Abstract
Apoptosis signal-regulating kinase 1 (ASK1) is a member of the mitogen-activated protein 3-kinase family that activates both c-Jun NH2-terminal kinase and p38 pathways in response to inflammatory cytokines and physicochemical stress. We report that ASK1 deficiency in mice results in dramatic retardation of wounding-induced hair regrowth in skin. Oligonucleotide microarray analysis revealed that expression of several chemotactic and activating factors for macrophages, as well as several macrophage-specific marker genes, was reduced in the skin wound area of ASK1-deficient mice. Intracutaneous transplantation of cytokine-activated bone marrow-derived macrophages strongly induced hair growth in both wild-type and ASK1-deficient mice. These findings indicate that ASK1 is required for wounding-induced infiltration and activation of macrophages, which play central roles in inflammation-dependent hair regrowth in skin.
Introduction
Apoptosis signal-regulating kinase (ASK) 1 is a MAP3K family member that activates both the JNK and p38 MAPK signaling cascades and is activated in response to various stimuli, including oxidative stress, endoplasmic reticulum stress, calcium influx, and inflammatory cytokines (Ichijo et al., 1997; Hayakawa et al., 2006; Sekine et al., 2006). Expression of ASK1 protein has been reported to be strongly induced surrounding wounds in rat palatal epithelium (Funato et al., 1998). It has also been demonstrated that ASK1 induces keratinocyte differentiation and regulates the innate immunity of the skin (Sayama et al., 2001, 2005). These findings have suggested that ASK1 may play an important role in epithelial wound healing.
Mammalian skin is composed of three differentiated epithelial compartments: the interfollicular epidermis, sebaceous glands, and hair follicles (Stenn and Paus, 2001). A bulge within each hair follicle contains stem cells, which in turn proliferate and differentiate into new hair follicles (Taylor et al., 2000; Fuchs et al., 2004). Wounding of skin has been reported to induce hair growth (Argyris, 1956). It was recently shown that the pattern of expression of epithelial stem cells in hair follicles around wound areas is similar to that in spontaneous hair cycling (Ito and Kizawa, 2001). This suggested that understanding of wound-induced hair regrowth may elucidate the general mechanisms of hair growth. Furthermore, it is known that onset of the developmental program in epithelial stem cells is triggered by environmental signals (Fuchs et al., 2004). However, the hair regrowth factors and mechanisms by which wounding induces hair regrowth remain to be determined.
In this study, we found that ASK1-deficient (ASK1 −/−) mice exhibited marked delay of wounding-induced hair regrowth. We also found that infiltration and activation of macrophages were strongly impaired in the wound area of ASK1 −/− mice, and that transplantation of activated macrophages induced hair regrowth in both wild-type (WT) and ASK1 −/− mice. These findings demonstrate the critical involvement of macrophages in hair growth and the need for regulation of infiltration and activation of macrophages in skin wounds by ASK1 for macrophage-dependent hair regrowth.
Results and discussion
ASK1-deficient mice display delayed wounding-induced hair regrowth
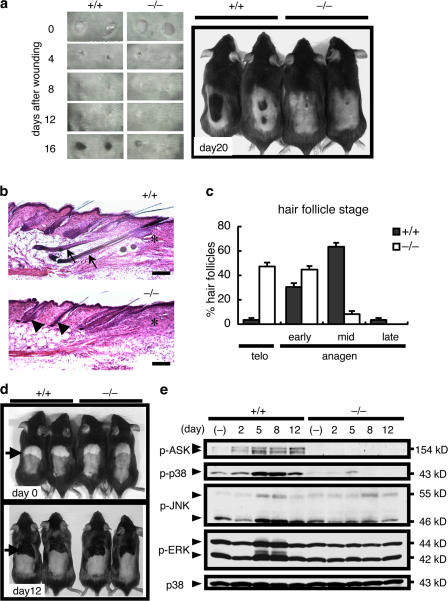
To explore the roles of ASK1 in wound healing, full-thickness 5-mm incisional wounds were produced on the shaved back skin of WT and ASK1 −/− mice, and wound areas were monitored for up to 20 d. During the healing process, the macroscopic appearances of wound closure and histological reepithelialization were similar in WT and ASK1 −/− mice (Fig. 1 a and not depicted). However, we found that wounding-induced hair regrowth was dramatically delayed in ASK1 −/− mice (Fig. 1 a). Although it has been reported that hair regrowth is induced by wounding, the reason for this has remained unclear (Argyris, 1956; Stenn and Paus, 2001). Hair follicles undergo repeated cycles of proliferation and differentiation (anagen stage), apoptosis (catagen stage), and resting (telogen stage) of epithelial cells (Stenn and Paus, 2001). Quantitative evaluation of hair follicle stages on the basis of well-defined morphological criteria revealed that the percentage of hair follicles in telogen stage was very high in ASK1 −/− mice at 12 d after wounding (Fig. 1, b and c; Muller-Rover et al., 2001). These findings suggested that lack of ASK1 delays wounding-induced anagen initiation. On the other hand, we have been unable to find histological differences between WT and ASK1 −/− in the normal development of hair follicles of 14.5-d-old embryos or the spontaneous anagen-stage hair follicles of 18-d-old neonatal mice (unpublished data). Moreover, plucking-induced hair regrowth, which is another experimental model of induced hair growth, the mechanism of which is unknown (Morris and Potten, 1999; Ito and Kizawa, 2001), occurred normally in ASK1 −/− mice (Fig. 1 d). These findings suggested that ASK1 deficiency does not affect the hair-regenerating activity of epithelial cells, per se.
Figure 1.
Impairment of wounding-induced hair regrowth in ASK1-deficient mice. (a) Macroscopic changes associated with wound closure and wounding-induced hair regrowth in the dorsal skin of WT and ASK1 −/− mice. Day 0 photographs were taken immediately after wounding. Representative results from >20 mice in each group are shown. (b and c) Evaluation of hair follicle stages at 12 d after wounding. (b) Skin paraffin sections of WT and ASK1 −/− mice were processed for hematoxylin and eosin staining. *, wound edge; arrow, hair follicles in anagen stage; arrowhead, hair follicles in telogen stage. Bars, 300 μm. (c) Percentages of hair follicles in defined stages at 12 d after wounding. Morphogenesis of a total of 112 hair follicles of 14 mice was evaluated by quantitative histomorphometry according to well-defined morphological criteria. telo, telogen; early anagen, anagen I–II; mid (middle) anagen, anagen III–VI; late anagen, anagen V–IV. (d) Macroscopic changes associated with plucking-induced hair regrowth in the dorsal skin of WT and ASK1 −/− mice. Day 0 photographs were taken immediately after plucking (indicated by arrows). Representative results from >20 mice in each group are shown. (e) Activation of ASK1 and MAP kinases (p38, JNK, and ERK) in postwounding skin. States of activation of these kinases in wounded skin on the indicated day were determined by immunoblotting of total skin lysates using antibodies to phospho-ASK1 (p-ASK1), -p38 (p-p38), -JNK (p-JNK), or -ERK (p-ERK). Total amounts of p38 are also presented as loading controls.
ASK1 is required for activation of p38, JNK, and extracellular signal-regulated kinase (ERK) in wound areas
We next examined the status of activation of ASK1 and MAPKs, including p38, JNK, and ERK, in wounded skin of WT and ASK1 −/− mice (Fig. 1 e). Wounding clearly induced activation of ASK1, p38, JNK, and ERK in WT mice. Activation of p38 and, to a lesser extent, JNK was reduced in the wound area of ASK1 −/− mice, indicating that ASK1 is required for activation of p38 and JNK in wounded skin (Fig. 1 e). ERK activation, which is not induced by ASK1 (Ichijo et al., 1997), was also reduced in wound areas of ASK1 −/− mice. Because ERK is widely activated by various growth factors and cytokines, it is possible that ERK activation is indirectly reduced in ASK1 −/− mice because of reduction of activity of p38 and JNK, which are required for the production of various inflammatory cytokines (see next section).
ASK1 is required for regulation of immune responses after wounding
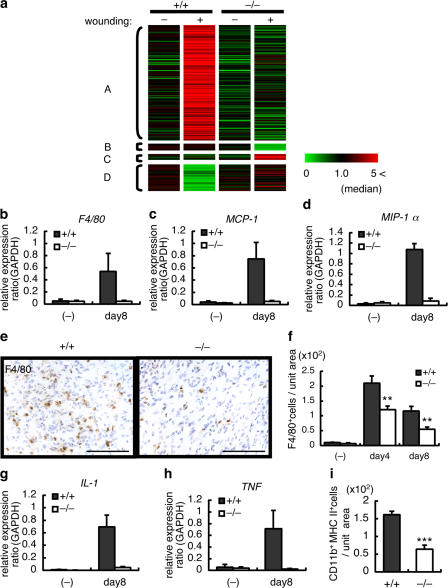
To identify the genes responsible for ASK1-dependent hair regrowth, skin samples from four groups (untreated and wounded skin 8 d after operation from WT or ASK1 −/− mice) were collected, and gene expression in each group was monitored using high-density Affymetrix GeneChips. We identified 185 annotated genes of the 45,102 represented on the array, which exhibited at least a fourfold difference in expression between WT and ASK1 −/− wounded skin. The levels of expression of these 185 genes in unwounded skin were similar in WT and ASK1 −/− mice. A total of 138 of the 185 genes (76%) were up-regulated in an ASK1-dependent fashion by wounding (Fig. 2 a, cluster A). Altogether, 53 of the 138 genes (38%) were classified in the category of “immune-response regulation,” including various cytokines and chemokines using the Gene Ontology Consortium classifications (Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200611015/DC1). Together with the results shown in Fig. 1 e, these findings suggested that ASK1 is required for regulation of postwounding immune responses, such as cytokine and chemokine production in wound areas.
Figure 2.
ASK1 is required for regulation of immune response in wound areas. (a) Gene expression is influenced in an ASK1-dependent manner by wounding. 185 genes exhibited differential expression in wounded skin of WT mice or ASK1 −/− mice at 8 d after wounding. Each gene is represented by a single row of colored boxes. For every gene, the median value of expression for the four samples shown was calculated, and its relative expression in one sample is indicated by color. Green, transcript abundance below median; black, transcript abundance near median; red, transcript abundance above median. Cluster A contains 138 genes that were induced in the wounded skin of WT mice to a greater extent than in ASK1 −/− mice. Cluster B contains eight genes with less expression in the wounded skin of ASK1 −/− mice than in that of WT mice. Cluster C contains seven genes, expression of which was induced to a greater extent in the wounded skin of ASK1 −/− than in that of WT mice. Cluster D contains 32 genes, expression of which was induced to a lesser extent in the wounded skin of WT mice than in that of ASK1 −/− mice. (b–d) Reduction of expression of a macrophage-specific marker and chemotactic factors in wounded skin of ASK1 −/− mice. Quantification of levels of mRNA expression in untreated skin and wounded skin (8 d after wounding) of WT and ASK1 −/− mice are as follows: F4/80 (b); MCP-1 (c); and MIP-1α (d). Relative mRNA expressions of these genes were measured by real-time RT-PCR with specific primers. Results are the mean ± the SEM. n = 3 for each group. (e and f) Decrease in number of macrophages in wounded skin of ASK1 −/− mice. (e) Immunohistochemical analysis with anti-F4/80 antibody at 8 d after wounding. Representative staining in WT and ASK1 −/− samples is shown. Bars, 100 μm. (f) Number of F4/80+ cells/unit area was quantified in wound areas at days 4 and 8 after wounding. (−), nontreated skin. Results are the mean ± the SEM. n = 8 fields around wound edge of four independent mice for each time point and group. **, P < 0.01, t test. (g and h) Reduction of expression of macrophage-activating factors in wounded skin of ASK1 −/− mice. Quantification of levels of mRNA expression in untreated or wounded skin of WT and ASK1 −/− mice at day 8 after wounding are as follows: IL-1β (g) and TNFα (h). Relative mRNA expressions of these genes were measured by real-time RT-PCR with specific primers. Results are the mean ± the SEM. n = 3 for each group. (i) Decrease in the number of activated macrophages in wounded skin of ASK1 −/− mice. Number of CD11b+MHC II+ cells per unit area was quantified in wound areas at day 8 after wounding. Results are the mean ± the SEM. n = 5 fields around wound edge of three independent mice for each group. ***, P < 0.001, t test.
ASK1 is required for the recruitment of macrophages into wound areas
GeneChip analysis also revealed that expression of several genes uniquely or selectively expressed in macrophages was reduced in the wounded skin of ASK1 −/− mice (Table S2, available at http://www.jcb.org/cgi/content/full/jcb.200611015/DC1). To confirm altered expression of macrophage-related genes, relative mRNA expressions of a macrophage-specific marker (F4/80) and chemotactic factors (monocyte chemoattractant protein-1 [MCP-1] and macrophage inflammatory protein-1α [MIP-1α]) were measured by real-time RT-PCR in the same RNA samples used for GeneChip analysis. Expression of these transcripts was confirmed to be strongly reduced in the wounded skin of ASK1 −/− mice (Fig. 2, b–d). Immunohistochemical analysis of day 4 and 8 wounds with F4/80 antibody further confirmed that recruitment of macrophages into the wound area of ASK1 −/− mice was significantly reduced compared with that in WT mice (Fig. 2, e and f). These findings suggested that ASK1 is required for infiltration of macrophages into wound areas, which may be caused by ASK1-mediated postwounding immune responses, such as the production of macrophage-specific chemotactic factors.
ASK1 is required for activation of macrophages in wound areas
Expression of interleukin (IL)-1β and TNFα in the wound area was also found by microarray and real-time RT-PCR analyses to be increased in an ASK1-dependent manner (Table S2 and Fig. 2, g and h). IL-1β and TNFα are typical macrophage-activating factors, which may be expressed in wounded skin and in activated macrophages. Double-staining with antibodies to macrophage marker CD11b and the activation marker major histocompatability complex (MHC) class II revealed that activated macrophages (double-positive cells) were significantly reduced in number in the wound area of ASK1 −/− mice compared with WT mice (Fig. 2 i). These findings suggested that ASK1 is also required for activation of macrophages, which may be mediated by macrophage-activating factors, such as IL-1β and TNFα, in the wound area.
Intracutaneous transplantation of macrophages is sufficient for induction of hair growth
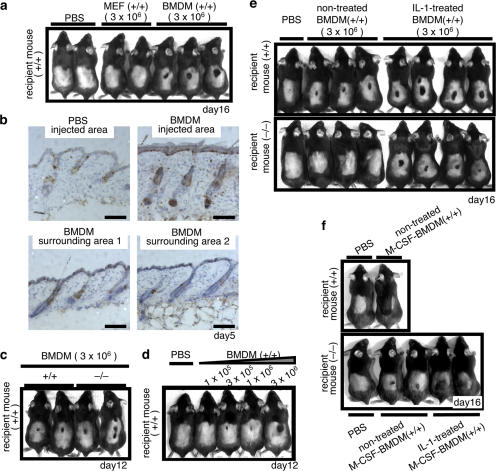
The aforementioned findings suggested that macrophages may be involved in wounding-induced hair regrowth, and that the delay of hair regrowth in ASK1 −/− mice may be caused by reduction of infiltration of activated macrophages in wounds. To examine whether macrophages promote hair growth, we performed intracutaneous transplantation of bone marrow–derived macrophages (BMDMs) into the dorsal skin of WT mice. Hair growth was strongly induced by transplantation of BMDMs derived from WT mice, but by neither mouse embryonic fibroblasts (MEFs) nor dendritic cells (DCs; Fig. 3 a and Fig. S1 c, available at http://www.jcb.org/cgi/content/full/jcb.200611015/DC1). Staining for Ki67 (a cell proliferation marker) in the area of transplantation of BMDMs revealed that proliferation of epidermal basal cells and hair follicle cells, including bulge stem cells, was accelerated by macrophages, but not by PBS control, only in the injected area (Fig. 3 b and Fig. S1, a and b). These findings clearly indicated that macrophages possess the ability to induce hair growth. Furthermore, hair regrowth was induced by BMDMs derived from ASK1 −/− mice, as well as those derived from WT mice (Fig. 3 c), indicating that ASK1 is not required for the macrophage function by itself to induce hair growth.
Figure 3.
Transplantation of macrophages induces hair growth. (a) Transplantation of BMDMs, but not MEFs, derived from WT mice into the dorsal skin of WT mice induced hair growth. Photographs at day 16 after transplantation. (b) Transplantation of BMDMs, but not of PBS control, induced proliferation of bulge stem cells only in the injection site. Immunohistochemical analysis with Ki67 antibody at day 5 after transplantation. Representative stainings are shown. Bars, 100 μm. For the orientation of isolated skin areas and the quantification analysis, see Fig. S1 (a and b). (c) BMDMs derived from ASK1 −/− mice induced hair growth to an extent comparable to that induced by BMDMs from WT mice. Photograph was taken at day 12 after transplantation. (d) Hair growth is enhanced in proportion to the number of transplanted BMDMs. Photograph at day 12 after transplantation. (e) Activation-dependent hair growth by BMDMs in ASK1 −/− mice. Transplantation of BMDMs induced hair growth in WT, but not in ASK1 −/−, mice. Transplantation of IL-1β–treated BMDMs induced hair growth in ASK1 −/− mice, as well as in WT mice. Photograph at day 16 after transplantation. (f) Transplantation of M-CSF–BMDMs also induced hair growth in WT, but not in ASK1 −/− mice, whereas transplantation of IL-1β–treated M-CSF–BMDMs induced hair growth in ASK1 −/− mice, as well as in WT mice. Photograph was taken at day 16 after transplantation. Fig. S1 is available at http://www.jcb.org/cgi/content/full/jcb.200611015/DC1
As shown in Fig. 2, ASK1 was required for the recruitment and activation of macrophages into wound areas, which appears to be caused by reduced production of chemotactic and activating factors for macrophages in ASK1 −/− skin wounds. To confirm that infiltration of macrophages is required for hair regrowth, we examined whether hair growth depends on the number of transplanted macrophages. Hair growth was enhanced in proportion to the number of transplanted BMDMs (Fig. 3 d), providing further evidence for the existence of a causal relationship between reduction of the number of macrophages infiltrating ASK1 −/− wounded skin and the delay of hair regrowth.
ASK1 is required for activation, as well as recruitment, of macrophages
Despite the aforementioned findings, we found that transplantation of BMDMs barely induced hair growth in ASK1 −/− mice (Fig. 3 e). As shown in Fig. 2 (g–i), ASK1 was also required for activation of macrophages, which may be induced by macrophage-activating factors in the wound area. These findings suggested that infiltration of macrophages into the wound area is not sufficient for induction of hair growth, and that activation of macrophages is also required for the induction of hair growth. BMDMs transplanted into ASK1 −/− mice may not be sufficiently activated, which is due in part to reduced production of macrophage-activating factors. Therefore, we performed transplantation of IL-1β–stimulated, and thus activated, BMDMs into the dorsal skin of WT and ASK1 −/− mice. IL-1β–treated BMDMs induced hair in WT mice more effectively than did nontreated BMDMs. Moreover, transplantation of IL-1β– stimulated BMDMs, but not of IL-1β–stimulated MEFs, was sufficient for induction of hair growth in ASK1 −/− mice, as well as in WT mice (Fig. 3 e and Fig. S1 d).
We also performed intracutaneous transplantation of macrophages of a different type, which were differentiated by monocyte colony-stimulating factor (M-CSF–BMDMs). Robust hair growth was induced by transplantation of M-CSF–BMDMs in WT mice (Fig. 3 f, top). M-CSF–BMDMs induced less hair growth in ASK1 −/− mice than in WT mice, but IL-1β–stimulated M-CSF–BMDMs induced hair growth in ASK1 −/− mice that was almost equivalent to that in WT mice (Fig. 3 f, bottom). These findings indicated that activated macrophages possess the ability to induce hair growth, and that regulation of both recruitment and activation of macrophages by ASK1 is required for macrophage-dependent hair regrowth.
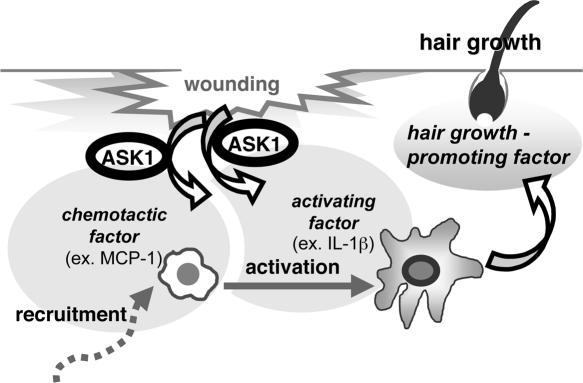
In this study, we found that macrophages play a crucial role in wounding-induced hair regrowth, and that regulation of infiltration and activation of macrophages in wound areas by ASK1 is required for macrophage-dependent hair regrowth (Fig. 4). No difference was found in the ability to induce hair regrowth between WT and ASK1 −/− BMDMs (Fig. 3 c). Epithelial cell functions, such as proliferation and differentiation, were also normal in ASK1 −/− mice (Fig. 1 d and not depicted). These findings suggested that ASK1 is selectively required for infiltration and subsequent activation of macrophages in wound areas (Fig. 4, left and middle), but not for the induction by macrophages of hair growth (Fig. 4, right). It is likely that infiltration of macrophages and activation of them are induced by macrophage-specific chemotactic factors and macrophage-activating factors, including MCP-1, MIP-1α, TNFα, and IL-1β, which are produced around the wound area in an ASK1-dependent fashion (Fig. 4).
Figure 4.
A schematic model of the involvement of ASK1 in macrophage-dependent hair regrowth. ASK1 is required for infiltration (left) and subsequent activation (middle) of macrophages in the wounded area, but not for the ability of macrophages by themselves to induce hair regrowth (right). Production of macrophage-specific chemotactic chemokines and macrophage-activating factors may be induced around wounded areas in an ASK1-dependent fashion.
Although it has been reported that hair growth is induced by wounding, the reasons for this have remained unclear (Argyris, 1956; Stenn and Paus, 2001). Synchronized hair follicle cycling in mice has been reported to be related to the number and activation of perifollicular macrophages (Paus et al., 1998). This study is the first to provide direct evidence that infiltration and activation of macrophages are critically involved in postwounding hair regrowth, in which ASK1 plays an essential role. We are currently identifying hair growth–promoting factors produced by activated BMDMs, which appear to be thermosensitive proteinaceous factors (unpublished data). It has recently been found that macrophage-stimulating factor (MSP), which was originally identified as a chemotactic factor for macrophages, could induce hair growth, and that RON, the receptor for MSP, is strongly expressed in hair follicles (McElwee et al., 2004). We therefore examined whether ASK1-dependent up-regulation of MSP and/or RON is responsible for macrophage-induced hair growth. However, no significant difference was found between WT and ASK1 −/− in levels of expression of MSP and RON after wounding (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200611015/DC1), suggesting that MSP and RON may not be responsible for ASK1-dependent hair growth.
Although further understanding of postwounding hair regrowth is needed, identification of macrophage-dependent hair growth–promoting factors or determination of a method of synthetic activation of ASK1 in skin may be of therapeutic benefit in accelerating impaired hair growth.
Materials and methods
Mice
ASK1 −/− (Tobiume et al., 2001) and WT mice were derived from heterozygote crosses of ASK1 +/− mice and constantly housed in a specific pathogen-free facility with a 12-h light/dark schedule and constant temperature. All experiments were performed using 8-wk-old female mice whose dorsal skin hair follicles were all in telogen stage. ASK1 +/− mice used in this study have been backcrossed on the C57BL/6J strain for 12 generations. All experiments were in accordance with protocols approved by the Animal Research Committee of the Graduate School of Pharmaceutical Sciences (University of Tokyo, Tokyo, Japan).
Wound-healing experiments
Before injury, mice were anaesthetized and the dorsal hair was shaved. Two equidistant 5-mm full-thickness incisional wounds were punched in the middle of the dorsum as previously described (Ashcroft et al., 1999). Each wound region was digitally photographed (DSC-D700; Sony) at the indicated time points.
RNA isolation
Total RNA extraction was performed using the Isogen Reagent (Nippon Gene Co. Ltd.). These RNA extracts were used for oligonucleotide microarray analysis and RT-PCR analysis.
Oligonucleotide microarray analysis
The levels of expression of over 45,102 transcripts and variants were analyzed by oligonucleotide microarray (GeneChip Mouse Genome 430 2.0 Arrays; Affymetrix). Sequence clusters were created from the UniGene database (Build 107, June 2002). Analysis was performed essentially as previously described (Hippo et al., 2002). The cutoff value was set at >50 for mean level of expression and >4 for the ratio (wounded skin of ASK1 −/− mice/WT mice) and ≤2 for the ratio (untreated skin of ASK1 −/− mice/WT mice). Classification of functions of genes was performed by Gene Ontology bioinformatics analysis using information from annotation files (2004.4 version).
Real-time RT-PCR amplification
Single-stranded cDNA was synthesized with oligo (dT) primers from total RNA using SuperScript III reverse transcriptase (Invitrogen). The abundance of transcripts in cDNA samples was measured by real-time PCR with specific primers. Real-time RT-PCR amplifications of cDNA were performed using the Quantitation kit on the ABI Prism 7000 Sequence Detection System (Applied Biosystems).
Immunoblot analysis
Skin extracts from each mouse were resolved by SDS-PAGE and analyzed by immunoblotting as previously described (Nishitoh et al., 1998).
Histology, immunohistochemistry, and image analysis
For morphological analysis, 5-μm sections of paraffin-embedded wounded skin were stained with hematoxylin and eosin. 7-μm sections of fresh-frozen wounded skin were immunostained with antibodies to F4/80 (A3-1; Serotec), CD11b (M1/70; Bioscience), and MHC class II (ER-TR3; BMA) at 4°C. 8-μm sections of fresh-frozen transplanted skin were immunostained with antibodies to Ki67 (TEC-3; DAKO Cytomation) at 4°C. Each staining was performed according to the manufacturer's instructions. For image acquisition, a microscope (DM4000B; Leica) equipped with PL FLUOTAR objective lenses (5×/0.15 NA, 20×/0.50 NA, and 40×/0.75 NA) and a digital camera (DC300FX; Leica) were used. Images were processed with IM50 image manager (Leica) and Photoshop CS software (Adobe).
Cell culture
A crude population of BMDMs was generated in vitro from mouse bone marrow, as previously described (Akagawa et al., 1996), but with some modifications. Bone marrow cells were cultured in RPMI 1640 medium containing 10% heat-inactivated FBS, 100 units/ml of penicillin G, 10 ng/ml of recombinant mouse granulocyte–monocyte colony-stimulating factor (Strathmann Biotech GmbH), and 5 ng/ml of recombinant mouse IL-4 (Genzyme/Techne) or 10 ng/ml of recombinant human M-CSF (PeproTech EC). The medium was replaced on days 2 and 4, and the nonadherent granulocytes were rinsed away. On day 7 of culture, the firmly adherent cells were obtained as a macrophage population characterized by certain features of morphology and phenotype, such as expression of high levels of F4/80 but low or undetectable levels of MHC class II and CD86 molecules (as determined by flow cytometry), and loosely adherent cells were obtained as DCs. We generated two types of BMDMs, which were cultured with granulocyte–monocyte colony-stimulating factor and IL-4 (BMDMs) or with M-CSF (M-CSF–BMDMs), and used as BMDMs in subsequent experiments. For activation of cells, the BMDMs, M-CSF–BMDMs, and MEFs obtained as described in this section were stimulated with media containing 10 ng/ml of recombinant human IL-1β (Roche) for 24 h. Cells were then washed five times with PBS and subjected to transplantation as described in the next section.
Transplantation
For hair regeneration experiments, BMDMs, M-CSF–BMDMs, DCs, and MEFs removed from WT and ASK1 −/− mice were resuspended in 50 μl PBS, and cells or vehicle alone were injected intracutaneously in the dorsal skin, as previously described (Ashcroft et al., 1999), but with some modifications. The number of transplanted BMDMs was similar to the number of macrophages that had infiltrated into the wound area (∼106 cells), as estimated by counting macrophages in sections of wounded skin stained immunohistochemically with an antibody to the macrophage-specific marker F4/80.
Online supplemental material
Fig. S1 (a and b) shows that the number of Ki67-positive cells increased only in the injection site of BMDMs, but not of those PBS control. Fig. S1 c shows that transplantation of DCs did not induce hair growth in WT mice. Fig. S1 d shows that transplantation of IL-1β–treated MEFs did not induce hair growth in ASK1 −/− mice. Fig. S2 shows levels of expression of MSP and RON in wounded skin. Table S1 shows Gene Ontology Consortium classifications. Table S2 shows the results of GeneChip analysis.
Acknowledgments
We thank N. Higashi for technical support in FACS analysis, and all members of the Laboratory of Cell Signaling.
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and Core Research for Evolutional Science and Technology (CREST), of the Japan Science and Technology Corporation.
The authors declare that they have no competing financial interests.
Abbreviations used in this paper: ASK1, apoptosis signal-regulating kinase 1; BMDM, bone marrow–derived macrophage; DC, dendritic cell; ERK, extracellular signal-regulated kinase; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MEF, mouse embryonic fibroblast; MHC, major histocompatability complex; MIP-1α, macrophage inflammatory protein-1α; MSP, macrophage-stimulating factor; WT, wild-type.
References
- Akagawa, K.S., N. Takasuka, Y. Nozaki, I. Komuro, M. Azuma, M. Ueda, M. Naito, and K. Takahashi. 1996. Generation of CD1+RelB+ dendritic cells and tartrate-resistant acid phosphatase-positive osteoclast-like multinucleated giant cells from human monocytes. Blood. 88:4029–4039. [PubMed] [Google Scholar]
- Argyris, T.S. 1956. The effect of wounds on adjacent growing or resting hair follicles in mice. AMA Arch. Pathol. 61:31–36. [PubMed] [Google Scholar]
- Ashcroft, G.S., X. Yang, A.B. Glick, M. Weinstein, J.L. Letterio, D.E. Mizel, M. Anzano, T. Greenwell-Wild, S.M. Wahl, C. Deng, and A.B. Roberts. 1999. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1:260–266. [DOI] [PubMed] [Google Scholar]
- Fuchs, E., T. Tumbar, and G. Guasch. 2004. Socializing with the neighbors: stem cells and their niche. Cell. 116:769–778. [DOI] [PubMed] [Google Scholar]
- Funato, N., K. Moriyama, M. Saitoh, Y. Baba, H. Ichijo, and T. Kuroda. 1998. Evidence for apoptosis signal-regulating kinase 1 in the regenerating palatal epithelium upon acute injury. Lab. Invest. 78:477–483. [PubMed] [Google Scholar]
- Hayakawa, T., A. Matsuzawa, T. Noguchi, K. Takeda, and H. Ichijo. 2006. The ASK1-MAP kinase pathways in immune and stress responses. Microbes Infect. 8:1098–1107. [DOI] [PubMed] [Google Scholar]
- Hippo, Y., H. Taniguchi, S. Tsutsumi, N. Machida, J.M. Chong, M. Fukayama, T. Kodama, and H. Aburatani. 2002. Global gene expression analysis of gastric cancer by oligonucleotide microarrays. Cancer Res. 62:233–240. [PubMed] [Google Scholar]
- Ichijo, H., E. Nishida, K. Irie, P. ten Dijke, M. Saitoh, T. Moriguchi, M. Takagi, K. Matsumoto, K. Miyazono, and Y. Gotoh. 1997. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 275:90–94. [DOI] [PubMed] [Google Scholar]
- Ito, M., and K. Kizawa. 2001. Expression of calcium-binding S100 proteins A4 and A6 in regions of the epithelial sac associated with the onset of hair follicle regeneration. J. Invest. Dermatol. 116:956–963. [DOI] [PubMed] [Google Scholar]
- McElwee, K.J., A. Huth, S. Kissling, and R. Hoffman. 2004. Macrophage-stimulating protein promotes hair growth ex vivo and induces anagen from telogen stage hair follicles in vivo. J. Invest. Dermatol. 123:34–40. [DOI] [PubMed] [Google Scholar]
- Morris, R.J., and C.S. Potten. 1999. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J. Invest. Dermatol. 112:470–475. [DOI] [PubMed] [Google Scholar]
- Muller-Rover, S., B. Handjiski, C. van der Veen, S. Eichmuller, K. Foitzik, I.A. McKay, K.S. Stenn, and R. Paus. 2001. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 117:3–15. [DOI] [PubMed] [Google Scholar]
- Nishitoh, H., M. Saitoh, Y. Mochida, K. Takeda, H. Nakano, M. Rothe, K. Miyazono, and H. Ichijo. 1998. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell. 2:389–395. [DOI] [PubMed] [Google Scholar]
- Paus, R., C. van der Veen, S. Eichmuller, T. Kopp, E. Hagen, S. Muller-Rover, and U. Hofmann. 1998. Generation and cyclic remodeling of the hair follicle immune system in mice. J. Invest. Dermatol. 111:7–18. [DOI] [PubMed] [Google Scholar]
- Sayama, K., Y. Hanakawa, Y. Shirakata, K. Yamasaki, Y. Sawada, L. Sun, K. Yamanishi, H. Ichijo, and K. Hashimoto. 2001. Apoptosis signal-regulating kinase 1 (ASK1) is an intracellular inducer of keratinocyte differentiation. J. Biol. Chem. 276:999–1004. [DOI] [PubMed] [Google Scholar]
- Sayama, K., H. Komatsuzawa, K. Yamasaki, Y. Shirakata, Y. Hanakawa, K. Ouhara, S. Tokumaru, X. Dai, M. Tohyama, P. Ten Dijke, et al. 2005. New mechanisms of skin innate immunity: ASK1-mediated keratinocyte differentiation regulates the expression of beta-defensins, LL37, and TLR2. Eur. J. Immunol. 35:1886–1895. [DOI] [PubMed] [Google Scholar]
- Sekine, Y., K. Takeda, and H. Ichijo. 2006. The ASK1-MAP kinase signaling in ER stress and neurodegenerative diseases. Curr. Mol. Med. 6:87–97. [DOI] [PubMed] [Google Scholar]
- Stenn, K.S., and R. Paus. 2001. Controls of hair follicle cycling. Physiol. Rev. 81:449–494. [DOI] [PubMed] [Google Scholar]
- Taylor, G., M.S. Lehrer, P.J. Jensen, T.T. Sun, and R.M. Lavker. 2000. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 102:451–461. [DOI] [PubMed] [Google Scholar]
- Tobiume, K., A. Matsuzawa, T. Takahashi, H. Nishitoh, K. Morita, K. Takeda, O. Minowa, K. Miyazono, T. Noda, and H. Ichijo. 2001. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]