Abstract
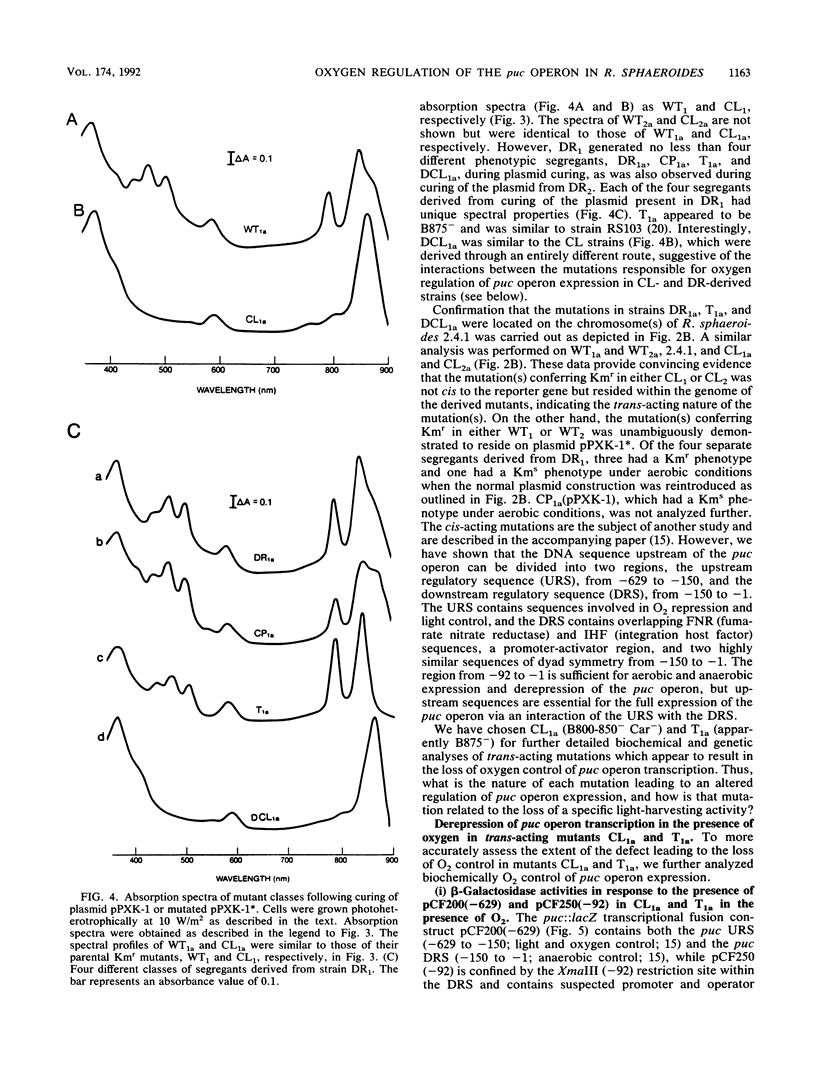
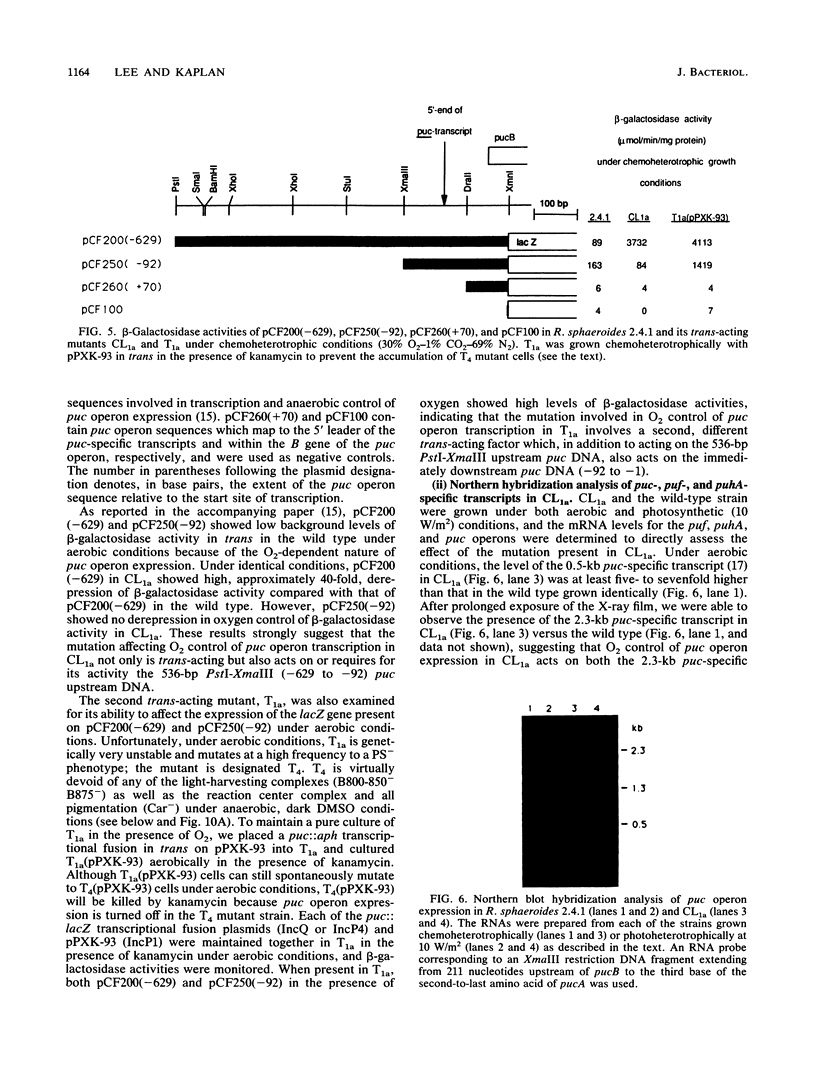
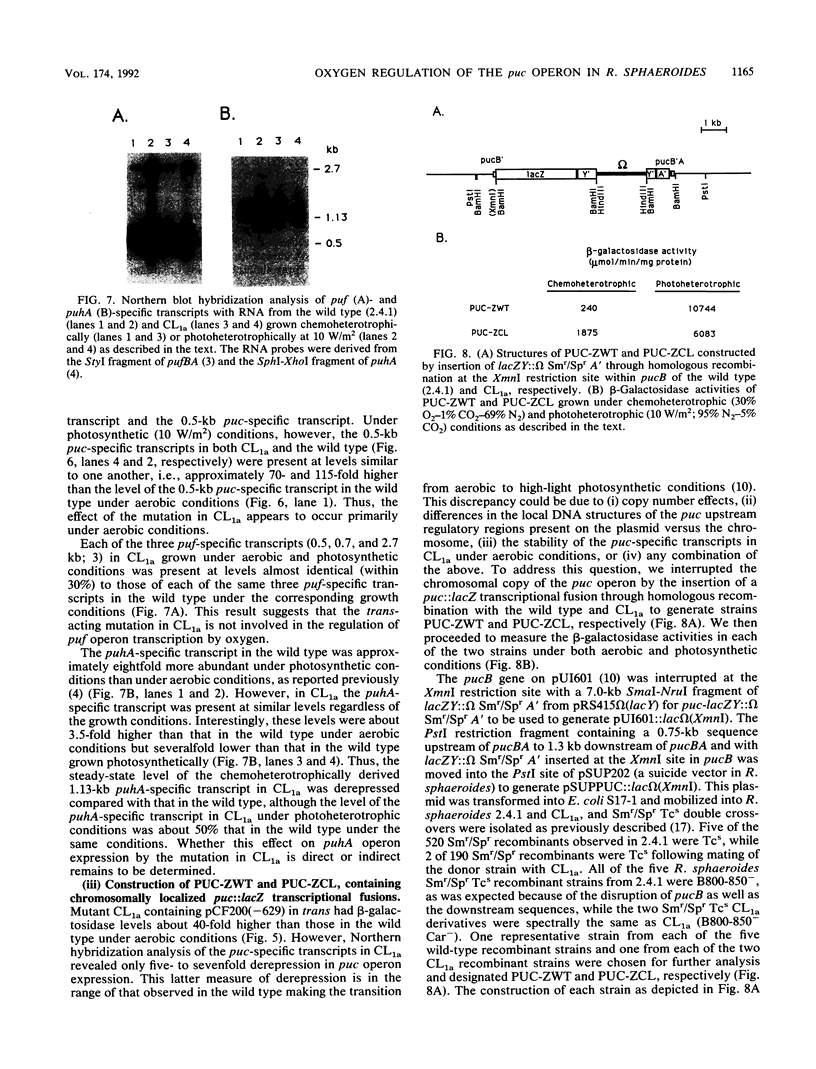
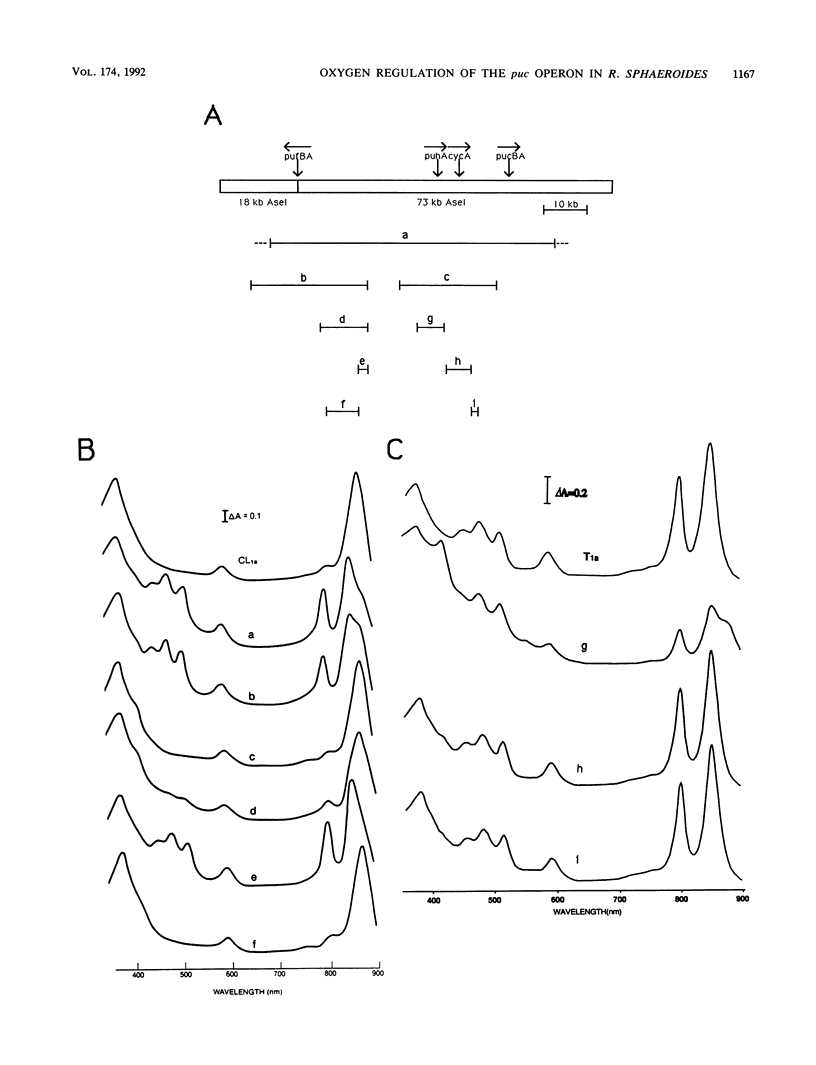
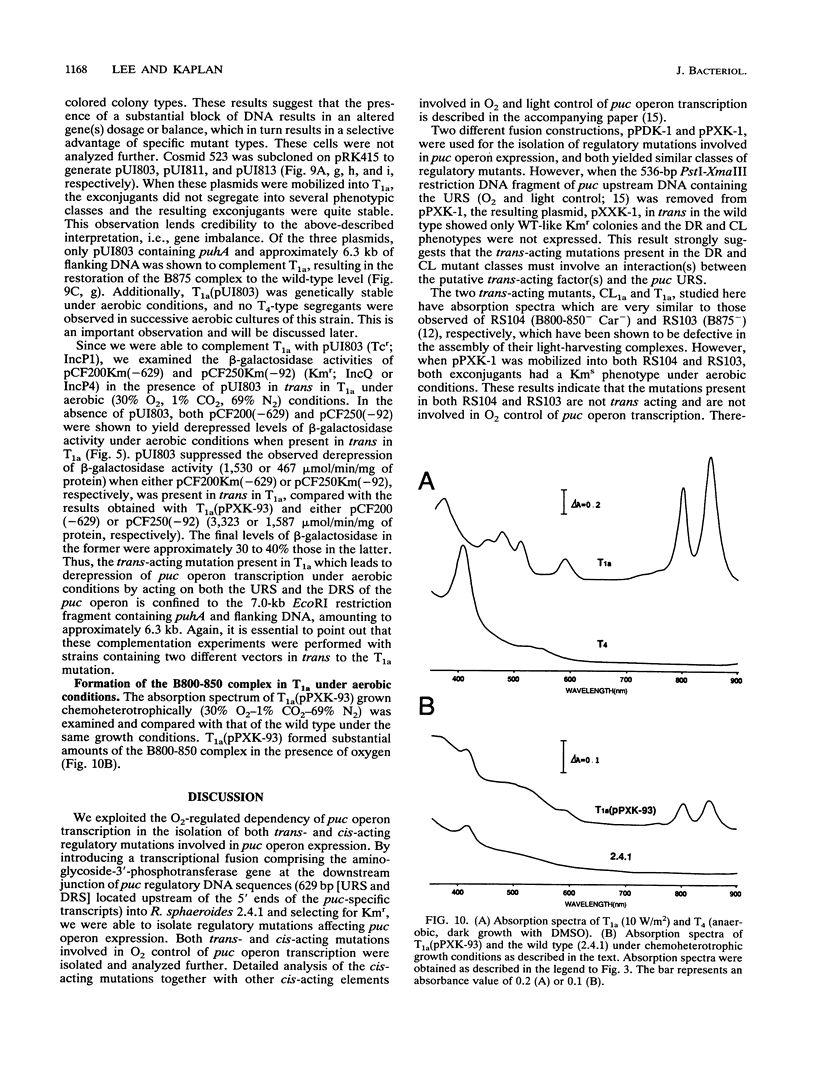
Transcriptional expression of the puc operon in Rhodobacter sphaeroides 2.4.1 is dependent on the partial pressure of oxygen. By using transcriptional fusions in trans of a promoterless fragment derived from the aminoglycoside-3'-phosphotransferase gene of Tn903 to puc operon-specific DNA containing a 629-bp 5' cis-acting regulatory region involved in the expression of puc-specific mRNA, we selected Kmr colonies under aerobic conditions. Two broad classes of mutations, trans and cis, which are involved in O2 control of puc operon transcription, fall into several distinct phenotypic classes. The cis-acting regulatory mutations are characterized in detail elsewhere (J.K. Lee and S. Kaplan, J. Bacteriol. 174:1146-1157, 1992). Two trans-acting regulatory mutants, CL1a and T1a, which are B800-850- Car- and apparently B875-, respectively, were shown to derepress puc operon transcription in the presence of oxygen. The mutation giving rise to CL1a has been shown to act at the puc operon-specific cis-acting upstream regulatory region (-629 to -92). On the other hand, the mutation giving rise to T1a, identifying a second trans-acting regulatory factor(s), appears to act at both the upstream (-629 to -92) and the downstream (-92 to -1) regulatory regions of the puc operon as well as at the level(s) of bacteriochlorophyll and carotenoid biosyntheses, as revealed by the presence of the B800-850 complex under chemoheterotrophic growth conditions. Both the B800-850- Car- phenotype and the trans-acting effect on puc operon expression in mutant CL1a were complemented with a 2.2-kb DNA fragment located within the carotenoid gene cluster. Mutant T1a was complemented with a 7.0-kb EcoRI restriction fragment containing the puhA gene and its flanking DNA (6.3 kb) to restore expression of the B875 complex and to suppress the trans-acting effect resulting in the loss of 02 control. Under chemoheterotrophic conditions, mutant T1a was highly unstable, segregating into a PS- mutant designated T4.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. A., Alberti M., Leach F., Hearst J. E. Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet. 1989 Apr;216(2-3):254–268. doi: 10.1007/BF00334364. [DOI] [PubMed] [Google Scholar]
- Davis J., Donohue T. J., Kaplan S. Construction, characterization, and complementation of a Puf- mutant of Rhodobacter sphaeroides. J Bacteriol. 1988 Jan;170(1):320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHoff B. S., Lee J. K., Donohue T. J., Gumport R. I., Kaplan S. In vivo analysis of puf operon expression in Rhodobacter sphaeroides after deletion of a putative intercistronic transcription terminator. J Bacteriol. 1988 Oct;170(10):4681–4692. doi: 10.1128/jb.170.10.4681-4692.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., Hoger J. H., Kaplan S. Cloning and expression of the Rhodobacter sphaeroides reaction center H gene. J Bacteriol. 1986 Nov;168(2):953–961. doi: 10.1128/jb.168.2.953-961.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., McEwan A. G., Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides cytochrome c2 gene. J Bacteriol. 1986 Nov;168(2):962–972. doi: 10.1128/jb.168.2.962-972.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden S. C., Kaplan S. Localization and structural analysis of the ribosomal RNA operons of Rhodobacter sphaeroides. Nucleic Acids Res. 1990 Dec 25;18(24):7267–7277. doi: 10.1093/nar/18.24.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama S., Leppik R. A., Rekik M., Mermod N., Lehrbach P. R., Reineke W., Timmis K. N. Gene order of the TOL catabolic plasmid upper pathway operon and oxidation of both toluene and benzyl alcohol by the xylA product. J Bacteriol. 1986 Aug;167(2):455–461. doi: 10.1128/jb.167.2.455-461.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides light-harvesting B800-850-alpha and B800-850-beta genes. J Bacteriol. 1987 Jul;169(7):3268–3275. doi: 10.1128/jb.169.7.3268-3275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988 Mar;52(1):50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Varga A., Kaplan S. Physiological and structural analysis of light-harvesting mutants of Rhodobacter sphaeroides. J Bacteriol. 1988 Mar;170(3):1103–1115. doi: 10.1128/jb.170.3.1103-1115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G. A DNA sequence upstream of the puf operon of Rhodobacter capsulatus is involved in its oxygen-dependent regulation and functions as a protein binding site. Mol Gen Genet. 1991 Apr;226(1-2):167–176. doi: 10.1007/BF00273600. [DOI] [PubMed] [Google Scholar]
- Klug G., Jock S. A base pair transition in a DNA sequence with dyad symmetry upstream of the puf promoter affects transcription of the puc operon in Rhodobacter capsulatus. J Bacteriol. 1991 Oct;173(19):6038–6045. doi: 10.1128/jb.173.19.6038-6045.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K., Kaplan S. cis-acting regulatory elements involved in oxygen and light control of puc operon transcription in Rhodobacter sphaeroides. J Bacteriol. 1992 Feb;174(4):1146–1157. doi: 10.1128/jb.174.4.1146-1157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K., Kiley P. J., Kaplan S. Posttranscriptional control of puc operon expression of B800-850 light-harvesting complex formation in Rhodobacter sphaeroides. J Bacteriol. 1989 Jun;171(6):3391–3405. doi: 10.1128/jb.171.6.3391-3405.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoff A. J., Smallwood A. E. The use of two-cistron constructions in improving the expression of a heterologous gene in E. coli. Nucleic Acids Res. 1990 Apr 11;18(7):1711–1718. doi: 10.1093/nar/18.7.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Meinhardt S. W., Kiley P. J., Kaplan S., Crofts A. R., Harayama S. Characterization of light-harvesting mutants of Rhodopseudomonas sphaeroides. I. Measurement of the efficiency of energy transfer from light-harvesting complexes to the reaction center. Arch Biochem Biophys. 1985 Jan;236(1):130–139. doi: 10.1016/0003-9861(85)90612-5. [DOI] [PubMed] [Google Scholar]
- Narro M. L., Adams C. W., Cohen S. N. Isolation and characterization of Rhodobacter capsulatus mutants defective in oxygen regulation of the puf operon. J Bacteriol. 1990 Aug;172(8):4549–4554. doi: 10.1128/jb.172.8.4549-4554.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Summerton J., Atkins T., Bestwick R. A rapid method for preparation of bacterial plasmids. Anal Biochem. 1983 Aug;133(1):79–84. doi: 10.1016/0003-2697(83)90224-5. [DOI] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. A self-transmissible, narrow-host-range endogenous plasmid of Rhodobacter sphaeroides 2.4.1: physical structure, incompatibility determinants, origin of replication, and transfer functions. J Bacteriol. 1992 Feb;174(4):1124–1134. doi: 10.1128/jb.174.4.1124-1134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Chromosome transfer in Rhodobacter sphaeroides: Hfr formation and genetic evidence for two unique circular chromosomes. J Bacteriol. 1992 Feb;174(4):1135–1145. doi: 10.1128/jb.174.4.1135-1145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: presence of two unique circular chromosomes. J Bacteriol. 1989 Nov;171(11):5850–5859. doi: 10.1128/jb.171.11.5850-5859.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T. N., Havelka W. A., Kaplan S. A broad-host-range vector system for cloning and translational lacZ fusion analysis. Plasmid. 1988 May;19(3):175–188. doi: 10.1016/0147-619x(88)90037-6. [DOI] [PubMed] [Google Scholar]
- Tichy H. V., Albien K. U., Gad'on N., Drews G. Analysis of the Rhodobacter capsulatus puc operon: the pucC gene plays a central role in the regulation of LHII (B800-850 complex) expression. EMBO J. 1991 Oct;10(10):2949–2955. doi: 10.1002/j.1460-2075.1991.tb07845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichy H. V., Oberlé B., Stiehle H., Schiltz E., Drews G. Genes downstream from pucB and pucA are essential for formation of the B800-850 complex of Rhodobacter capsulatus. J Bacteriol. 1989 Sep;171(9):4914–4922. doi: 10.1128/jb.171.9.4914-4922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. Q., MacGregor B. J., Donohue T. J., Kaplan S., Yen B. Genetic and physical mapping of the Rhodobacter sphaeroides photosynthetic gene cluster from R-prime pWS2. Plasmid. 1991 May;25(3):163–176. doi: 10.1016/0147-619x(91)90010-t. [DOI] [PubMed] [Google Scholar]
- Zucconi A. P., Beatty J. T. Posttranscriptional regulation by light of the steady-state levels of mature B800-850 light-harvesting complexes in Rhodobacter capsulatus. J Bacteriol. 1988 Feb;170(2):877–882. doi: 10.1128/jb.170.2.877-882.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]