a 1:200 dilution of overnight culture (500 μl of culture into 100 ml of TSB in a 1 l flask) and incubated at 37°C with shaking (250 rpm). All strains were cultured to mid-exponential (OD600 = 0.75) or stationary (OD600 = 2.0) phases of growth and placed on ice until used. Bacteria were removed from culture media by two sequential rounds of centrifugation at 2851 g for 10 min at 4°C. This procedure yielded clarified culture supernatants for subsequent proteomic analyses.
Precipitation and preparation of culture supernatant proteins
Precipitation of proteins from clarified culture supernatants was performed in polypropylene flasks to reduce protein loss. One hundred millilitres of clarified supernatant was added to 300 ml of 100% ethanol (Molecular Grade, Sigma-Aldrich, St Louis, MO) and chilled at −20°C overnight. Precipitated protein was transferred to Oakridge centrifuge tubes and sedimented by centrifugation at 27 216 g for 30 min at 4°C. To optimize yield, protein in polypropylene flasks (residual) and centrifuge tubes was air-dried for approximately 1 h. Protein in the flasks were solubilized with 3 ml of 2-D solubilization solution (7 M urea, 2 M thiourea, 4% CHAPS) with gentle swirling. These samples were transferred to Oakridge tubes containing precipitated protein pellets and swirled gently to dissolve pellets. Polypropylene flasks were rinsed with an additional 600 μl of 2-D solubilization solution.
Culture supernatant proteins were clarified further with a second precipitation in 30% trifluoroacetic acid (Sigma-Aldrich) and incubated on ice for a minimum of 30 min. Precipitated proteins were collected by centrifugation at 14 100 g for 5 min at room temperature. The protein pellet was dispersed by vortexing in 25 μl of distilled H2O for 10 s followed by the addition of 1 ml of acetone (−20°C). Proteins were washed in acetone for a minimum of 30 min at −20°C and then pelleted by centrifugation at 14 100 g for 5 min at room temperature. Proteins were air dried for ∼1 h or until pellets appeared dry. Pellets were solubilized in 400 μl of Destreak Rehydration Solution (GE Healthcare, Piscataway, NJ) containing tributylphosphine (200 mM) and ampholytes (pH 3–10, 4 μl of 100× solution) (Bio-Rad, Hercules, CA) at room temperature for 1 h with constant swirling. Samples were used immediately or stored briefly at −20°C.
Isolation of human neutrophils
Human polymorphonuclear leucocytes were isolated from fresh venous blood of healthy individuals using a published method (Kobayashi et al., 2002; Burlak et al., 2006). Studies were performed in accordance with a protocol approved by the Institutional Review Board for Human Subjects, National Institute of Allergy and Infectious Diseases. PMN preparations typically contained ∼94% neutrophils, with the remaining cells being predominantly eosinophils. All reagents used contained < 25.0 pg ml−1 endotoxin (Limulus Amebocyte Lysate assay, Fisher Scientific, Suwannee, GA).
Neutrophil phagocytosis
MW2 and LAC were cultured to mid-exponential phase of growth and phagocytosis experiments were performed with serum-opsonized bacteria as described (Kobayashi et al., 2003; Voyich et al., 2005). At the indicated times, phagocytosis was terminated either by chilling PMNs on ice or adding cold paraformaldehyde (4%) to assay wells.
Generation of immune sera
Female Crl:SKH1-hrBR mice (Charles River Laboratories, Wilmington, MA) were anaesthetized with isoflurane and inoculated with 50 μl of DPBS containing 107 cfu of MW2 or LAC by subcutaneous injection in the right flank. Abscesses typically formed within 4 days and resolved 14 days after infection (Voyich et al., 2006). At 28 days post infection, mice were euthanized and blood was collected from 10 to 15 mice to prepare pooled immune serum. Blood from uninfected animals was processed in parallel (non-immune serum). All studies conformed to guidelines set forth by the National Institutes of Health and were reviewed and approved by the Animal Use Committee at Rocky Mountain Laboratories, NIAID.
Isoelectric focusing and second-dimension SDS-PAGE
Culture supernatant proteins were precipitated in cold acetone and then solubilized with isoelectric focusing (IEF) buffer (7% urea, 2% thiourea, 4% CHAPS and 200 mM tributylphosphine) as described (Burlak et al., 2006). Protein concentration was measured with the 2-D Quant kit (GE Healthcare) and purified proteins were stored at −80°C.
For IEF, samples were treated with Destreak Rehydration solution (25% of total sample volume) (GE Healthcare), 200 mM tributylphosphine and 1% ampholytes. IEF was performed with 11 cm IPG Ready Strips for 40 kVh. IPG Ready Strips were rehydrated actively at 50 V overnight prior to first dimension separation. Moistened filter paper wicks (Whatman no. 1 paper, Whatman, Florham Park, NJ) were added between each electrode and strip prior to focusing (after rehydration). Wicks were changed four times in the first 4 h of IEF, after which the voltage was maintained at 8000 V (11 cm IPG Ready Strips). Following IEF, IPG Ready Strips were stored at −80°C until used for SDS-PAGE.
Second-dimension SDS-PAGE was performed essentially as described (Burlak et al., 2006), except electrophoresis was performed at 50 mA per gel until the dye front reached the bottom of each gel (∼1 h for 11 cm gels). Protein spots were excised and peptides were prepared for ADI-MS/MS analysis as described previously for liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Burlak et al., 2006). For simplicity, the combination of IEF and second-dimension SDS-PAGE is abbreviated as 2-DGE.
Mass spectrometry
Peptide samples (tryptic digests, as described/referenced above) were analysed by automated direct infusion (ADI) using Nanomate (Advion BioSciences, Ithaca, NY), an automated chip-based nano-electrospray interface source, coupled to a quadrupole-time of flight mass spectrometer, QStarXL MS/MS System (Applied Biosystems/Sciex, Framingham, MA). Computer-controlled data-dependent automated switching to MS/MS provided peptide sequence information. AnalystQS software (Applied Biosystems/Sciex) was used for data acquisition. Data processing and databank searching were performed with Mascot software version 2.1 (Matrix Science, Beachwood, OH). The National Center for Biotechnology Information non-redundant protein database (NCBInr, updated 12 May, 2006 at 18:01:48 GMT), National Library of Medicine, NIH was used for the search analysis. Search criteria were limited to double- and triple-charge ions and included monoisotopic masses, analysis of peptides for carbamidomethylation and/or propionamidylation of cysteine, oxidation of methionine, peptide and MS/MS tolerances of 0.2 Da and 0.8 Da respectively, and a maximum of one missed tryptic cleavage. Significance threshold for positive identification was determined by the Mascot Search program.
Amino acid sequence analysis
Proteins involved in virulence/defence mechanisms, stress response proteins, toxins, haemolysins and proteins of unknown function were evaluated for presence of an LPXTG motif, which predicts a cell wall anchor, and/or for sequences that predict an N-terminal signal peptide or transmembrane region(s). We used the NCBInr database to query the full sequence of each protein identified by ADI/MS/MS for the presence of LPXTG motifs. The presence of membrane spanning domains and N-terminal signal peptide sequences was deduced by searching protein sequences with PSORT software provided by the PSORT WWW server (http://psort.nibb.ac.jp/). Although none of the 20 hypothetical proteins identified in this study contain LPXTG motifs, eight proteins contain probable N-terminal signal peptide sequences and five have predicted transmembrane regions (Table 1 and Table S1).
In addition, sequences of the hypothetical proteins were compared to 902 genomes (Bacterial, Archaea, Viral, and Eukarya) using NCBI and ERGO, a curated NIAID database containing public and proprietary DNA.
SDS-PAGE and immunoblotting
Proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and membranes were blocked with 10% normal goat serum in Tris buffered saline 150 mM NaCL, 50 mM Tris base, pH 7.5, 1% Tween 20 and 0.02% sodium azide (Sigma-Aldrich) overnight at 4°C. Blots were probed with immune or non-immune mouse sera for 1–2 h at ambient temperature or overnight at 4°C. Blots were washed in buffer containing 250 mM NaCl, 10 mM Hepes and 2% Tween 20 (Sigma-Aldrich) and incubated with secondary antibodies conjugated to horseradish peroxidase for 1–2 h at ambient temperature. Immunoreactive proteins were visualized with enhanced chemiluminescence (SuperSignal West Pico, Pierce Biotechnology, Rockford, IL) using Kodak X-Omat film (Eastman Kodak, Rochester, NY).
Immunofluorescence and confocal laser-scanning microscopy
Acid washed coverslips (No. 1, 13 mm, round) were flamed and coated with 100% normal human serum in 24 well tissue culture plates for 1 h. Coverslips were washed twice with DPBS and synchronized phagocytosis was performed in 24 well plates as described above. Fixed PMNs were washed three times in DPBS and then permeabilized with 0.2% Triton X-100 for 5 min. After three more washes in DPBS, cells were incubated with blocking buffer (DPBS containing 5% BSA, 0.02% sodium azide) for 1 h. Samples were labelled with a 1:1000 dilution of rabbit antiserum containing antibodies specific for Hla (Sigma-Aldrich), SspA, SspB, Aur and 2 μg ml−1 of goat polyclonal antibodies specific for human actin-related protein 2 μg ml−1 (Santa Cruz Biotechnologies, Santa Cruz, CA) overnight at 4°C. Samples were washed and subsequently labelled with donkey anti-rabbit antibody conjugated with phycoerythrin 1:1000 (Jackson Immunoresearch, West Grove, PA) or donkey anti-goat antibody conjugated to AlexaFluor488 (1:1000) (Molecular Probes, Eugene, OR). PMNs were stained with DAPI (300 nM in DPBS, Molecular Probes) and DRAQ5 (1.25 μM in DPBS, Biostatus Limited, Leicestershire, UK) prior to mounting coverslips onto slides. Slides were analysed with a Zeiss LSM510 confocal laser-scanning microscope coupled to an Axiovert 200M inverted microscope (Carl Zeiss, Thornwood, NY). Images were acquired using a 100× Plan-Apochromat oil immersion objective (1.4 NA) at 512 × 512 pixel resolution with 2.7× digital magnification. Images were adjusted equally for brightness and contrast in Adobe Photoshop CS (Adobe Systems Incorporated, San Jose, CA).
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institutes of Allergy and Infectious Diseases.
Supplementary material
The following supplementary material is available for this article online:
Inter-strain conservation of proteins of unknown function.
This material is available as part of the online article from http://www.blackwell-synergy.com
References
- Adem PV, Montgomery CP, Husain AN, Koogler TK, Arangelovich V, Humilier M, et al. Staphylococcus aureus sepsis and the Waterhouse–Friderichsen syndrome in children. N Engl J Med. 2005;353:1245–1251. doi: 10.1056/NEJMoa044194. [DOI] [PubMed] [Google Scholar]
- Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- de Bentzmann S, Tristan A, Etienne J, Brousse N, Vandenesch F, Lina G. Staphylococcus aureus isolates associated with necrotizing pneumonia bind to basement membrane type I and IV collagens and laminin. J Infect Dis. 2004;190:1506–1515. doi: 10.1086/424521. [DOI] [PubMed] [Google Scholar]
- Bernardo K, Pakulat N, Fleer S, Schnaith A, Utermohlen O, Krut O, et al. Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob Agents Chemother. 2004;48:546–444. doi: 10.1128/AAC.48.2.546-555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S, Valeva A, Walev I, Zitzer A, Palmer M. Pore-forming bacterial cytolysins. Symp Ser Soc Appl Microbiol. 1998;27:15S–25S. doi: 10.1046/j.1365-2672.1998.0840s115s.x. [DOI] [PubMed] [Google Scholar]
- Burlak C, Whitney AR, Mead DJ, Hackstadt T, DeLeo FR. Maturation of human neutrophil phagosomes includes incorporation of molecular chaperones and endoplasmic reticulum quality control machinery. Mol Cell Proteomics. 2006;5:620–634. doi: 10.1074/mcp.M500336-MCP200. [DOI] [PubMed] [Google Scholar]
- Carneiro CR, Postol E, Nomizo R, Reis LF, Brentani RR. Identification of enolase as a laminin-binding protein on the surface of Staphylococcus aureus. Microbes Infect. 2004;6:604–608. doi: 10.1016/j.micinf.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus– Minnesota and North Dakota, 1997–99. JAMA. 1999;282:1123–1125. [PubMed] [Google Scholar]
- Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers HF. Community-associated MRSA – resistance and virulence converge. N Engl J Med. 2005;352:1485–1487. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- Chaussee MS, Watson RO, Smoot JC, Musser JM. Identification of Rgg-regulated exoproteins of Streptococcus pyogenes. Infect Immun. 2001;69:822–831. doi: 10.1128/IAI.69.2.822-831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SR, Brummell KJ, Horsburgh MJ, McDowell PW, Mohamad SA, Stapleton MR, et al. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis. 2006;193:1098–1108. doi: 10.1086/501471. [DOI] [PubMed] [Google Scholar]
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–99. Clin Infect Dis. 2001;32(Suppl. 2):S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- Diep BA, Sensabaugh GF, Somboona NS, Carleton HA, Perdreau-Remington F. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J Clin Microbiol. 2004;42:2080–2084. doi: 10.1128/JCM.42.5.2080-2084.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–1015. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A, Sjoquist J. ‘Protein A’ from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966;97:822–827. [PubMed] [Google Scholar]
- Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- Greene C, McDevitt D, Francois P, Vaudaux PE, Lew DP, Foster TJ. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- Imamura T, Tanase S, Szmyd G, Kozik A, Travis J, Potempa J. Induction of vascular leakage through release of bradykinin and a novel kinin by cysteine proteinases from Staphylococcus aureus. J Exp Med. 2005;201:1669–1676. doi: 10.1084/jem.20042041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko J, Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci Biotechnol Biochem. 2004;68:981–1003. doi: 10.1271/bbb.68.981. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Saravia-Otten P, Tegmark K, Morfeldt E, Arvidson S. Decreased amounts of cell wall-associated protein A and fibronectin-binding proteins in Staphylococcus aureus sarA mutants due to up-regulation of extracellular proteases. Infect Immun. 2001;69:4742–4748. doi: 10.1128/IAI.69.8.4742-4748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakova SV, Hageman JC, Matava M, Srinivasan A, Phelan L, Garfinkel B, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352:468–475. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- Kobayashi SD, Voyich JM, Buhl CL, Stahl RM, DeLeo FR. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc Natl Acad Sci USA. 2002;99:6901–6906. doi: 10.1073/pnas.092148299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi SD, Braughton KR, Whitney AR, Voyich JM, Schwan TG, Musser JM, DeLeo FR. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc Natl Acad Sci USA. 2003;100:10948–10953. doi: 10.1073/pnas.1833375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk-Balska A, Bielecki J. Listeria monocytogenes listeriolysin O and phosphatidylinositol-specific phospholipase C affect adherence to epithelial cells. Can J Microbiol. 2005;51:745–751. doi: 10.1139/w05-058. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- Lei B, Mackie S, Lukomski S, Musser JM. Identification and immunogenicity of group A Streptococcus culture supernatant proteins. Infect Immun. 2000;68:6807–6818. doi: 10.1128/iai.68.12.6807-6818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- Llewelyn M, Sriskandan S, Peakman M, Ambrozak DR, Douek DC, Kwok WW, et al. HLA class II polymorphisms determine responses to bacterial superantigens. J Immunol. 2004;172:1719–1726. doi: 10.4049/jimmunol.172.3.1719. [DOI] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin MJ, Zahradka C, Rice K, Scott JE. Modification of the Staphylococcus aureus fibronectin binding phenotype by V8 protease. Infect Immun. 1997;65:2621–2628. doi: 10.1128/iai.65.7.2621-2628.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchiodi EL, Eisenstein E, Fields BA, Ohlendorf DH, Schlievert PM, Karjalainen K, Mariuzza RA. Superantigen binding to a T cell receptor beta chain of known three-dimensional structure. J Exp Med. 1995;182:1833–1845. doi: 10.1084/jem.182.6.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimi I, Park E, Rice K, Muller-Esterl W, Sauder D, McGavin MJ. Identification of a novel maturation mechanism and restricted substrate specificity for the SspB cysteine protease of Staphylococcus aureus. J Biol Chem. 2002;277:41770–41777. doi: 10.1074/jbc.M207162200. [DOI] [PubMed] [Google Scholar]
- Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- Nakano M, Kawano Y, Kawagish M, Hasegawa T, Iinuma Y, Oht M. Two-dimensional analysis of exoproteins of methicillin-resistant Staphylococcus aureus (MRSA) for possible epidemiological applications. Microbiol Immunol. 2002;46:11–22. doi: 10.1111/j.1348-0421.2002.tb02671.x. [DOI] [PubMed] [Google Scholar]
- Orwin PM, Leung DY, Tripp TJ, Bohach GA, Earhart CA, Ohlendorf DH, Schlievert PM. Characterization of a novel staphylococcal enterotoxin-like superantigen, a member of the group V subfamily of pyrogenic toxins. Biochemistry. 2002;41:14033–14040. doi: 10.1021/bi025977q. [DOI] [PubMed] [Google Scholar]
- Panizzi P, Friedrich R, Fuentes-Prior P, Richter K, Bock PE, Bode W. Fibrinogen substrate recognition by staphylocoagulase (pro) thrombin complexes. J Biol Chem. 2006;281:1179–1187. doi: 10.1074/jbc.M507956200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti JM, Jonsson H, Guss B, Switalski LM, Wiberg K, Lindberg M, Hook M. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J Biol Chem. 1992;267:4766–4772. [PubMed] [Google Scholar]
- Qoronfleh MW, Weraarchakul W, Wilkinson BJ. Antibodies to a range of Staphylococcus aureus and Escherichia coli heat shock proteins in sera from patients with S. aureus endocarditis. Infect Immun. 1993;61:1567–1570. doi: 10.1128/iai.61.4.1567-1570.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qoronfleh MW, Bortner CA, Schwartzberg P, Wilkinson BJ. Enhanced levels of Staphylococcus aureus stress protein GroEL and DnaK homologs early in infection of human epithelial cells. Infect Immun. 1998;66:3024–3027. doi: 10.1128/iai.66.6.3024-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999;37:3556–3563. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost M, Wehmhoner D, Karst U, Dieterich G, Wehland J, Jansch L. Comparative proteome analysis of secretory proteins from pathogenic and nonpathogenic Listeria species. Proteomics. 2005;5:1544–1557. doi: 10.1002/pmic.200401024. [DOI] [PubMed] [Google Scholar]
- Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- Vytvytska O, Nagy E, Bluggel M, Meyer HE, Kurzbauer R, Huber LA, Klade CS. Identification of vaccine candidate antigens of Staphylococcus aureus by serological proteome analysis. Proteomics. 2002;2:580–590. doi: 10.1002/1615-9861(200205)2:5<580::AID-PROT580>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Walev I, Martin E, Jonas D, Mohamadzadeh M, Muller-Klieser W, Kunz L, Bhakdi S. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun. 1993;61:4972–4979. doi: 10.1128/iai.61.12.4972-4979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Schnupf P, Poussin MA, Zenewicz LA, Shen H, Goldfine H. Characterization of Listeria monocytogenes expressing anthrolysin O and phosphatidylinositol-specific phospholipase C from Bacillus anthracis. Infect Immun. 2005;73:6639–6646. doi: 10.1128/IAI.73.10.6639-6646.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005;5:275–286. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jacobsson K, Vasi J, Lindberg M, Frykberg L. A second IgG-binding protein in Staphylococcus aureus. Microbiology. 1998;144:985–991. doi: 10.1099/00221287-144-4-985. [DOI] [PubMed] [Google Scholar]
- Ziebandt AK, Weber H, Rudolph J, Schmid R, Hoper D, Engelmann S, Hecker M. Extracellular proteins of Staphylococcus aureus and the role of SarA and sigma B. Proteomics. 2001;1:480–493. doi: 10.1002/1615-9861(200104)1:4<480::AID-PROT480>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ziebandt AK, Becher D, Ohlsen K, Hacker J, Hecker M, Engelmann S. The influence of agr and sigmaB in growth phase dependent regulation of virulence factors in Staphylococcus aureus. Proteomics. 2004;4:3034–3047. doi: 10.1002/pmic.200400937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inter-strain conservation of proteins of unknown function.