Abstract
Elastic fibers are required for the elasticity and integrity of various organs. We and others previously showed that fibulin-5 (also called developing arteries and neural crest EGF-like [DANCE] or embryonic vascular EGF-like repeat–containing protein [EVEC]) is indispensable for elastogenesis by studying fibulin-5–deficient mice, which recapitulate human aging phenotypes caused by disorganized elastic fibers (Nakamura, T., P.R. Lozano, Y. Ikeda, Y. Iwanaga, A. Hinek, S. Minamisawa, C.F. Cheng, K. Kobuke, N. Dalton, Y. Takada, et al. 2002. Nature. 415:171–175; Yanagisawa, H., E.C. Davis, B.C. Starcher, T. Ouchi, M. Yanagisawa, J.A. Richardson, and E.N. Olson. 2002. Nature. 415:168–171). However, the molecular mechanism by which fiblin-5 contributes to elastogenesis remains unknown. We report that fibulin-5 protein potently induces elastic fiber assembly and maturation by organizing tropoelastin and cross-linking enzymes onto microfibrils. Deposition of fibulin-5 on microfibrils promotes coacervation and alignment of tropoelastins on microfibrils, and also facilitates cross-linking of tropoelastin by tethering lysyl oxidase-like 1, 2, and 4 enzymes. Notably, recombinant fibulin-5 protein induced elastogenesis even in serum-free conditions, although elastogenesis in cell culture has been believed to be serum-dependent. Moreover, the amount of full-length fibulin-5 diminishes with age, while truncated fibulin-5, which cannot promote elastogenesis, increases. These data suggest that fibulin-5 could be a novel therapeutic target for elastic fiber regeneration.
Introduction
Elastic fibers play a crucial role in the structural integrity and function of various organs (Rosenbloom et al., 1993). The loss of elasticity leads to many age-related phenotypes, such as wrinkled skin, emphysema, and arteriosclerosis (Pasquali-Ronchetti and Baccarani-Contri, 1997; Bailey, 2001). Therapeutic intervention for these age-related changes has not been realized because the mechanisms of the development and maintenance of elastic fibers remain unclear. Elastic fibers comprise two distinct components: an amorphous core of cross-linked elastin surrounded by a peripheral mantle of microfibrils. In the process of elastic fiber assembly, elastin precursors (tropoelastin) are deposited on microfibrils, aligned in an orderly way, and cross-linked by lysyl oxidase (LOX) enzymes to form mature elastin, which confers the elastic properties to elastic fibers (Rosenbloom et al., 1993). However, the molecular mechanism of this multistep process remains largely unknown (Kielty et al., 2002).
Tropoelastin, which is a major constituent of the elastic fiber, can interact with other tropoelastin molecules and self-aggregate at physiological temperatures. Thus, tropoelastins undergo a phase transition, which is termed coacervation, from soluble monomers to insoluble aggregates (Urry, 1988). In elastic fiber assembly, coacervation is considered to play a critical role through concentrating and aligning tropoelastin before cross-linking. However, coacervation of tropoelastins alone is not sufficient to explain the assembly process and the variable forms of elastic fibers in different tissues. For fibrillar deposition of tropoelastin, microfibrils, which are mainly composed of fibrillin-1 and -2, are assumed to act as scaffolds, and to direct the morphogenesis of elastic fibers. In fact, mice deficient in both fibrillin-1 and -2 were recently reported to exhibit impaired elastogenesis (Carta et al., 2006), indicating that fibrillins are required for the initial assembly of elastic fibers.
The final cross-linking step is initiated by oxidative deamination of tropoelastins, which is catalyzed by LOXs (Kagan and Li, 2003). LOXs are copper-containing monoamine oxidases secreted by fibrogenic cells, such as fibroblasts and smooth muscle cells. Five LOX family members have been identified so far: LOX, LOX-like 1 (LOXL1), 2, 3, and 4 (Molnar et al., 2003). LOX-deficient mice were reported to die perinatally because of insufficient cross-linking of elastin and collagens (Maki et al., 2002; Hornstra et al., 2003). LOXL1-deficient mice were reported to live to adulthood, but to have degenerated elastic fibers, suggesting that LOXL1 is also important for the development and/or maintenance of elastic fibers (Liu et al., 2004). Yet, little is known about how LOXs are recruited to catalyze tropoelastins on microfibrils.
Thus, the elastic fiber assembly involves recruitment and organized deposition of various molecules, and seems to be tightly regulated and finely tuned to achieve the appropriate elastic properties in each organ. However, whether there are any specific molecules organizing the assembly of elastic fiber components remains unknown. Recently, we and others showed that the elastic fiber assembly process requires fibulin-5 (also known as developing arteries and neural crest EGF-like [DANCE] or embryonic vascular EGF-like repeat–containing protein [EVEC]) by thoroughly investigating fibulin-5–deficient mice (Nakamura et al., 2002; Yanagisawa et al., 2002). These mice recapitulate human aging phenotypes, such as loose skin, emphysematous lungs, and stiff arteries because of disorganized elastic fibers. Fibulin-5 has an Arg-Gly-Asp motif at the N-terminal domain and interacts with cell surface integrins, such as αvβ5, αvβ3, and α9β1 (Nakamura et al., 1999, 2002). Fibulin-5 also has tandem arrays of calcium-binding EGF-like domains and a characteristic C-terminal fibulin domain, and these structures enable it to interact with several elastic fiber components, such as elastin (Yanagisawa et al., 2002; Freeman et al., 2005), EMILIN (Zanetti et al., 2004), and LOXL1 (Liu et al., 2004). Thus, fibulin-5 is a good candidate for an organizer of elastogenesis that scaffolds elastic fiber components in the vicinity of cell surfaces. However, the specific role of fibulin-5 in elastogenesis, whether fibulin-5 can promote elastogenesis, and whether there is a correlation of aging-associated alterations of fibulin-5 with aging phenotypes, remains unknown.
We report that recombinant fibulin-5 protein potently induces elastic fiber assembly, even in serum-free cell culture, without changing the expression of elastic fiber components, suggesting that fibulin-5 serves as an organizer molecule for elastogenesis. Fibulin-5 deposits on microfibrils, promotes aggregation of tropoelastin molecules through coacervation, and also interacts with LOXL1, 2, and 4, which are enzymes that cross-link elastin. We propose a model in which fibulin-5 tethers LOXL enzymes to microfibrils, thus facilitating aggregation and cross-linking of elastin on microfibrils. Intriguingly, a much lower level of full-length fibulin-5, and a higher level of a truncated form of fibulin-5 were detected in aged mouse loose skin than in young mouse skin because of proteolytic cleavage of the N-terminal domain. The cleavage of fibulin-5 abrogates fibulin-5–microfibril interaction, leading to loss of the elastic fiber–organizing activity of fibulin-5.
Results
Fibulin-5 promotes assembly of elastic fiber components
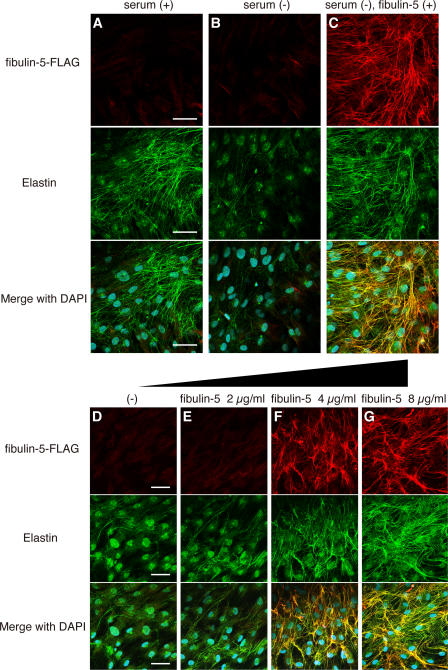
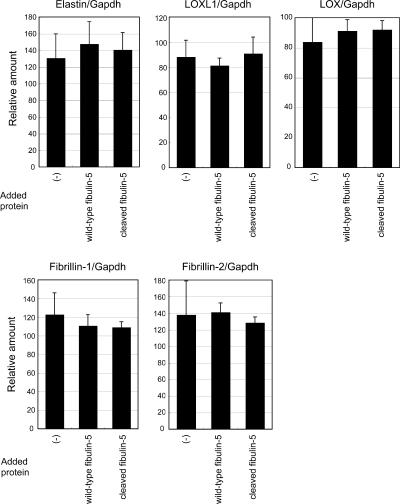
To elucidate the molecular role of fibulin-5 in elastogenesis, we established an in vitro system to evaluate elastic fiber assembly, using human skin fibroblast cultures. In the presence of fetal bovine serum, skin fibroblasts developed an abundant meshwork of elastic fibers, which were stained with antielastin antibody (Fig. 1 A), as previously reported (Mecham, 1987). In the absence of serum, they did not develop elastic fibers (Fig. 1 B). Investigation of elastogenic factors has been hampered by the serum dependency of elastogenesis in cell culture, as serum contains various proteins of known and unknown functions (Mecham, 1987). Surprisingly, we found that skin fibroblasts developed an abundant meshwork of elastic fibers, even in serum-free media, when they were cultured with purified recombinant fibulin-5 protein (Fig. 1 C). Staining with anti-FLAG antibody revealed that added fibulin-5 protein colocalized with elastin. Recombinant fibulin-5 protein induced elastic fiber assembly in a dose-dependent manner in serum-free medium (Fig. 1, D–G). We scarcely detected endogenous fibulin-5 in serum-free culture, whereas we detected abundant deposition of fibulin-5 in serum-containing culture (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200611026/DC1). Addition of fibulin-5 protein did not affect the mRNA expression of elastic fiber components (e.g., elastin and fibrillins; Fig. 2), indicating that a sufficient amount of elastic fiber components was produced even in serum-free culture, but that they could not be organized into elastic fibers without the addition of fibulin-5 protein. These data suggest that fibulin-5 is not a signaling molecule that changes gene expression, but an organizer molecule that promotes assembly of elastic fiber components.
Figure 1.
Fibulin-5 potently induces elastic fiber development and promotes tropoelastin coacervation. (A–C) Human skin fibroblasts were cultured in 10% fetal bovine serum–containing medium (A), in serum-free medium (B), and in serum-free medium with 8 μg/ml of purified recombinant FLAG-tagged fibulin-5 protein (C). Cultures were stained with anti-FLAG (top) or anti–human elastin (middle) antibody. The bottom images were produced by superimposition of the top and middle images, together with DAPI nuclear staining. (D–G) Human skin fibroblasts were cultured in serum-free medium with 0, 2, 4, or 8 μg/ml of purified FLAG-tagged recombinant fibulin-5 protein, to examine the dose-dependency of fibulin-5 elastogenic activity. Cultures were stained as in A–C. Bars, 50 μm.
Figure 2.
Quantitative PCR analysis in skin fibroblasts after addition of recombinant full-length fibulin-5 or truncated form of fibulin-5. Total RNA from skin fibroblasts was extracted 2 d after addition of recombinant proteins in the serum-free culture media. cDNA was synthesized and was subjected to quantitative real-time PCR for the expression of elastin, LOXL1, LOX, fibrillin-1, -2, and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) transcripts. A graphic presentation of results obtained by real-time PCR is shown. Levels of Gapdh transcript were used to normalize cDNA levels. The relative amount of PCR product amplified from control fibroblasts was set at 100. Data are presented as triplicates, and the means ± the SD are shown. The primers used are shown in Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200611026/DC1.
Fibulin-5 promotes coacervation of tropoelastin
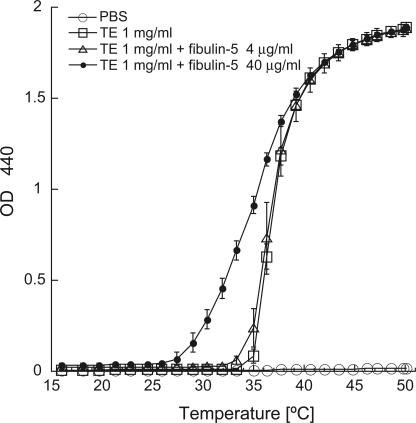
Tropoelastin, which is the precursor of elastin, can interact with other tropoelastin molecules and self-aggregate at physiological temperatures through a phase transition termed coacervation (Urry, 1988; Vrhovski et al., 1997). Coacervation is considered to be an important step before cross-linking of tropoelastin molecules for elastic fiber assembly (Urry, 1988; Vrhovski et al., 1997). Because fibulin-5 can directly interact with tropoelastin (Yanagisawa et al., 2002), we examined whether fibulin-5 can affect the coacervation of tropoelastin. Purified recombinant tropoelastin protein coacervated at 35–40°C, causing the solution to become turbid (Fig. 3). In the presence of recombinant fibulin-5 protein, tropoelastin started to coacervate at lower temperatures (Fig. 3), suggesting that fibulin-5 promotes coacervation of tropoelastin.
Figure 3.
Coacervation assay using purified recombinant tropoelastin and fibulin-5 proteins. 1 mg/ml of soluble tropoelastin with 0, 4, or 40 μg/ml of fibulin-5 protein was induced to coacervate by increasing the temperature, and the turbidity of the solution was measured. Data were obtained in duplicate, and the mean ± the SD values are shown.
The switching of fibulin-5 subtypes occurs with age, whereas elastic matrices are deteriorated
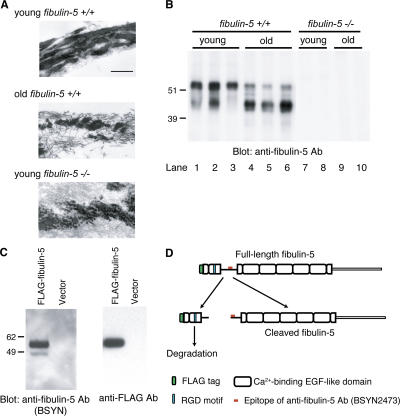
Fibulin-5–deficient mice exhibit aging-related characteristics, including loose skin, emphysema, and stiff arteries, because of disorganized elastic fiber development (Nakamura et al., 2002; Yanagisawa et al., 2002). Because tissue elastic properties are gradually reduced with aging (Pasquali-Ronchetti and Baccarani-Contri, 1997; Bailey, 2001), we examined the age-related changes of elastic matrices by observing skin tissues of young and old mice with transmission electron microscopy. As shown in Fig. 4 A, elastic fibers were disorganized and fragmented in old mice, whereas they were organized into thick fibrous structures in young mice (Fig. 4 A, middle and top). The deteriorated elastic fibers in old mice closely resemble the disorganized and fragmented elastic fibers found even in young fibulin-5–deficient mice (Fig. 4 A, middle and bottom). Next, we investigated whether the expression of fibulin-5 is altered during aging, as we inferred from the data in the previous sections that suggested fibulin-5 is not only necessary for elastogenesis but also able to promote elastic fiber organization. Using skin tissues of young and old mice, we performed Western blotting with anti–fibulin-5 antibody (BSYN2473). As shown in Fig. 4 B, we detected two specific bands in wild-type mice, while we did not detect any band in fibulin-5–deficient mice. Notably, the intensity of the upper bands was considerably decreased in the old mice compared with the young mice (Fig. 4 B, compare lanes 1–3 with lanes 4–6). On the other hand, the intensity of the bottom bands increased remarkably in the old mice (Fig. 4 B, compare lanes 1–3 with lanes 4–6). From these results, we hypothesized that the switching of fibulin-5 subtypes may affect the elastogenic activity of fibulin-5 and be correlated with the deterioration of elastic matrices during aging.
Figure 4.
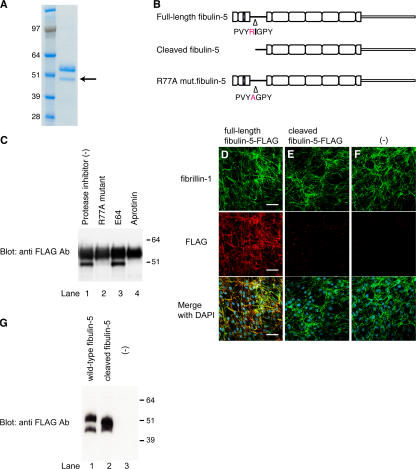
The N-terminal domain cleavage of fibulin-5 is found in aged mouse skin, as well as in cell cultures. (A) Fine structure of elastic fibers in skin tissues observed by transmission electromicroscopy. Elastin was stained with tannic acid, and therefore appears as black amorphous material. The fine fibers surrounding elastin are microfibrils. Bar, 0.4 μm. (B) Skin tissues were harvested from wild-type or fibulin-5–deficient young (3-mo-old) and old (22-mo-old) mice. Proteins were extracted from skin tissues with 8 M urea and dialyzed against PBS. 10 μg each of these extracts were resolved by SDS-PAGE, and analyzed by Western blotting with anti–fibulin-5 antibody (BSYN2473). Two specific bands of 45 and 55 kD were detected in wild-type mice with anti–fibulin-5 antibody (lanes 1–3). The 55-kD band markedly decreased with age, whereas the 45-kD band markedly increased with age (lanes 4–6). (C) 293T cells were transiently transfected with an expression vector encoding fibulin-5 cDNA with a signal peptide and a FLAG tag at the N terminus. Conditioned medium was subjected to SDS-PAGE, followed by Western blotting analysis with either anti– fibulin-5 or anti-FLAG antibody. Two bands of 55 and 45 kD were detected with anti–fibulin-5 antibody, whereas only a 55-kD band was detected with anti-FLAG antibody. (D) Anti–fibulin-5 antibody (BSYN2473) was raised against a peptide corresponding to amino acids 76–98 (red mark). These findings suggest that fibulin-5 is cleaved at a more N-terminal position than the recognition site of anti–fibulin-5 antibody.
Fibulin-5 is partially cleaved in vitro
Because only one major transcript of fibulin-5 was detected by Northern blot analysis (Nakamura et al., 1999) and no mRNA or EST that would code for a protein ∼10 kD smaller or larger than the major form of fibulin-5 protein was found in public databases, the two subtypes of fibulin-5 protein we observed were not thought to be splice variants, but were rather considered to be indicative of posttranslational modification. To investigate this modification, we transfected an expression vector containing fibulin-5 cDNA with a signal peptide and a FLAG tag at the N terminus into 293T cells. The culture medium of these cells was collected and subjected to Western blotting. As shown in Fig. 4 C, we detected 2 specific bands of 45 and 55 kD with an anti–fibulin-5 antibody (BSYN2473; Fig. 4 C, left) that recognizes amino acids 76–98 (Fig. 4 D, red mark). On the other hand, we detected only one 55-kD band with anti-FLAG antibody (Fig. 4 C, right). These results suggest that fibulin-5 may be cleaved at a more N-terminal position than the recognition site of anti–fibulin-5 antibody (Fig. 4 D). We deduced that the lower 45-kD band is the cleaved form of fibulin-5, while the upper 55-kD band is the full-length fibulin-5. The cleaved N-terminal fragment of fibulin-5 appears to be easily degraded because we did not detect a 10-kD band corresponding to this fragment with anti-FLAG antibody.
Fibulin-5 is specifically cleaved after arginine at position 77 by a serine protease
To identify the cleavage site of fibulin-5, we stably transfected the C-terminal–histidine-tagged expression vector encoding fibulin-5 cDNA into 293T cells. We purified recombinant fibulin-5 protein from the conditioned medium of these cells with immobilized metal affinity chromatography, and examined the purified protein by SDS-PAGE. As expected, we detected two bands stained with Coomassie blue, and the lower 45-kD band was assumed to be the cleaved form of fibulin-5. We sequenced the N-terminal end of the lower band using Edman degradation (Fig. 5 A, arrow), and determined that fibulin-5 is cleaved after the arginine at position 77 (Fig. 5 B, top and middle). The upper 55-kD band was also confirmed by protein sequencing to be the full-length fibulin-5, as the N-terminal sequence coincided with the predicted cleavage site after the signal peptide. As many proteases are arginine-specific, we examined whether the arginine at position 77 is necessary for the cleavage of fibulin-5. We constructed an expression vector encoding R77A mutant fibulin-5, which was mutated from arginine to alanine at position 77 (Fig. 5 B, bottom), and transfected this R77A mutant fibulin-5 vector into 293T cells, followed by Western blotting. As shown in Fig. 5 C, we detected only the upper 55-kD band, whereas the lower 45-kD band disappeared (lane 2), indicating that the R77A mutant fibulin-5 is resistant to cleavage. These results indicate that fibulin-5 is specifically cleaved after the arginine at position 77 (Fig. 5 C, compare lanes 1 and 2), although we cannot rule out the possibility that fibulin-5 might not be cleaved in vivo at the same site as in vitro cell culture. Next, we examined the nature of the protease that cleaves fibulin-5. We transiently transfected the expression vector encoding C-terminal FLAG-tagged fibulin-5 cDNA into 293T cells, and subsequently added different types of protease inhibitors to the culture medium, which was subjected to Western blotting. Whereas a cysteine protease inhibitor, E64, did not inhibit the cleavage of fibulin-5 at all, a serine protease inhibitor, aprotinin, completely inhibited the cleavage (Fig. 5 C, compare lanes 3 and 4). These results indicate that fibulin-5 is cleaved by a serine protease in vitro. We have not yet succeeded in identifying the serine protease that cleaves fibulin-5.
Figure 5.
Fibulin-5 is cleaved after the arginine at position 77 by serine protease and loses the microfibril-associating activity. (A) 293T cells were stably transfected with an expression vector encoding C-terminal-FLAG– and 6× histidine-tagged fibulin-5 cDNA. Recombinant fibulin-5 protein was purified by chelating chromatography from the culture media of these cells, subjected to SDS-PAGE, and stained with Coomassie blue. The lower band (arrow) was subjected to N-terminal sequencing. (B) The N-terminal sequencing of the lower band identified the specific cleavage site of fibulin-5 at the arginine at position 77. (C) 293T cells were transiently transfected with the expression vector encoding C-terminal-FLAG– and 6× histidine-tagged fibulin-5 or R77A mutant fibulin-5, which had a mutation from arginine to alanine at position 77. Transfected cells were cultured for 2 d with or without a cysteine protease inhibitor, E64, or a serine protease inhibitor, aprotinine, added to the culture media. Culture media were then harvested, concentrated by chelating chromatography, and subjected to Western blotting with anti-FLAG antibody. (D–G) Human skin fibroblasts were cultured for 4 d in serum-free medium in the presence of recombinant fibulin-5 (D) or recombinant cleaved fibulin-5 (E) proteins at a concentration of 4 μg/ml in the medium, or without recombinant protein (F). The cells were then double-stained with anti–fibrillin-1 polyclonal antibody (top) and with anti-FLAG monoclonal antibody (middle). Bottom images were produced by superimposition of the top and middle images, together with DAPI nuclear staining. Bars, 60 μm. At the same time, the conditioned medium was immunoblotted with anti-FLAG antibody to confirm that neither fibulin-5 nor cleaved fibulin-5 was degraded during the culture period (G).
Full-length fibulin-5 can deposit on microfibrils, whereas the truncated form of fibulin-5 cannot
To assess the functional consequence of fibulin-5 cleavage, we studied the interaction of fibulin-5 and microfibrils. Microfibrils, which are mainly composed of fibrillin-1 and -2, are considered to serve as scaffolds for tropoelastin deposition and subsequent elastic fiber assembly. Fibroblast cell cultures developed fine meshworks of fibrillin-1 microfibrils, even under serum-free conditions, at days 4–7 of culture (Fig. 5, D–F); this is much earlier than elastin deposition, which requires >10 d of culture in serum-containing medium. When we added recombinant fibulin-5 protein to the culture, fibulin-5 colocalized with fibrillin-1 microfibrils. However, when we added recombinant truncated fibulin-5 protein to the culture, the added truncated fibulin-5 did not deposit on fibrillin-1 microfibrils. This was not because the truncated form of fibulin-5 is susceptible to degradation, as Western blot analysis of the culture medium showed that truncated form of fibulin-5 was as stable as the full-length fibulin-5 in the medium (Fig. 5 G). These data indicate that full-length fibulin-5 can deposit on microfibrils, whereas the truncated form of fibulin-5 cannot.
The cleavage of fibulin-5 causes inactivation of the elastogenic activity of fibulin-5
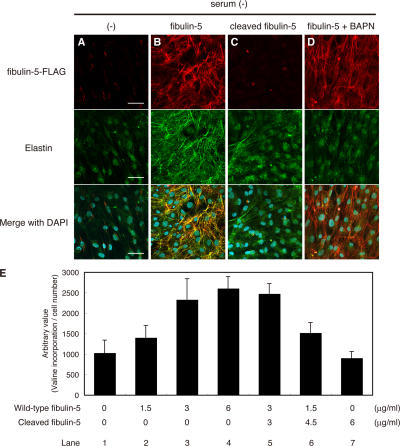
Next, we investigated the consequences of the functional change caused by the cleavage of fibulin-5 in elastic fiber assembly, using the in vitro elastogenesis assay shown in Fig. 1 A with recombinant fibulin-5 and the truncated form of fibulin-5 proteins. Whereas wild-type fibulin-5 potently induced elastic fiber development, the truncated form of fibulin-5 did not induce elastic fiber development at all (Fig. 6, B and C). Therefore, we concluded that the cleavage of fibulin-5 causes inactivation of the elastogenic activity of fibulin-5.
Figure 6.
Fibulin-5 loses its elastogenic activity upon proteolytic cleavage. (A–E) Human skin fibroblasts were cultured in serum-free media without addition of proteins (A), with wild-type fibulin-5 (B), with the cleaved form of fibulin-5 (C), or with wild-type fibulin-5 and 500 μM BAPN (D). Each FLAG-tagged recombinant protein was added to the culture at a final concentration of 4 μg/ml. Cultures were stained with anti-FLAG antibody (top) and anti–human elastin antibody (middle). The bottom images were produced by superimposition of the top and middle images, together with DAPI nuclear staining. Bars, 50 μm. (E) Quantitation of insoluble (i.e., cross-linked and mature) elastin produced by cells cultured with various amounts of wild-type and cleaved fibulin-5 added to the medium. Cultured skin fibroblasts were metabolically labeled with [3H]valine during the culture period, and the radioactivity of the NaOH-insoluble fractions was quantitated. The radioactivity count was corrected by the relative cell number of separate wells measured with a modified MTT assay, although neither wild-type nor cleaved fibulin-5 significantly affected the cell number (not depicted). Data were obtained as quadruplicates, and the mean ± the SD is shown.
Cross-linking of elastin is promoted by prealigned fibulin-5 on microfibrils
The assembly of functional elastic fibers requires not only fibrillar deposition and coacervation of tropoelastin, but also cross-linking of tropoelastin. The aforementioned data suggest that deposition of fibulin-5 onto microfibrils accelerates fibrillar tropoelastin deposition, but it was not clear if the tropoelastin molecules are cross-linked to form mature functional elastic fibers. To examine this, we added β-aminopropionitrile (BAPN), an irreversible inhibitor of LOXs (Tang et al., 1983), to serum-free skin fibroblast culture containing recombinant fibulin-5 protein. It is known that LOXs, which are cross-linking enzymes for elastin monomers, are necessary for elastic fiber maturation (Hornstra et al., 2003; Liu et al., 2004; Maki et al., 2002). As shown in Fig. 6 D, elastins showed only a dotlike deposition when elastin cross-linking was largely inhibited by BAPN, whereas fibrillar deposition of fibulin-5 on microfibrils was not affected by BAPN. This punctuate distribution of elastin is displayed by tropoelastin coacervates before cross-linking (Clarke et al., 2006). These data indicate that elastic fibers organized by the addition of fibulin-5 protein are cross-linked and mature fibers, and that not only deposition and aggregation but also cross-linking of elastin is promoted by prealigned fibulin-5 on microfibrils.
The N-terminal domain-truncated fibulin-5 loses the elastogenic activity, but does not interfere with elastic fiber assembly
We next examined whether cleaved fibulin-5 is merely inactive or works against elastogenesis in a dominant-negative manner. For this purpose, we quantified the amount of elastic fibers induced by full-length fibulin-5 with or without cleaved fibulin-5. We metabolically labeled newly synthesized elastin with [3H]valine (Keeley, 1976), and measured the incorporation of [3H]valine in the NaOH-insoluble fraction of these cells, which reflects the amount of mature elastic fibers (Hinek et al., 2000). As shown in Fig. 6 E, we detected substantial, dose-dependent incorporation of [3H]valine upon adding recombinant fibulin-5 to the medium (Fig. 6 E, compare lanes 1–4). On the other hand, cleaved fibulin-5 showed neither an additive nor a dominant-negative effect on fibulin-5–induced elastic fiber development (Fig. 6 E, compare lanes 1–7). These results indicate that fibulin-5 simply loses its elastogenic activity as a result of cleavage of the N-terminal domain.
LOXL1, 2, and 4 proteins mainly interact with the C-terminal domain of fibulin-5
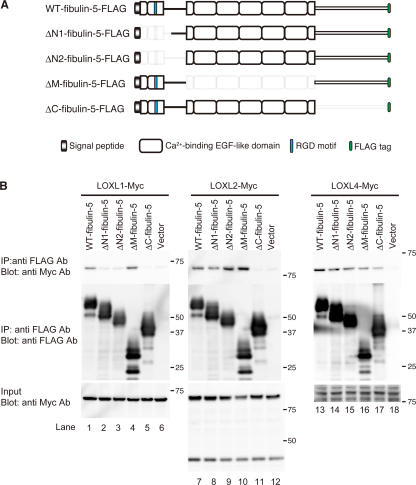
To develop mature elastic fibers, tropoelastin molecules need to be highly cross-linked by LOXs. Five LOX family members have been identified so far, LOX, LOXL1, 2, 3, and 4 (Molnar et al., 2003). Mice lacking LOX and mice lacking LOXL1 were recently reported to show profound fragmentation of elastic fibers (Maki et al., 2002; Hornstra et al., 2003; Liu et al., 2004). Moreover, LOXL1 has been reported to interact with fibulin-5 (Liu et al., 2004). To investigate whether fibulin-5 interacts with other LOX family members, we performed in vitro binding assays using Myc-tagged LOX and LOXLs, and the set of FLAG-tagged fibulin-5 deletion mutant constructs shown in Fig. 7 A. As shown in Fig. 7 B, we detected the specific interaction of LOXL1, 2, and 4 proteins with fibulin-5 protein (top, lanes 1, 7, and 13). The interaction of fibulin-5 and these LOXL proteins was eliminated or considerably diminished by the C-terminal deletion of fibulin-5 (Fig. 7 B, top, lanes 5, 11, and 17). These results indicate that LOXL1, 2, and 4 proteins mainly interact with the C-terminal domain of fibulin-5. We also detected substantial, but weak, interaction of fibulin-5 with LOX and LOXL3 in in vitro binding assays (unpublished data). However, we could not determine the specificity of these interactions because all of the fibulin-5 deletion mutants tested seemed to weakly interact with LOX or LOXL3. Intriguingly, the fibulin-5–LOXL1 interaction was markedly diminished by N-terminal deletion of fibulin-5 (Fig. 7 B, top left, lanes 2 and 3). Thus, fibulin-5 does not interact with LOXL1 after cleavage of fibulin-5, and this defect might contribute to the loss of elastogenic activity.
Figure 7.
Fibulin-5 interacts with LOXL enzymes. (A) Domain structures of the full-length fibulin-5 and the fibulin-5 deletion mutants used for in vitro binding assays. ΔN1-fibulin-5 corresponds to the naturally cleaved form of fibulin-5. These mutants were expressed as C-terminal-FLAG–tagged proteins. (B) Fibulin-5 binds to LOXL1, 2, and 4 through the C-terminal domain. 293T cells were transiently transfected with the vectors shown in A or a mock vector. Expression vectors for Myc-tagged LOX or LOXLs were also independently transfected into 293T cells. The conditioned media were harvested, and mixed. Each mixture was subjected to immunoprecipitation with anti-FLAG antibody, separated by SDS-PAGE, and analyzed by Western blotting with a monoclonal anti-Myc antibody.
Discussion
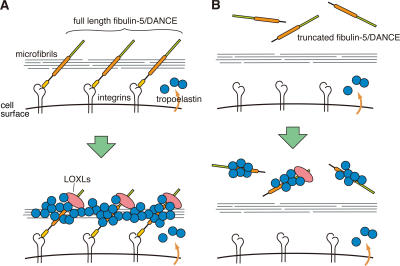
We have identified an elastic fiber–organizing activity of fibulin-5/DANCE protein that is abrogated by proteolytic cleavage of the N-terminal domain in cell culture and in vivo. Our proposed molecular mechanism of elastic fiber organization by fibulin-5/DANCE protein is illustrated in Fig. 8 A. We call this the “Line DANCE model.” According to this model, full-length fibulin-5 protein associates with microfibrils. Direct interaction of fibulin-5 and fibrillin-1 (Freeman et al., 2005) may be important for this association. As fibulin-5 serves as an integrin ligand through its N-terminal domain (Nakamura et al., 1999, 2002), integrin–fibulin-5 interaction, in addition to integrin–fibrillin interaction, may help the assembly to occur in the proximity of the cell surface. After fibulin-5 interacts with microfibrils, tropoelastins accumulate on fibulin-5 proteins and coacervate. LOXL enzymes tethered on the C-terminal domain of fibulin-5 promote cross-linking of the aggregated tropoelastins, to form mature elastic fibers. In contrast, as illustrated in Fig. 8 B, the truncated form of fibulin-5 cannot associate with microfibrils or integrins, and therefore is unable to promote the organization of the elastic fiber assembly, although the truncated form of fibulin-5 itself has the ability to bind tropoelastin and enhance coacervation of tropoelastin as well (unpublished data).
Figure 8.
The Line DANCE model. (A) This model illustrates how fibulin-5/DANCE promotes fibrillar deposition and cross-linking of tropoelastin molecules on microfibrils to promote the development of mature elastic fibers. (B) Truncated fibulin-5 cannot be deposited on microfibrils, and therefore cannot promote elastic fiber assembly.
In this study, we first succeeded in inducing the development of elastic fibers in serum-free skin fibroblast culture by adding purified fibulin-5 protein. Tropoelastin and other elastic fiber components were not organized into elastic fibers without addition of fibulin-5 protein, suggesting that fibulin-5 is not only a necessary component of elastic fibers (Nakamura et al., 2002; Yanagisawa et al., 2002) but also an organizer that can induce elastic fiber assembly. Skin fibroblasts are known to develop abundant elastic fibers in serum-containing media. However, serum contains various known and unknown factors, such as cytokines and growth factors, and also causes increases of total cell number and cell density, making it difficult to identify specific molecules that are important for elastogenesis. Indeed, we detected a significant amount of fibulin-5 in serum using immunoprecipitation and ELISA (unpublished data), which might contribute to the elastogenic activity of the serum to some extent. We detected abundant deposition of fibulin-5 that may be derived from fibroblasts or from serum in serum-containing culture of skin fibroblasts, whereas endogenous fibulin-5 was scarcely detected in serum-free culture (Fig. S1). This indicates that the dose of fibulin-5 is crucial for elastic fiber organization in this serum-free cell culture, and that the endogenous expression of fibulin-5 alone is insufficient. Our serum-free elastogenesis system, in combination with gene knockdown by RNAi, would be a useful tool to identify other factors that regulate elastic fiber assembly, as unknown factors in the serum need not be taken into account.
It has been reported that fibulin-5 can strongly interact with tropoelastin (Yanagisawa et al., 2002). We found that fibulin-5 not only binds with tropoelastin, but also promotes coacervation of tropoelastins, and thus plays a more positive role in the alignment and deposition of tropoelastin. Fibrillin-1 has also recently been reported to promote coacervation (Clarke et al., 2005). Because fibulin-5 interacts with both fibrillin-1 and tropoelastins (Yanagisawa et al., 2002; Freeman et al., 2005), a fibulin-5–fibrillin-1 complex might cooperatively promote coacervation. Coacervated tropoelastin molecules need to be cross-linked by LOX family enzymes for the development of mature elastic fibers. Our data show that fibulin-5 also promotes cross-linking of tropoelastin, which may be mediated by recruiting LOXL1, 2, and 4 in the vicinity of coacervated tropoelastin (Fig. 7 B).
We found that a much higher level of the truncated form of fibulin-5 was present in aged mouse skin than in young mouse skin (Fig. 4 B). There are several possible interpretations of this result: (a) fibulin-5 might be cleaved because of more protease activity in aged tissues; (b) truncated fibulin-5 might be stable and accumulate during life; or (c) truncated fibulin-5 might be more easily extracted from aged elastic fibers than from young elastic fibers. In any case, the truncated form of fibulin-5 cannot contribute to new elastogenesis, and therefore the decrease in full-length fibulin-5 may directly reflect a decrease in the elastogenic activity of aged tissues.
In this study, we could not exclude the possibility that the truncated form of fibulin-5 might play some roles in elastogenesis in vivo. Although truncated fibulin-5 cannot contribute to new elastogenesis as it cannot interact with microfibrils, fibulin-5 protein cleaved after deposition onto microfibrils might take part in subsequent processes of elastogenesis, as binding with tropoelastin and facilitating coacervation of tropoelastin are not compromised by the N-terminal domain cleavage (unpublished data). To clarify the physiological consequences of the cleavage of fibulin-5, further studies will be needed using gene-targeted mice in which a cleavage-resistant mutant form of fibulin-5 is knocked-in.
So far, elastic matrices have been considered to be designed to maintain elastic function for a lifetime (Kielty et al., 2002). Elastin is reported to have an exceptionally long half-life, and to have a turnover rate which approaches our lifespan (Shapiro et al., 1991). On the other hand, elastase activity is provoked by exposure to sunlight and by smoking, causing degradation of the elastic fibers of the skin and lung, respectively (Fisher et al., 1996; Hautamaki et al., 1997). This implies that elastogenic activity needs to be maintained even in the adult tissues to regenerate elastic fibers. Indeed, we detected a significant amount of fibulin-5 in the serum of aged mice (unpublished data), and we and others reported that the expression of fibulin-5 is up-regulated in some pathological conditions, such as in atherosclerotic lesions (Kowal et al., 1999; Nakamura et al., 1999; Nguyen et al., 2004), lung injury by elastase (Kuang et al., 2003), and pulmonary hypertension (Merklinger et al., 2005). The potent elastogenic activity of fibulin-5 might provide future therapeutic applications for aging-associated diseases, such as emphysema and arteriosclerosis, or cosmetic applications for wrinkled skin. Application of fibulin-5 might be extended to tissue engineering, to make elastic skin or vessels by using cell cultures. Further studies are needed to examine whether fibulin-5 might improve age-related changes of elastic matrices in vivo.
In summary, we demonstrated the elastic fiber organizing activity of fibulin-5 that is abrogated by age-associated proteolytic cleavage. We demonstrated that the addition of recombinant fibulin-5 protein induces elastic fiber development, and that a decrease in full-length fibulin-5 caused by proteolytic cleavage may contribute to the loss of the elastogenic potential in aged elastic tissues. Our findings might provide a molecular pathway to future therapeutic applications of the elastic fiber organizing activity of fibulin-5.
Materials and methods
Cell culture
293T cells and human skin fibroblasts (HSFs) were maintained in DME (Sigma-Aldrich) supplemented with 2 mM glutamine, 10% penicillin/streptomycin, and 10% FBS at 37°C in 5% CO2. HSFs were provided by M. Naito (Kyoto University, Kyoto, Japan).
Plasmid construction
Human full-length fibulin-5 cDNA was cloned as previously described (Nakamura et al., 1999). pEF6/V5 (Invitrogen) was modified by incorporation of a C-terminal FLAG-tag or Myc-tag (pEF6/FLAG, pEF6/Myc). The fibulin-5 ΔN1- (Δnt 247–399), ΔN2- (Δnt 247–504), ΔM- (Δnt 505–1,110), and ΔC- (Δnt 1, 114–1,512) fibulin-5 cDNAs were subcloned into pEF6/FLAG. Human fibulin-5 cDNA sequences are numbered according to GenBank accession no. AF112152. Human full-length LOXL1, 2, 3, and 4 cDNAs were obtained from IMAGE (available from GenBank under accession no. BC015090, BC000594, BC071865, and BC013153, respectively), and subcloned into pEF6/Myc. All constructs were confirmed by sequencing (ABI Prism 3100).
Protein purification
pEF6/V5 (Invitrogen) was modified by incorporation of a C-terminal histidine and FLAG-tag. Human wild-type and each mutant fibulin-5 cDNA were subcloned into pEF6/FLAG-His. The respective stable blasticidin-resistant 293T cell clones were then obtained by transfection with these expression vectors. Recombinant proteins were purified from the serum-free conditioned medium of stable lines using Ni-NTA metal affinity resin (QIAGEN), and desalted with a Hi-Trap desalting column (GE Healthcare). Protein concentrations were determined with Coomassie Plus-200 Protein Assay Reagent (Pierce Chemical Co.). Human tropoelastin cDNA was cloned from human skin fibroblasts by RT-PCR. Of the four splice variants obtained, the longest cDNA, which codes one of the splicing variants of the elastin gene (ELNc; available from GenBank under accession no. BC035570), was subcloned into pTrcHis vector (Invitrogen) and expressed in bacteria, followed by protein purification with Ni-NTA column (GE Healthcare), as previously described (Wachi et al., 2005).
In vitro elastogenesis assay and immunofluorescence
HSFs were subconfluently plated on microscope cover glasses (Fisher Scientific). 3 d after plating, culture media were changed to serum-free DME/F12 (Sigma-Aldrich), and purified recombinant fibulin-5 proteins were added, and the incubation was continued for another 10 d. The cells were fixed with 100% methanol, and blocked with 2% BSA. The primary antibodies used were anti-FLAG M2 monoclonal (1/100; Sigma-Aldrich), anti–human elastin polyclonal (1/100; EPC, PR533), anti–human fibulin-5 monoclonal (1/100; 10A), and anti–human fibrillin-1 polyclonal (1/100; EPC, PR217) antibodies. The secondary antibodies used were Alexa Fluor 488, 546, or 647 anti–rabbit or –mouse IgG (1/100; Invitrogen). After staining with DAPI, stained cells were mounted with a Prolong Gold Antifade Kit (Invitrogen). Fluorescence images were sequentially collected using a confocal microscope featuring 405-, 488-, 543-, and 633-nm laser lines with an open pinhole setting operated by the built-in software; either a microscope (LSM510 META; Carl Zeiss MicroImaging, Inc.) with a C-Apo 40×/1.2 NA lens (Figs. 1 and 6) or a microscope (FV-1000; Olympus) with a UPlanSApo 40×/0.9 NA lens (Figs. 5 and S1). Image files were converted to TIFF format using the operating software, merged, linearly contrast stretched (with the same setting in each set of experiments) using Photoshop CS2 (Adobe), and imported into Illustrator CS2 (Adobe) for assembly. Anti–human fibulin-5 monoclonal antibody (10A) was raised by Iwaki Co., Ltd. by immunization of fibulin-5–deficient mice with purified recombinant human fibulin-5 protein.
Coacervation assay
Coacervation of tropoelastin with or without fibulin-5 protein was assessed using the method described (Clarke et al., 2005). Soluble tropoelastin and fibulin-5 were prepared in PBS and mixed on ice. Each reaction mixture was transferred to an 8-channel quartz cuvette for light scattering measurements at 440 nm. The temperature of a spectrophotometer (UV-1650; Shimazu) fitted with a heating/cooling block was regulated by a circulating water bath. All channels were simultaneously cooled and heated in the heating/cooling block by the circulating water.
Transfection, in vitro binding assay, and Western blotting
293T cells were transfected using Lipofectamine PLUS (Invitrogen). The mixtures of conditioned media were subjected to immunoprecipitation with anti-FLAG M2 affinity gel (Sigma-Aldrich). After washing, immune complexes were resolved by SDS-PAGE, transferred to PVDF membranes (Bio-Rad Laboratories), and reacted with HRP-conjugated anti-Myc monoclonal (9E10; 1/500; Santa Cruz Biotechnology) or anti-FLAG M2 monoclonal (1/1,000; Sigma-Aldrich) antibody. Signals were detected using Western Lightning Chemiluminescence Reagent PLUS (Perkin Elmer).
Tissue extraction and Western blotting
Proteins were extensively extracted from skin tissues with 8 M urea solution. After dialysis against PBS, the protein concentration was measured with Coomassie Plus-200 Protein Assay Reagent. Protein samples of the same amount were subjected to SDS-PAGE (Invitrogen), followed by Western blotting with anti–fibulin-5 antibody (1/400, BSYN2473). HRP-conjugated anti–rabbit antibody (1/2,000; Santa Cruz Biotechnology) was used as a secondary antibody.
Transmission electron microscopy
For transmission electron microscopy, skin tissues were fixed with 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1% tannic acid in 0.1 M cacodylate buffer. Sections were then stained with uranyl acetate.
Quantitative measurement of insoluble elastin
HSFs were subconfluently plated on 60-mm dishes in quadruplicates. 3 d after plating, 20 μCi [3H]valine was added to each dish, together with purified recombinant fibulin-5 protein or cleaved fibulin-5 protein. The cultures were then incubated at 37°C in 5% CO2 for 10 d. The cells were harvested in 0.1 M acetic acid on ice. After centrifugation, the pellets were boiled in 0.1 N NaOH for 1 h. Subsequently, the NaOH-insoluble pellets were boiled with 5.7 N HCl for 1 h, mixed with scintillation fluid, and measured for radioactivity with a scintillation counter (Beckman Coulter).
Reverse transcription-polymerase chain reaction and quantitative PCR
Total RNAs were extracted using RNeasy Plus Mini Kit (QIAGEN) and transcribed to cDNA with random hexamers using the SuperScript III First-Strand Synthesis System (Invitrogen). For quantitative PCR, the reaction was performed with a QuantiTect SYBR Green PCR kit (QIAGEN), and the products were analyzed with the Mx3000P QPCR System (Stratagene). Primers used for quantitative PCR are provided in Table S1 (available at http://www.jcb.org/cgi/content/full/jcb.200611026/DC1).
Online supplemental material
Fig. S1 shows that serum induces fibulin-5 deposition on microfibrils. Table S1 shows primers used for quantitative PCR. The online version of this article is available at http://www.jcb.org/cgi/content/full/jcb.200611026/DC1.
Acknowledgments
We thank Ms. N. Tomikawa for excellent technical assistance.
This work was supported in part by Japan Health and Labour Sciences Research Grants, the Japan Society for the Promotion of Science, the Japan Science and Technology Agency, the Takeda Science Foundation, Sakakibara Memorial Foundation, and Japan Heart Foundation Research Grant to T. Nakamura, by grants from the Ministry of Education, Science and Culture of Japan to T. Kita, and by grants from the Japan Society for the Promotion of Science, Japan Heart Foundation Research Grant on Arteriosclerosis Update to M. Hirai.
Abbreviations used in this paper: BAPN, β-aminopropionitrile; DANCE, developing arteries and neural crest EGF-like; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HSF, human skin fibroblast; LOX, lysyl oxidase; LOXL, LOX-like.
References
- Bailey, A.J. 2001. Molecular mechanisms of ageing in connective tissues. Mech. Ageing Dev. 122:735–755. [DOI] [PubMed] [Google Scholar]
- Carta, L., L. Pereira, E. Arteaga-Solis, S.Y. Lee-Arteaga, B. Lenart, B. Starcher, C.A. Merkel, M. Sukoyan, A. Kerkis, N. Hazeki, et al. 2006. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J. Biol. Chem. 281:8016–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, A.W., S.G. Wise, S.A. Cain, C.M. Kielty, and A.S. Weiss. 2005. Coacervation is promoted by molecular interactions between the PF2 segment of fibrillin-1 and the domain 4 region of tropoelastin. Biochemistry. 44:10271–10281. [DOI] [PubMed] [Google Scholar]
- Clarke, A.W., E.C. Arnspang, S.M. Mithieux, E. Korkmaz, F. Braet, and A.S. Weiss. 2006. Tropoelastin massively associates during coacervation to form quantized protein spheres. Biochemistry. 45:9989–9996. [DOI] [PubMed] [Google Scholar]
- Fisher, G.J., S.C. Datta, H.S. Talwar, Z.Q. Wang, J. Varani, S. Kang, and J.J. Voorhees. 1996. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 379:335–339. [DOI] [PubMed] [Google Scholar]
- Freeman, L.J., A. Lomas, N. Hodson, M.J. Sherratt, K.T. Mellody, A.S. Weiss, A. Shuttleworth, and C.M. Kielty. 2005. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem. J. 388:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautamaki, R.D., D.K. Kobayashi, R.M. Senior, and S.D. Shapiro. 1997. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 277:2002–2004. [DOI] [PubMed] [Google Scholar]
- Hinek, A., A.C. Smith, E.M. Cutiongco, J.W. Callahan, K.W. Gripp, and R. Weksberg. 2000. Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein. Am. J. Hum. Genet. 66:859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstra, I.K., S. Birge, B. Starcher, A.J. Bailey, R.P. Mecham, and S.D. Shapiro. 2003. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J. Biol. Chem. 278:14387–14393. [DOI] [PubMed] [Google Scholar]
- Kagan, H.M., and W. Li. 2003. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem. 88:660–672. [DOI] [PubMed] [Google Scholar]
- Keeley, F.W. 1976. A convenient method for the identification and estimation of soluble elastin synthesis in vitro. Connect. Tissue Res. 4:193–203. [DOI] [PubMed] [Google Scholar]
- Kielty, C.M., M.J. Sherratt, and C.A. Shuttleworth. 2002. Elastic fibres. J. Cell Sci. 115:2817–2828. [DOI] [PubMed] [Google Scholar]
- Kowal, R.C., J.A. Richardson, J.M. Miano, and E.N. Olson. 1999. EVEC, a novel epidermal growth factor-like repeat-containing protein upregulated in embryonic and diseased adult vasculature. Circ. Res. 84:1166–1176. [DOI] [PubMed] [Google Scholar]
- Kuang, P.P., R.H. Goldstein, Y. Liu, D.C. Rishikof, J.C. Jean, and M. Joyce-Brady. 2003. Coordinate expression of fibulin-5/DANCE and elastin during lung injury repair. Am. J. Physiol. Lung Cell. Mol. Physiol. 285:L1147–L1152. [DOI] [PubMed] [Google Scholar]
- Liu, X., Y. Zhao, J. Gao, B. Pawlyk, B. Starcher, J.A. Spencer, H. Yanagisawa, J. Zuo, and T. Li. 2004. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 36:178–182. [DOI] [PubMed] [Google Scholar]
- Maki, J.M., J. Rasanen, H. Tikkanen, R. Sormunen, K. Makikallio, K.I. Kivirikko, and R. Soininen. 2002. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 106:2503–2509. [DOI] [PubMed] [Google Scholar]
- Mecham, R.P. 1987. Modulation of elastin synthesis: in vitro models. Methods Enzymol. 144:232–246. [DOI] [PubMed] [Google Scholar]
- Merklinger, S.L., R.A. Wagner, E. Spiekerkoetter, A. Hinek, R.H. Knutsen, M.G. Kabir, K. Desai, S. Hacker, L. Wang, G.M. Cann, et al. 2005. Increased fibulin-5 and elastin in S100A4/Mts1 mice with pulmonary hypertension. Circ. Res. 97:596–604. [DOI] [PubMed] [Google Scholar]
- Molnar, J., K.S. Fong, Q.P. He, K. Hayashi, Y. Kim, S.F. Fong, B. Fogelgren, K.M. Szauter, M. Mink, and K. Csiszar. 2003. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim. Biophys. Acta. 1647:220–224. [DOI] [PubMed] [Google Scholar]
- Nakamura, T., P. Ruiz-Lozano, V. Lindner, D. Yabe, M. Taniwaki, Y. Furukawa, K. Kobuke, K. Tashiro, Z. Lu, N.L. Andon, et al. 1999. DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J. Biol. Chem. 274:22476–22483. [DOI] [PubMed] [Google Scholar]
- Nakamura, T., P.R. Lozano, Y. Ikeda, Y. Iwanaga, A. Hinek, S. Minamisawa, C.F. Cheng, K. Kobuke, N. Dalton, Y. Takada, et al. 2002. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 415:171–175. [DOI] [PubMed] [Google Scholar]
- Nguyen, A.D., S. Itoh, V. Jeney, H. Yanagisawa, M. Fujimoto, M. Ushio-Fukai, and T. Fukai. 2004. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ. Res. 95:1067–1074. [DOI] [PubMed] [Google Scholar]
- Pasquali-Ronchetti, I., and M. Baccarani-Contri. 1997. Elastic fiber during development and aging. Microsc. Res. Tech. 38:428–435. [DOI] [PubMed] [Google Scholar]
- Rosenbloom, J., W.R. Abrams, and R. Mecham. 1993. Extracellular matrix 4: the elastic fiber. FASEB J. 7:1208–1218. [PubMed] [Google Scholar]
- Shapiro, S.D., S.K. Endicott, M.A. Province, J.A. Pierce, and E.J. Campbell. 1991. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Invest. 87:1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, S.S., P.C. Trackman, and H.M. Kagan. 1983. Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J. Biol. Chem. 258:4331–4338. [PubMed] [Google Scholar]
- Urry, D.W. 1988. Entropic elastic processes in protein mechanisms. I. Elastic structure due to an inverse temperature transition and elasticity due to internal chain dynamics. J. Protein Chem. 7:1–34. [DOI] [PubMed] [Google Scholar]
- Vrhovski, B., S. Jensen, and A.S. Weiss. 1997. Coacervation characteristics of recombinant human tropoelastin. Eur. J. Biochem. 250:92–98. [DOI] [PubMed] [Google Scholar]
- Wachi, H., F. Sato, H. Murata, J. Nakazawa, B.C. Starcher, and Y. Seyama. 2005. Development of a new in vitro model of elastic fiber assembly in human pigmented epithelial cells. Clin. Biochem. 38:643–653. [DOI] [PubMed] [Google Scholar]
- Yanagisawa, H., E.C. Davis, B.C. Starcher, T. Ouchi, M. Yanagisawa, J.A. Richardson, and E.N. Olson. 2002. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 415:168–171. [DOI] [PubMed] [Google Scholar]
- Zanetti, M., P. Braghetta, P. Sabatelli, I. Mura, R. Doliana, A. Colombatti, D. Volpin, P. Bonaldo, and G.M. Bressan. 2004. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol. Cell. Biol. 24:638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]