Abstract
In this study, we establish that the tyrosine kinase Syk is essential for osteoclast function in vitro and in vivo. Syk−/− osteoclasts fail to organize their cytoskeleton, and, as such, their bone-resorptive capacity is arrested. This defect results in increased skeletal mass in Syk−/− embryos and dampened basal and stimulated bone resorption in chimeric mice whose osteoclasts lack the kinase. The skeletal impact of Syk deficiency reflects diminished activity of the mature osteoclast and not impaired differentiation. Syk regulates bone resorption by its inclusion with the αvβ3 integrin and c-Src in a signaling complex, which is generated only when αvβ3 is activated. Upon integrin occupancy, c-Src phosphorylates Syk. αvβ3-induced phosphorylation of Syk and the latter's capacity to associate with c-Src is mediated by the immunoreceptor tyrosine-based activation motif (ITAM) proteins Dap12 and FcRγ. Thus, in conjunction with ITAM-bearing proteins, Syk, c-Src, and αvβ3 represent an essential signaling complex in the bone-resorbing osteoclast, and, therefore, each is a candidate therapeutic target.
Introduction
The osteoclast is a polykaryon whose capacity to mobilize bone requires the organization of its unique cytoskeleton. Thus, upon mineralized matrix recognition, the osteoclast polarizes its fibrillar actin, eventuating in the formation of an acidified extracellular microenvironment wherein the resorptive machinery of the cell is activated. Failure to undergo this polarization event results in osteoclast dysfunction and, consequently, in varying degrees of osteopetrosis (Teitelbaum and Ross, 2003).
Soriano et al. (1991) made the surprising observation that the principal phenotype of c-Src–deleted mice resides in the skeleton. These animals develop severe osteopetrosis despite an abundance of osteoclasts. Thus, the disorder does not reflect the failure of osteoclast recruitment but defective osteoclast function: specifically, disorganization of the cell's actin cytoskeleton (Boyce et al., 1992). In fact, c-Src is the dominant Src family kinase (SFK) expressed in the osteoclast and regulates the cell's cytoskeleton as an adaptor protein as well as through its kinase activity (Schwartzberg et al., 1997; Miyazaki et al., 2004).
Syk is another tyrosine kinase that modulates osteoclast function, at least in vitro (Faccio et al., 2003c; Mocsai et al., 2004). The discovery of Syk in this regard was a consequence of the observation that immunoregulatory adaptor molecules such as Dap12 and FcRγ (Koga et al., 2004; Mocsai et al., 2004), which bear immunoreceptor tyrosine-based activation motifs (ITAMs), are central to osteoclastogenesis, as mice lacking both proteins are osteopetrotic because of the failure to generate osteoclasts (Koga et al., 2004). Alternatively, whether ITAM proteins impact the resorptive capacity of mature osteoclasts is unknown. The signaling events emanating from these proteins are activated by SFKs, which phosphorylate tyrosine residues within the ITAM (Pitcher and van Oers, 2003). These ITAM phosphotyrosines bind to and activate Syk (Futterer et al., 1998; Brdicka et al., 2005). However, the maintenance of ITAM-initiated Syk activity is under the aegis of the associated SFK (Brdicka et al., 2005). Activated ITAM-bound Syk targets guanine nucleotide exchange factors such as Vavs, leading to induction of the Rho GTPase Rac (Faccio et al., 2005).
Integrins are αβ-transmembrane heterodimers and the principal cell/matrix recognition molecules. The osteoclast is particularly rich in the αvβ3 integrin, which mediates the capacity of the cell to polarize, spread, and optimally degrade bone. Although less pronounced than that of c-Src–deficient mice, those lacking αvβ3 develop enhanced bone mass as a result of osteoclast dysfunction, again reflecting a failure to undergo cytoskeletal organization (McHugh et al., 2000; Faccio et al., 2003a).
The β3-integrin subunit is also expressed by platelets, wherein it associates with the αIIb chain. Similar to ITAM-bearing proteins, αIIbβ3 recognizes Syk (Woodside et al., 2001), which is also essential for integrin-mediated signaling in neutrophils (Mocsai et al., 2002). Interestingly, Syk binds directly to the β3 subunit of αIIbβ3 via the kinase SH2 domain but, unlike its interaction with ITAMs, does so independently of the phosphorylation of integrin tyrosine residues (Woodside et al., 2001). In contrast to its association with phosphorylated ITAMs, recognition of the β3 subunit itself has no immediate impact on Syk activation, which occurs upon integrin/ligand occupancy and is presumably secondary to the induction of an associated SFK (Woodside et al., 2001). As a result of αvβ3-induced Syk phosphorylation in the osteoclast, Vav3, which regulates the cell's cytoskeleton, is activated (Faccio et al., 2005). Thus, Syk is downstream of immunoregulatory proteins such as Dap12 and FcRγ, which govern osteoclastogenesis, and of integrins, which regulate the cytoskeleton.
Because αvβ3 and its associated proteins are therapeutic targets in the context of pathological bone resorption (Murphy et al., 2005), we explored the mechanism by which the integrin regulates Syk in the osteoclast. We find that Syk, c-Src, and αvβ3 form a ternary complex in the cell. Syk–c-Src binding requires the terminal three amino acids of the β3 subunit and the kinase and C-terminal SH2 domains of Syk. The consequence of αvβ3-ligand occupancy is the activation of c-Src, which, in an ITAM-dependent manner, phosphorylates Syk, thus organizing the osteoclast cytoskeleton. In keeping with these observations, Syk−/− osteoclasts have a disorganized cytoskeleton leading to subnormal bone resorption in vitro and in vivo.
Results
Generation of Syk−/− radiation chimeras
To overcome the perinatal lethality of Syk−/− mice, we generated bone marrow chimeras by transplanting Syk−/− fetal liver cells into lethally irradiated wild-type (WT) recipients (Mocsai et al., 2002). Syk+/? (nongenotyped Syk+/+ or Syk+/− mice, which are phenotypically identical) fetal liver cells, which were injected into similar hosts, served as a control. These animals are designated Syk−/− and Syk+/? chimeras.
Donor and recipient cells are distinguished on the basis of surface antigen Ly-5.1 expression. Specifically, recipient mice (B6.SJL) express Ly-5.1, whereas donors (129.sv) do not. Purified osteoclast precursors in the form of bone marrow macrophages (BMMs) that recovered from Syk chimeras consist almost exclusively of donor cells (no Ly-5.1; Fig. S1 A, available at http:///www.jcb.org/cgi/content/full/jcb.200611083/DC1). Furthermore, Syk−/− chimeric BMMs express normal levels of receptor activator of nuclear factor κB (RANK) and the macrophage colony–stimulating factor (MCSF) receptor c-Fms (Fig. S1 B).
Syk is required for osteoclast function but not differentiation
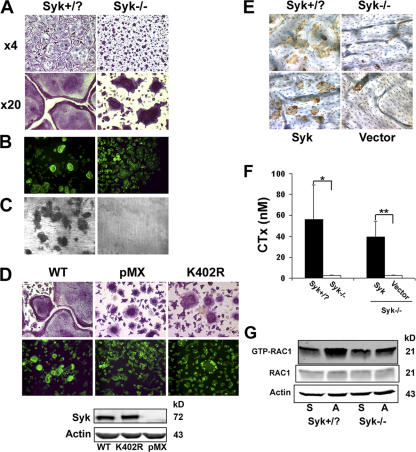
Syk is essential for T cell development, and we asked whether the same is true regarding the osteoclast. To this end, we cultured Syk+/? and Syk−/− BMMs with MCSF and RANK ligand (RANKL) for 5 d. Syk-deficient osteoclasts, which were generated in culture, are distinctly abnormal. Both genotypes differentiate into tartrate-resistant acidic phosphatase (TRAP)–expressing multinucleated cells. However, Syk+/? BMMs form sheets of characteristic well-spread osteoclasts, whereas those lacking Syk are small with an irregular crenated appearance, suggesting a cytoskeletal defect (Fig. 1 A). To ensure that these morphological abnormalities of Syk−/− osteoclasts do not reflect arrested differentiation, we measured a series of markers of osteoclastogenesis in BMMs exposed to MCSF and RANKL with time. The expression of characteristic osteoclastogenic proteins is not delayed in the Syk−/− cells (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200611083/DC1) nor are intracellular signaling events required for efficient osteoclast differentiation (Fig. S2 B). These pathways include RANKL activation of nuclear factor κB, which is assessed by IκB-α phosphorylation and degradation, as well as JNK, ERK1/2, and p-38 phosphorylation. Furthermore, MCSF-driven ERK1/2 and AKT phosphorylation is normal in Syk−/− osteoclasts (Fig. S2 C).
Figure 1.
Syk deficiency impairs osteoclast function but not differentiation in vitro. (A) BMMs derived from Syk+/? or Syk−/− chimeras were cultured with RANKL and MCSF for 5 d. Cells were stained for TRAP activity (red reaction product). (B) BMMs derived from Syk+/? or Syk−/− chimeras were cultured with RANKL and MCSF on dentin for 6 d. Actin ring formation was determined by immunofluorescence after FITC-phalloidin staining. (C) After 6 d, Syk+/? or Syk−/− osteoclasts were removed, and the dentin was stained with Coomassie brilliant blue to visualize resorption lacunae. (D) Mature Syk−/− osteoclasts transduced with either WT Syk, kinase-inactive SykK402R, or pMX vector were generated on dentin for 5 d with RANKL and MCSF. Cells were stained for TRAP activity and/or with FITC-phalloidin. The expression of Syk was determined by immunoblot assay of TCL Syk−/−. (E) BMMs were retrovirally transduced with WT Syk or empty vector. Syk+/? and Syk−/− cells serve as positive and negative controls, respectively. The cells were placed on bone slices and differentiated into osteoclasts by exposure to RANKL and MCSF. After 5 d, the osteoclasts were removed. Resorption pits were visualized by incubation of the specimen with 20 μg/ml peroxidase-conjugated wheat germ agglutinin. (F) Medium was collected and assayed for CTx concentration. Actin serves as a loading control. Error bars represent SD. *, P < 0.01; **, P < 0.001. (G) Syk+/? or Syk−/− BMMs were cultured with MCSF and RANKL for 3 d. The cells were then lifted and either maintained in suspension (S) or plated on vitronectin (A) for 30 min. GTP-bound Rac1 and total Rac1 expression were determined, and the immunoblot was densitometrically quantitated.
These data establish that Syk deficiency does not impair osteoclast differentiation, and, therefore, we turned to osteoclast function. To this end, we plated BMMs on dentin, added MCSF and RANKL for 6 d, and stained the actin cytoskeleton with FITC-phalloidin. Documenting that the abnormal shape of the Syk−/− polykaryons reflects deranged cytoskeletal organization, they are incapable of forming actin rings (Fig. 1 B). In keeping with their dysfunctional cytoskeleton, Syk−/− osteoclasts are also unable to degrade mineralized matrix in vitro, as indicated by a complete absence of dentin-resorptive lacunae (Fig. 1 C). As expected, the failure of Syk-deficient osteoclasts to spread and form actin rings is completely normalized by retroviral reconstitution of the WT tyrosine kinase (Fig. 1 D) as is the cells' resorptive capacities (Fig. 1, E and F). In contrast, the kinase-inactive mutant SykK402R fails to rescue the mutant cell's spreading defect (Fig. 1 D). Thus, the kinase activity of Syk is central to its capacity to organize the osteoclast cytoskeleton.
Rac is a downstream effector of αvβ3-mediated cytoskeletal organization (Faccio et al., 2005). To determine whether Rac signals downstream of αvβ3-activated Syk in committed osteoclast precursors, we cultured Syk+/? or Syk−/− BMMs in osteoclastogenic conditions for 3 d. The cells were lifted and maintained in suspension or replated on αvβ3 ligand for 30 min. Densitometric analysis of Fig. 1 G reveals that αvβ3 occupancy increases Rac activation 1.8-fold in Syk+/? cells, whereas no detectable change (1.1-fold) occurs in the absence of the tyrosine kinase.
Syk deficiency impairs osteoclast function in vivo
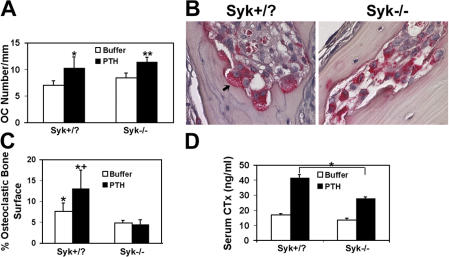
Having established that Syk deficiency impacts osteoclastic bone resorption in vitro, we asked whether the same obtains in vivo. Similar to the in vitro situation, osteoclast number in Syk−/− chimeric mice 2 mo after the generation of the animals is indistinguishable from that of irradiated controls transplanted with WT liver cells whether in the basal state or after stimulation with the resorptive agonist parathyroid hormone (PTH) (1–34) (Fig. 2 A). On the other hand, Syk-deficient osteoclasts are small and appear incapable of adhering to the bone surface or forming well-demarcated resorption lacunae after receiving the bone-resorptive hormone (Fig. 2 B). Not only does Syk deficiency reduce the percentage of bone surface covered by osteoclasts adherent to bone, but, unlike WT, the proportion of bone-apposed cells does not increase in response to PTH (Fig. 2 C). Confirming an osteoclast defect in these Syk−/− chimeras, PTH(1–34)-enhanced serum levels of the global bone-resorptive marker CTx are blunted (Fig. 2 D).
Figure 2.
Syk deficiency results in abnormal osteoclast function in vivo. (A) PTH(1–34) or vehicle was administered to Syk+/? or Syk−/− chimeras for 4 d. Calvariae were fixed and stained for TRAP activity, and osteoclast (OC) number was histomorphometrically determined, *, P < 0.05; **, P < 0.01; PTH versus buffer. (B) TRAP-stained histological sections of calvaria of PTH-treated Syk+/? or Syk−/− chimeric mice. Osteoclasts (red reaction product) of PTH-treated Syk+/? mice reside in resorption lacunae and contain ruffled membranes (arrow), whereas most Syk−/− osteoclasts are smaller and are not juxtaposed to the bone. (C) Percentage of bone surface juxtaposed to osteoclasts in control and PTH-treated Syk+/? or Syk−/− mice. *, P < 0.05 versus Syk−/−; +, P < 0.05 versus buffer. (D) Serum was collected from vehicle and PTH-treated Syk+/? and Syk−/− chimeras. CTx was measured by ELISA. *, P < 0.01. Error bars represent SD.
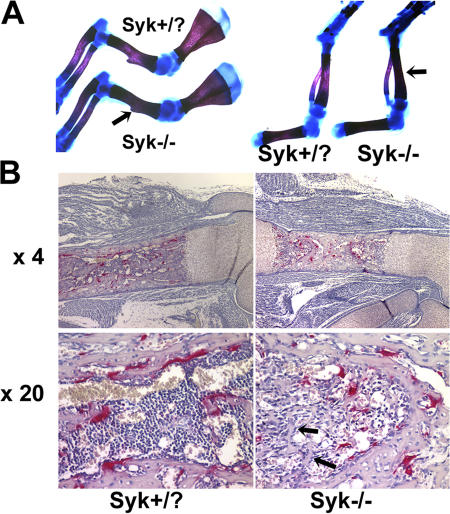
For reasons unknown, Syk−/− chimeras survive only 1–2 mo after transplantation, preventing the meaningful assessment of skeletal mass with age. On the other hand, 18.5-d Syk−/− embryos have increased bone density as compared with Syk+/? littermates (Fig. 3 A and Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200611083/DC1). Moreover, osteoclasts resident in Syk-deleted embryos have the same crenated appearance as their counterparts in radiation chimeras (Fig. 3 B). In keeping with increased bone density, the marrow space of Syk−/− mice contains a network of trabeculae that is absent in their Syk+/? counterparts. Alternatively, growth plate morphology of the mutant mice is unremarkable.
Figure 3.
Bone density is increased in Syk−/− embryos. (A) Skeletons of 18.5-d Syk+/? and Syk−/− embryos were fixed in 95% ethanol and stained with Alcian blue and Alizarin red. Syk−/− forelimb and hindlimb bones are denser as indicated by increased Alizarin staining (arrows). (B) TRAP-stained (red reaction product) histological sections of day 18.5 Syk+/? and Syk−/− embryo femurs. Arrows indicate the trabecular network in Syk−/− but not Syk+/? marrow space.
Syk deficiency does not impact osteoblast function
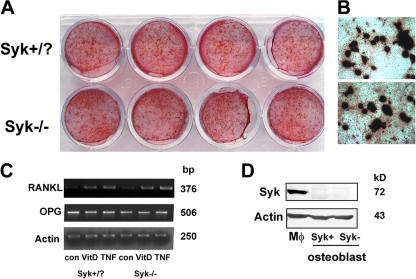
Osteoblasts may increase skeletal mass by accelerating bone formation or arresting osteoclast formation and function as a result of reduced RANKL or increased osteoprotegrin (OPG) expression. To address this issue in the context of Syk deficiency, we cultured Syk+/? and Syk−/− calvarial osteoblasts in osteogenic conditions for 20 d, after which they were stained with Alizarin red. As seen in Fig. 4 (A and B), there is no apparent difference in the capacity of the two genotypically distinct osteoblasts to generate mineralized bone nodules.
Figure 4.
Syk does not regulate osteoblast function. (A and B) Syk+/? or Syk−/− primary calvaria osteoblasts were cultured with differentiation media (α-MEM containing 50 μg/ml ascorbic acid and 2 mM β-glycerophosphate) for 20 d. Bone nodule formation was visualized by Alizarin red staining. (C) Syk+/? or Syk−/− primary calvarial osteoblasts were cultured in α-MEM media (con), 10 nM 1,25-dihydroxyvitamin D3 (VitD), or 10 ng/ml TNF-α for 24 h. RANKL and OPG expression were analyzed by RT-PCR. Actin serves as loading control. (D) Syk+/? or Syk−/− primary calvarial osteoblasts were lysed, and Syk expression was determined by immunoblotting. WT macrophage (Mφ) lysate serves as a positive control.
Next, we treated the same combination of osteoblasts with 1,25-dihydroxyvitamin D3 or TNF-α, as each is a modulator of RANKL synthesis. Similar to their bone-forming capacity, Syk+/? and Syk−/− osteoblasts express equivalent amounts of RANKL and OPG mRNA in response to both agents (Fig. 4 C). Finally, and in keeping with the unaltered biological activity of Syk−/− osteoblasts, Syk protein is also not detectable by immunoblotting in their Syk+/? counterparts (Fig. 4 D).
Syk, c-Src, and the αvβ3 integrin form a trimolecular complex in osteoclasts
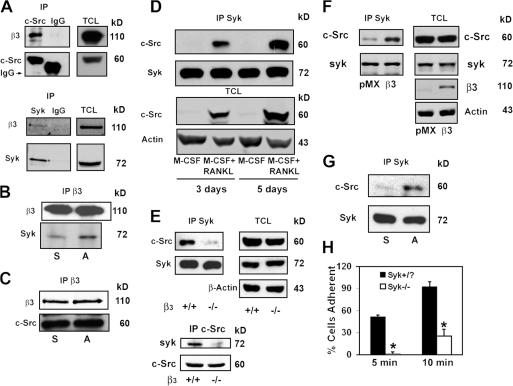
Osteoclasts deficient in Syk, the αvβ3 integrin (McHugh et al., 2000), or c-Src (Soriano et al., 1991) each have striking cytoskeletal defects eventuating in subnormal bone resorption. Furthermore, c-Src (Arias-Salgado et al., 2003) or Syk (Woodside et al., 2001) in other circumstances associates with the β3-integrin cytoplasmic domain, prompting us to ask whether the three molecules form a complex in osteoclasts. To address this issue, we turned to β3−/− BMMs transduced with human β3 (hβ3). These transductants express physiological levels of αvβ3 (Feng et al., 2001). Human constructs of the integrin were used because the WT cytoplasmic component is identical to its mouse counterpart, and an antibody that effectively recognizes its extracellular domain is available. The cells were placed in MCSF and RANKL for 5 d to generate mature, adherent osteoclasts. Total cell lysates (TCLs) were then immunoprecipitated with anti–c-Src, anti-Syk antibodies, or irrelevant IgG. Both tyrosine kinases associate with αvβ3 in mature osteoclasts (Fig. 5 A).
Figure 5.
Syk, c-Src, and αvβ3 form a complex in osteoclasts. (A) β3−/− BMMs retrovirally transduced with hβ3WT were cultured with RANKL and MCSF for 5 d. Total cell lysate (TCL) was immunoprecipitated with anti-Syk or ant–c-Src antibodies or irrelevant IgG. Immunoprecipitates and TCLs were probed by Western blotting for β3-integrin, Syk, and c-Src content. (B and C) WT BMMs were cultured with MCSF and RANKL for 3 d. The cells were then lifted and either maintained in suspension (S) or plated on vitronectin (A) for 30 min. β3-integrin immunoprecipitates from each group of cells were probed by immunoblotting for β3-integrin and Syk content (B) or for c-Src content (C). (D) WT BMMs were cultured with MCSF alone or MCSF and RANKL for 3 or 5 d. TCL was immunoprecipitated with anti-Syk antibody followed by c-Src and Syk immunoblotting. (E) TCL from WT or β3−/− osteoclasts was immunoprecipitated with anti-Syk (top) or anti–c-Src (bottom) antibodies. Immunoprecipitates and TCLs were immunoblotted for c-Src and Syk content. (F) β3−/− BMMs were retrovirally transduced with hβ3 or pMX vector. The transductants were cultured in RANKL and MCSF for 5 d. Syk immunoprecipitates and TCLs were then immunoblotted for c-Src, Syk, or β3-integrin content. (G) WT preosteoclasts, which were generated by culturing BMMs in MCSF and RANKL for 3 d, were lifted and either plated on vitronectin (A) or maintained in suspension (S) for 30 min. c-Src and Syk content in Syk immunoprecipitates was determined by immunoblotting. (H) Suspended Syk+/? or Syk−/− preosteoclasts were plated on vitronectin. The percentage of spread cells was determined after 5 and 10 min. *, P < 0.001 versus Syk+/?. Error bars represent SD.
To determine whether formation of the kinase–integrin complexes depends on αvβ3 occupancy, we generated mononuclear preosteoclasts by exposing BMMs to MCSF and RANKL for 3 d. In this circumstance, the cells are committed to the osteoclast phenotype as they express TRAP, αvβ3, and c-Src (Faccio et al., 2003b) but, unlike mature osteoclasts, are easily suspended. The cells were maintained in suspension or were adherent to the αvβ3 ligand vitronectin for 30 min. Reciprocal immunoprecipitation and immunoblotting show that Syk associates with the integrin in an adhesion-dependent manner (Fig. 5 B). In contrast, β3–c-Src recognition is constitutive and is not impacted by integrin occupancy (Fig. 5 C).
The interaction of both Syk and c-Src with αvβ3 in osteoclasts suggests that the two kinases may coimmunoprecipitate. To address this issue, we cultured WT BMMs in MCSF with or without RANKL for 3 or 5 d. Syk immunoprecipitates and TCLs were then immunoblotted for c-Src content. Consistent with the progressive expression of c-Src as cells assume the osteoclast phenotype, the kinase is increasingly abundant in Syk immunoprecipitates derived from MCSF- and RANKL-containing cultures and is absent in those treated with MCSF alone (Fig. 5 D). Also, in contrast to c-Src, whose expression by macrophages is specific to the osteoclast phenotype, the production of Syk is unaffected by time or RANKL.
To determine the functional role of the β3 integrin in c-Src–Syk association, WT or β3−/− BMMs were cultured with RANKL and MCSF for 5 d, after which we immunoprecipitated c-Src or Syk and reciprocally immunoblotted the other. As shown in Fig. 5 E, association of the two tyrosine kinases is substantially reduced in the absence of αvβ3, although the expression of neither c-Src nor Syk is altered. Confirming its role in regulating c-Src–Syk recognition, retroviral reconstitution of β3-deficient osteoclasts with the integrin subunit restores coimmunoprecipitation of the kinases (Fig. 5 F).
We next asked whether the integrin's state of occupancy impacts c-Src–Syk association. Committed preosteoclasts were once again generated by incubating WT BMMs in MCSF and RANKL for 3 d. The cells were lifted and either maintained in suspension or plated on vitronectin for 30 min. Syk immunoprecipitates were then immunoblotted for c-Src and Syk content. Adhesion of preosteoclasts to αvβ3 ligand enhances c-Src–Syk coprecipitation in circumstances of equal Syk content (Fig. 5 G).
These data suggest that Syk mediates αvβ3 function in osteoclasts. Thus, we asked whether an essential property of the integrin, namely rapid spreading on αvβ3 ligand, is disturbed in the absence of Syk. To this end, committed preosteoclasts were suspended and replated on vitronectin-coated plates. In keeping with disturbed αvβ3 function, Syk deficiency markedly reduces the capacity of preosteoclasts to rapidly spread on the integrin's ligand(Fig. 5 H and Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200611083/DC1).
The terminal three amino acid residues of the β3-integrin cytoplasmic domain regulate c-Src–Syk association
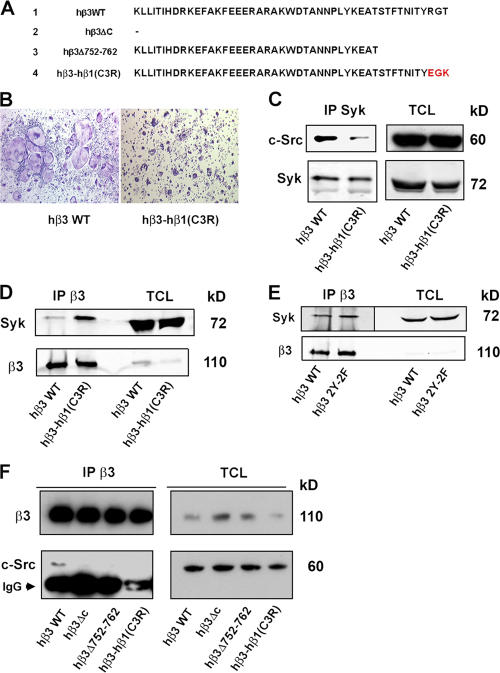
c-Src directly associates with the distal three amino acids of the β3-subunit cytoplasmic domain in vitro and in transformed cells (Arias-Salgado et al., 2003, 2005). To determine whether this motif regulates c-Src–Syk interaction in the osteoclast, we substituted these residues with the C-terminal three amino acids of the β1-integrin chain to generate hβ3–hβ1(C3R) (Fig. 6 A; Arias-Salgado et al., 2005). hβ3–hβ1(C3R) or hβ3WT was retrovirally transduced into β3−/− BMMs, which were then placed in osteoclastogenic conditions. TRAP staining of the cultures after 5 d demonstrates that the mutated construct yields small, poorly spread osteoclasts that are indistinguishable from those generated from naive β3−/− BMMs, whereas the WT subunit completely rescues the osteoclast phenotype (Fig. 6 B). This observation and the diminished coprecipitation of c-Src and Syk in the hβ3–hβ1(C3R)-bearing cells (Fig. 6 C) establish that the three terminal β3 amino acids are required for optimal association of the two kinases and their ability to organize the osteoclast cytoskeleton.
Figure 6.
The distal three amino acids of the β3-integrin cytoplasmic domain mediate Syk–c-Src association. (A) Amino acid sequences of human (h) β3-integrin constructs used in this study. Letters in red refer to substituted β1-integrin subunit residues. (B–D) β3−/− BMMs transduced with hβ3WT or hβ3–hβ1(C3R) were cultured in the presence of RANKL and MCSF for 5 d. (B) Cells were stained for TRAP activity. (C) Syk immunoprecipitates and TCLs were immunoblotted for c-Src and Syk content. (D) β3-integrin immunoprecipitates and TCLs were immunoblotted for Syk and β3-integrin content. (E) β3−/− BMMs transduced with hβ3WT or hβ3Y747F/Y752F (hβ3 2Y-2F) were cultured in the presence of RANKL and MCSF for 5 d. β3-integrin immunoprecipitates and TCLs were immunoblotted for Syk and β3-integrin content. (F) β3−/− BMMs transduced with hβ3WT, hβ3ΔC, hβ3Δ752–762, or hβ3–hβ1(C3R) were cultured in the presence of RANKL and MCSF for 5 d. β3-integrin immunoprecipitates and TCLs were immunoblotted for β3-integrin and c-Src content.
To further explore this issue, we asked whether the three terminal β3 residues regulate the integrin's interaction with Syk and/or c-Src in the osteoclast. Thus, we transduced β3−/− BMMs with hβ3WT or hβ3–hβ1(C3R). After 5 d in MCSF and RANKL, the integrin constructs were immunoprecipitated, and the immunoprecipitates were blotted with anti-Syk or β3 antibodies. Unlike transformed cells expressing αIIbβ3 (Woodside et al., 2001), Syk binds hβ3–hβ1(C3R) in the osteoclast and, for reasons unknown, does so more effectively than to hβ3WT (Fig. 6 D). However, this conundrum does not reflect the enhanced affinity of Syk for the β1 integrin in the absence of β3 (Fig.5, available at http://www.jcb.org/cgi/content/full/jcb.200611083/DC1).
Syk phosphorylation in the context of αIIbβ3 does not depend on the presence of the two Y residues in the β3 cytoplasmic domain (Gao et al., 1997). On the other hand, αIIbβ3–Syk binding is arrested by the alanine mutation of β3Y747 (Woodside et al., 2001). We find that unlike the platelet integrin, in which β3Y747F/Y752F promotes a bleeding dyscrasia (Law et al., 1999), the same double mutant does not impact the osteoclast (Feng et al., 2001). In keeping with this observation, Syk association with β3 is unaltered by β3Y747F/Y752F (Fig. 6 E). In contrast to Syk, the binding of c-Src to hβ3 is arrested by the hβ3–hβ1(C3R) mutation (Fig. 6 F). Consistent with this observation, no c-Src–β3 association is evident upon deletion of the entire integrin cytoplasmic domain (hβ3ΔC) or its termination at residue 751 (hβ3Δ752–762).
c-Src mediates αvβ3-induced Syk activation in osteoclasts
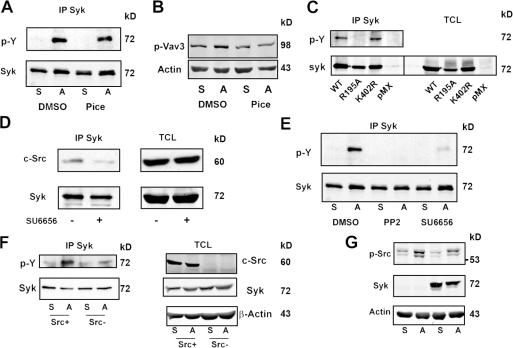
Syk must be phosphorylated to induce its downstream targets and, when bound to the ITAM domain of immune response receptors, is activated via autophosphorylation (Pitcher and van Oers, 2003). To determine whether such is the case regarding its association with αvβ3 in the osteoclast, we suspended WT preosteoclasts in the presence of the Syk kinase inhibitor piceatannol (Pice; Calbiochem) or a carrier (DMSO). After 20 min, the cells were either maintained in suspension or replated on the αvβ3 ligand vitronectin. 30 min later, Syk immunoprecipitates were analyzed for phosphotyrosine content by immunoblotting. Fig. 7 A shows that Pice does not detectably impact adhesion-induced Syk phosphorylation. Confirming that it is an effective Syk kinase inhibitor in osteoclasts, Pice arrests integrin-mediated phosphorylation of the Syk downstream target Vav3 (Fig. 7 B; Faccio et al., 2005).
Figure 7.
c-Src mediates αvβ3-induced Syk activation in osteoclasts. (A and B) WT BMMs were cultured in RANKL and MCSF for 3 d. The preosteoclasts were lifted and preincubated in 10 μM piceatannol (Pice) or DMSO for 20 min. They were then plated on vitronectin (A) or maintained in suspension (S) for 30 min, after which tyrosine-phosphorylated Syk (A) or Vav3 (B) was determined by immunoblotting. (C) Syk−/− BMMs transduced with WT Syk, SykR195A, SykK402R, or pMX vector were cultured in RANKL and MCSF for 3 d. The preosteoclasts were lifted and plated on vitronectin for 30 min, after which phosphorylated tyrosine in Syk immunoprecipitates and TCLs was determined by immunoblotting. (D) WT BMMs were cultured in RANKL and MCSF for 3 d. The preosteoclasts were then suspended and preincubated in 2 μM SU6656 (+) or DMSO (−) for 20 min. After 30-min adhesion to vitronectin, Syk immunoprecipitates and TCLs were immunoblotted for c-Src and Syk. (E) WT BMMs cultured in RANKL and MCSF for 3 d were lifted and preincubated for 20 min in 10 μM PP2, 2 μM SU6656, or DMSO for 20 min. The cells were then plated on vitronectin (A) or maintained in suspension (S) for 30 min, after which phosphorylated tyrosine in Syk immunoprecipitates was determined by immunoblotting. (F) WT or c-Src−/− spleen cells were cultured in RANKL and MCSF for 3 d. The cells were lifted and either replated on vitronectin or maintained in suspension for 30 min, after which phosphorylated tyrosine in Syk immunoprecipitates was determined by immunoblotting. Syk, c-Src, and β-actin (loading control) content was also assessed in TCLs. (G) WT or Syk−/− BMMs were cultured in RANKL and MCSF for 3 d. The cells were lifted and either replated on vitronectin or maintained in suspension for 30 min. The plated cells were then rinsed with PBS to remove nonadherent cells. c-SrcY416 and β-actin (loading control) content was assessed by immunoblotting in TCLs of both suspended and adherent cells.
We next transduced WT or kinase-inactive Syk (SykK402R) into Syk−/− BMMs. The cells were plated on vitronectin, and tyrosine-phosphorylated Syk was measured. Consistent with our Pice-based studies, adherent Syk−/− preosteoclasts bearing kinase-inactive Syk undergo normal adhesion-associated tyrosine phosphorylation (Fig. 7 C). These data indicate that although Syk kinase activity is necessary for organization of the osteoclast cytoskeleton (Fig. 1 D), αvβ3-associated Syk is itself phosphorylated by another kinase. Given that αvβ3 induces Syk–c-Src interaction, the latter is a likely Syk kinase. In fact, SFK inhibitors diminish c-Src–Syk association (Fig. 7 D) and adhesion-induced Syk phosphorylation (Fig. 7 E). More specifically, Syk activation in response to αvβ3 occupancy is markedly reduced in Src−/− preosteoclasts (Fig. 7 F).
Having established that c-Src activates Syk in the osteoclast, we asked the reciprocal question: does αvβ3 occupancy activate c-Src, and, if so, is the event mediated by Syk? We have shown that the absence of Syk attenuates c-Src activity in the context of an entire population of preosteoclasts (Faccio et al., 2003c). However, these previous experiments included both αvβ3 ligand–adherent and –nonadherent cells, the latter being abundant in the absence of Syk. As our present aim involved the impact of Syk on αvβ3-stimulated c-Src activity, we maintained Syk+/? or Syk−/− preosteoclasts on vitronectin for 30 min, after which nonadherent cells were removed. TCLs of the remaining adherent preosteoclasts, representing the population with occupied αvβ3, were then immunoblotted for activated c-Src. Adhesion to the αvβ3 ligand phosphorylates the activating residue c-SrcY416 equally in the presence or absence of Syk (Fig. 7 G). Thus, although αvβ3-associated c-Src activates Syk, the reciprocal is not true.
ITAM-bearing proteins mediate αvβ3-stimulated Syk activation
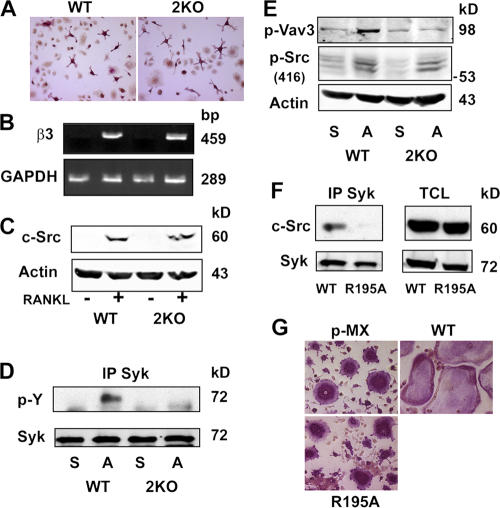
Syk and c-Src recognize and signal through ITAM-bearing immunoreceptors as well as the αvβ3 integrin (Woodside, 2002). To determine whether interdependency exists between these two receptor-mediated signaling pathways in osteoclasts, we turned to osteopetrotic mice deleted of two essential osteoclastogenic ITAM immunoreceptors, namely Dap12 and FcRγ (Koga et al., 2004). In keeping with previous studies (Koga et al., 2004; Mocsai et al., 2004), we find that terminal osteoclastogenesis, which was induced in our system by 5-d exposure of spleen cells to RANKL and MCSF, is attenuated in Dap12−/−/FcRγ−/− mice (unpublished data). Surprisingly, however, the appearance of TRAP-stained mutant cells after only 3 d in osteoclastogenic medium is similar to WT (Fig. 8 A). Furthermore, the expression of β3-integrin mRNA (Fig. 8 B) and c-Src (Fig. 8 C) is also indistinguishable in the two genotypes at this earlier time. Next, Dap12−/−/FcRγ−/− day 3 preosteoclasts were lifted and maintained in suspension or replated on αvβ3 ligand for 30 min. Subsequent immunoprecipitation and phosphotyrosine immunoblotting shows that the adhesion-mediated activation of Syk is obviated in the absence of the two ITAM proteins (Fig. 8 D). Furthermore, integrin occupancy of Dap12−/−/FcRγ−/− preosteoclasts fails to phosphorylate the Syk kinase target Vav3 but continues to activate c-Src, which is upstream of Syk in the αvβ3 signaling cascade (Fig. 8 E).
Figure 8.
ITAM proteins mediate αvβ3-induced Syk activation in osteoclasts. (A–C) WT or Dap12−/−/FcRγ−/− (2KO) spleen cells were cultured in RANKL and MCSF for 3 d, after which the cells were stained for TRAP activity (A), analyzed for β3-integrin mRNA content by RT-PCR (B), and analyzed for c-Src expression by immunoblotting (C). (D and E) WT or Dap12−/−/FcRγ−/− (2KO) spleen cells were cultured in RANKL and MCSF for 3 d. The cells were lifted and either replated on vitronectin (A) or maintained in suspension (S) for 30 min, after which Syk immunoblots were probed for phosphotyrosine content (D) or TCLs were immunoblotted for phosphorylated Vav3 and c-Srcp-Y416 (E). (F) Syk−/− BMMs transduced with WT Syk or SykR195A were cultured in RANKL and MCSF for 5 d. Syk immunoprecipitates and TCLs were then immunoblotted for c-Src and Syk content. (G) Syk−/− BMMs were transduced with WT Syk, SykR195A, or empty vector (pMX). After 5-d exposure to RANKL and MCSF, the cells were stained for TRAP activity.
These data indicate that integrin activation of Syk requires ITAM immunoreceptors. If so, one would expect the disruption of Syk–ITAM association to blunt the tyrosine kinase's capacity to organize the osteoclast cytoskeleton. To address this issue, we turned to SykR195A, which continues to bind the β3 integrin but not ITAM proteins (Gao et al., 1997; Woodside et al., 2001). We expressed WT Syk or this mutant of the C-terminal SH2 domain in Syk−/− preosteoclasts generated by 3-d exposure to RANKL and MCSF. The cells were suspended and replated on vitronectin for 30 min. In this circumstance, the WT protein but not SykR195A is phosphorylated (Fig. 7 C), and Syk and c-Src no longer meaningfully associate (Fig. 8 F). These collective observations indicate that ITAM proteins mediate αvβ3-stimulated Syk activation. Importantly, WT Syk rescues the Syk−/− osteoclast cytoskeleton, but SykR195A fails to do so (Fig. 8 G). Therefore, αvβ3- and ITAM-bearing immunoreceptors function in tandem regarding the impact of c-Src–Syk on the osteoclast.
Discussion
Osteoclasts are characterized by a unique cytoskeleton that mediates the resorptive process. Upon contact with bone, the cell generates two polarized structures enabling it to degrade skeletal tissue. These include a villous organelle unique to the resorbing osteoclast, which is known as the ruffled membrane, and the actin ring or sealing zone, which isolates the resorptive microenvironment from the general extracellular space (Teitelbaum and Ross, 2003).
The β3-integrin knockout mouse serves as an important tool for determining the role of the osteoclast's most abundant integrin in its capacity to resorb bone (McHugh et al., 2000). Failure to express αvβ3 results in dramatic alterations of the cell's actin cytoskeleton, including failure to form actin rings or normal ruffled membranes in vivo. These abnormalities result in attenuated bone resorption.
The mechanisms by which c-Src exerts its effects in osteoclasts are complex. In the first instance, it functions as an adaptor protein independent of its capacity to induce tyrosine phosphorylation (Schwartzberg et al., 1997). Alternatively, complete reversal of the functional abnormalities of c-Src–deficient osteoclasts also requires its kinase activity (Schwartzberg et al., 1997; Miyazaki et al., 2004).
The similar cytoskeletal phenotypes of c-Src and αvβ3-deficient osteoclasts suggest a commonality of intracellular signaling. c-Src binds directly to the terminal three amino acids of the β3 subunit in the context of the platelet integrin αIIbβ3 (Arias-Salgado et al., 2003), and we have established that the same residues regulate αvβ3–c-Src association in the osteoclast. However, the means by which c-Src is recruited to αvβ3 in the osteoclast is not fully understood. A current model holds that Pyk2 and c-Cbl mobilize c-Src to the integrin (Sanjay et al., 2001). In this paradigm, αvβ3 occupancy induces the phosphorylation of Pyk2Y402, which then activates c-Src by occupying its SH2 domain. Although provocative, these data await documentation that Cbl or Pyk2 deletion prompts a meaningful bone phenotype. Furthermore, although c-Src and c-Cbl phosphorylation depends on αvβ3 occupancy, this is not the case regarding Pyk2 (Faccio et al., 2003a). On the other hand, there is substantial evidence that Syk partners with c-Src, and, therefore, we asked whether these two nonreceptor tyrosine kinases impact the osteoclast in the context of the αvβ3 integrin.
Differentiation of Syk-deficient BMMs into osteoclasts is not delayed as determined by the progressive expression of markers of the mature resorptive cell in the presence of RANKL and MCSF. Moreover, activation of many intracellular signals induced by the two osteoclastogenic cytokines is not retarded in the mutant cells. Like those lacking c-Src or αvβ3, however, Syk−/− osteoclasts have a deranged cytoskeleton, as they fail to spread or form actin rings in vitro and are largely unattached to the bone surface in vivo. Failure of a kinase-inactive Syk mutant to rescue Syk−/− osteoclasts establishes that activated Syk is required to organize the cell's cytoskeleton. Confirming the functional significance of these abnormalities, Syk−/− embryos have increased bone mass, and basal and PTH-stimulated bone resorption are attenuated in radiation chimeric mice whose osteoclasts lack the kinase. The morphological similarity of osteoclasts lacking c-Src, αvβ3, or Syk raises the possibility that these molecules work in tandem in the osteoclast. In fact, we find that each associates with the other two in the mature resorptive cell and that c-Src–Syk interaction requires the presence of the liganded integrin.
The β3-integrin cytoplasmic domain consists of 47 amino acids, and we previously noted that of six candidate substitutions, only one, the human mutation β3S752P, fails to rescue the dysfunctional β3-deficient osteoclast (Feng et al., 2001). In the present study, we extend these data to include the terminal three residues of the subunit as essential for osteoclast cytoskeletal organization. The fact that the absence of this motif arrests both β3–c-Src and c-Src–Syk association provides compelling evidence that the two tyrosine kinases partner with the integrin to regulate the osteoclast cytoskeleton. On the other hand, neither substitution of the three C-terminal β3 residues nor the absence of c-Src dampens Syk's capacity to bind the integrin. Therefore, in osteoclasts, Syk binds to the β3 subunit independently of c-Src and at a site that is probably distinct from the SFK.
In contrast to the platelet (Law et al., 1999), mutation of the two β3 cytoplasmic domain tyrosine residues to phenylalanine does not impact the osteoclast. This observation may be a result of the fact that although the β3 chain recognizes Syk via the latter's N-terminal SH2 domain, it does so in a phosphotyrosine-independent manner (Gao et al., 1997). This is in keeping with our finding that mutation of the two tyrosine residues in the β3 cytoplasmic domain does not alter its recognition of Syk in osteoclasts. This circumstance permits Syk in association with αvβ3 to continue to activate downstream cytoskeletal-organizing molecules such as Vav3 and Rac and explains the capacity of β3Y747F/Y752F to completely rescue β3−/− osteoclasts (Feng et al., 2001).
The earliest insights into the mechanisms by which Syk impacts the platelet and the osteoclast relate to its capacity to interact with ITAM-containing proteins (Woodside, 2002). In the osteoclast, these ITAM proteins include Dap12 and FcRγ (Koga et al., 2004; Mocsai et al., 2004). Although the deletion of each enhances bone mass, the elimination of both promotes severe osteopetrosis, an event mediated, at least in part, by Syk dysfunction. In contrast to the means by which its N-terminal SH2 domain associates with β3 integrin, Syk's C-terminal SH2 motif binds ITAM proteins via the classic mechanism of phosphotyrosine recognition (Futterer et al., 1998). Given the individual flexibility of each Syk SH2 domain (Futterer et al., 1998), these observations suggest that Syk associates with αvβ3 and ITAM proteins in the same complex.
Deletion of both Dap12 and FcRγ arrests terminal osteoclastogenesis as a result of the failed expression of NFATc1 (Koga et al., 2004). However, the absence of Syk does not impact the osteoclastogenic transcription factor. This observation is consistent with the fact that osteoclasts lacking the kinase, like those deleted of the integrin, mature normally but fail to organize their cytoskeleton. Thus, although Syk is regulated by both αvβ3 and ITAM proteins, the impact of each on the osteoclast differs.
On the other hand, we find that ITAM proteins are not required for early commitment to the osteoclast phenotype, enabling us to explore the role of Dap12 and FcRγ in αvβ3 activation of Syk and its downstream targets. Syk activation in osteoclasts is independent of its own kinase and occurs under the aegis of c-Src. Furthermore, Syk's ability to undergo phosphorylation and associate with c-Src in the bone-resorptive cell depends on an intact ITAM-interactive C-terminal SH2 domain (Gao et al., 1997; Woodside et al., 2001). These data suggest a model in which Syk is first recruited to the activated integrin at a site other than the three terminal amino acids (Fig. 9). Syk, in turn, functions as an adaptor for the integrin and ITAM proteins wherein the kinase interacts with the latter in an SH2 domain–dependent manner. c-Src binds independently of integrin occupancy to the distal β3 cytoplasmic domain, where it associates with and phosphorylates Syk upon αvβ3 occupancy. Importantly, these observations establish for the first time that ITAM proteins not only regulate osteoclast function but the capacity of the mature resorptive cell to resorb bone as well. Thus, our data show that Syk is a nexus of a novel signaling pathway regulating osteoclast function and, like c-Src (Kamath et al., 2003) and αvβ3 (Murphy et al., 2005), is a candidate anti–bone-resorptive therapeutic target.
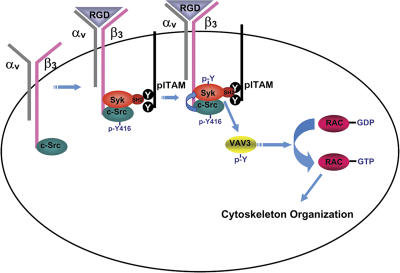
Figure 9.
Model of Syk-mediated organization of the osteoclast cytoskeleton. c-Src constitutively associates with the β3-integrin subunit. αvβ3 is activated upon ligand (RGD) occupancy, which recruits Syk bound to the Y-phosphorylated ITAM proteins FcRγ and Dap12 in an SH2 domain–dependent manner to the integrin–c-Src complex. αvβ3 occupancy also activates c-Src by phosphorylating Y416. In turn, activated c-Src phosphorylates Syk. Activated Syk phosphorylates Vav3, which shuttles Rac to its GTP-bound form, thereby organizing the actin cytoskeleton.
Materials and methods
Mice
Animals were housed in the animal care unit of the Washington University School of Medicine and were maintained according to guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care. All animal experimentation was approved by the Animal Studies Committee of the Washington University School of Medicine.
Generation of Syk−/− bone marrow chimeras
Syk+/− mice (Turner et al., 1995) were maintained on a 129.sv background (which does not carry the Ly-5.1 allele). Mice were genotyped by PCR using 5′-AGAGAAGCCCTGCCCATGGAC-3′ (Syk+) and 5′-CCTTGGGAAAAGCGCCTCCCCTACCC-3′ (Syk−) as forward primers in combination with the 5′-GTCCAGGTAGACCTCTTTGGGC-3′ reverse primer. The products (86 and 120 bp for Syk+ and Syk−, respectively) were resolved on a 2.5% agarose gel. Syk−/− and littermate Syk+/? fetal liver cells were obtained from embryonic day 15–17 embryos from timed matings of Syk+/− carriers. Bone marrow chimeras were generated by intravenous injection of unfractionated fetal liver cells into 6–8-wk-old lethally irradiated congenic recipient B6.SJL mice (carrying the Ly-5.1 allele). To evaluate marrow engraftment, chimeric marrow cells were incubated for 30 min with FITC-conjugated anti-Ly-5.1 mAb IgG. The cells were washed and subjected to FACS analysis. Chimeras were used 4–8 wk after the bone marrow transplantation.
Macrophage isolation and osteoclast culture
Primary BMMs were prepared as described previously (Faccio et al., 2003c) with slight modification. Marrow was extracted from femora and tibia of 6–8-wk-old mice with α-MEM and cultured in α-MEM containing 10% inactivated FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin (a-10 medium) with 100 ng/ml MCSF (Zhao et al., 1999) in petri dishes. In c-Src−/− and Dap12−/−/FcRγ−/− mice, macrophages were cultured from their spleen cells. Cells were incubated at 37°C in 6% CO2 for 3 d. Cells were washed with PBS and lifted with 1× trypsin/EDTA (Invitrogen) in PBS. A total of 5 × 103 cells were cultured in 200 μl α-MEM containing 10% heat-inactivated FBS with 100 ng/ml GST-RANKL and 26 ng/ml of mouse recombinant MCSF in 96-well tissue culture plates, some of which contained a sterile whale dentin slice. Cells were fixed and stained for TRAP activity after 5 d in culture using a commercial kit (387-A; Sigma-Aldrich). For actin ring staining, cells were fixed in 4% PFA, permeabilized in 0.1% Triton X-100, rinsed in PBS, and immunostained with AlexaFluor488 phalloidin (Invitrogen). To quantitate resorption lacunae, cells were removed from the dentin slices with 2 N NaOH and mechanical agitation. Dentin slides were stained with Coomassie brilliant blue. For preosteoclast generation, 1.2 × 106 BMMs were plated per 10-cm tissue culture dish. After a 3-d culture in 26 ng/ml MCSF and 100 ng/ml GST-RANKL, TRAP-expressing preosteoclasts were lifted with 0.02% EDTA in PBS.
Retroviral transduction
WT human Syk, hβ3 integrin subunit, and their mutants in pMX retrovirus vector were transiently transfected into Plat-E packaging cells using FuGENE 6 Transfection Reagent (Roche). The virus was collected 48 h after transfection. BMMs were infected with the virus for 24 h in the presence of 26 ng/ml MCSF and 4 μg/ml polybrene (Sigma-Aldrich). Cells were selected in the presence of MCSF and 1 μg/ml blasticidin (Calbiochem) for 3 d before analysis of osteoclastogenesis.
Western blotting and immunoprecipitation
Cultured cells were washed twice with ice-cold PBS and lysed in radioimmunoprecipitation buffer containing 20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM NaF, and 1× protease inhibitor mixture (Roche). After incubation on ice for 10 min, the cell lysates were clarified by centrifugation at 15,000 rpm for 10 min. 40 μg of total lysates were subjected to 8 or 12% SDS-PAGE and transferred onto nitrocellulose membrane. The filters were blocked in 5% milk/TBS and 0.1% Tween 20 for 1 h and incubated with primary antibodies at 4°C overnight followed by probing with secondary antibodies coupled with HRP (Santa Cruz Biotechnology, Inc.). The proteins were visualized using SuperSignal West Dura Extended Duration Substrate (Pierce Chemical Co.). Goat anti–β3-integrin pAb was purchased from Santa Cruz Biotechnology, Inc. Mouse anti-Syk mAb was purchased from Abcam, and antiphosphotyrosine mAb 4G10 was purchased from Upstate Biotechnology. Antiphospho-Vav3 (pY173) antibody was purchased from Biosource International. mAb 327 directed against the c-Src protein and full-length c-Src cDNA were gifts from A. Shaw (Department of Pathology, Washington University School of Medicine, St. Louis, MO). Anti–c-Srcp-Y416 mAb was purchased from Cell Signaling. Anti–β1-integrin mAb was a gift from R. Hynes (Massachusetts Institute of Technology, Cambridge, MA). For immunoprecipitation, BMMs were cultured with MCSF and RANKL in culture dishes for 3 d. Preosteoclasts were lifted and plated onto 5-μg/ml vitronectin-precoated dishes or cultured in suspension. Cells were washed in cold PBS and lysed on ice in radioimmunoprecipitation lysis buffer. 800 μg of protein was incubated with 2 μg monoclonal anti–human β3-integrin antibody (7G2; gift from E. Brown, University of California, San Francisco, San Francisco, CA) or polyclonal anti-Syk antibody (N19; Santa Cruz Biotechnology, Inc.) at 4°C overnight with rotation. Protein A/G agarose (Santa Cruz Biotechnology, Inc.) was then added and incubated with rotation for 3 h at 4°C. Immunoprecipitates were washed three times in lysis buffer, and the beads were boiled in 2× SDS sample buffer for 5 min. After centrifugation, proteins were separated by 8 or 10% SDS polyacrylamide gels.
Osteoblast assays
Primary calvaria-derived osteoblasts were isolated in collagen-gel culture from 17.5-d Syk+/? or Syk−/− embryos (Udagawa et al., 2000). 5 × 104 primary osteoblasts were maintained in 48-well plates in α-MEM for 3 d, after which osteoblastogenic medium consisting of α-MEM containing 50 μg/ml ascorbic acid and 2 mM β-glycerophosphate was added. 20 d later, bone nodules were stained with Alizarin red.
In vitro adhesion assay
1.2 × 106 Syk+/? or Syk−/− BMMs were cultured with 100 ng/ml RANKL and 25 ng/ml MCSF for 3 d. Preosteoclasts were lifted with 0.02% EDTA in PBS and washed twice with α0 media. 1.5 × 105 preosteoclasts were plated on vitronectin (BD Biosciences)-precoated six-well nontissue culture-treated plates for 5 or 10 min.
Rac activation assay
WT or Syk−/− preosteoclasts were lifted and plated onto 5-μg/ml vitronectin-precoated dishes or were cultured in suspension. Cells were washed in cold PBS and lysed with lysis buffer. Levels of Rac1-bound GTP were determined according to the protocol of the Rac1 activation kit (Pierce Chemical Co.).
PTH-induced osteoclastogenesis in vivo
10 μg synthetic human PTH(1–34) (Bachem California Inc.) in 25 μl of vehicle (1 mM HCl and 0.1% BSA) or vehicle alone were injected four times daily for 4 d into the subcutaneous tissue overlying the calvariae using a Hamilton syringe. After killing by CO2 narcosis, calvariae were removed intact, soft tissues were gently dissected, and the calvariae were fixed in 10% phosphate-buffered formalin for 24 h and were further processed as described previously (Zhao et al., 1999). Osteoclast number was determined using the Bioquant System (BIOQUANT Image Analysis). The mouse TRAP assay kit was purchased from Immunodiagnostic Systems Ltd. Serum TRAcP 5b was determined according to the manufacturer's instructions of the mouse TRAP assay kit (Immunodiagnostic Systems Ltd). All in vivo experiments were approved by the Washington University Animal Studies Committee.
Analysis of mouse embryos
For whole-mount skeletal staining, 18-d embryos were eviscerated, fixed in 95% ethanol, and stained with Alcian blue and Alizarin red according to standard protocols (McLeod, 1980). For histological analyses, embryonic bones were fixed in 10% formalin, and decalcified sections were stained for TRAP activity.
Statistics
All data are expressed as means ± SD, and statistical significance was calculated by t test.
Image acquisition and manipulation
Images from Figs. 1 (A–E), 2 B, 3 B, 4 B, 6 B, and 8 (A and G) were acquired using a microscope (Eclipse E400; Nikon) with plan Fluor lenses at RT. All magnifications are 20× except for Fig. 2 B, which is 60×. Zimmerson oil (Fisher Scientific) served as the imaging medium in Fig. 2 B. Fluorochrome FITC was used in Fig. 1 (B and D). Images from Fig. 3 A were acquired using a microscope (MZFLIII; Leica) with a plan Apo 1× lens (model 10447157; Leica) at RT. No imaging media or fluorochromes were used. Photographs were taken with a camera (MagnaFire S99802; Optronics) and displayed with MagnaFire software (version 2.1B; Meyer Instruments) in all circumstances except Fig. 4 A. The images were organized in Photoshop (version 7.0.1; Adobe). Fig. 4 A was acquired with a digital camera (Powershot A75; Canon) and organized in Photoshop.
Online supplemental material
Fig. S1 shows the generation of Syk chimeras. Fig. S2 shows that Syk deficiency does not delay osteoclast differentiation or arrest intracellular signaling. Fig. S3 shows that bone density is increased in Syk−/− embryos. Fig. 4 shows that Syk−/− preosteoclasts do not rapidly spread on αvβ3 ligand. Fig. S5 shows that the deletion of β3 integrin does not enhance Syk–β1 integrin association in osteoclasts. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200611083/DC1.
Acknowledgments
We wish to thank Dr. Marco Colonna for the Dap12−/−/FcRγ−/− spleen cells.
This study was supported by the following grants from the National Institutes of Health: AR032788, AR046523, AR048853, DK056341 (Clinical Nutrition Research Unit; to S.L. Teitelbaum), AR046852, AR048812 (to F.P. Ross), HL57900, HL78784 (to S.J. Shattil), and HL-078784 (to M.H. Ginsberg).
Abbreviations used in this paper: BMM, bone marrow macrophage; ITAM, immunoreceptor tyrosine-based activation motif; MCSF, macrophage colony–stimulating factor; OPG, osteoprotegrin; PTH, parathyroid hormone; RANK, receptor activator of nuclear factor κB; RANKL, RANK ligand; SFK, Src family kinase; TCL, total cell lysate; TRAP, tartrate-resistant acidic phosphatase; WT, wild type.
References
- Arias-Salgado, E.G., S. Lizano, S. Sarkar, J.S. Brugge, M.H. Ginsberg, and S.J. Shattil. 2003. Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc. Natl. Acad. Sci. USA. 100:13298–13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Salgado, E.G., S. Lizano, S.J. Shattil, and M.H. Ginsberg. 2005. Specification of the direction of adhesive signaling by the integrin β cytoplasmic domain. J. Biol. Chem. 280:29699–29707. [DOI] [PubMed] [Google Scholar]
- Boyce, B.F., T. Yoneda, C. Lowe, P. Soriano, and G.R. Mundy. 1992. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J. Clin. Invest. 90:1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdicka, T., T.A. Kadlecek, J.P. Roose, A.W. Pastuszak, and A. Weiss. 2005. Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol. Cell. Biol. 25:4924–4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccio, R., D.V. Novack, A. Zallone, F.P. Ross, and S.L. Teitelbaum. 2003. a. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by β3 integrin. J. Cell Biol. 162:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccio, R., A. Zallone, F.P. Ross, and S.L. Teitelbaum. 2003. b. c-Fms and the αvβ3 integrin collaborate during osteoclast differentiation. J. Clin. Invest. 111:749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccio, R., W. Zou, G. Colaianni, S.L. Teitelbaum, and F.P. Ross. 2003. c. High dose M-CSF partially rescues the Dap12−/− osteoclast phenotype. J. Cell. Biochem. 90:871–883. [DOI] [PubMed] [Google Scholar]
- Faccio, R., S.L. Teitelbaum, K. Fujikawa, J.C. Chappel, A. Zallone, V.L. Tybulewicz, F.P. Ross, and W. Swat. 2005. Vav3 regulates osteoclast function and bone mass. Nat. Med. 11:284–290. [DOI] [PubMed] [Google Scholar]
- Feng, X., D.V. Novack, R. Faccio, D.S. Ory, K. Aya, M.I. Boyer, K.P. McHugh, F.P. Ross, and S.L. Teitelbaum. 2001. A Glanzmann's mutation of the β3 integrin gene specifically impairs osteoclast function. J. Clin. Invest. 107:1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterer, K., J. Wong, R.A. Grucza, A.C. Chan, and G. Waksman. 1998. Structural basis for syk tyrosine kinase ubiquity in signal transduction pathways revealed by the crystal structure of its regulatory SH2 domains bound to a dually phosphorylated ITAM peptide. J. Mol. Biol. 281:523–537. [DOI] [PubMed] [Google Scholar]
- Gao, J., K.E. Zoller, M.H. Ginsberg, J.S. Brugge, and S.J. Shattil. 1997. Regulation of the pp72syk protein tyrosine kinase by platelet integrin αIIbß3. EMBO J. 16:6414–6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath, J.R., R. Liu, A.M. Enstrom, Q. Lou, and K.S. Lam. 2003. Development and characterization of potent and specific peptide inhibitors of p60c-src protein tyrosine kinase using pseudosubstrate-based inhibitor design approach. J. Pept. Res. 62:260–268. [DOI] [PubMed] [Google Scholar]
- Koga, T., M. Inui, K. Inoue, S. Kim, A. Suematsu, E. Kobayashi, T. Iwata, H. Ohnishi, T. Matozaki, T. Kodama, et al. 2004. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 428:758–763. [DOI] [PubMed] [Google Scholar]
- Law, D.A., F.R. DeGuzman, P. Heiser, K. Ministri-Madrid, N. Killeen, and D.R. Phillips. 1999. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 401:808–811. [DOI] [PubMed] [Google Scholar]
- McHugh, K.P., K. Hodivala-Dilke, M.H. Zheng, N. Namba, J. Lam, D. Novack, X. Feng, F.P. Ross, R.O. Hynes, and S.L. Teitelbaum. 2000. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 105:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod, M.J. 1980. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 22:299–301. [DOI] [PubMed] [Google Scholar]
- Miyazaki, T., A. Sanjay, L. Neff, S. Tanaka, W.C. Horne, and R. Baron. 2004. Src kinase activity is essential for osteoclast function. J. Biol. Chem. 279:17660–17666. [DOI] [PubMed] [Google Scholar]
- Mocsai, A., M. Zhou, F. Meng, V.L. Tybulewicz, and C.A. Lowell. 2002. Syk is required for integrin signaling in neutrophils. Immunity. 16:547–548. [DOI] [PubMed] [Google Scholar]
- Mocsai, A., M.B. Humphrey, J.A.G. Van Ziffle, Y. Hu, A. Burghardt, S.C. Spusta, S. Majumdar, L.L. Lanier, C.A. Lowell, and M.C. Nakamura. 2004. The immunomodulatory adapter proteins DAP12 and Fc receptor γ-chain (FcRγ) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. USA. 101:6158–6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, M.G., K. Cerchio, S.A. Stoch, K. Gottesdiener, M. Wu, R. Recker, and the L-000845704 Study Group. 2005. Effect of L-000845704, an αvβ3 integrin antagonist, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J. Clin. Endocrinol. Metab. 90:2022–2028. [DOI] [PubMed] [Google Scholar]
- Pitcher, L.A., and N.S.C. van Oers. 2003. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 24:554–560. [DOI] [PubMed] [Google Scholar]
- Sanjay, A., A. Houghton, L. Neff, E. Didomenico, C. Bardelay, E. Antoine, J. Levy, J. Gailit, D. Bowtell, W.C. Horne, and R. Baron. 2001. Cbl associates with Pyk2 and Src to regulate Src kinase activity, αvβ3 integrin-mediated signaling, cell adhesion, and osteoclast motility. J. Cell Biol. 152:181–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg, P.L., L. Xing, O. Hoffmann, C.A. Lowell, L. Garrett, B.F. Boyce, and H.E. Varmus. 1997. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev. 11:2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano, P., C. Montgomery, R. Geske, and A. Bradley. 1991. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 64:693–702. [DOI] [PubMed] [Google Scholar]
- Teitelbaum, S.L., and F.P. Ross. 2003. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4:638–649. [DOI] [PubMed] [Google Scholar]
- Turner, M., P.J. Mee, P.S. Costello, O. Williams, A.A. Price, L.P. Duddy, M.T. Furlong, R.L. Geahlen, and V.L. Tybulewicz. 1995. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 378:298–302. [DOI] [PubMed] [Google Scholar]
- Udagawa, N., N. Takahashi, H. Yasuda, A. Mizuno, K. Itoh, Y. Ueno, T. Shinki, M. Gillespie, T. Martin, K. Higashio, and T. Suda. 2000. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology. 141:3478–3484. [DOI] [PubMed] [Google Scholar]
- Woodside, D.G. 2002. Dancing with multiple partners. Sci. STKE. 10.1126/stke.2002.124.pe14. [DOI] [PubMed]
- Woodside, D.G., A. Obergfell, L. Leng, J.L. Wilsbacher, C.K. Miranti, J.S. Brugge, S.J. Shattil, and M.H. Ginsberg. 2001. Activation of Syk protein tyrosine kinase through interaction with integrin [beta] cytoplasmic domains. Curr. Biol. 11:1799–1804. [DOI] [PubMed] [Google Scholar]
- Zhao, W.G., M.H. Byrne, B.F. Boyce, and S.M. Krane. 1999. Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J. Clin. Invest. 103:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]