Abstract
We initially identified a nuclear protein, prothymosin-α1 (ProTα), as a key protein inhibiting necrosis by subjecting conditioned media from serum-free cultures of cortical neurons to a few chromatography steps. ProTα inhibited necrosis of cultured neurons by preventing rapid loss of cellular adenosine triphosphate levels by reversing the decreased membrane localization of glucose transporters but caused apoptosis through up-regulation of proapoptotic Bcl2-family proteins. The apoptosis caused by ProTα was further inhibited by growth factors, including brain-derived neurotrophic factor. The ProTα-induced cell death mode switch from necrosis to apoptosis was also reproduced in experimental ischemia-reperfusion culture experiments, although the apoptosis level was markedly reduced, possibly because of the presence of growth factors in the reperfused serum. Knock down of PKCβII expression prevented this cell death mode switch. Collectively, these results suggest that ProTα is an extracellular signal protein that acts as a cell death mode switch and could be a promising candidate for preventing brain strokes with the help of known apoptosis inhibitors.
Introduction
Stroke is a major cause of death and a major factor behind people spending their lives confined to bed, as the consequences of a stroke include loss of functions such as memory, sensory perception, and motor skills. These symptoms are caused by various kinds of ischemia, which drive brain neurons toward death. In most cases with brain ischemia, neuronal death is composed of necrosis and apoptosis, which remove all damaged neurons (Dirnagl et al., 1999; Lipton, 1999). Necrosis occurs first in the ischemic core, whereas apoptosis occurs several days later in the region surrounding the core, called the penumbra. Both cell death modes after ischemia are initiated by the rapid loss of cellular ATP, followed by disturbances in cellular signaling mechanisms, including Ca2+ homeostasis (Lipton, 1999; White et al., 2000). The apoptosis machinery is accelerated after reperfusion, which partially supplies blood flow to produce the ATP required for the execution of apoptosis (Ferri and Kroemer, 2001; Danial and Korsmeyer, 2004; Ueda and Fujita, 2004). Many studies have revealed that several compounds that inhibit apoptosis in cells have protective roles against ischemic damage in vivo, although their potencies are limited (Cheng et al., 1998; Brines et al., 2000; Gilgun-Sherki et al., 2002; Gladstone et al., 2002). This may be related to the possibility that rapid and expanding necrosis largely contributes to the total loss of brain neurons after ischemia. Thus, rapid treatments are currently the focus of investigations into cures for brain strokes (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995; Gladstone et al., 2002; Borsello et al., 2003). Compared with the machinery of apoptosis, necrosis is a more passive process in which energy failure leads to mitochondrial swelling, accompanied by cristae disruption. These processes then lead to rupture of the plasma membrane with concomitant loss of intracellular proteins and ions. However, little is known about how to develop compounds that inhibit necrosis.
We recently demonstrated that cultured cortical neurons die by necrosis under low-density (LD) and starvation stress without serum or any supplements (Fujita et al., 2001; Fujita and Ueda, 2003a,b). Of particular interest are the findings that neuronal death in high-density (HD) cultures is markedly inhibited and that addition of conditioned medium (CM) from HD cultures prevents necrosis in LD cultures (Fujita and Ueda, 2003b). Here, we report the identification of a CM molecule, prothymosin-α1 (ProTα), that mediates necrosis inhibition and note the clinical potential of this protein to prevent brain strokes.
Results
As previously reported (Fujita et al., 2001; Fujita and Ueda, 2003a,b), rat embryonic cortical neurons in serum-free LD (105 cells/cm2) cultures rapidly died by necrosis. As early as 6 h, but not at 3 h, after the start of serum-free culture, neurons under LD conditions showed many pores on their surfaces by scanning EM analysis (Fig. 1 a). At 12 h, the cell surface membranes were largely destroyed and only the nuclei remained. By transmission EM analysis, typical necrotic features, such as membrane destruction, loss of cytoplasmic electron density, and swollen mitochondria with a disrupted cristae structure, were observed at 6 h (Fujita and Ueda, 2003a,b). Necrotic features were also observed by staining with propidium iodide (PI). PI staining was substantially observed after 3 h of LD culture and showed a time course that was parallel to the decrease in survival activity (Fig. 1 b). Addition of CM derived from 72-h HD (5 × 105 cells/cm2) cultures delayed the cell death in LD cultures in a concentration-dependent manner, with the concentration dependency also being parallel to the decrease in survival activity (Fig. 1 c). When the factor mediating this survival activity was purified from prefractionated extracts, 6.3 μg of an ∼20-kD protein was obtained by molecular weight cutoff ultrafiltration, ion-exchange filtration, and SDS-PAGE from 20 ml of the CM (Fig. 1 d–f; and Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200608022/DC1). After SDS-PAGE, this 20-kD protein was analyzed by matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectrometry (MS), and a search in the nonredundant National Center for Biotechnology Information protein database for matching peptide mass fingerprints revealed 17 peptides that were unique to rat ProTα. Moreover, tandem MS analysis confirmed that the N terminus of purified ProTα was an acetylated serine (129.612 vs. Ser 87.343 m/z; Fig. 1 g), in agreement with a previous report (Pineiro et al., 2000).
Figure 1.
Purification and identification of ProTα. (a) Scanning EM (SEM) analysis of neuronal necrosis. Cortical neurons were cultured at LD (105 cell/cm2) under the serum-free condition. (b) Parallel time-dependent decreases in survival activity and increases in PI staining (10 μg/ml) after the start of LD and serum-free cultures. Survival activity was evaluated by the WST-8 reduction activity. (c) Parallel CM concentration-dependent increases in survival activity and decreases in PI staining. The activities were measured at 12 h after the start of LD and serum-free cultures with various concentrations of CM. Error bars indicate mean ± SEM. (d and e) Purification of ProTα. Vivaspin 2, and Vivapure Q mini were used for ultrafiltration (d) and ion-exchange spin column chromatography (e), respectively. The samples indicated by asterisks in panels d and e were used for further separation in panels e and f, respectively. (f) SDS-PAGE analysis of the final purified material. (g) Predicted amino acid sequence.
For biological identification, we performed several experiments using an anti-ProTα IgG, which had already been characterized (Figs. S1 and S2, available at http://www.jcb.org/cgi/content/full/jcb.200608022/DC1). When CM factors were applied to anti-ProTα IgG-conjugated beads, the eluates obtained from acid-treated beads exhibited a single band that corresponded to recombinant ProTα on SDS-PAGE and an “acidic blot,” with no substantial signal in the flow-through, whereas the ProTα signal was observed in the flow-through from preimmune IgG-conjugated beads, but not in the control eluates (Fig. 2 a). After pretreatment of the CM with anti-ProTα IgG-conjugated beads, but not preimmune IgG-conjugated beads, ∼80% of the original CM-induced survival activity was lost (Fig. 2 b). Thus, it is evident that a large proportion of the survival activity in the CM can be attributed to the action of ProTα.
Figure 2.
ProTα is a major CM factor. (a) Immunoblot (“acidic blot”) identification of ProTα in the CM. (b) Functional absorption of the survival activity of CM factors by anti-rat ProTα IgG (α-ProTα IgG)–conjugated or preimmune IgG–conjugated beads. CM (20%) was added to cortical neurons at the start of LD (105 cells/cm2) culture, and the survival activity was evaluated after 12 h. Next, α-ProTα IgG (1 μg/ml)–conjugated or preimmune IgG–conjugated beads were incubated with the CM at 4°C for 8–12 h. *, P < 0.05 versus preimmune IgG–treated flow-through. (c) Time course of ProTα secretion in serum-free HD culture. In total, 2.75 × 107 cells were used for each sample. The protein (ProTα) was purified by acid-phenol extraction and detected by blue staining. The protein was identified as ProTα using MALDI-TOF MS. (d) Inhibition of survival activity in HD (5 × 105 cells/cm2) cultures after addition of α-ProTα IgG. *, P < 0.05 versus 0 μg/ml ProTα treatment. (e) Comparison of the survival activities of purified ProTα, recombinant ProTα, and ProTα deletion mutants after 12 h in serum-free LD culture. The amounts of ProTα precoated on the culture plates at 2 h before the LD culture are represented in pmol/cm2. (f) Equivalent ProTα-induced survival activities after precoating or addition. The amounts of ProTα precoated on the culture plates correspond to the initial concentrations indicated for ProTα addition (in nM). The results represent the mean ± SEM from six independent experiments.
For quantitative analysis, ProTα was extracted from the CM by acid phenol (Fig. S1 and supplemental text), subjected to SDS-PAGE, and directly detected by the highly sensitive blue stain method without a blotting procedure, as transfer of ProTα to a blotting membrane is unstable because of its acidity. ProTα was detected in the CM as early as 1 h after the onset of serum-free culture, and the level was maintained for up to 12 h, whereas the intracellular ProTα level was reduced (Fig. 2 c). The amount of ProTα in the CM of HD culture (72 h) was determined to be 66 pmol/cm2. This release into the CM was observed in serum-free, but not in serum-containing (serum-plus), cultures. Because cortical neurons showed no substantial plasma membrane damage at 12 h after the start of serum-free HD culture in terms of PI staining or transmision EM analysis (Fujita and Ueda, 2003a,b), the ProTα in the CM is likely to have been released from neurons whose membranes have not yet been disrupted. ProTα lacking a signal peptide sequence is probably released in a nonvesicular manner (unpublished data), as seen in the case of FGF-1 (LaVallee et al., 1998; Matsunaga and Ueda, 2006). When anti-ProTα IgG was simply added to HD cultures, a concentration-dependent decrease in the survival activity was observed, despite no extra incubation for immunoabsorption (Fig. 2 d). This finding provides strong evidence that ProTα released under serum-free stress plays a substantial neuroprotective role.
ProTα purified to homogeneity exhibited a concentration dependency equivalent to that of the recombinant protein and had a maximum survival activity in LD cultures that was equal to that in HD cultures (Fig. 2 e). Furthermore, addition of ProTα mutants that lacked the N-terminal region (Δ1–29), including thymosin-α1 (Pineiro et al., 2000), or the C-terminal region (Δ102–112), including the nuclear localization signal TKKQKK, retained the original activity of ProTα. As the culture plates were precoated with ProTα in the aforementioned experiments, the site of ProTα action seems to be through unidentified cell surface membrane targets, but not through thymosin-α1 receptors or nuclear binding sites. In this experiment, the survival activities of ProTα were equivalent when the same amount of protein (25 pmol/cm2) was used to precoat culture plates or added directly to cultures (initial medium concentration: 80 nM; Fig. 2 f).
Recombinant ProTα reversed the rapid decrease in survival activity in cortical neurons caused by the serum-free or permanent ischemia model (Fig. 3 a). The addition of ProTα abolished all the typical necrotic features, such as disrupted plasma membranes and swollen mitochondria, but no damage to the nucleus, at 6 h in the transmission EM analysis, and instead caused apoptosis, as observed by nuclear fragmentation, at 12 h (Fig. 3 b). A similar cell death mode switch from necrosis to apoptosis was observed by pretreatment with CM factors (20%) derived from HD cultures, whereas treatment with 1 μg/ml anti-ProTα IgG inhibited the cell death mode switch (Fig. 3 b). When the cell death mode was evaluated by double staining with necrosis (PI) and apoptosis (annexin V, caspase-3, and TUNEL) markers, 69, 86, and 92% of neurons, respectively, died by necrosis under serum-free stress, whereas only 15, 22, and 5% of neurons, respectively, died by apoptosis (Fig. 3 c). Addition of ProTα or CM totally switched the cell death mode from necrosis to apoptosis, and the CM-induced changes were abolished by further addition of anti-ProTα IgG. These findings suggest that the cell death mode switch induced by CM factors is largely attributable to the action of ProTα. A pharmacological study using various inhibitors revealed that the survival activity of recombinant ProTα was mediated through activation of PLC and PKC (Fig. 3 d), consistent with a previous report regarding CM factors (Fujita and Ueda, 2003b). In the present study, we used 1 μM U73122, a PLC inhibitor, and GF109203X, a pan-type PKC inhibitor. These findings were supported by a biochemical study, in which addition of ProTα significantly increased the PKC activity and the effect was reversed by U73122 (Fig. 3 e). This survival activity at 12 h was inhibited by Go6976, a PKCα/β inhibitor, but not by HBDDE, a PKCα/γ inhibitor, or Rottlerin, a PKCα/δ/θ inhibitor (Fig. 3 d). Therefore, the PKCβ isoform is likely to be involved.
Figure 3.
ProTα induced cell death mode switch. (a) Time course of the survival activity of cortical neurons throughout serum-free (SFree) and LD cultures. The survival activity of ProTα was evaluated by the WST-8 reduction activity as the percentage relative to the 0 time activity immediately after the start of the cultures using 96-well culture plates precoated with 0 or 25 pmol/cm2 ProTα at 2 h before the culture. (b) Transmission EM analysis at 12 h. Necrosis is characterized by membrane destruction and loss of electron density in the cytosol. Apoptotic features of nuclear fragmentation, but no substantial necrotic features, are observed in neurons treated with 25 pmol/cm2 ProTα or 20% CM. The CM, which had been preabsorbed with α-ProTα IgG, shows no apoptosis induction. (c) Double staining of LD cultures with PI (red) and annexin V (green), PI (red) and activated caspase-3 IgG (green), and PI (red) and TUNEL (green) after incubation for 3, 12, and 24 h, respectively. (d) Effects of various inhibitors of PKC and PLC on ProTα-induced survival activity. All the inhibitors were used at 1 μM. The survival activity is inhibited by U73122, a PLC inhibitor; GF109203X, a pan-type PKC inhibitor; and Go6976, a PKCα/β inhibitor, but not by U73343, an inactive isomer of U73122; HBDDE, a PKCα/γ inhibitor; or Rottlerin, a PKCα/δ/θ inhibitor. None of the inhibitors had any significant effects alone (not depicted). (e) ProTα induced PKC activation through PLC. The results represent the mean ± SEM from six independent experiments (a, d, and e). *, P < 0.05 versus vehicle; #, P < 0.05 versus ProTα alone.
Significant ProTα-induced survival activity was observed after 12 h of serum-free culture, but not at 24 h (Fig. 4 a). However, more potent and long-lasting survival activity was observed in the low-oxygen and low-glucose (LOG) ischemia and reperfusion model. It should be noted that no change in the survival activity was observed between 24 and 48 h in the latter condition. The incidence of apoptosis in ProTα-treated samples was markedly lower in the latter reperfusion model (38.4 ± 6.16%; n = 4) than in the serum-free model (86.0 ± 8.25%; n = 6), suggesting that this difference could be attributed to the action of antiapoptotic serum factors. This view was clearly confirmed when antiapoptotic growth factors and ProTα were added to the serum-free culture (Fig. 4 b). At 48 h after the start of serum-free culture, the survival activity was as low as 5%, even in the presence of ProTα alone. However, further addition of NGF, brain-derived neurotrophic factor (BDNF), basic FGF, or interleukin-6 rescued the survival activity to >80% of the control level, whereas these factors alone had no effects on the survival. There was no mitochondrial cytochrome c (cyto c) release, which induces apoptosis through the formation of the apoptosome with Apaf- 1 and caspase-9, whereas addition of ProTα caused cyto c release (Fig. 4 c). It should be noted that ProTα-induced cyto c release was abolished by further addition of BDNF or BIP-V5, which blocks the translocation of Bax to mitochondria (Yoshida et al., 2004), but not by zVAD-fmk, a pan-type caspase inhibitor.
Figure 4.
ProTα induced cell death mode switch and improvement of survival by neurotrophic factors. (a) Comparison of 80 nM ProTα-induced survival activity under serum-free and LOG stress conditions. The concentration (80 nM) corresponds to the initial concentration when ProTα was used at 25 pmol/cm2 for precoating culture plates (Fig. 2 f). (b) Complete neuroprotection after the addition of various neurotrophic factors. Each neurotrophic factor (100 ng/ml) was added with 25 pmol/cm2 ProTα to serum-free cultures. (c) 25 pmol/cm2 ProTα induced cyto c release and its blockade by BDNF or BIP-V5. Double staining with the mitochondrial marker CMXRos and anti–cyto c IgG was performed as described previously (Fujita and Ueda, 2003a). ProTα and 100 ng/ml BDNF, 100 μM zVAD-fmk, or 100 μM BIP-V5 were added at the beginning of serum-free culture. (d) Effects of various apoptosis inhibitors on the cell death modes of 25 pmol/cm2 ProTα-treated cells. Results represent the time-dependent changes in cell death modes after the start of serum-free culture. TUNEL- and PI-positive cells were evaluated as apoptotic and necrotic cells, respectively, whereas TUNEL- and PI-negative ones were as living cells. Data are expressed as the mean ± SEM from three independent experiments. *, P < 0.05 versus vehicle treatment; #, P < 0.05 versus ProTα alone.
Fig. 4 d demonstrates the time-dependent changes in cell death status when the culture was performed in the presence of ProTα and BDNF, zVAD-fmk, or BIP-V5. The addition of ProTα alone inhibited necrosis throughout 48 h and increased the number of living cells (or necrosis, apoptosis double negative) more prominently at the early stage (12 h), but not at the later stage (24 or 48 h). On the contrary, the number of cells showing apoptosis time-dependently increased in the presence of ProTα. Further addition of BDNF or BIP-V5 showed complete survival by inhibiting apoptosis throughout 48 h. However, zVAD-fmk caused a marked cell death by necrosis at the later stage, though it showed complete survival at the early stage.
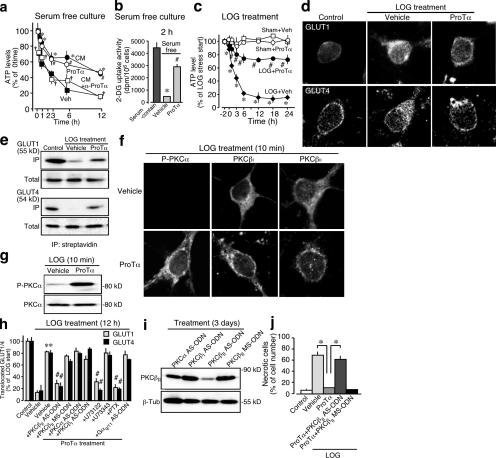
It is generally accepted that necrosis is caused by energy failure because of the loss of cellular ATP (Eguchi et al., 1997; Fujita and Ueda, 2003a,b; Zong and Thompson, 2006). The cellular ATP levels of cortical neurons rapidly decreased immediately after the start of serum-free culture (Fig. 5 a). This decrease was markedly inhibited by the addition of ProTα or CM, and further addition of anti-ProTα IgG abolished the CM effects. As previously reported (Fujita and Ueda, 2003b), this rapid decrease and its reversal by ProTα seem to be parallel to the activity of glucose transport, as [3H]-2-DG uptake was markedly decreased by serum-free treatment and reversed by ProTα (Fig. 5 b). Similar changes were also observed in the ischemia-reperfusion model of culture (Fig. 5 c). Addition of ProTα reversed the rapid decrease in the cellular ATP levels of cortical neurons after LOG ischemic stress and reperfusion with serum-containing medium (Ueda and Fujita, 2004). We previously revealed that the membrane translocation of the glucose transporters GLUT1 and -4 is largely inhibited in serum-free cultures of cortical neurons, which leads to necrotic cell death (Fujita and Ueda, 2003a). In the present study, inhibition of GLUT1 and -4 membrane translocation was also observed under LOG stress by immunocytochemistry (Fig. 5 d). Biochemical evidence was also found when the cell surface proteins were biotinylated before Western blot analysis (Fig. 5 e).
Figure 5.
ProTα induced inhibition of necrosis through PKCβII. (a and b) ProTα induced reversal of the decreases in the cellular ATP level (a) and [3H]2-DG uptake (b) after serum-free stress. [3H]2-DG uptake was performed for 2 h immediately after the start of the culture. (c) Time course of the ProTα-induced reversal of the decrease in the cellular ATP level after LOG stress. 80 nM ProTα was added to the culture from the time of LOG stress to the end of experiments. (d and e) Decreased translocation of GLUT1 and -4 to the plasma membrane at 2 h after LOG stress and its reversal by ProTα. All the proteins on the outer surface of cortical neurons (LD cultures) were biotinylated and subjected to immunoprecipitation by streptavidin (e). (f) ProTα induced PKC activation in terms of phosphorylation of PKCα (p-PKCα) and translocation of PKCβI and -βII at 10 min after ProTα treatment in serum-free culture. No substantial ProTα-induced activation of other PKC isoforms was observed in experiments using a rabbit anti-PKCγ antibody (1:100), goat anti-PKCɛ antibody (1:100), rabbit anti–phosphorylated PKCδ antibody (1:100), or rabbit anti–phosphorylated PKCζ antibody (1:100; not depicted). (g) Immunoblot analysis of the protein expression of phosphorylated or total PKCα at 2 h after LOG stress. (h) Signal transduction for ProTα-induced reversal of the LOG stress–induced decrease in GLUT1/4 membrane translocation (n = 3). The method for quantifying the GLUT1/4 membrane localization is described in Fig. S2 (available at http://www.jcb.org/cgi/content/full/jcb.200608022/DC1). Cells were treated with 1 μM U73122, 1 μM U73343, or 100 ng/ml pertussis toxin (PTX) from 30 min before the LOG treatment. Treatment of LD cultures with AS-ODNs for PKCα, PKCβI, PKCβII, or Gαq/11 was started 3 d before the LOG treatment. Selective down-regulation of Gαq/11 by its AS-ODN was confirmed by Western blot analysis (Fig. S3). (i) Selective down-regulation of PKCβII by its AS-ODN. The specificities of the other AS-ODNs are shown in Fig. S3. (j) ProTα induced inhibition of necrotic cell death through PKCβII activation. PI was added to the cells at 12 h after reperfusion and incubated for 30 min. 80 nM ProTα was added to the culture from the time of LOG stress. *, P < 0.05 versus vehicle; #, P < 0.05 versus ProTα. Error bars indicate mean ± SEM.
An immunocytochemical study revealed that ProTα activated PKCα, -βI, and -βII at 10 min (Fig. 5, f and g). A knockdown study using antisense oligodeoxynucleotides (AS-ODNs) for these kinases demonstrated that only PKCβII, not PKCα or -βI, plays roles in the ProTα-induced GLUT1/4 translocation (Fig. 5, h and i). Further characterization revealed that ProTα induced GLUT1/4 translocation by activation of PLC through pertussis toxin–sensitive Gαi/o, but not Gαq/11, suggesting that a putative Gαi/o-coupled ProTα receptor may be involved in this action. Furthermore, the PKCβII AS- ODN treatment reversed the ProTα-induced necrotic PI staining (Fig. 5 j).
The molecular machineries for apoptosis are relatively better characterized than those for necrosis. In terms of the activation of various caspases, caspase-3 is believed to be the final execution molecule for apoptotic cell death linked to DNA breakdown and nuclear fragmentation (Ferri and Kroemer, 2001; Danial and Korsmeyer, 2004). ProTα activated caspase-3 in the serum-free or permanent ischemia model (Fig. 6 a). Similar activation was also observed for caspase-9, but not for caspase-8 or -12. These findings suggest that ProTα causes apoptosis through a caspase-9–mediated mitochondrial pathway. This view was clearly confirmed by the findings that ProTα increased the expression of proapoptotic Bax and Bim, but slightly decreased the expression of antiapoptotic Bcl-2 and -xL, which regulate mitochondrial apoptotic signaling (Fig. 6 b). On the other hand, the PKCβII AS-ODN also reversed the ProTα-induced proapoptotic Bax expression in the LOG stress model (Fig. 6 c). However, it should be noted that the Bax expression was also abolished by treatment with the AS-ODN for PKCβ1, but not that for PKCα.
Figure 6.
ProTα induced stimulation of apoptosis through PKCβI and -βII. (a and b) ProTα induced activation of caspase family proteins (a) and up-regulation of apoptosis, and down-regulation of anti-apoptotic Bcl-2 family proteins (b), in serum-free stress cultures. (c) PKCβI and -βII AS-ODNs block the ProTα-induced up-regulation of Bax at 12 h after LOG stress. (d) The blockade of ProTα-induced apoptosis by Bax siRNA treatment at 24 h after the LOG stress of cultured cortical neurons. Apoptosis and necrosis were evaluated by the immunostaining of active caspase-3 or by PI staining, respectively. The cells indicated by arrows represent Bax-negative ones. (e) Quantification of Bax siRNA–induced blockade of apoptosis in the presence of ProTα. Double caspase-3– and PI-negative cells were counted as living cells. The Bax-positive and -negative cells represent the cells without and with Bax down-regulation by the siRNA treatment, respectively. Results are expressed as the mean ± SEM from three independent experiments. *, P < 0.05 versus vehicle treatment; #, P < 0.05 versus Bax-positive group.
To examine the role of Bax and Bim in the ProTα-induced apoptosis, we performed the experiments using siRNA in the LOG ischemic stress and reperfusion model. As shown in Fig. 6 d, the ProTα treatment markedly up-regulated the Bax expression in all cells. The pretreatment of Bax siRNA 24 h before ProTα treatment caused a complete loss of Bax in 10–18% of total cells, and these Bax-negative cells did not show any apoptotic active caspase-3 or necrotic PI staining. This finding was also confirmed by the quantification of incidence of apoptotic, necrotic, and living cells in experiments without and with Bax siRNA treatment. As mentioned in Fig. 3 (b and c), the ProTα treatment alone abolished the necrosis, whereas it increased the survival with some apoptosis (Fig. 6 e, left). A similar cell death ratio was observed in Bax-positive cells, which are unlikely to be transfected with siRNA. However, Bax-negative cells were all alive, or apoptosis and necrosis negative. However, the down-regulation of Bim showed less significant changes in the number of cells showing apoptosis (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200608022/DC1). These results strongly suggest that ProTα causes apoptosis through an up-regulation of Bax.
Discussion
ProTα is a highly acidic nuclear protein of the α-thymosin family and is widely distributed throughout the body (Haritos et al., 1985; Clinton et al., 1991). It is generally thought to be an oncoprotein that is correlated with cell proliferation by sequestering anticoactivator, a repressor of estrogen receptor activity, in various cells (Martini et al., 2000; Bianco and Montano, 2002). On the other hand, ProTα has also been reported to act as an extracellular signaling molecule, as observed in the activation of macrophages, natural killer cells, and lymphokine-activated killer cells, and in the production of interleukin-2 and TNF-α (Pineiro et al., 2000). Here, we isolated ProTα from CM of primary cultures of cortical neurons as a molecule providing protection against neuronal necrosis (Fujita and Ueda, 2003b). By using a specific antibody, ProTα was proven to be the major CM factor involved in density-dependent survival under conditions of serum-starvation stress.
The identity of the target of ProTα in respect to cell death regulation is a very interesting issue. ProTα was reported to inhibit apoptosome formation in NIH3T3 cells (Jiang et al., 2003). This observation is in good contrast with the present finding that addition of ProTα to neuronal cultures caused apoptosis, suggesting that ProTα has opposite functions inside and outside of the cell. Furthermore, as a ProTα deletion mutant lacking the nuclear localization signal retained the full survival activity, it is unlikely to be the aforementioned genomic action. The most probable candidate would be a cell surface receptor. Indeed, the presence of a cell surface ProTα receptor has been reported in lymphoid cells (Pineiro et al., 2001; Salgado et al., 2005), and we confirmed this in cortical neurons by using ProTα–Alexa 488 (Fig. S5, available at http://www.jcb.org/cgi/content/full/jcb.200608022/DC1). Further strong evidence to support the presence of a cell surface ProTα receptor is the fact that ProTα-induced membrane transport of GLUT1/4 was mediated through a Gαi/o-coupled receptor, which activated PLC and PKCβII. Because ProTα-induced translocation of PKCβII was observed within 10 min, it is evident that this signaling can be attributed to a direct action through a membrane receptor.
The distinctive advantage of ProTα-induced neuroprotection can be attributed to the inhibition of necrosis. Necrosis is characterized by bioenergetics failure and rapid loss of plasma membrane integrity, which may result from decreased glucose transport (Fujita and Ueda, 2003a), as well as enzymatic destruction of cofactors required for ATP production, increased mitochondrial reactive oxygen species production, and channel-mediated calcium uptake (Nordberg and Arner, 2001; Xiong et al., 2004; Zong and Thompson, 2006). In our serum-free or LOG stress model, most cortical neurons died by necrosis. We found that rapid cell death or necrosis was accompanied by decreased glucose uptake and cellular ATP levels. The glucose transport mechanism is one of the most important sets of molecules for maintaining cell survival. Various species of GLUT have been identified in different cell types (McEwen and Reagan, 2004). Because GLUT3, which is most abundant in neurons, is constitutively localized in membranes, its function is unlikely to be regulated by environmental factors. In contrast, it was reported that some survival factors induce translocation of GLUT1 and -4 into plasma membranes through activation of protein kinases, including AKT and PKCs (Perrini et al., 2004; Ishiki and Klip, 2005; Welsh et al., 2005). This is consistent with our previous study showing that serum-free stress reduces GLUT1/4 translocation (Fujita and Ueda, 2003a). Here, we successfully demonstrated that ProTα prevented the stress-induced reduction of GLUT1/4 transport through PKCβII activation.
The second important issue is that ProTα switches the cell death mode by causing apoptosis. Because serum-free stress alone did not cause mitochondrial cyto c release, this stress by itself is unlikely to induce the machinery for apoptosis as well as that for necrosis. This view is supported by our previous report that addition of pyruvate to serum-free cultures to maintain the cellular ATP levels prevented necrosis but did not induce apoptosis (Fujita and Ueda, 2003a). Although the possibility still remains that pyruvate has an unidentified mechanism to remove the trigger for apoptosis, it is very likely that apoptosis does not always occur after the prevention of necrosis. This finding strongly supports the view that ProTα induces apoptosis. In the present study, we have demonstrated that ProTα up-regulates proapoptotic Bax and Bim, and down-regulates antiapoptotic Bcl-2 and -xL. Because the treatment with Bax siRNA blocked the ProTα-induced apoptosis, and the treatment with BIP-V5 blocked the ProTα-induced cyto c release and apoptosis, it is evident that the up-regulation of Bax plays an important role in ProTα-induced apoptosis. On the other hand, the caspase inhibitor zVAD-fmk blocked the ProTα-induced apoptosis and caused necrosis. This may be explained by the view that the up-regulation of Bax by ProTα causes a cyto c depletion from mitochondria, followed by the necrosis induction through a damage of mitochondrial ATP production (Chipuk et al., 2006; Malhi and Gores, 2006), as apoptosis is inhibited by zVAD-fmk.
ProTα-induced up-regulation of Bax was found to be mediated by PKCβI and -βII activation, consistent with previous reports that PKCβ activates the I-κB kinase complex, IKK (Mattson and Camandola, 2001; Herrmann et al., 2005), leading to NF-κB activation followed by Bax up-regulation. Thus, PKCβII is likely to be an important switch molecule to determine the cell death mode. The lack of contribution of PKCβ1 to the ProTα-induced necrosis inhibition may be related to the deficiency of the membrane-anchoring C-terminal peptide of PKCβII (Ono et al., 1986).
By use of acid-phenol extraction and blue staining, the amount of ProTα in the CM was determined to be 66 pmol/cm2. The data of Fig. 2 c revealed that CM amounts are ∼50% of the total (CM + cells) at 6–12 h after the start of serum-free HD culture. As this value in CM (∼30 pmol/cm2 of ProTα) corresponds to the concentration of ProTα (25 pmol/cm2) required to make the conversion of necrosis to apoptosis, this mechanism seems to be physiologically relevant.
The possible in vivo roles of ProTα in brain stroke represent the most important issue to be discussed. ProTα inhibits the rapid cell death of neurons after serum-free ischemic stress by inhibiting necrosis. This property seems to be beneficial, as the representative growth factors used in the present study had no effects on the necrosis, although they have potent antiapoptosis activities. Furthermore, ProTα is a unique cell death regulatory molecule in that it converts the intractable cell death necrosis into the controllable apoptosis. Because this apoptosis would be inhibited by growth factors secreted upon ischemic stress, it is expected that ProTα may have neuroprotective roles in brain stroke. As mentioned in Fig. 4 (b–d), the combined use of ProTα with growth factors, but not caspase inhibitors, may have a potential clinical availability. In conclusion, we have identified the survival factor secreted from cortical cultures as the nuclear protein ProTα. We have also demonstrated that this protein plays an in vivo neuroprotective role in brain ischemic events. Moreover, it has the potential for clinical use against brain strokes.
Materials and methods
Materials
Cell culture medium and FCS were purchased from Invitrogen. The antibodies used in the present study were GULT1 and -4; BDNF; phosphorylated PKCα, -βI, -βII, -γ, -ɛ, and -δ (all from Santa Cruz Biotechnology, Inc.); activated caspase-3 and phosphorylated PKCζ (Cell Signaling); and cyto c (BD Biosciences). The reagents for staining were PI (Sigma-Aldrich), TUNEL, Hoechst 33342 (Invitrogen), and Gelcode blue stain reagent (Pierce Chemical Co.).
Purification of ProTα
After several trials, we optimized our procedures for purifying ProTα. Purification was started with 20 ml CM, which had been collected at 72 h after the start of HD culture, as previously reported (Fujita and Ueda, 2003b). The CM was first subjected to ultrafiltration (Vivaspin 2; Sartorius KK), and the active materials observed in the >5-kD fraction were applied to an ion-exchange membrane spin column (Vivapure Q Mini; Sartorius KK), which had been equilibrated with 20 mM sodium acetate, pH 5.2. The sample was eluted with different concentrations of NaCl (0.2–1 M), and the active fraction was finally separated by SDS-PAGE and stained with Gelcode blue stain. The appropriate band was excised from the gel, washed with 50 mM NH4HCO3 and 50% acetonitrile, and incubated with 100% acetonitrile for 10 min. The gel segment was rehydrated in 50 mM NH4HCO3 and then dehydrated in 100% acetonitrile. The resulting gel plug was incubated overnight with 5 ng/μl trypsin in 25 mM NH4HCO3. The digested peptide mixture was diluted with the matrix 4-hydroxy-α-cyanocinnamic acid (HCCA) in 1:1 acetonitrile/0.1% TFA (vol/vol), deposited on a target, and dried to allow MALDI-TOF MS analysis (Bruker Daltonik).
Preparation and detection of recombinant proteins
Purification of recombinant rat ProTα was performed as described previously (Evstafieva et al., 1995). This procedure using acid phenol was also available for simple purification of endogenous ProTα for SDS-PAGE analysis. In the recombinant protein preparation, the genes for ProTα and its deletion mutants (Δ1–29 and Δ102–112) were first amplified from cDNAs derived from rat embryonic brain using specific primers (rat and mouse 5′-primer, 5′-AACATATGTCAGACGCGGCAGTGGA-3′; rat 3′-primer, 5′-AAGGATCCAGTGGAGGGTGAATAGGTCAC-3′; rat Δ1–29 5′-primer, 5′-AAGAATTCGGAAGAGACGCACCTGCC-3′; rat 3′-primer, 5′-GAGTCGACCTAGTCATCCTCATCAGTCTTC-3′; rat Δ102–112 5′-primer, 5′-AAGAATTCATGTCAGACGCGGCAGTG-3′; rat 3′-primer, 5′-GAGTCGACCTACTCAACATCATCATCCTCATC-3′). The PCR products were cloned into pGEM-T Easy and subcloned into pET16b. BL21 (DE3) cells were transformed with pET16b-ProTα. Recombinant rat ProTα and its derivatives were induced by 0.1 mM IPTG, purified (Biophoresis; ATTO), and dialyzed against PBS for later use. Recombinant and endogenous ProTα isolated by the acid-phenol extraction procedure were detected as described previously (Evstafieva et al., 1995).
Primary culture
Primary culture of the cerebral cortex from 17 d of embryonic rats was performed according to the previously reported protocol (Fujita et al., 2001; Fujita and Ueda, 2003b). They were seeded onto 96-well culture dishes, 8-well Lab-Tek chambers (Nunc), and 3.5- and 9.0-cm culture dishes that had been all coated with poly-dl-ornithine (Sigma-Aldrich) and cultured in DME/F-12 medium at 37°C in 5%-CO2 atmosphere. For ProTα coating, recombinant ProTα was added to culture dishes and incubated for 2 h at 25°C. The dishes were washed twice with PBS for immediate use.
In vitro ischemia-reperfusion stress model
Primary cultures of 17-d-old embryonic rat cerebral cortex were prepared as described previously (Fujita and Ueda, 2003b). After being cultured for 3 d, cortical neurons were washed twice with glucose-free balanced salt solution (BSS; 116 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 1 mM NaH2PO4, pH 7.3), which had been deaerated using a vacuum. After replacement of the BSS with fresh BSS containing 1 mM glucose, neurons were exposed to hypoxia (<0.4% O2, 5% CO2, and 95% N2) for 2 h at 37°C in a commercially available culture incubator (Nuair). After the ischemic stress, the culture medium was exchanged for fresh DME/F-15 medium (1:1) containing 5% horse serum and 5% FBS, and the neurons were further incubated for the indicated periods in a 5% CO2 atmosphere (reperfusion).
Characterization of the modes of cell death
Survival activity was determined by the WST-8 reduction assay throughout the experiments. The modes of cell death were determined by various means, including PI staining, activated caspase-3, GLUT1, GLUT4, TUNEL, ATP measurement, and scanning and transmission EM analyses, as previously reported (Fujita et al., 2001; Fujita and Ueda, 2003a,b). In the GLUT translocation analyses, the cortical neurons were biotinylated (Pierce Chemical Co.), lysed, immunoprecipitated with streptavidin-conjugated beads, and subjected to Western blot analysis. Characterization of the modes of cell death and the immunocytochemistry analysis are described in the supplemental text.
Immunocytochemistry and immunoblot analysis for PKCs
Cortical cells on 8-well Lab-Tek chamber slides were fixed with 4% PFA in PBS for 30 min at 25°C, followed by permeabilization with 50 and 100% methanol for 5 min each at 25°C. The cells were then rinsed twice with PBS and preincubated in blocking buffer (2% BSA with 0.1% Tween 20 in PBS) for 1 h at 25°C. Next, the cells were incubated with each primary antibodies in blocking buffer overnight at 4°C, rinsed with PBS, and incubated with FITC-conjugated anti-rabbit IgG (1:200; Santa Cruz Biotechnology, Inc.) or FITC-conjugated anti-goat IgG (1:200; Rockland) for 2 h at 25°C. The immunolabeled cells were mounted with Permafluor (Thermo Scientific). For imaging cells, a laser-scanning confocal microscope imaging system consisting of a microscope (Axiovert 200 M; Carl Zeiss MicroImaging, Inc.) and a scan module (LSM 510 META and LSM 5 PASCAL; Carl Zeiss MicroImaging, Inc.) with image browser software (Carl Zeiss MicroImaging, Inc.) were used at ambient temperature, equipped with 40×/1.3 and 63×/1.4 oil-immersion lens and nonimaging photodetection device (photomultiplier tube; Carl Zeiss MicroImaging, Inc.). The imaging medium used was immersion oil (Immersol 518; Carl Zeiss MicroImaging, Inc.). A dynamic range adjustment was used to optimize the signal for the fluorophores, and images were collected in multitrack mode (Carl Zeiss MicroImaging, Inc.). Any brightness and contrast adjustments were performed in Photoshop (Adobe).
Western blot analysis
SDS-PAGE using 10–15% polyacrylamide gels and immunoblot analyses were performed as described previously (Fujita and Ueda, 2003a). The primary antibodies were an anti-phosphorylated PKCα antibody, anti-PKCα antibody, rabbit anti-PKCβI antibody, and rabbit anti-PKCβII antibody (1:1,000; Santa Cruz Biotechnology, Inc.). Visualization of immunoreactive bands was performed using an enhanced chemiluminescent substrate (Super Signaling Substrate; Pierce Chemical Co.) for HRP detection.
AS-ODN treatments
To determine the activation of various PKC isoforms and G protein in the mechanism of GLUT translocation, cultures were grown in the presence of AS-ODNs for PKCα, PKCβI, PKCβII, or Gαq/11. The AS-ODNs were diluted in water to a concentration of 20 μM and added to the cultures at a final volume of 1/50 of the culture medium every 12 h after seeding for 3 d. In parallel, some cultures were treated with the corresponding missense ODNs containing the same bases as the AS-ODNs but in a random order. None of the ODNs resembled any other sequences in the GenBank database. Using Western blot and immunocytochemical analyses, we demonstrated that treatment of cortical neurons in culture with these AS-ODNs, but not the missense ODNs, reduced the levels of the target proteins. The probes had the following sequences: PKCα AS-ODN, 5′-CGGGTAAACGTCAGC-3′ (Fleming et al., 1998); PKCβI AS-ODN, 5′-GTTTTAAGCATTTCG-3′; PKCβII AS-ODN, 5′-GTTGGAGGTGTCTCT-3′; PKCβII missense ODN, 5′-ACGAGCCCGAACCACCGT-3′ (Simpson et al., 1998); and Gαq/11 AS-ODN, 5′-ATGGACTCCAGAGT-3′ (Mizota et al., 2005). All the ODNs were purchased from QIAGEN.
Bax and Bim gene silencing by siRNA
Bax and Bim siRNA constructs were purchased from Ambion (siRNA ID 49750 and 47149). Gene silencing was attained by transfection of siRNA into cells using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's instructions. The gene silencing was verified by detecting protein with immunocytochemical analysis 48 h after the transfection of primary cortical neurons with siRNA. In brief, cells (1 × 105 cells/cm2) grown in an 8-well Lab-Tek chamber slide were transiently transfected with 50 nM siRNA using 20 μl/ml Lipofectamine 2000 in a total transfection volume of 0.2 ml DME (Invitrogen). After incubation at 37°C in 5% CO2 for 6 h, the medium was replaced by fresh serum–containing medium. 2 d after the incubation, treated neurons were used for the characterization of cell death modes, as described.
Statistical analysis
Multiple comparisons of analysis of variance followed by t test were used for statistical analysis of the data. The criterion of significance was set at P < 0.05. All the results are expressed as the mean ± SEM.
Online supplemental material
The supplemental text contains additional methodological details on characterization of anti-ProTα IgG used, as well as protocols used for cell survival activity, intracellular ATP levels, [3H]-2-DG uptake, PKC kinase assay, and immunostaining protocol. Table S1 shows a summary of the procedures for purifying ProTα from CM. Fig. S1 shows a characterization of anti-ProTα IgG. Fig. S2 shows an evaluation of membrane localization of GLUT1/4 by fluorescence imaging. Fig. S3 shows specific down-regulation of Gαq/11 and PKC isoforms by treatment with AS-ODNs. Fig. S4 shows immunostaining of ProTα-induced apoptosis under the Bim siRNA–treated LOG stress condition. Fig. S5 shows ProTα–Alexa 488 binding to cell membranes. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200608022/DC1.
Acknowledgments
We are grateful to N. Fukushima and H. Kawasaki for technical advice; M. Niwa and T. Suematsu for help with the scanning and transmission EM studies; K. Fujiwara for help with the anti-ProTα antibody preparation; and N. Takayama, W. Hamabe, and N. Kiguchi for technical assistance.
Parts of this study were supported by grants-in-aid and special coordination funds from the Ministry of Education, Culture, Sports, Science and Technology.
Abbreviations used in this paper: AS-ODN, antisense ODN; BDNF, brain-derived neurotrophic factor; CM, conditioned medium; HD, high-density; LD, low-density; LOG, low-oxygen and low-glucose; MALDI-TOF, matrix-assisted laser desorption/ionization–time of flight; MS, mass spectrometry; ODN, oligodeoxynucleotide; PI, propidium iodide; ProTα, prothymosin-α1.
References
- Bianco, N.R., and M.M. Montano. 2002. Regulation of prothymosin alpha by estrogen receptor alpha: molecular mechanisms and relevance in estrogen-mediated breast cell growth. Oncogene. 21:5233–5244. [DOI] [PubMed] [Google Scholar]
- Borsello, T., P.G. Clarke, L. Hirt, A. Vercelli, M. Repici, D.F. Schorderet, J. Bogousslavsky, and C. Bonny. 2003. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 9:1180–1186. [DOI] [PubMed] [Google Scholar]
- Brines, M.L., P. Ghezzi, S. Keenan, D. Agnello, N.C. de Lanerolle, C. Cerami, L.M. Itri, and A. Cerami. 2000. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc. Natl. Acad. Sci. USA. 97:10526–10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y., M. Deshmukh, A. D'Costa, J.A. Demaro, J.M. Gidday, A. Shah, Y. Sun, M.F. Jacquin, E.M. Johnson, and D.M. Holtzman. 1998. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J. Clin. Invest. 101:1992–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk, J.E., L. Bouchier-Hayes, and D.R. Green. 2006. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 13:1396–1402. [DOI] [PubMed] [Google Scholar]
- Clinton, M., L. Graeve, H. el-Dorry, E. Rodriguez-Boulan, and B.L. Horecker. 1991. Evidence for nuclear targeting of prothymosin and parathymosin synthesized in situ. Proc. Natl. Acad. Sci. USA. 88:6608–6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial, N.N., and S.J. Korsmeyer. 2004. Cell death: critical control points. Cell. 116:205–219. [DOI] [PubMed] [Google Scholar]
- Dirnagl, U., C. Iadecola, and M.A. Moskowitz. 1999. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 22:391–397. [DOI] [PubMed] [Google Scholar]
- Eguchi, Y., S. Shimizu, and Y. Tsujimoto. 1997. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 57:1835–1840. [PubMed] [Google Scholar]
- Evstafieva, A.G., N.V. Chichkova, T.N. Makarova, A.B. Vartapetian, A.V. Vasilenko, V.M. Abramov, and A.A. Bogdanov. 1995. Overproduction in Escherichia coli, purification and properties of human prothymosin alpha. Eur. J. Biochem. 231:639–643. [PubMed] [Google Scholar]
- Ferri, K.F., and G. Kroemer. 2001. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 3:E255–E263. [DOI] [PubMed] [Google Scholar]
- Fleming, I., S.J. MacKenzie, R.G. Vernon, N.G. Anderson, M.D. Houslay, and E. Kilgour. 1998. Protein kinase C isoforms play differential roles in the regulation of adipocyte differentiation. Biochem. J. 333:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, R., and H. Ueda. 2003. a. Protein kinase C-mediated cell death mode switch induced by high glucose. Cell Death Differ. 10:1336–1347. [DOI] [PubMed] [Google Scholar]
- Fujita, R., and H. Ueda. 2003. b. Protein kinase C-mediated necrosis-apoptosis switch of cortical neurons by conditioned medium factors secreted under the serum-free stress. Cell Death Differ. 10:782–790. [DOI] [PubMed] [Google Scholar]
- Fujita, R., A. Yoshida, K. Mizuno, and H. Ueda. 2001. Cell density-dependent death mode switch of cultured cortical neurons under serum-free starvation stress. Cell. Mol. Neurobiol. 21:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgun-Sherki, Y., Z. Rosenbaum, E. Melamed, and D. Offen. 2002. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol. Rev. 54:271–284. [DOI] [PubMed] [Google Scholar]
- Gladstone, D.J., S.E. Black, and A.M. Hakim. 2002. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 33:2123–2136. [DOI] [PubMed] [Google Scholar]
- Haritos, A.A., R. Blacher, S. Stein, J. Caldarella, and B.L. Horecker. 1985. Primary structure of rat thymus prothymosin alpha. Proc. Natl. Acad. Sci. USA. 82:343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann, O., B. Baumann, R. de Lorenzi, S. Muhammad, W. Zhang, J. Kleesiek, M. Malfertheiner, M. Kohrmann, I. Potrovita, I. Maegele, et al. 2005. IKK mediates ischemia-induced neuronal death. Nat. Med. 11:1322–1329. [DOI] [PubMed] [Google Scholar]
- Ishiki, M., and A. Klip. 2005. Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology. 146:5071–5078. [DOI] [PubMed] [Google Scholar]
- Jiang, X., H.E. Kim, H. Shu, Y. Zhao, H. Zhang, J. Kofron, J. Donnelly, D. Burns, S.C. Ng, S. Rosenberg, and X. Wang. 2003. Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science. 299:223–226. [DOI] [PubMed] [Google Scholar]
- LaVallee, T.M., F. Tarantini, S. Gamble, C. Mouta Carreira, A. Jackson, and T. Maciag. 1998. Synaptotagmin-1 is required for fibroblast growth factor-1 release. J. Biol. Chem. 273:22217–22223. [DOI] [PubMed] [Google Scholar]
- Lipton, P. 1999. Ischemic cell death in brain neurons. Physiol. Rev. 79:1431–1568. [DOI] [PubMed] [Google Scholar]
- Malhi, H., and G.J. Gores. 2006. Review article: the modern diagnosis and therapy of cholangiocarcinoma. Aliment. Pharmacol. Ther. 23:1287–1296. [DOI] [PubMed] [Google Scholar]
- Martini, P.G., R. Delage-Mourroux, D.M. Kraichely, and B.S. Katzenellenbogen. 2000. Prothymosin alpha selectively enhances estrogen receptor transcriptional activity by interacting with a repressor of estrogen receptor activity. Mol. Cell. Biol. 20:6224–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga, H., and H. Ueda. 2006. Evidence for serum-deprivation-induced co-release of FGF-1 and S100A13 from astrocytes. Neurochem. Int. 49:294–303. [DOI] [PubMed] [Google Scholar]
- Mattson, M.P., and S. Camandola. 2001. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J. Clin. Invest. 107:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B.S., and L.P. Reagan. 2004. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur. J. Pharmacol. 490:13–24. [DOI] [PubMed] [Google Scholar]
- Mizota, K., A. Yoshida, H. Uchida, R. Fujita, and H. Ueda. 2005. Novel type of Gq/11 protein-coupled neurosteroid receptor sensitive to endocrine disrupting chemicals in mast cell line (RBL-2H3). Br. J. Pharmacol. 145:545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. 1995. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 333:1581–1588. [DOI] [PubMed] [Google Scholar]
- Nordberg, J., and E.S. Arner. 2001. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 31:1287–1312. [DOI] [PubMed] [Google Scholar]
- Ono, Y., T. Kurokawa, T. Fujii, K. Kawahara, K. Igarashi, U. Kikkawa, K. Ogita, and Y. Nishizuka. 1986. Two types of complementary DNAs of rat brain protein kinase C. Heterogeneity determined by alternative splicing. FEBS Lett. 206:347–352. [DOI] [PubMed] [Google Scholar]
- Perrini, S., A. Natalicchio, L. Laviola, G. Belsanti, C. Montrone, A. Cignarelli, V. Minielli, M. Grano, G. De Pergola, R. Giorgino, and F. Giorgino. 2004. Dehydroepiandrosterone stimulates glucose uptake in human and murine adipocytes by inducing GLUT1 and GLUT4 translocation to the plasma membrane. Diabetes. 53:41–52. [DOI] [PubMed] [Google Scholar]
- Pineiro, A., O.J. Cordero, and M. Nogueira. 2000. Fifteen years of prothymosin alpha: contradictory past and new horizons. Peptides. 21:1433–1446. [DOI] [PubMed] [Google Scholar]
- Pineiro, A., M. Begona Bugia, M. Pilar Arias, O.J. Cordero, and M. Nogueira. 2001. Identification of receptors for prothymosin alpha on human lymphocytes. Biol. Chem. 382:1473–1482. [DOI] [PubMed] [Google Scholar]
- Salgado, F.J., A. Pineiro, A. Canda-Sanchez, J. Lojo, and M. Nogueira. 2005. Prothymosin alpha-receptor associates with lipid rafts in PHA-stimulated lymphocytes. Mol. Membr. Biol. 22:163–176. [DOI] [PubMed] [Google Scholar]
- Simpson, R.U., T.D. O'Connell, Q. Pan, J. Newhouse, and M.J. Somerman. 1998. Antisense oligonucleotides targeted against protein kinase Cbeta and CbetaII block 1,25-(OH)2D3-induced differentiation. J. Biol. Chem. 273:19587–19591. [DOI] [PubMed] [Google Scholar]
- Ueda, H., and R. Fujita. 2004. Cell death mode switch from necrosis to apoptosis in brain. Biol. Pharm. Bull. 27:950–955. [DOI] [PubMed] [Google Scholar]
- Welsh, G.I., I. Hers, D.C. Berwick, G. Dell, M. Wherlock, R. Birkin, S. Leney, and J.M. Tavare. 2005. Role of protein kinase B in insulin-regulated glucose uptake. Biochem. Soc. Trans. 33:346–349. [DOI] [PubMed] [Google Scholar]
- White, B.C., J.M. Sullivan, D.J. DeGracia, B.J. O'Neil, R.W. Neumar, L.I. Grossman, J.A. Rafols, and G.S. Krause. 2000. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J. Neurol. Sci. 179:1–33. [DOI] [PubMed] [Google Scholar]
- Xiong, Z.G., X.M. Zhu, X.P. Chu, M. Minami, J. Hey, W.L. Wei, J.F. MacDonald, J.A. Wemmie, M.P. Price, M.J. Welsh, and R.P. Simon. 2004. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 118:687–698. [DOI] [PubMed] [Google Scholar]
- Yoshida, T., I. Tomioka, T. Nagahara, T. Holyst, M. Sawada, P. Hayes, V. Gama, M. Okuno, Y. Chen, Y. Abe, et al. 2004. Bax-inhibiting peptide derived from mouse and rat Ku70. Biochem. Biophys. Res. Commun. 321:961–966. [DOI] [PubMed] [Google Scholar]
- Zong, W.X., and C.B. Thompson. 2006. Necrotic death as a cell fate. Genes Dev. 20:1–15. [DOI] [PubMed] [Google Scholar]