Abstract
During mitosis, the inner centromeric region (ICR) recruits protein complexes that regulate sister chromatid cohesion, monitor tension, and modulate microtubule attachment. Biochemical pathways that govern formation of the inner centromere remain elusive. The kinetochore protein Bub1 was shown to promote assembly of the outer kinetochore components, such as BubR1 and CENP-F, on centromeres. Bub1 was also implicated in targeting of Shugoshin (Sgo) to the ICR. We show that Bub1 works as a master organizer of the ICR. Depletion of Bub1 from Xenopus laevis egg extract or from HeLa cells resulted in both destabilization and displacement of chromosomal passenger complex (CPC) from the ICR. Moreover, soluble Bub1 controls the binding of Sgo to chromatin, whereas the CPC restricts loading of Sgo specifically onto centromeres. We further provide evidence that Bub1 kinase activity is pivotal for recruitment of all of these components. Together, our findings demonstrate that Bub1 acts at multiple points to assure the correct kinetochore formation.
Introduction
Attachment of chromosomes to spindle microtubules (MTs) is performed by kinetochores, which are large proteinaceous structures that assemble at the centromeric regions of each sister chromatid. According to the topology of its components, the kinetochore may be subdivided to two domains: the outer kinetochore (OKt) and the inner centromeric region (ICR). The OKt consists of several electron-dense zones, and it contains proteins that are involved in MT capture and regulation of the spindle assembly checkpoint (Andrews et al., 2003; Cleveland et al., 2003; Chan et al., 2005). The ICR is positioned between sister centromeres, and contains protein complexes that are involved in regulation of sister chromatid cohesion and modulation of MT attachment. These proteins include chromosomal passenger complex (CPC), mitotic centromere-associated kinesin (MCAK), and Shugoshin (Sgo; Maiato et al., 2004; Rivera and Losada, 2006). The CPC consists of Aurora B, INCENP, Survivin, and Dasra/Borealin (Vagnarelli and Earnshaw, 2004). Aurora B phosphorylates and inhibits the MT depolymerase MCAK, thus, controlling the polymerization/depolymerization state of tubulin filaments to achieve correct end-on attachment of MTs to the kinetochore (Andrews et al., 2004; Lan et al., 2004). The inner centromere protein (INCENP) subunit of the CPC can directly bind MTs, and a recent study suggests that the CPC can function as a bridge between the centromere and kinetochore MTs (Sandall et al., 2006). The CPC itself localizes along chromosomes in prophase, and then concentrates at the ICR in prometaphase and metaphase (Andrews et al., 2003). The mechanism that regulates CPC relocalization is unknown. Sgo plays critical roles in both cohesion and MT dynamics during metazoan mitosis. Sgo functions as an adaptor protein for phosphatase PP2A, recruiting it to the ICR. PP2A dephosphorylates SA2 subunit of the cohesin complex, preventing the latter from Plk1-dependent release during the G2–M transition, thus, maintaining centromeric cohesion until anaphase (Kitajima et al., 2006; Riedel et al., 2006). Localization of Sgo has been reported to depend on Bub1 activity (Kitajima et al., 2005).
Bub1 was first isolated in a screen for budding yeast mutants that were sensitive to benomyl, which is an inhibitor of MT polymerization (Hoyt et al., 1991). It was later characterized as a protein kinase that is involved in spindle checkpoint response in yeast and in vertebrates (Roberts et al., 1994; Taylor and McKeon, 1997). Bub1 is not only involved in control of the checkpoint (Tang et al., 2004a), but also regulates the loading of spindle checkpoint proteins to kinetochores. Bub1 is recruited to centromeres early in prophase and promotes binding of Plx1, BubR1, Mad1, Mad2, Cenp-E, and Cenp-F to the OKt (Sharp-Baker and Chen, 2001; Johnson et al., 2004; Wong and Fang, 2006). Interestingly, recruitment of these proteins does not require Bub1 kinase activity, suggesting that Bub1 plays a structural role in organization of the OKt. However, yeast Bub1 has additional functions in chromosome segregation that are independent of its ability to recruit the OKt components (Warren et al., 2002; Vanoosthuyse et al., 2004). Recent studies suggested that Bub1 kinase may play a role in localization of Sgo in the ICR, thus providing a possible link between Bub1 kinase activity and chromosome segregation (Kitajima et al., 2005).
We decided to analyze whether Bub1's function in ICR assembly is restricted to Sgo targeting. We show that Bub1 kinase works as a master organizer of the ICR in both Xenopus laevis egg extracts and mammalian cells. Bub1 controls both stability and correct positioning of the CPC to the ICR in a kinase-dependent manner. Moreover, we find that soluble Bub1 kinase mediates binding of Sgo to mitotic chromatin, whereas CPC directs relocalization of chromatin-bound Sgo specifically to the ICR. Together, our results indicate that Bub1's dual role in Sgo and CPC targeting to the ICR represents a novel and important new paradigm for its action at multiple levels of kinetochore assembly.
Results
Bub1 controls CPC localization
To assess a precise role of Bub1 in kinetochore formation, we immunodepleted Bub1 from meiotically arrested (cytostatic factor [CSF]) X. laevis egg extracts (Kornbluth, 2001). Quantitative Western blotting showed that immunodepletion removed Bub1 to undetectable levels (Figs. 1 A and S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200609044/DC1). As reported earlier (Sharp-Baker and Chen, 2001; Vigneron et al., 2004), we found that a sperm chromatin directly assembled into condensed chromosomes within Bub1-depleted CSF extracts was essentially devoid of BubR1 and the Dynein–Dynactin complex (Fig. S1 B and not depicted). To observe the effect of Bub1 depletion upon replicated chromatin (Maresca and Heald, 2006), we added sperm nuclei to mock- or Bub1-depleted CSF-arrested extracts that had been driven into interphase through the addition of 0.06 mM CaCl2. After completion of DNA replication, mitosis was induced with a fresh aliquot of corresponding CSF extract. Under these circumstances, although Bub1 depletion caused a substantial reduction in the kinetochore recruitment of BubR1, Mad2, Bub3, and Dynein–Dynactin complex in the chromatin-bound fraction, small amounts of these proteins were generally visible on kinetochores (Figs. 1 A and S1 C). This residual population may reflect a difference in the organization of the unreplicated and replicated sperm chromatin assembled in X. laevis extract (Sharp-Baker and Chen, 2001; Vigneron et al., 2004). Interestingly, depletion of Bub1 by RNAi in somatic cells similarly inhibited, but did not eliminate, recruitment of Mad2, CENP-E, and BubR1 to kinetochores (Ditchfield et al., 2003; Johnson et al., 2004). These findings support previous conclusions that Bub1 has an important role in kinetochore formation, and suggest that this function is modulated by the status of the mitotic chromatin.
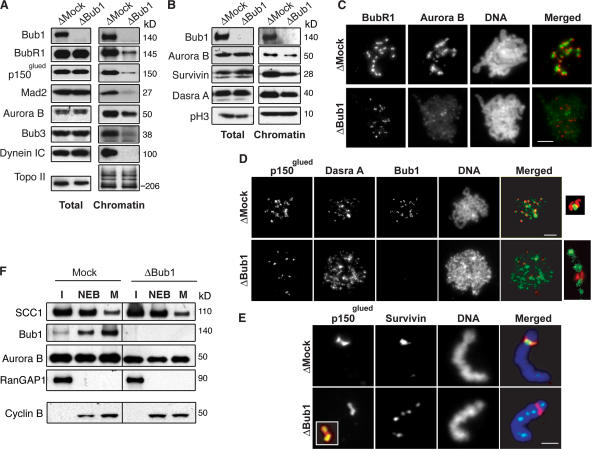
Figure 1.
Bub1 controls the localization of the CPC at the ICR in X. laevis egg extracts. Mock- or Bub1-depleted CSF extracts containing 1,000 sperm/μl were driven into interphase. After 60 min, mitosis was reestablished by the addition of an aliquot of corresponding CSF-arrested extracts containing nocodazole. (A and B) Samples of total extracts or purified mitotic chromatin were analyzed by Western blotting with antibodies against indicated proteins. (C and D) Chromatin assembled as in A was purified and analyzed by indirect immunofluorescence with antibodies against Aurora B and BubR1; Dasra A and p150glued and Bub1 (unmerged). DNA was visualized with Hoechst 33342 (unmerged). Insets show magnified image of individual kinetochore pairs. In the case of Bub1-depleted extracts, note that the acquired signals for Aurora B, BubR1, and Dasra A were slightly overexposed during the processing (see the text for explanation). (E) Individual chromosomes stained with antibodies against Survivin and p150glued are shown. Inset shows magnified image of the same kinetochore stained with antibodies against CENP-A (green) and p150glued (red). (F) Chromatin assembled in mock- or Bub1-depleted extracts was purified at interphase (I, 50 min after initiation), NEB (10 min after initiation of mitosis), and metaphase (M, 30 min after initiation of mitosis), and then probed for the presence of indicated proteins by Western blotting. Antibody against RanGap1 was used to detect NEB. Total lysates of egg extracts were probed with antibody against Cyclin B to estimate stability of mitosis (bottom). Bars: (C and D) 20 μm; (E) 3 μm.
It has been reported that Bub1 also regulates the recruitment of some (Kitajima et al., 2005), but not all (Johnson et al., 2004; Meraldi and Sorger, 2005), proteins associated with the ICR of the kinetochore. Therefore, we were curious to also examine the loading of proteins associated with the ICR. Surprisingly, we observed that Bub1 depletion reduced the amount of Aurora B and other CPC components (Survivin and Dasra A) into the chromatin fraction (Fig. 1, A and B), although no changes in the concentration of any CPC constituents were observed in total extracts. We examined the localization of the residual CPC bound to chromatin in the absence of Bub1. Although the amounts of BubR1 and p150glued were reduced after Bub1 depletion (Fig. 1, C and D), they were still conspicuous and properly positioned at kinetochores according to their colocalization with the centromeric protein CENP-A (Fig. S1 C and not depicted), thereby allowing the use of BubR1 and p150glued as kinetochore markers in Bub1-depleted extracts. In mock-depleted extract, Aurora B localized precisely on the ICR and partially colocalized with BubR1. Remarkably, Aurora B staining was no longer juxtaposed to BubR1 in Bub1-depleted extracts, suggesting that it was not properly targeted to the ICR (Fig. 1 C). Immunofluorescent analysis of Survivin and Dasra A localization in Bub1-depleted extracts similarly showed that these CPC components were displaced from kinetochore markers (Fig. 1, D and E). Notably, depletion of Bub1-related checkpoint kinase, BubR1, caused no changes in the Aurora B staining pattern (Fig. S2, B and C, available at http://www.jcb.org/cgi/content/full/jcb.200609044/DC1), showing that CPC localization is specifically regulated by Bub1.
Because Aurora B binds to chromosome arms during prophase (Vagnarelli and Earnshaw, 2004), we wished to test whether CPC mislocalization in Bub1-depleted extracts was a result of prophase-like arrest. To do this, we assayed whether Cohesin was released normally from chromosomes in extracts lacking Bub1. Cohesin complexes largely dissociate from chromosome arms at prophase/prometaphase, but a small portion is retained at centromeres (Sumara et al., 2000; Losada et al., 2002). To monitor Cohesin dynamics, we isolated chromatin at three different stages: at interphase (at the end of DNA replication), at nuclear envelope breakdown (NEB), and at metaphase (30 min after induction of mitosis), and probed it for the presence of Scc1, which is a component of the cohesin complex. As expected, the levels of chromatin-bound Scc1 gradually decreased throughout progression from interphase to metaphase in mock-depleted extracts (Fig. 1 F). The dynamics of dissociation and the levels of Scc1 bound to metaphase chromosomes were indistinguishable in mock- and Bub1-depleted extr acts, indicating that depletion of Bub1 does not affect prophase–prometaphase transition. Collectively, our results suggest Bub1 depletion disrupts metaphase recruitment of the CPC to the ICR in X. laevis egg extracts, but that this disruption does not reflect a defect in cell cycle progression.
To test whether Bub1 plays a comparable role in other systems, we depleted Bub1 from HeLa cells by RNAi and analyzed distribution of Aurora B in control (Lamin A/C RNAi) and Bub1-depleted cells. 24–48 h after transfection of siRNA duplexes, the cells were incubated with nocodazole for 1 h, immediately followed by fixation and staining. In control mitotic cells, Aurora B consistently localized to the ICR, as expected (Fig. 2 A, top). In contrast, cells treated with Bub1 siRNA showed mislocalization of Aurora B (Fig. 2 A, middle and bottom), similar to the displacement that we observed in X. laevis egg extracts lacking Bub1. Analysis of individual chromosomes indicated that Aurora B localized along the chromosome arms in the absence of Bub1, and did not display considerable colocalization with centromeric antigens (CREST; Fig. 2 B). Also consistent with our observation that CPC recruitment is quantitatively reduced in Bub1-depleted egg extracts, the intensity of Aurora B staining was also reduced in Bub1-depleted cells (Fig. 2 A). Similar results were obtained using HeLa cells stably expressing Survivin-GFP; 24 h after Bub1 siRNA transfection, most prometaphase cells showed dispersed distribution of Survivin along chromosomes arms, whereas cells treated with Lamin A/C siRNA localized Survivin-GFP preferentially to the ICR (Fig. 2 C). Thus, it appears that the role of Bub1 in CPC recruitment to the ICR may be a general feature of metazoan systems.
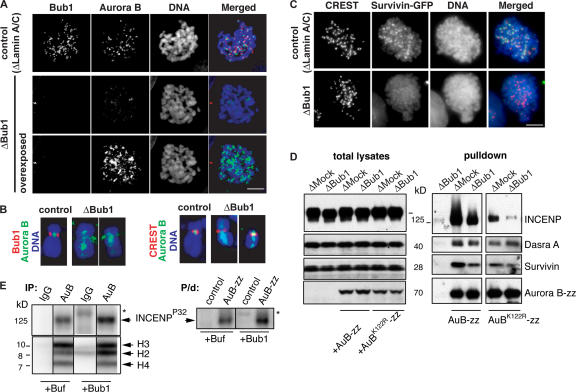
Figure 2.
Bub1 is required for the inner centromeric localization of CPC in somatic cells. HeLa cells were transfected with Lamin A/C or Bub1 siRNA duplexes for 24 h and treated with nocodazole for 1 h before fixation. (A) Cells were stained to detect Bub1 (red) and Aurora B (green). (B) Individual chromosomes are shown in higher magnification to allow comparison of the Aurora B staining pattern to that of Bub1 or to the centromere marker (CREST). DNA was visualized as in Fig. 1. Note that the acquired signal of Aurora B staining in Bub1-depleted cells was overexposed. (C) HeLa cells stably expressing GFP-Survivin were treated as in A, and the position of GFP signal pattern was compared with that of CREST. (D) Bub1 is required for stability of CPC. Recombinant wtAurora B-zz or Aurora BK122R–zz were added to control or Bub1-depleted CSF extracts at a concentration approximately equal to that of endogenous Aurora B, and CPC complexes that formed on Aurora B-zz were purified by IgG–Sepharose beads. Total extracts (left) and eluates (right) were probed for the presence of CPC components. (E) Bub1 phosphorylates INCENP. CPC was either precipitated from Bub1-depleted egg extracts using antibodies against Aurora B (IP; left) or was purified on IgG–Sepharose from extracts, supplemented with Aurora B-zz (P/d, right). Mock-treated beads and beads containing precipitates were incubated with baculovirus-expressed 6His-xBub1 (50 μM) or buffer in the presence of γ[32P]ATP. Phosphate incorporation was detected using a PhosphorImager. The asterisks show the position of 6His-xBub1 (top). Immunoprecipitates (as in Fig. 1) were also incubated with core histones as exogenous substrates in the presence or absence of 6His-xBub1 to monitor Aurora B activity (bottom).
Bub1 modulates CPC stability
We reasoned that the decreased association of the CPC to chromatin in egg extracts or in cells lacking Bub1 might be linked to some properties of the complex that were altered in the absence of Bub1. Notably, depletion of Bub1 from egg extracts did not affect phosphorylation of histone H3, which is a well-known substrate of Aurora B (Murnion et al., 2001), arguing that mislocalized Aurora B was not inactivated as a kinase (Fig. 1 B).
To test whether CPC stability was compromised, we produced recombinant xAurora B fused with a zz tag (Aurora B-zz) by translation of its mRNA in Aurora B (CPC)-depleted egg extracts. Recombinant protein was added to control or Bub1-depleted CSF extracts at concentration approximately equal to that of endogenous Aurora B, and CPC complexes that formed on Aurora B-zz were purified by IgG–Sepharose beads. Extracts lacking Bub1 did not promote efficient binding of CPC constituents, such as INCENP, Survivin, and Dasra A, to Aurora B-zz beads (Fig. 2 D). CPC stability is regulated by Aurora B kinase activity (Honda et al., 2003). To understand whether impaired CPC formation is mediated by Bub1-dependent modulation of Aurora B itself, we performed the same kind of assay, but using a kinase-dead version of Aurora B (Aurora BK122R-zz) as bait. Both Survivin and INCENP bound to Aurora BK122R-zz less efficiently than to Aurora B-zz, as expected. However, the absence of Bub1 further exacerbated CPC formation so that Survivin and INCENP became barely detectable on Aurora BK122R-zz beads (Fig. 2 D). These data suggest that Bub1 controls CPC stability in a manner that is independent of Aurora B activity. It is formally possible that in the absence of Bub1, CPC becomes more stable; this is an alternative explanation for why exogenous Aurora B-zz accumulated less CPC components (Fig. 2 D). However, because it is known that down-regulation of the single CPC component compromises residual CPC recruitment to the chromatin (Gassmann et al., 2004; Sampath et al., 2004), the phenomena that resembles our observations in Bub1-depleted extracts (Figs. 1 and 2), we believe that Bub1 stabilizes the CPC complex. Because Bub1 controls stability of the CPC, we reasoned that Bub1 might phosphorylate one or several of its subunits. To address this issue, we performed a kinase assay using recombinant Bub1 and CPC purified from Bub1-depleted extracts by immunoprecipitation (Fig. 2 E, left) or by Aurora B-zz pulldown (Fig. 2 E, right). The INCENP subunit of the CPC is phosphorylated by Aurora B in the absence of Bub1 (Fig. 2 E; Honda et al., 2003). Strikingly, however, addition of Bub1 enhanced INCENP phosphorylation levels (Fig. 2 E). Only the INCENP subunit of the CPC appears to be phosphorylated by Bub1, as we could not detect any [32P]-containing band corresponding to Dasra, Survivin, or Aurora B (unpublished data). Moreover, Bub1 appears to phosphorylate INCENP directly because similar assays that also included core histones as substrates showed that the level of histone H3 phosphorylation was not affected by the presence of Bub1, arguing that Bub1 does not stimulate Aurora B activity (Fig. 2 E). Our results suggest that Bub1 controls stability of the CPC by phosphorylating its INCENP subunit.
Bub1 kinase activity is essential for the targeting of CPC to the inner centromere
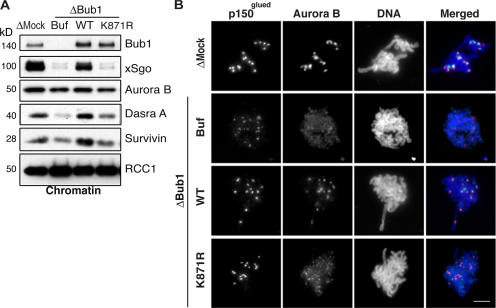
Bub1 recruits several proteins onto kinetochores through protein–protein interactions that are independent of its kinase activity (Sharp-Baker and Chen, 2001). To determine whether Bub1 kinase activity is similarly dispensable for CPC localization, we expressed wild-type Bub1 and a kinase-dead mutant (Bub1K871R; Sharp-Baker and Chen, 2001). Recombinant Bub1 and Bub1K871R were added to Bub1-depleted extracts. Consistent with previous observations, we found that Bub1 kinase activity was not required for restoration of BubR1, Mad2, or p150glued recruitment to kinetochores (Fig. 3 B and not depicted; Sharp-Baker and Chen, 2001). Moreover, exogenous wild-type Bub1 quantitatively restored recruitment of Aurora B, Dasra A, and Survivin to chromatin, as well as their localization to the ICR (Fig. 3, A and B; and not depicted). In striking contrast to its ability to restore localization of the OKt components, however, Bub1K871R failed to rescue mislocalization of CPC components caused by Bub1 depletion (Fig. 3, A and B). These observations indicate that Bub1 kinase activity is absolutely necessary for the regulation of CPC localization during prometa- and metaphase. Collectively, our results demonstrate that Bub1 controls localization of the chromosome passenger complex in the ICR during mitosis in vertebrates in a kinase-dependent manner.
Figure 3.
Bub1 kinase activity is required for ICR assembly. (A and B) Bub1-depleted extracts were supplemented either with buffer (Buf) or with in vitro–translated wild-type (WT) or kinase-dead (K871R) versions of X. laevis Bub1. (A) Mitotic chromatin assembled in these and mock-depleted extracts were analyzed for the abundance of Bub1, Sgo, and the components of the CPC by Western blotting. Antibody against RCC1 was used as a loading control. (B) Assembled chromatin was analyzed by indirect immunofluorescence with antibodies against Aurora B (green), p150glued (red), and Bub1 (blue). Bar, 20 μm.
Bub1 is required for the centromeric localization of MCAK and Sgo
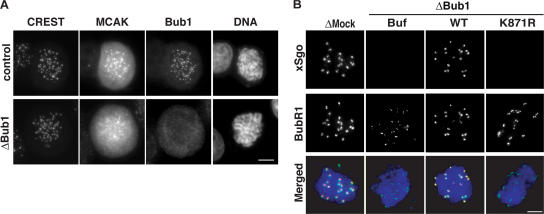
We examined two additional ICR components, MCAK and Sgo, to assess whether ICR structure was generally disrupted in the absence of Bub1, or whether this effect was limited to CPC. Remarkably, Bub1 depletion by RNAi caused a substantial reduction in the amount of MCAK associated with kinetochores (Fig. 4 A). Because localization of MCAK to the ICR depends on Aurora B activity (Andrews et al., 2004; Lan et al., 2004; Ohi et al., 2004), it is highly possible that this defect in its recruitment is a secondary consequence of Aurora B displacement. In this scenario, it is notable that Aurora B kinase activity alone appears to be insufficient for MCAK targeting, suggesting that CPC localization and/or interactions among CPC members may also be critical for its full biological function.
Figure 4.
Bub1 controls localization of MCAK and xSgo to the ICR. (A) Cells treated as in Fig. 2 were stained with antibodies against Bub1, MCAK, and CREST. (B) Chromatin assembled as in Fig. 3 was analyzed by indirect immunofluorescence with antibodies against xSgo (red) and anti-BubR1 (green). DNA was stained as in A (blue).
Bub1 is essential for kinetochore localization of Sgo in both yeast and mammals (Kitajima et al., 2004; Tang et al., 2004b). It has also been shown that Bub1 kinase activity is required in fission yeast for the centromeric localization of spSgo1 and spSgo2 (Kitajima et al., 2004). Consistent with these studies, removal of Bub1 from X. laevis egg extract prevented binding of X. laevis Sgo (xSgo) to mitotic chromatin (Fig. 4 B), although total xSgo levels within depleted extract remained unchanged. We further sought to determine whether Sgo targeting required Bub1 kinase activity, as the CPC does, or is kinase-independent, as is the case for OKt components. Remarkably, the ability of xSgo to bind mitotic chromatin in Bub1-depleted extracts could be rescued by addition of wild-type Bub1, but not Bub1K871R (Figs. 4 B). In combination with our earlier finding on the CPC (Fig. 3), these data strongly argue that Bub1 kinase activity is critical for general organization of a functional ICR.
Soluble Bub1 kinase activity is sufficient for Sgo loading onto mitotic chromatin
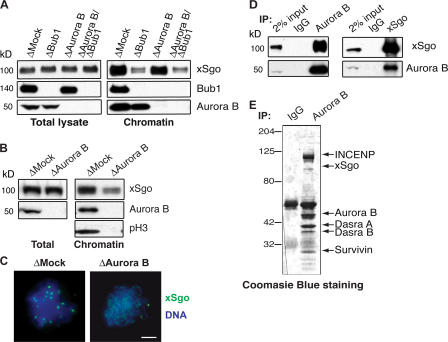
Because it is the kinase activity of Bub1 that is generally required for targeting of ICR components, we wondered whether Bub1 itself must localize at kinetochores to perform its function. Depletion of Aurora B from egg extracts has been reported to prevent Bub1 binding to chromosomes (Vigneron et al., 2004). We confirmed this observation and examined Bub1 after Aurora B depletion (Fig. 5 A and not depicted). We found that neither its total levels nor its kinase activity were substantially affected. In the absence of Aurora B, xSgo still bound to chromatin, albeit at slightly reduced levels (Figs. 5 A and S2 B). As expected, simultaneous depletion of Aurora B and Bub1 resulted in loss of xSgo from mitotic chromatin, suggesting that Aurora B depletion does not bypass the requirement for Bub1 in recruiting xSgo to chromatin (Fig. 5 A). These data demonstrate that Aurora B and other CPC components are not required for recruitment of xSgo to chromatin. Additionally, they indicate that although Bub1 kinase activity is essential for xSgo recruitment to mitotic chromatin, its own association to chromosomes is dispensable.
Figure 5.
Soluble Bub1 is able to promote xSgo binding to the mitotic chromatin, whereas the CPC directs xSgo to the ICR. (A) Total extracts and mitotic chromatin purified from control extracts or extracts lacking Bub1, Aurora B, or both Bub1 and Aurora B, were analyzed by Western blotting with indicating antibodies. (B–E) Mock- or Aurora B-depleted extracts were treated as in Fig. 1 A and analyzed by Western blotting with the indicated antibodies (B) or by immunofluorescence with antibodies against xSgo (C; green). DNA, stained as in Fig. 1, is shown in blue. (D) Aurora B and xSgo were immunoprecipitated from CSF-arrested egg extracts by the corresponding antibodies. Immunoprecipitates were subjected to Western blotting with antibodies against Aurora B and xSgo. (E) Chromosome passenger complex precipitated with anti–Aurora B antibodies was analyzed by SDS-PAGE and CBB staining. Arrows indicate the positions of the CPC components and xSgo.
CPC targets Sgo onto the inner centromere
We examined whether xSgo loaded onto chromatin in the absence of CPC was correctly localized. In contrast to the well- defined kinetochore staining of xSgo in control extracts, xSgo was diffusely distributed throughout chromosomes assembled in CPC-depleted extracts (Fig. 5 C). Although Bub1 was thus sufficient to establish xSgo chromatin binding, the CPC appears to be essential for restriction of xSgo to the ICR. To determine whether the capacity of the CPC to restrict xSgo to the ICR might involve direct interactions, we made reciprocal immunoprecipitation using Aurora B and xSgo antibodies. We found that xSgo could be coprecipitated using anti-Aurora B antibody and Aurora B could be coprecipitated with xSgo (Fig. 5, D and E), clearly demonstrating that the CPC and xSgo interact with each other. Notably, xSgo did not appear to be a stochiometric component of the CPC on Coomassie-stained gels (Fig. 5 E). In addition, we could co-deplete neither xSgo from egg extracts through Aurora B depletion nor Aurora B through xSgo (Fig. 5 B and Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.2006090r4/DC1).
Our findings suggest that both Bub1 and CPC regulate localization of xSgo. To determine whether there is a feedback loop between xSgo and Bub1 or Aurora B, we depleted xSgo from egg extract. Depletion of xSgo affected neither Aurora B nor Bub1 levels on chromatin. Moreover, kinetochores devoid of xSgo were still able to recruit Aurora B and Bub1 (Fig. S3). These results support the argument that xSgo is strictly a downstream target for both Bub1 and CPC, rather than a component of a regulatory loop.
Formation of the inner and outer centromere occurs independently of chromatin condensation
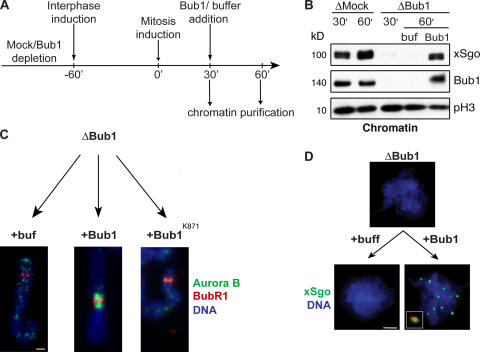
We had observed that addition of recombinant Bub1 to Bub1-depleted egg extracts, either at the start of reaction (Fig. 3 B) or at the induction of mitosis (not depicted), fully rescued CPC and xSgo localization. Finally, we wished to determine the execution point of Bub1's role in ICR assembly, and, specifically, whether Bub1's kinase activity is essential during the interval of mitotic chromosome assembly. To answer this question, we first assembled fully condensed mitotic chromosomes in Bub1-depleted extracts, and then added recombinant Bub1 or Bub1K871R (Fig. 6 A).
Figure 6.
The assembly of the ICR is independent of mitotic chromosome formation. (A) Schematic overview of the experiment. Mock- or Bub1-depleted extracts were driven into interphase. After 60 min, mitosis was reestablished by addition of an aliquot of mock- or Bub1-depleted CSF-arrested extracts together with nocodazole. 30 min after induction of mitosis, baculovirus-expressed 6His-tagged wtBub1, Bub1K871R, or buffer were added, followed by incubation for an additional 30 min. (B) Chromatin was analyzed by Western blotting for the abundance of xSgo, Bub1, and phosphorylated histone H3 (pH3). (C and D) Purified chromatin was stained with antibody against Aurora B (C, green) and BubR1 (C, red) or xSgo (D, green). DNA was visualized as in Fig. 1 (blue). The inset in D shows a magnified image of an individual kinetochore pair stained with antibody against Bub1 (red) and xSgo (green). Bars: (C) 2 μm; (D) 20 μm.
Consistent with our previous results, Aurora B was displaced from the ICR and xSgo was unable to bind to the mitotic chromatin in Bub1-depleted extracts. Moreover, even allowing a prolonged interval for mitotic chromatin assembly did not cause accumulation of Aurora B at the ICR and loading of xSgo onto chromatin in the extracts lacking Bub1 (Fig. 6, C and D). Addition of exogenous wild-type Bub1, but not Bub1K871R, to egg extracts 30 min after mitotic induction also rescued proper localization of Aurora B and xSgo in the ICR (Fig. 6). These findings clearly demonstrate that Bub1 can promote the formation of the ICR in preformed mitotic chromosomes. Furthermore, they suggest that the assembly of the ICR is independent of many other aspects of chromosome condensation.
Discussion
We have shown that Bub1 plays a central role in ICR formation, acting at multiple points in this assembly pathway. First, Bub1 controls CPC localization to the ICR. In the absence of Bub1, the CPC can bind to chromosome arms, albeit with reduced efficiency, but it does not become associated to the ICR (Figs. 1 and 2). Although the activity of Aurora B as a histone H3 kinase was not lost under these circumstances, the stability of the CPC was markedly altered (Fig. 2). Second, as in earlier studies, we found that Bub1 mediates xSgo recruitment to the ICR (Figs. 3 and 4). In addition, we found that Bub1 acts primarily by promoting xSgo binding to mitotic chromatin (Fig. 5); Bub1 can accomplish this function even when it is not being stably associated to mitotic chromosomes or kinetochores. Third, in contrast to chromatin binding of xSgo, Bub1 by itself is insufficient to direct xSgo to the ICR in the absence of the CPC (Fig. 5). Together, these findings suggest that Bub1 regulates localization of ICR components through mechanisms that are both CPC-dependent and -independent. Remarkably, we find that Bub1's kinase activity is essential for all of its roles in ICR assembly. It is also notable that Bub1 kinase can accomplish its essential roles in ICR formation in a manner that is not coupled to chromosome condensation or the OKt formation because it was able to fully restore ICR assembly on completely condensed replicated chromosomes (Fig. 6).
Our data suggest that Bub1 plays an indispensable role in localizing the CPC to the ICR. In X. laevis egg extracts, Bub1 depletion completely prevented CPC recruitment to the ICR in a manner that could be fully rescued with wild-type Bub1, but not with kinase-dead mutant Bub1 (Fig. 3). We similarly observed mistargeting of the CPC to chromosome arms in prometaphase-arrested HeLa cells that had been depleted of Bub1 through RNAi (Fig. 2), arguing that Bub1's role in controlling CPC distribution may be a general feature of metazoan systems. Notably, our results do not agree with those of earlier studies, which concluded that the CPC could localize to centromeres in a Bub1-independent manner (Johnson et al., 2004; Meraldi and Sorger, 2005). There are two possible sources of this discrepancy. First, our finding that soluble Bub1 can promote xSgo localization suggests that it does not need to achieve a high level of kinase activity on kinetochores to execute this function. If a limited level of soluble (or kinetochore-associated) Bub1 activity is able to promote CPC recruitment, then partial RNAi-mediated depletion of Bub1 should not cause redistribution of the CPC. Indeed, we were also able to find cells that had substantially reduced levels of Bub1 on kinetochores after Bub1 siRNA, but which contained several chromosomes with the proper localization of Aurora B in the ICR, as might be expected in this case (unpublished data). Second, even after depletion of Bub1 to immeasurable levels, we continue to observe loading of the CPC throughout chromosome arms in both X. laevis egg extracts and HeLa cells (Figs. 1 and 2), implying that recruitment of the CPC to prophase chromosomes is independent of Bub1. Because costaining with centromere markers was not provided in the earlier studies, it is conceivable that arm-associated foci of CPC staining might have been incorrectly attributed to ICR- associated populations.
Our results strongly suggest that formation of the OKt and the ICR differs by their sensitivity to Bub1's activity. Recruitment of such OKt components as Plx1, BubR1, Mad1, Mad2, Cenp-E, and Cenp-F depends on Bub1 itself, but not on its kinase activity (Sharp-Baker and Chen, 2001; Johnson et al., 2004; Wong and Fang, 2006). One notable exception is Mps1, whose localization to the OKt is controlled by Bub1's kinase function (Wong and Fang, 2006). On the other hand, localization of all of the ICR elements tested (CPC and xSgo) absolutely requires Bub1's kinase activity (Figs. 1–4 ). Together, our data indicate that initiation of OKt assembly relies on physical interaction of their elements with Bub1, but that the formation of the functional ICR requires only kinase activity of Bub1.
Our result that Sgo localization requires both Bub1 and Aurora B is consistent with data obtained in other model systems (Tang et al., 2004b; Kitajima et al., 2005; Resnick et al., 2006; Riedel et al., 2006). However, our findings address several key issues that were not predicted. First, we show that Bub1 mediates binding of xSgo to the mitotic chromatin, by itself, not to the kinetochore. Second, we show that soluble Bub1 kinase can promote binding of xSgo to mitotic chromatin, whereas Aurora B (CPC) directs chromatin-bound xSgo to the ICR. It is also worth mentioning that localization of both Aurora B (CPC) and Bub1 to centromeres depend on each other (Figs. 1, 2, and 5; Johnson et al., 2004). Based on these observations, we would like to propose a scheme for Bub1-mediated events in kinetochore assembly. During prophase, Aurora B kinase initiates kinetochore formation, probably by phosphorylation of centromeric proteins like CENP-A (Zeitlin et al., 2001). This results in recruitment of Bub1 onto kinetochores and assembly of the spindle checkpoint components at the OKt. In return, Bub1 kinase, while soluble or kinetochore bound, controls formation of the ICR by two pathways. First, it promotes relocalization of the CPC from chromosome arms to the ICR. It is feasible that Bub1 controls not only stability of the CPC but also its association with yet unidentified component that is essential for CPC targeting to the ICR (Fig. 2). This idea is supported by the observation that a mixture of recombinant CPC components (Aurora B, Dasra A, INCENP, and Survivin) does not rescue CPC depletion in X. laevis egg extracts (Sampath et al., 2004), implying that the in vivo CPC is built up of more than these four constituents. Second, Bub1 may phosphorylate Sgo or its mitotic chromatin binding sites to promote its recruitment. This chromatin-bound xSgo requires the CPC to further direct its localization at the ICR; then Bub1 itself or Bub1-mediated accumulation of the CPC targets MCAK into the ICR.
In summary, the ICR is a dynamic structure whose assembly is independent from many other aspects of chromosome condensation and is controlled by Bub1 kinase through a web of interactions. Bub1 promotes binding of xSgo to chromatin and mediates relocalization of CPC from chromosome arms.
Materials and methods
Recombinant proteins and antibodies
A cDNA encoding the X. laevis Bub1K871R kinase-dead mutant and X. laevis Aurora BK122R kinase-dead mutant was generated by PCR. Wild-type and kinase-dead mutant of Bub1 were cloned into modified pGEM transcription vector that contains 5′ and 3′ UTR regions of X. laevis β-globin; wild-type and kinase-dead mutant of Aurora B, both fused with zz-tag, were cloned into similarly modified SP6-based vector (both vectors were provided by Y.-B. Shi, National Institutes of Health, Bethesda, MD). RNA transcripts were produced using mMessage mMACHINE T7 or SP6 transcription kit correspondingly (Ambion). Production of proteins in egg extract was performed as previously described (Sharp-Baker and Chen, 2001). His-tagged wild-type xBub1 was expressed in High Five cells (baculovirus was provided by J. Maller, University of Colorado, Denver, CO) and purified as described previously (Schwab et al., 2001).
Antibodies against the following proteins were used: X. laevis Sgo, RCC1, PIASy, and topoisomerase II have been described previously (Saitoh et al., 1996; Azuma et al., 2003, 2005; Salic et al., 2004); Dynein IC (clone 74.1; Abcam); p150glued; Aurora B (Beckman Dickinson); Survivin (R&D Systems); phosphorylated histone H3 (Millipore); X. laevis Cenp-A (either a gift from A. Straight [Stanford University, Stanford, CA] or raised in rabbits against peptide MRPGSTPPSRRKSRPPRRVS-C); X. laevis Bub3 and Mad2 (a gift from R.H. Chen, Institute of Molecular Biology, Taipei, Taiwan); X. laevis Dasra A (a gift from H. Funabiki, The Rockefeller University, New York, NY); X. laevis INCENP (a gift from P.T. Stukenberg, University of Virginia, Charlottesville, VA); and human Bub1 (either a gift from S.S. Taylor [University of Manchester, Manchester, UK] or purchased from Sigma-Aldrich, clone 14H5). CREST sera were a gift from I. Ouspensky (National Institutes of Health, Bethesda, MD). Polyclonal anti–X. laevis Bub1 (aa 274–467), anti–X. laevis Aurora B, anti–X. laevis RanGAP1, anti-Scc1 (EPYSDIIATPGPRFH), anti–X. laevis BubR1 (aa 189–359), anti-hMCAK (C-IQKQKRRSVNSKIPA), and anti-xSgo (aa 1–663) were raised in rabbits or chickens and affinity purified. All secondary antibodies conjugated with Alexa Fluor 488, 568, or 647 were obtained from Invitrogen.
X. laevis egg extract preparation, immunoprecipitation, and immunodepletion
X. laevis sperm nuclei and low-speed extracts of X. laevis eggs arrested by CSF were prepared as previously described (Kornbluth, 2001).
For immunoprecipitation, protein A–conjugated Sepharose beads coupled to corresponding antibodies were prepared. CSF-arrested extract diluted fivefold with CSF-XB buffer (5 mM Hepes-KOH, pH 7.7, 100 mM KCl, 2 mM MgCl2, 10 μm CaCl2, and 5 mM EGTA) supplemented with 20 mM β-glycerophosphate and 10 μg/ml each of leupeptin, pepstatin, and chymostatin was incubated with antibody-coated beads for 1.5 h at 4°C. After incubation, beads were washed four times with CSF-XB buffer supplemented with 20 mM β-glycerophosphate and 0.5% Triton X-100, and the precipitates were eluted from beads by addition of 0.1 M glycine, pH 2.3.
For immunodepletion, protein A–conjugated magnetic beads (Dynal) were incubated overnight with indicated antibodies or rabbit IgG (Vector Laboratories) at 4°C and then covalently coupled using Dimethyl pimelimidate 2 HCl (Pierce Chemical Co.) according to the manufacturer's protocol. Beads were blocked with 10% gelatin hydrolysate (Sigma-Aldrich) in CSF-XB buffer for 20 min, washed with CSF-XB buffer and incubated with extracts for 1 h at 23°C or at 4°C. Beads were removed by magnetic separation, and supernatants were used for the experiments. Interphase was induced by addition of CaCl2 at a final concentration 0.06 mM to CSF-arrested egg extracts. Sperm chromatin was added at concentration 1,000–3,000 nuclei/μl. After DNA replication, 2/3 vol of correspondent CSF-arrested extract was added to induce mitosis. Nocodazole (Sigma-Aldrich) at a final concentration 20 μg/ml was added along with CSF extracts, where indicated.
For pulldown assay, extracts supplemented with either Aurora B-zz or Aurora BK122R-zz were diluted five times with CSF-XB buffer (containing 20 mM β-glycerophosphate and 10 μg/ml LPC) and incubated with IgG–Sepharose beads for 2 h at 4°C. After incubation, beads were washed four times with CSF-XB buffer supplemented with 20 mM β-glycerophosphate and 0.5% Triton X-100, and the precipitates were eluted from IgG–Sepharose beads by addition of 0.1 M glycine, pH 2.3.
For in vitro kinase assays, the CPC was precipitated from Bub1-depleted X. laevis egg extracts using antibody against Aurora B, or purified on IgG–Sepharose through affinity to exogenously added Aurora B-zz. Control beads and beads containing precipitates were incubated with addition of either baculovirus-expressed xBub1 or buffer alone (0.8× CSF-XB buffer containing 20 mM β-glycerophosphate, 1 mM DTT, 5 mM MgCl2, 1 mM ATP, and 1 μCi of γ[32P]ATP). To analyze activity of Aurora B toward exogenous substrates, core histones (a gift from R. Kamakaka, University of California, Santa Cruz, Santa Cruz, CA) were added to the kinase assay reaction at a final concentration of 70 μg/ml. After 35-min incubation at 23°C, the reactions were stopped by the addition of SDS sample buffer. Protein samples were separated by SDS-PAGE and phosphate incorporation was determined by PhosphorImager (GE Healthcare).
Chromatin purification
100-μl aliquots of each reaction were diluted fivefold with 0.8× CSF-XB buffer containing 20 mM β-glycerophosphate, 5% glycerol, and 0.5% Triton X-100, and were incubated for 1 min at RT. The samples were then layered onto a 35% glycerol-containing CSF-XB cushion and centrifuged at 10,000 g for 5 min at 4°C. The pellets were resuspended in the same buffer, and the centrifugation was repeated. 60 μl of SDS-PAGE sample buffer was added to the resulting pellet, and the samples were heated to 100°C for 5 min, followed by vortexing. For purification of interphase chromatin, 100-μl aliquots of extract were diluted with 0.8× CSF-XB buffer containing 20 mM β-glycerophosphate and 5% glycerol and incubated for 1 min at RT, followed by centrifugation through the cushion at 10,000 g for 5 min at 4°C.
For immunofluorescence, 30 μl of each reaction were diluted 10-fold with 0.8× CSF-XB buffer supplemented with 250 mM sucrose. Chromosomes were fixed by addition of an equal volume of the same buffer containing 4% PFA. After 30-min incubation at 23°C, chromosomes were spun down through a 40% glycerol cushion onto glass coverslips. For the staining with anti–CENP-A antibodies, chromatin was fixed by addition of five volumes of 0.8× CSF-XB buffer containing 4% PFA for 5 min, spun down through the cushion, and postfixed with ice-cold methanol for 5 min.
Cell culture and RNAi
HeLa and HeLaEGFP-Survivin cells were cultured in DME containing 10% FBS (BioWest) at 37°C. EGFP-Survivin plasmid was provided by S. Dimitrov (Institut Albert Bonniot, La Tronche Cedex, France). HeLa cells stably expressing EGFP-Survivin were made using Effectene (QIAGEN) according to the manufacturer's protocol. siRNA duplexes designed to repress Lamin A/C (Dharmacon) or Bub1 (corresponding to nt 273–295 of the Bub1 coding region; QIAGEN; Tang et al., 2004a), were transfected using Oligofectamine (Invitrogen) according to the manufacturer's instructions. Cells were analyzed 24–48 h after transfection.
Immunofluorescence and image analysis
Cells on coverslips were washed with PBS containing 1 mM MgCl2 and immediately fixed with 4% PFA. After fixation, cells were washed in TBS-T and permeabilized with 0.2% Triton X-100. Chromatin or cells were blocked with 3% BSA for 30 min, and then stained with corresponded primary antibody for 40 min at RT, followed by the staining with secondary antibodies for 40 min. DNA was counterstained by 4 μg/ml Hoechst 33342 (Sigma-Aldrich). Samples were mounted in medium (Vectashield; Vector Laboratories) and sealed. Specimens were observed by a fluorescent microscope (Axioskop; Carl Zeiss MicroImaging, Inc.) with Iris 100×/1.4 NA objective (Carl Zeiss MicroImaging, Inc.). Images were taken with a charge-coupled device camera (Orca II; Hamamatsu) operated by Openlab software (Improvision). 0.9-μm-wide slices with 0.1 μm distance were taken. Flattened stacks of images were taken for the same exposure and processed in the same manner. For Fig. 2, images were acquired with a LSM 510 Meta system (Carl Zeiss MicroImaging, Inc.). The scale bar is 20 μm throughout, unless otherwise specified.
Online supplemental material
Fig. S1 shows that Bub1 is undetectable in Bub1-depleted X. laevis egg extracts, and that BubR1 does or does not localize to kinetochores in such extracts, depending on the status of mitotic chromatin. Fig. S2 shows that depletion of BubR1 does not affect formation of the ICR and that depletion of Aurora B co-depletes CPC components. Fig. S3 shows that depletion of Sgo does not alter localization of the CPC in the ICR. The online version of this article is available at http://www.jcb.org/cgi/content/full/jcb.200609044/DC1.
Acknowledgments
We thank R.H. Chen, S. Dimitrov, H. Funabiki, R. Kamakaka, J. Maller, I. Ouspensky, Y-B. Shi, P.T. Stuckenberg, and S.S. Taylor for providing reagents.
This work was supported through National Institute of Child Health and Human Development Intramural funds (Z01-HD008740).
Abbreviations used in this paper: CPC, chromosomal passenger complex; CSF, cytostatic factor; ICR, inner centromeric region; INCENP, inner centromere protein; MCAK, mitotic centromere-associated kinesin; MT, microtubule; NEB, nuclear envelope breakdown; OKt, outer kinetochore; Shugoshin, Sgo; xSgo, X. laevis Shugoshin.
References
- Andrews, P.D., E. Knatko, W.J. Moore, and J.R. Swedlow. 2003. Mitotic mechanics: the auroras come into view. Curr. Opin. Cell Biol. 15:672–683. [DOI] [PubMed] [Google Scholar]
- Andrews, P.D., Y. Ovechkina, N. Morrice, M. Wagenbach, K. Duncan, L. Wordeman, and J.R. Swedlow. 2004. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 6:253–268. [DOI] [PubMed] [Google Scholar]
- Azuma, Y., A. Arnaoutov, and M. Dasso. 2003. SUMO-2/3 regulates topoisomerase II in mitosis. J. Cell Biol. 163:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma, Y., A. Arnaoutov, T. Anan, and M. Dasso. 2005. PIASy mediates SUMO-2 conjugation of topoisomerase-II on mitotic chromosomes. EMBO J. 24:2172–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, G.K., S.T. Liu, and T.J. Yen. 2005. Kinetochore structure and function. Trends Cell Biol. 15:589–598. [DOI] [PubMed] [Google Scholar]
- Cleveland, D.W., Y. Mao, and K.F. Sullivan. 2003. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 112:407–421. [DOI] [PubMed] [Google Scholar]
- Ditchfield, C., V.L. Johnson, A. Tighe, R. Ellston, C. Haworth, T. Johnson, A. Mortlock, N. Keen, and S.S. Taylor. 2003. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann, R., A. Carvalho, A.J. Henzing, S. Ruchaud, D.F. Hudson, R. Honda, E.A. Nigg, D.L. Gerloff, and W.C. Earnshaw. 2004. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, R., R. Korner, and E.A. Nigg. 2003. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell. 14:3325–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt, M.A., L. Totis, and B.T. Roberts. 1991. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 66:507–517. [DOI] [PubMed] [Google Scholar]
- Johnson, V.L., M.I. Scott, S.V. Holt, D. Hussein, and S.S. Taylor. 2004. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J. Cell Sci. 117:1577–1589. [DOI] [PubMed] [Google Scholar]
- Kitajima, T.S., S.A. Kawashima, and Y. Watanabe. 2004. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature. 427:510–517. [DOI] [PubMed] [Google Scholar]
- Kitajima, T.S., S. Hauf, M. Ohsugi, T. Yamamoto, and Y. Watanabe. 2005. Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr. Biol. 15:353–359. [DOI] [PubMed] [Google Scholar]
- Kitajima, T.S., T. Sakuno, K. Ishiguro, S. Iemura, T. Natsume, S.A. Kawashima, and Y. Watanabe. 2006. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 441:46–52. [DOI] [PubMed] [Google Scholar]
- Kornbluth, S., J. Yang, and M. Powers. 2001. Analysis of the cell cycle using Xenopus egg extracts. In Current Protocols in Cell Biology. John Wiley and Sons, Inc. 11.11.11–11.11.13. [DOI] [PubMed]
- Lan, W., X. Zhang, S.L. Kline-Smith, S.E. Rosasco, G.A. Barrett-Wilt, J. Shabanowitz, D.F. Hunt, C.E. Walczak, and P.T. Stukenberg. 2004. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 14:273–286. [DOI] [PubMed] [Google Scholar]
- Losada, A., M. Hirano, and T. Hirano. 2002. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 16:3004–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato, H., J. DeLuca, E.D. Salmon, and W.C. Earnshaw. 2004. The dynamic kinetochore-microtubule interface. J. Cell Sci. 117:5461–5477. [DOI] [PubMed] [Google Scholar]
- Maresca, T.J., and R. Heald. 2006. The long and the short of it: linker histone H1 is required for metaphase chromosome compaction. Cell Cycle. 5:589–591. [DOI] [PubMed] [Google Scholar]
- Meraldi, P., and P.K. Sorger. 2005. A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J. 24:1621–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnion, M.E., R.R. Adams, D.M. Callister, C.D. Allis, W.C. Earnshaw, and J.R. Swedlow. 2001. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J. Biol. Chem. 276:26656–26665. [DOI] [PubMed] [Google Scholar]
- Ohi, R., T. Sapra, J. Howard, and T.J. Mitchison. 2004. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol. Biol. Cell. 15:2895–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick, T.D., D.L. Satinover, F. MacIsaac, P.T. Stukenberg, W.C. Earnshaw, T.L. Orr-Weaver, and M. Carmena. 2006. INCENP and Aurora B promote meiotic sister chromatid cohesion through localization of the Shugoshin MEI-S332 in Drosophila. Dev. Cell. 11:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel, C.G., V.L. Katis, Y. Katou, S. Mori, T. Itoh, W. Helmhart, M. Galova, M. Petronczki, J. Gregan, B. Cetin, et al. 2006. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature. 441:53–61. [DOI] [PubMed] [Google Scholar]
- Rivera, T., and A. Losada. 2006. Shugoshin and PP2A, shared duties at the centromere. Bioessays. 28:775–779. [DOI] [PubMed] [Google Scholar]
- Roberts, B.T., K.A. Farr, and M.A. Hoyt. 1994. The Saccharomyces cerevisiae checkpoint gene BUB1 encodes a novel protein kinase. Mol. Cell. Biol. 14:8282–8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh, H., C.A. Cooke, W.H. Burgess, W.C. Earnshaw, and M. Dasso. 1996. Direct and indirect association of the small GTPase ran with nuclear pore proteins and soluble transport factors: studies in Xenopus laevis egg extracts. Mol. Biol. Cell. 7:1319–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic, A., J.C. Waters, and T.J. Mitchison. 2004. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell. 118:567–578. [DOI] [PubMed] [Google Scholar]
- Sampath, S.C., R. Ohi, O. Leismann, A. Salic, A. Pozniakovski, and H. Funabiki. 2004. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 118:187–202. [DOI] [PubMed] [Google Scholar]
- Sandall, S., F. Severin, I.X. McLeod, J.R. Yates III, K. Oegema, A. Hyman, and A. Desai. 2006. A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell. 127:1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab, M.S., B.T. Roberts, S.D. Gross, B.J. Tunquist, F.E. Taieb, A.L. Lewellyn, and J.L. Maller. 2001. Bub1 is activated by the protein kinase p90(Rsk) during Xenopus oocyte maturation. Curr. Biol. 11:141–150. [DOI] [PubMed] [Google Scholar]
- Sharp-Baker, H., and R.H. Chen. 2001. Spindle checkpoint protein Bub1 is required for kinetochore localization of Mad1, Mad2, Bub3, and CENP-E, independently of its kinase activity. J. Cell Biol. 153:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumara, I., E. Vorlaufer, C. Gieffers, B.H. Peters, and J.M. Peters. 2000. Characterization of vertebrate cohesin complexes and their regulation in prophase. J. Cell Biol. 151:749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Z., H. Shu, D. Oncel, S. Chen, and H. Yu. 2004. a. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol. Cell. 16:387–397. [DOI] [PubMed] [Google Scholar]
- Tang, Z., Y. Sun, S.E. Harley, H. Zou, and H. Yu. 2004. b. Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc. Natl. Acad. Sci. USA. 101:18012–18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, S.S., and F. McKeon. 1997. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell. 89:727–735. [DOI] [PubMed] [Google Scholar]
- Vagnarelli, P., and W.C. Earnshaw. 2004. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 113:211–222. [DOI] [PubMed] [Google Scholar]
- Vanoosthuyse, V., R. Valsdottir, J.P. Javerzat, and K.G. Hardwick. 2004. Kinetochore targeting of fission yeast Mad and Bub proteins is essential for spindle checkpoint function but not for all chromosome segregation roles of Bub1p. Mol. Cell. Biol. 24:9786–9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron, S., S. Prieto, C. Bernis, J.C. Labbe, A. Castro, and T. Lorca. 2004. Kinetochore localization of spindle checkpoint proteins: who controls whom? Mol. Biol. Cell. 15:4584–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, C.D., D.M. Brady, R.C. Johnston, J.S. Hanna, K.G. Hardwick, and F.A. Spencer. 2002. Distinct chromosome segregation roles for spindle checkpoint proteins. Mol. Biol. Cell. 13:3029–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, O.K., and G. Fang. 2006. Loading of the 3F3/2 antigen onto kinetochores is dependent on the ordered assembly of the spindle checkpoint proteins. Mol. Biol. Cell. 17:4390–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin, S.G., R.D. Shelby, and K.F. Sullivan. 2001. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J. Cell Biol. 155:1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]