Abstract
The ubiquitin (Ub) domain protein Herp plays a crucial role in the maintenance of calcium homeostasis during endoplasmic reticulum (ER) stress. We now show that Herp is a substrate as well as an activator of the E3 Ub ligase POSH. Herp-mediated POSH activation requires the Ubl domain and exclusively promotes lysine-63–linked polyubiquitination. Confocal microscopy demonstrates that Herp resides mostly in the trans-Golgi network, but, shortly after calcium perturbation by thapsigargin (Tpg), it appears mainly in the ER. Substitution of all lysine residues within the Ubl domain abolishes lysine-63–linked polyubiquitination of Herp in vitro and calcium-induced Herp relocalization that is also abrogated by the overexpression of a dominant-negative POSHV14A. A correlation exists between the kinetics of Tpg-induced Herp relocalization and POSH-dependent polyubiquitination. Finally, the overexpression of POSH attenuates, whereas the inhibition of POSH by the expression of POSHV14A or by RNA interference enhances Tpg-induced calcium burst. Altogether, these results establish a critical role for POSH-mediated ubiquitination in the maintenance of calcium homeostasis through the spatial control of Herp.
Introduction
POSH (plenty of SH3s) was initially identified as a Rac-binding protein and an activator of the JNK and nuclear factor κB signaling pathways (Tapon et al., 1998). Subsequently, POSH was shown to activate JNK signaling by acting as a scaffold for mixed lineage kinases (Xu et al., 2003b), a function negatively regulated by the protein kinase Akt2 (Figueroa et al., 2003). As a regulator of JNK, POSH is mainly implicated in the activation of apoptosis and differentiation of neuronal cells (Xu et al., 2003b; Kim et al., 2005a; Zhang et al., 2005). Apoptotic stimuli increase the expression of POSH, mixed lineage kinases, JNK, and Siah1, and the latter is a POSH-interacting E3 ligase and a known activator of the JNK pathway (Xu et al., 2006). Conversely, siRNA-mediated silencing of POSH confers neuroprotection (Zhang et al., 2005). In contrast to its proapoptotic function in mammalian neurons, the neuronal-specific expression of POSH extends the longevity of adult fruit flies (Drosophila melanogaster; Seong et al., 2001).
POSH contains an N-terminal RING finger domain, which is a hallmark of many ubiquitin (Ub) E3 ligases. The E3 Ub ligase family of proteins consists of hundreds of structurally diverse enzymes that determine the specificity of Ub conjugation through specific recognition of substrates and the recruitment of cognate E2 Ub-conjugating enzymes (Hershko and Ciechanover, 1998). Indeed, recent studies implicate the Ub ligase function of POSH in the production of infectious HIV-1 (Alroy et al., 2005) in degradation of the early endosome resident sorting factor Hrs (hepatocyte growth factor–regulated tyrosine kinase substrate; Kim et al., 2006) and in control of the Drosophila immune system via degradation of the JNK activator TAK-1 (Tsuda et al., 2005).
We now report the identification of Herp (homocysteine-inducible ER protein) as a novel ubiquitination substrate and regulator of POSH. Herp, which contains a Ub-like domain, is an ER stress–inducible protein critical for cell survival under stress. The underlying mechanism by which Herp exerts its protective function has been obscure, although it likely involves the control of calcium homeostasis during ER stress (Chan et al., 2004). We previously showed that POSH is a TGN-associated protein despite lacking a detectable transmembrane domain (Alroy et al., 2005). In the present study, we show that POSH associates with the TGN membrane through an association with Herp. Within minutes of the perturbation of intracellular calcium by the calcium-perturbing agent thapsigargin (Tpg) or induction of ER stress by the glycosylation inhibitor tunicamycin (Tm), Herp is redistributed from the TGN to the ER. The increased ER expression of Herp occurs long before the ER stress–induced enhancement of Herp expression and is dependent on the POSH-mediated conjugation of lysine-63–linked poly-Ub chains to Herp. Thus, we provide evidence that POSH regulates calcium homeostasis by increasing the levels of Herp in the ER.
Results
Herp is a POSH-interacting protein
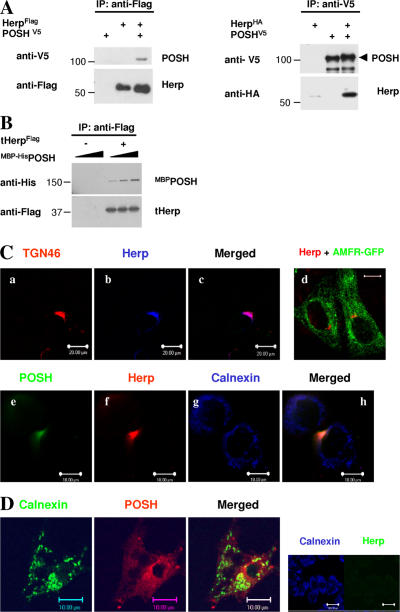
Herp was identified as a POSH-interacting protein through a yeast two-hybrid screen of a HeLa cDNA expression library. The POSH construct used as bait in the screen lacked the RING domain. To confirm the interaction between POSH and Herp, detergent extracts from cells transiently coexpressing epitope-tagged POSH and Herp were subjected to immunoprecipitation followed by Western blot analysis. POSH and Herp were both coprecipitated by antibodies to the complementary protein (Fig. 1 A). Similar coimmunoprecipitation was observed in vitro after the incubation of bacterially expressed maltose-binding protein (MBP) POSH fusion protein (MBPPOSH) and truncated Herp (tHerp; amino acids 1–272), which lacked the putative membrane-associated domain (Fig. 1 B). Therefore, the interaction between POSH and Herp is direct.
Figure 1.
POSH and Herp are interacting proteins. (A) In vivo interaction. HeLa cells were transfected with vectors encoding Flag-tagged Herp and V5-tagged POSH. Cells were subsequently lysed, and either Herp (left) or POSH (right) immune complexes were isolated and subjected to Western blot analyses with anti-V5 or anti-Flag for the detection of POSH and Herp, respectively. (B) In vitro interaction. His-tagged bacterially expressed MBP-POSH. (MBP-HisPOSH) was incubated either alone or with increasing concentrations of tHerp-Flag. Herp immune complex isolated with anti-Flag was subsequently subjected to Western blot analysis with anti-His. (C) Intracellular localization of Herp and POSH. (a–c) Herp localizes with TGN46. Immunostaining: TGN46 sheep anti-TGN46 and Cy3-conjugated donkey anti–sheep; Herp 25B and Cy5-conjugated goat anti–rabbit (for specificity of 25B to Herp, see abolishment of the signal upon silencing of Herp by siRNA; right panel in D). (d) Immunofluorescence analysis of Herp and gp78/AMFR. Cells were transfected with a plasmid encoding AMFR-GFP fusion protein and subsequently subjected to confocal microscopy. Immunostaining: Herp 25B and Cy3-conjugated goat anti–rabbit. Bar, 10 μm. (e–h) Immunofluoresence analysis of POSH and Herp. Cells were initially stained for POSH with rabbit anti-POSH (Alroy et al., 2005) and Cy2-conjugated goat anti–rabbit and for calnexin with anticalnexin followed by Cy5-conjugated goat anti–mouse. After the initial staining, cells were blocked with a 10-fold excess of unconjugated goat anti–rabbit IgG and then were stained for Herp with 25B and Cy3-conjugated goat anti–rabbit. (D) POSH in Herp-depleted cells. Immunostaining: calnexin mouse anticalnexin and Cy2-conjugated goat anti–mouse; POSH rabbit anti-POSH and Cy3-conjugated anti–rabbit. (right) Immunofluorescence analysis of Herp-depleted cells. Immunostaining: calnexin anticalnexin and Cy5-conjugated anti–mouse; Herp 25B and Cy2-conjugated anti–rabbit. (D, right) Bars, 100 μm.
POSH is associated exclusively with the TGN membrane (Alroy et al., 2005), whereas Herp was reported as an ER resident protein (Kokame et al., 2000). If the interaction between POSH and Herp is physiologically relevant, it would require that both proteins be present in the same intracellular compartment. To resolve this issue, we used immunofluorescence microscopy to determine the intracellular localization of endogenous Herp. The results indicate a polar distribution of endogenous Herp around the nucleus and colocalization with the TGN marker TGN46 (Fig. 1 C, a–c). To further confirm the TGN localization of Herp, we determined the intracellular distribution of Herp in cells expressing a fusion between autocrine motility factor receptor (AMFR) and GFP. The E3 ligase gp78/AMFR is an integral ER membrane protein (Fang et al., 2001). Consequently, AMFR-GFP is detected in a characteristic ER reticular network structure throughout the cell, from which Herp, which retains its polar distribution, is mostly excluded (Fig. 1 C, d). A similar distribution of Herp is observed in cells stained with POSH and Herp antibodies. Herp colocalizes with POSH in a typical TGN appearance, and both proteins are mostly excluded from the ER based on costaining with an antibody against the ER resident chaperone calnexin (Fig. 1 C, e–h).
The discrepancy between our findings and previous studies (Kokame et al., 2000; Sai et al., 2003) showing mainly ER localization for Herp is likely an outcome of different immunostaining protocols. The staining protocol used throughout this study excludes detergents, as we had noticed that Herp loses its TGN localization when 0.05% Tween 20 is used in the immunofluorescence staining procedure (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200611036/DC1). Related to this is the unresolved issue of the membrane topology of Herp. A previous study indicated that the majority of Herp faces the cytoplasm and predicted a short transmembrane segment (Sai et al., 2003; Chan et al., 2004). However, no physical evidence has been provided for membrane integration. The fact that the subcellular localization of Herp is sensitive to mild detergents suggests either peripheral membrane association or dynamic insertion of the protein into the TGN membrane. Nevertheless, we cannot exclude the possibility that Herp is also expressed at low levels in the ER.
POSH is a soluble protein associated with the TGN membrane. Because Herp expresses a hydrophobic C-terminal region implicated in membrane binding (Kokame et al., 2000), we tested whether Herp mediates POSH binding to the TGN. To this end, we determined the subcellular localization of POSH in cells treated with siRNA to knock down the expression of Herp (Fig. 1 D). Immunofluorescence analysis demonstrates that silencing of Herp expression causes a redistribution of POSH throughout the cell. Therefore, POSH association with the TGN membrane is indeed Herp dependent.
Herp is a POSH ubiquitination substrate
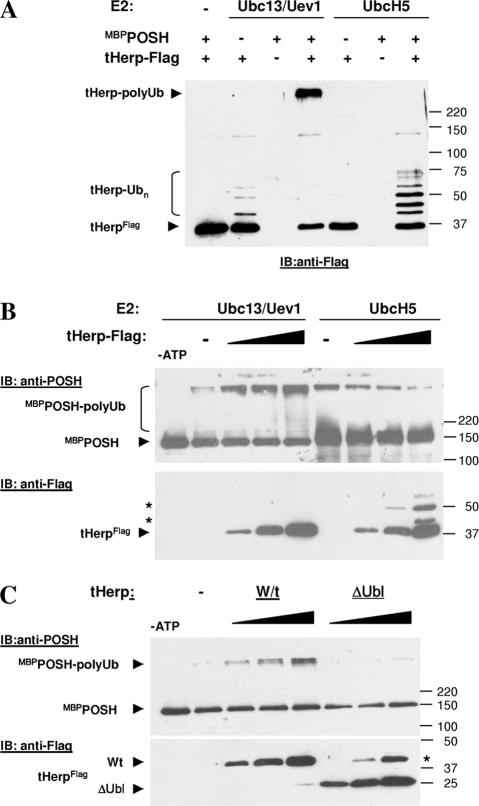
The association of Herp with POSH prompted us to test whether Herp is a POSH ubiquitination substrate. POSH ubiquitinates itself in the presence of the E2 ligases UbcH5 (Alroy et al., 2005) or Ubc13/Uev1A (Fig. 2 B). Therefore, we tested whether Herp is ubiquitinated by POSH in the presence of either E2 enzyme (Fig. 2 A). When tHerp-Flag was incubated in an in vitro ubiquitination reaction in the presence of Ubc13/Uev1a and MBPPOSH, high mol wt tHerp–poly-Ub conjugates were efficiently synthesized based on anti-Flag immunoblotting. Interestingly, very low mol wt Herp-Ub conjugates were also generated in the absence of POSH. In a ubiquitination reaction using UbcH5, Herp was also ubiquitinated, but only low mol wt species corresponding to one to approximately six conjugated Ub molecules per Herp protein were generated.
Figure 2.
Herp is a POSH ubiquitination substrate. (A) Ubiquitination of Herp in vitro. Bacterially expressed tHerp-Flag was incubated with MBPPOSH in an in vitro ubiquitination reaction either with Ubc13/Uev1a or UbcH5c as described in Materials and methods. Herp-Flag conjugates were subsequently detected by Western blot analysis with anti-Flag. (B) Herp activates POSH ubiquitination activity. MBPPOSH was incubated in vitro with either Ubc13/Uev1a or UbcH5c and with tHerp-Flag at POSH/Herp molar ratios of 1:1, 1:3, and 1:10. The reaction was resolved by SDS-PAGE and subsequently subjected to Western blot analysis with anti-POSH mAb, PT1 (top), and anti-Flag (bottom). Asterisks indicate the positions of mono- and di-Herp Ub. (C) An in vitro ubiquitination reaction was performed with Ubc13/Uev1 in the presence of either wild-type (Wt) or HerpΔUbl and subsequently analyzed by Western blotting as indicated in the figure. The asterisk indicates the position of the migration of residual, uncut HerpΔUbl-GST fusion protein.
Herp activates Ubc13/Uev1-dependent POSH self-ubiquitination
The heteromeric Ubc13/Uev1a E2 is exclusively involved in the generation of lysine K63–linked poly-Ub chains (Hofmann and Pickart, 2001; Pickart, 2001). Rather than target proteins for proteasomal degradation, K63-linked polyubiquitination serves regulatory functions mainly through the promotion of protein–protein interactions (Pickart and Fushman, 2004). Activation of the inhibitor of nuclear factor κB kinase requires the formation of K63-linked poly-Ub chains on the TNF receptor–associated factor (TRAF) 2 or 6 adaptor proteins. The TRAFs are also RING proteins that catalyze the formation of K63-linked Ub chains on themselves and on other proteins. This activity is promoted by distinct activators and coincides with activator-induced TRAF oligomerization (Ea et al., 2004; Sun et al., 2004). By analogy, the association of POSH with Herp and the fact that POSH also mediates the formation of K63-linked poly-Ub chains prompted us to test whether Herp also functions as a POSH activator in addition to constituting a POSH substrate. To this end, we performed an in vitro POSH self-ubiquitination assay in the absence or presence of Herp and subsequently detected POSH ubiquitination by Western blot analysis with a POSH mAb. High mol wt POSH-Ub adducts are substantially stimulated upon the addition of increasing concentrations of tHerp (Fig. 2 B). Activation of POSH by Herp is specific to Ubc13/Uev1a (Fig. 2 B) and requires the Herp Ubl domain (Fig. 2 C).
Herp-induced oligomerization activates POSH
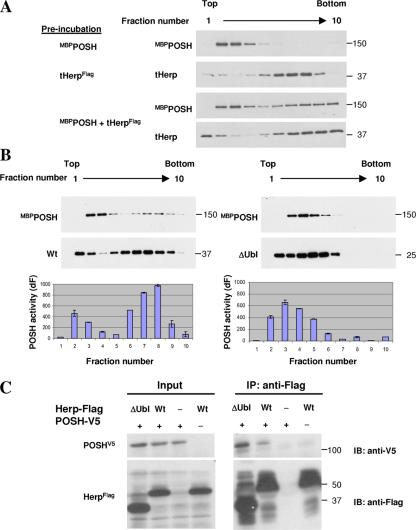
We next tested whether POSH activation also requires its oligomerization, as occurs with the activation of TRAF. First, we investigated whether Herp influences the oligomeric state of POSH by determining POSH sedimentation in glycerol density gradients in the presence or absence of Herp. The results indicate that when POSH is incubated alone, it migrates as a low mol wt species in fractions 2–4 (Fig. 3 A, top). tHerp migrates in two distinct positions in the gradient (a minor low mol wt peak and a major high mol wt peak), indicating that the bulk of tHerp is in an oligomeric state (Fig. 3 A, middle). Notably, when POSH is incubated together with Herp before centrifugation, a portion of POSH subsequently appears as a broad high mol wt peak in fractions 7–10, and this peak coincides with that of tHerp (Fig. 3 A, bottom). It is noteworthy that the peak of oligomeric Herp moves further toward the bottom of the gradient in the presence of POSH. These observations strongly suggest that POSH and Herp form a heterooligomeric complex.
Figure 3.
Herp-induced POSH oligomerization activates POSH. (A) Herp induces POSH oligomerization. MBPPOSH and tHerp were incubated either alone or together. The incubation mixture was then resolved by glycerol density centrifugation as described in Materials and methods. An aliquot of each gradient fraction was subsequently subjected to Western blot analysis with the indicated antibodies. (B) Oligomeric POSH is activated. MBPPOSH was incubated with either wild-type (Wt) or ΔUbl Herp. The incubation mixtures were subsequently resolved by glycerol density centrifugation. An aliquot of each gradient fraction was subjected to Western blot analysis with PT1 and anti-Flag for the detection of POSH and Herp, respectively (top). An 8-μl aliquot of each fraction was incubated in an in vitro ubiquitination reaction in the presence of Ubc13/Uev1a. POSH self-ubiquitination was determined (bottom) by the homogeneous time-resolved fluorescence method as described in Materials and methods. Error bars represent SD. (C) The Ubl domain of Herp is dispensable for interaction with POSH. Cells were cotransfected with Herp (either wild type or ΔUbl)-Flag and V5-POSH encoding plasmids as indicated. Herp immune complexes were then isolated and subjected to Western blot analysis with anti-V5.
Because Herp both activates POSH self-ubiquitination and induces POSH oligomerization, we asked whether these two Herp activities were related. The Ubl domain of Herp is essential for POSH activation, so we tested whether HerpΔUbl was also defective for inducing POSH oligomerization. Indeed, unlike tHerp, tHerpΔUbl failed to increase POSH sedimentation in a glycerol density gradient (Fig. 3 B, top). To confirm that oligomerization activates POSH, we determined the self-ubiquitination activity of POSH protein isolated from the gradient fractions (Fig. 3 B, bottom). Aliquots from each fraction were incubated with E1, Ub-activating enzyme, Ubc13/Uev1a, Ub, and ATP in an in vitro ubiquitination assay. In this reaction, Herp-Ub as well as POSH-Ub conjugates are synthesized. Therefore, ubiquitinated POSH was determined only after the removal of Herp-Ub conjugates by immunoprecipitation. The results indicated that after incubation of POSH with tHerp, two activity peaks were detected. The first at fractions 2 and 3 corresponded to low mol wt POSH, and the second at fractions 6–9 coincided with oligomeric POSH. The activity in the oligomerized POSH fractions was considerably higher than that in the monomeric POSH fractions despite the smaller amount of POSH in the high mol wt fractions, indicating that oligomerization substantially increases POSH-specific activity. As expected, only a single activity peak coinciding with monomeric POSH appeared in the presence of tHerpΔUbl. Together, these results indicate that the oligomerization of POSH is closely linked to Herp-induced POSH activation.
The failure of tHerpΔUbl to activate POSH does not result from an inability to interact with POSH. A Herp pull-down experiment indicated that POSH was present in Herp as well as in HerpΔUbl immune complexes (Fig. 3 C). This result is consistent with the decreased size of Herp oligomers as determined by glycerol density centrifugation (Fig. 3 B) and suggests that POSH oligomerizes through interaction with high mol wt Herp oligomers, the formation of which requires the Ubl domain.
Herp redistributes to the ER upon the induction of ER stress
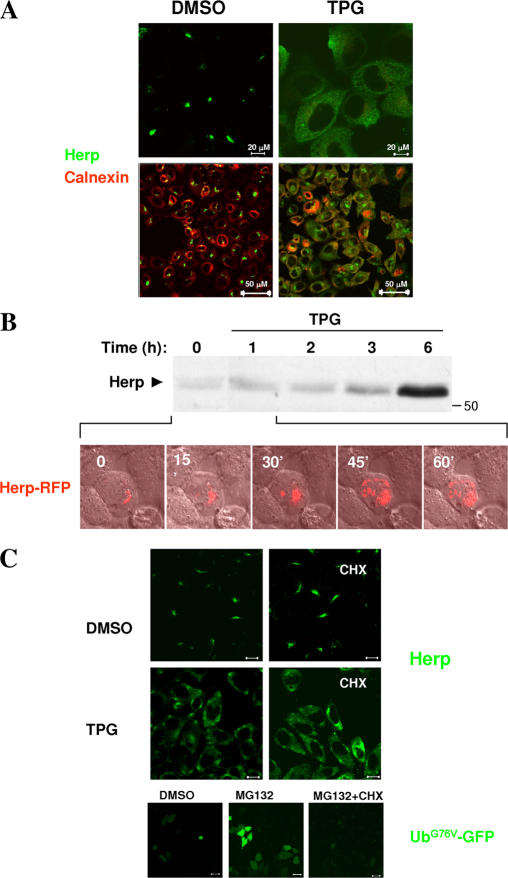
Herp is an ER stress–induced protein whose reported functions include the enhancement of ER-associated degradation (ERAD) via association with the E3 ligase HRD1/synoviolin (Schulze et al., 2005) and maintenance of low cytosolic calcium during ER stress (Chan et al., 2004). Both of these functions suggest that the Herp ER stress–protective functions are performed at the ER membrane. Because we find Herp associated primarily with the TGN, we postulated that Herp is deployed to the ER upon the induction of ER stress. To test this hypothesis, we determined the subcellular localization of Herp in resting and ER-stressed cells by immunofluorescence microscopy. ER stress was induced either by perturbation of calcium by the calcium ATPase inhibitor Tpg, which causes the rapid accumulation of calcium from the ER lumen to the cytosol, or by the protein glycosylation inhibitor Tm, which causes the accumulation of misfolded proteins in the ER. Immunostaining of endogenous Herp indicates that after a 1-h incubation with Tpg, Herp distribution changes dramatically from a focal to a diffuse appearance (Fig. 4 A, top). Confocal microscopy further reveals that after Tpg treatment, Herp staining generally overlaps with the ER marker calnexin (Fig. 4 A, bottom). Herp stress-induced redeployment is also observed after Tpg treatment of PC3 and SW480 prostate and colon cancer cell lines, respectively, indicating that this is a general response in multiple cell types (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200611036/DC1).
Figure 4.
Tpg induces Herp redistribution. (A) Endogenous Herp was visualized by immunofluorescence microscopy after incubation of HeLa cells for 1 h with either 2 μM Tpg or DMSO solvent. (B) Kinetic analysis of Herp induction and redistribution. (top) Endogenous Herp was detected by Western blot analysis of extracts from HeLa cells treated with Tpg for the indicated time periods. (bottom) HeLa cells transiently expressing Herp-RFP fusion protein were visualized at 5-min intervals (until 1 h) after the addition of Tpg. The images taken at the 15-min intervals are presented. (C, top) Immunofluorescence of Herp in cells incubated with either DMSO or Tpg in the absence (left) or presence (right) of 50 μM CHX. (bottom) CHX inhibits the stabilization of UbG76V-GFP by MG132. Cells transiently expressing UbG76V-GFP were incubated for 1 h with the indicated reagents and were subsequently visualized by fluorescence microscopy. Bars, 50 μm.
Upon ER stress, the expression of Herp is strongly elevated as a result of the induction of gene transcription (Kokame et al., 2000; Hori et al., 2004; Ma and Hendershot, 2004). Thus, we tested the possibility that Herp redistribution to the ER is caused by its strong overexpression. To resolve this issue, we compared the kinetics of stress-induced Herp redeployment with the induction of Herp expression. Western blot analysis of endogenous Herp demonstrates distinct kinetics for Herp redistribution and induction of Herp expression. Induction of expression occurs between 3 and 6 h after Tpg addition (Fig. 4 B, top). In contrast, live cell imaging of Herp-RFP indicates that deployment to the ER is initiated within 15 min of the addition of Tpg and is complete within 45 min. Similarly, Herp relocation is complete within 2 h, whereas Herp protein accumulation occurs only between 3 and 6 h after the initiation of Tm treatment (Fig. S2). Thus, Herp stress-induced redistribution clearly occurs on a much faster timescale than Herp protein induction, so increased Herp protein levels during ER stress cannot account for its rapid relocalization.
We also tested the possibility that stress-induced ER deployment of Herp results from the selective stabilization of a rapidly turning over (unstable) Herp population in the ER. Thus, we determined the effect of the protein synthesis inhibitor cycloheximide (CHX) on Herp redistribution. If there was a rapidly degraded pool of ER-localized Herp that was stabilized by Tpg treatment, the appearance of Herp in the ER would require continuous protein synthesis coupled with the inhibition of protein degradation. If the stabilization of Herp in the ER was the mechanism of Herp redistribution, CHX, by preventing new protein synthesis, would also prevent Herp ER appearance. Consequently, cells were incubated either with solvent or CHX alone or with Tpg for 1 h followed by visualization of Herp by immunofluorescence microscopy (Fig. 4 C, top). The result indicated that Tpg-induced Herp redistribution was indistinguishable in the presence and absence of CHX, establishing that new protein synthesis was not involved. In a parallel experiment, CHX completely abolished the accumulation of unstable UbG76V-GFP fusion protein (Dantuma et al., 2000) in the presence of the proteasome inhibitor MG132, confirming the potency of the CHX inhibition (Fig. 4 C, bottom). From these results, we conclude that immediately after the induction of ER stress, Herp relocalizes from the TGN to the ER.
Herp relocalization is dependent on POSH ubiquitination activity
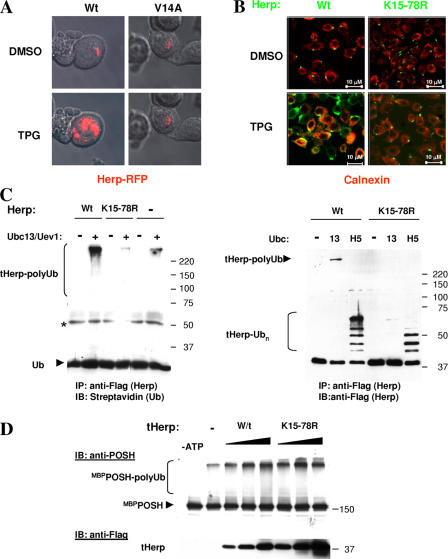
The fact that Herp is a POSH ubiquitination substrate prompted us to investigate whether POSH-mediated ubiquitination is involved in the stress-induced Herp redistribution. To this end, we tested whether redistribution is inhibited by expression of the RING finger mutant POSHV14A, which is inactive as a Ub ligase and was previously shown to function in a dominant-negative fashion (Alroy et al., 2005). Cells were transfected with Herp-RFP together with either native or the dominant-negative POSH. Tpg was subsequently added to each culture, and Herp-RFP was immediately visualized. The live fluorescence analysis revealed that although Tpg stimulated the redistribution of Herp when wild-type POSH was overexpressed, Herp was refractory to Tpg treatment and retained its polar distribution in the presence of the dominant-negative POSH mutant (Fig. 5 A).
Figure 5.
POSH-mediated ubiquitination of the Ubl domain is required for Tpg-induced translocation. (A) Expression of dominant-negative POSH inhibits Herp redistribution. HeLa cells transiently coexpressing Herp-RFP and POSH (either wild type [Wt] or V14A) were visualized after the addition of either DMSO or Tpg. (B) HeLa cells expressing either Herp-Flag or HerpK15-78R were incubated with either DMSO or Tpg. Cells were subsequently immunostained in tandem; initially for Herp-Flag (with anti-Flag and Cy2-conjugated anti–mouse), and then blocked with an excess of unconjugated anti–mouse and for calnexin (with mouse anticalnexin and Cy3-cojugated anti–mouse). (C) Conjugation of Herp in vitro. Herp wild type and K15-78R mutant were incubated in the presence of either Ubc13/Uev1a (13) or UbcH5c (H5) in an in vitro ubiquitination reaction containing N-terminal biotin-Ub. After the incubation, Herp was pulled down with anti-Flag, and the Herp immune complexes were resolved by SDS-PAGE. Herp-Ub conjugates were then detected by Western blot analysis either with streptavidin (left) or with anti-Flag (right). The asterisk indicates a nonspecific immunoreactive protein band. (D) Activation of POSH by wild-type and mutant Herp. In vitro ubiquitination reactions were performed in the presence of increasing amounts of Herp as described in Fig. 2. POSH-Ub conjugates were detected by Western blot analysis with PT1.
Ubiquitination of the Ubl domain is required for calcium-induced Herp redistribution
A previous study showed that Herp is rapidly degraded in a proteasome-dependent fashion (Sai et al., 2003). In the present study, we show that POSH promotes the conjugation of K63- but not K48-linked Herp–poly-Ub chains, suggesting that it is not directly involved in the degradation of Herp. Therefore, to further analyze the functional role of POSH and of K63 polyubiquitination, we compared the effect of Tpg on the redistribution of Herp and HerpK15-78R, a mutant in which all of the lysine residues within the Ubl domain had been substituted with arginines. As shown in Fig. 5 B, when cells were incubated with DMSO, Herp staining appeared as small speckles discrete from calnexin. As expected, upon Tpg treatment, wild-type Herp was redistributed and colocalized with calnexin, indicating ER localization (Fig. 5 B, left), whereas HerpK15-78R remained immobile (Fig. 5 B, right).
The inability of HerpK15-78R to redistribute in response to stress can result from either an inability to activate POSH or because it cannot be ubiquitinated as a result of the loss of the ubiquitination acceptor sites (or both). To distinguish between these possibilities, we compared the capacity of wild-type and mutant Herp to serve as substrates of POSH and as POSH activators in vitro. The comparison indicated that the lysine mutations completely abolished the Ubc13/Uev1a-dependent ubiquitination, whereas the UbcH5-dependent ubiquitination of both Herp proteins was essentially similar (Fig. 5 C). Both wild-type and mutant Herp demonstrate a similar ability to activate POSH self-ubiquitination (Fig. 5 D), indicating that HerpK15-78R can functionally interact with POSH. The correlation between the abolishment of K63-linked polyubiquitination in vitro and the failure to relocalize Herp in vivo suggests that POSH-mediated K63-linked polyubiquitination of the Ubl domain of Herp is essential for Herp relocalization to the ER.
HerpK15-78R ubiquitination in the presence of UbcH5c indicates that lysine residues downstream of the Ubl domain can also serve as ubiquitination sites. Herp ubiquitination at downstream lysine residues is consistent with the results of Sai et al. (2003), who showed that truncation of the Ubl domain inhibits the proteasomal degradation but does not eliminate ubiquitination. Thus, the functional significance of Herp ubiquitination downstream of the Ubl domain remains obscure.
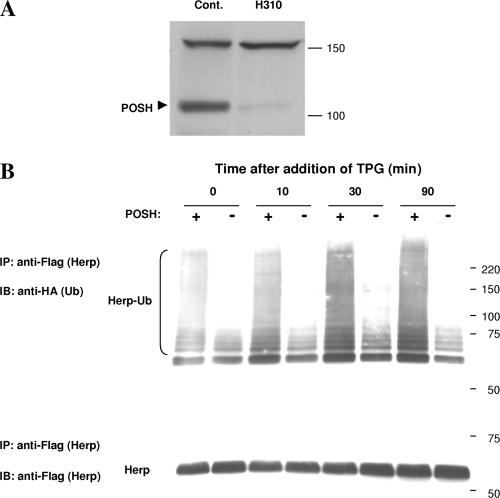
Herp ubiquitination in vivo is POSH dependent and is stimulated by calcium perturbation
The model by which POSH-mediated K63-linked polyubiquitination is essential for calcium-dependent Herp relocalization predicts that Herp is ubiquitinated in vivo upon the induction of ER stress. To test this prediction directly, we monitored the effects of Tpg and POSH on the kinetics of Herp ubiquitination in a stable POSH knockdown cell line that expresses substantially reduced POSH levels (Fig. 6 A). To this end, H310 cells were cotransfected with Herp-Flag and HA-tagged UbK63, a Ub derivative in which all lysine residues but lysine-63 are mutated to arginines, in the presence or absence of POSH overexpression. The cells were subsequently treated with Tpg for various time periods, after which Herp ubiquitination was analyzed by Western blot analysis of isolated Herp- Flag immune complexes with anti-HA. The results (Fig. 6 B, time 0) show that the overexpression of POSH markedly increased the ubiquitination of Herp in H310 cells, indicating that POSH is a rate-limiting factor for Herp K63-linked polyubiquitination in vivo. The level of Herp ubiquitination is further stimulated after the addition of Tpg: a considerable increase in Herp polyubiquitination is observed at 10 min and further intensifies at 30 min after Tpg addition. A slight decrease in Herp ubiquitination is subsequently observed after 90 min. Collectively, these results indicate that the ubiquitination of Herp is POSH dependent and is regulated by calcium. Furthermore, the kinetics of Herp ubiquitination in vivo is coincident with the kinetics of Tpg-induced Herp redistribution (Fig. 4 B) and, thus, supports a mechanism whereby POSH-mediated Herp ubiquitination is activated by calcium and regulates Herp ER localization.
Figure 6.
Herp K63-linked polyubiquitination is POSH dependent and is enhanced by Tpg. (A) Analysis of POSH expression in H310 cells constitutively expressing a POSH shRNA and in H314 cells (control) expressing a scrambled shRNA. Detection of POSH was performed by Western blot analysis with anti-POSH (PT1). (B) Analysis of Herp ubiquitination in H310 cells. Cells were cotransfected with Herp-Flag and HA-Ub-K63 either in the presence (+) or absence (−) of shRNA-resistant POSH. 24 h after the transfection, 2 μM Tpg was added to each culture, and the cells were further incubated for the indicated time periods, after which detergent lysates were prepared and Herp was isolated by immunoprecipitation with anti-Flag. For detection of Herp-Ub conjugates, the Herp immune complexes were resolved by SDS-PAGE (7.5% gels) and subsequently subjected to Western blot analysis with anti-HA (Ub; top) or with anti-Flag (Herp; bottom).
POSH-mediated ubiquitination is required for the restriction of free calcium
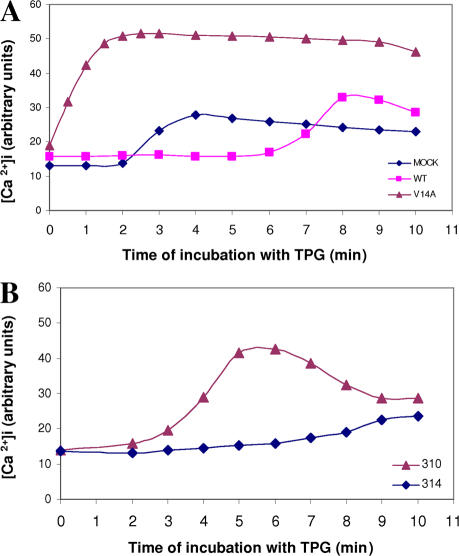
Herp restricts calcium flow in neuronal cells subjected to agents that transiently increase intracellular calcium such as Tpg and bradykinin (Chan et al., 2004). Maintenance of low cytosolic calcium likely requires the regulation of ER calcium channels by Herp. As POSH-mediated ubiquitination is obligatory for the recruitment of Herp to the ER, we predicted that inhibition of POSH activity would accelerate calcium release from the ER in the presence of Tpg. To test this hypothesis, we determined the initial rates of Tpg-induced calcium release in mock-transfected cells and in cells expressing either wild-type or dominant-negative POSH. Because during the experiment, cells were kept in a calcium-free medium, the observed elevation in intracellular calcium reflected the influx of ER calcium. The results (Fig. 7 A) demonstrate that in the control culture, calcium release was initiated ∼2 min after the addition of Tpg and reached a maximum after 4 min. Consistent with the hypothesis that POSH function is rate limiting for Herp relocalization, the initiation of calcium release was considerably delayed in cultures overexpressing native POSH and started only 6 min after Tpg addition. In contrast, in cultures expressing POSHV14A, the rate of calcium release was dramatically accelerated: it was initiated instantaneously, increased rapidly, and reached a twofold higher maximal value within 2 min. The initial rate of calcium release was also dramatically accelerated in Tpg-treated H310 cells relative to control cells (Fig. 7 B), further indicating the essential role of POSH in the regulation of cytosolic calcium. Together, these results establish a crucial role for POSH-mediated ubiquitination in the maintenance of calcium homeostasis through the regulation of Herp.
Figure 7.
POSH ubiquitination activity is required for restriction of calcium flow. Quantitative analysis of intracellular calcium before (time 0) and after the addition of 0.5 μM Tpg. (A) Cells transiently transfected with empty (mock), POSH (wild type [WT]), or dominant negative (V14A). (B) H310 and H314 cells. Intracellular free calcium ([Ca2+]i) was detected with the calcium-sensitive dye Fluo-4 and quantified as described in Materials and methods. Each point in the graph represents the Fluo-4 intensity of an image taken at the indicated time point (Fig. S4 shows calcium images of experiment A; available at http://www.jcb.org/cgi/content/full/jcb.200611036/DC1).
Discussion
Herp is an essential factor for ER stress resistance. A Herp knockout study in mice indicates that in Herp-null cells, ER stress signaling and ERAD are reduced, whereas ER stress–induced cell death is increased (Hori et al., 2004). The molecular basis for Herp function during stress has recently started to be addressed. Herp function was directly linked to the ERAD machinery and to the stabilization of calcium homeostasis, functions that both require ER localization. Nevertheless, all studies describing the role of Herp during stress are based on data obtained from cells that were subjected to prolonged stress periods. In this study, we report a regulatory mechanism that recruits Herp to the ER immediately after the induction of ER stress. Although we cannot exclude ER localization, we provide evidence that in resting cells, Herp is primarily associated with the TGN (Fig. 1 C) and that only after the induction of stress does it appear mainly in the ER (Figs. 4 A and 5 B). Stress-induced ER expression of Herp occurs within minutes and is independent of stress-induced Herp protein induction (Fig. 4 B), suggesting that Herp protein relocalization is involved.
The mechanism of Herp relocalization is unclear. Because Herp is a membrane-embedded protein, retrograde transport likely involves release from a TGN membrane retention factor followed by vesicular traffic, possibly mediated via COPI (coatomer complex I) vesicles (Rechards et al., 2003; Rabouille and Klumperman, 2005). Retention of Herp in the ER may be achieved through binding to an ER membrane protein. Recent data demonstrating a direct interaction between the Ubl domain of Herp and the integral ER membrane E3 ligase Hrd1 suggests that Hrd1 may function as a Herp ER retention factor (Schulze et al., 2005). Whether Herp interacts exclusively with Hrd1 or with additional integral membrane ERAD E3 ligases such as gp78/AMFR remains to be explored.
We establish a critical function for POSH-mediated ubiquitination in stress-induced Herp translocation by showing the inhibitory effect conferred by the expression of dominant- negative POSHV14A (Fig. 5 A). The role of POSH-mediated Herp K63 polyubiquitination is further strengthened by the observed correlation between the exclusive requirement for lysine residues within the Ubl domain for stress-induced translocation and for Ubc13/Uev1a-dependent Herp ubiquitination in vitro (Fig. 5, B and C). As would be anticipated if POSH-mediated Herp ubiquitination drives translocation, we find that Tpg-activated ubiquitination slightly precedes ER mobilization: Herp polyubiquitination is observed as early as 10 min, whereas Herp relocalization is distinguishable only 15 min after the addition of Tpg (Figs. 4 B and 6 B).
Regulation of signal-induced protein translocation by K63-linked polyubiquitination was initially demonstrated by Geetha et al. (2005), who showed an absolute requirement for TRAF6-mediated polyubiquitination for nuclear translocation of the neurotrophin receptor–interacting factor (NRIF) upon interaction of neurotrophin with its receptor. These investigators further demonstrated a correlation between the inhibition of TRAF6-induced NRIF ubiquitination and resistance to neurotrophin-induced apoptosis, thereby establishing a physiological role for TRAF6-mediated NRIF ubiquitination. Similarly, in our study, we establish the physiological role for POSH-mediated Herp ubiquitination by showing that the expression of dominant-negative POSHV14A inhibits ER stress–induced Herp relocalization to the ER (Fig. 5 A) and stimulates free calcium release (Fig. 7 A). The latter effect of POSH is consistent with the established function of Herp in restricting stress-induced calcium flow, a function that requires ER localization. Silencing of POSH expression by RNA interference also stimulates calcium release in the presence of Tpg (Fig. 7 B) and, thus, further confirmed the critical function of POSH in restricting cytosolic calcium upon the induction of ER stress.
Comparing the kinetics of Herp ER mobilization and restriction of free calcium upon Tpg treatment presents a major discrepancy: although the relocalization of Herp is observed after 15 min (Fig. 4 B), Tpg-induced calcium release is much faster and occurs within a few minutes (Fig. 7). How does Herp control calcium release from the ER if calcium release precedes translocation? This apparent paradox can be resolved if one considers the rapid action of Tpg, which elevates intracellular calcium within minutes (Chan et al., 2004). Therefore, the initial calcium concentration upon the addition of Tpg is most likely determined by the basal levels of Herp at the ER membrane rather than by de novo activation of Herp translocation. Constitutive POSH activity may release small amounts of Herp to the ER. Herp is short lived (Sai et al., 2003; Hori et al., 2004) and, thus, does not accumulate in the ER. However, the basal levels might be sufficient to attenuate initial stress-induced calcium leakage. According to this model, substantial mobilization of Herp to the ER and further restriction of free calcium flow is only facilitated when POSH is activated and allows the recruitment of additional Herp. The result of the in vivo Herp ubiquitination experiment that demonstrated basal POSH-dependent Herp ubiquitination that was further enhanced by Tpg (Fig. 6 B) is consistent with this model.
Because POSH activity is required for Herp ER localization, the relative levels of Herp at the ER membrane under basal conditions is determined by the relative POSH activity: it may be completely inhibited by the overexpression of POSHV14A, partially inhibited by RNA interference (Fig. 6, A and B), and obviously elevated by the overexpression of native POSH. Consequently, the Tpg-induced calcium burst is instantaneous in POSHV14A-overexpressing cells, accelerated in POSH-depleted cells, and markedly attenuated in the wild-type POSH- overexpressing cells (Fig. 7).
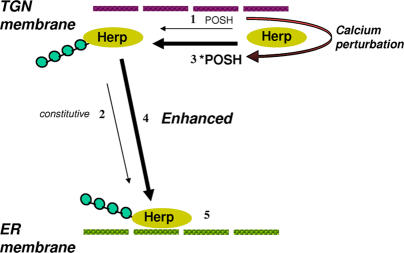
Based on the results presented in this study, we propose the following model for the regulation of Herp ER mobilization (Fig. 8): POSH constitutively polyubiquitinates Herp (1), releasing small amounts of Herp to the ER (2). Elevation of intracellular calcium activates the ubiquitination activity of the POSH–Herp complex at the TGN. As a result, Herp polyubiquitination is enhanced (3), resulting in the activation of Herp mobilization to the ER membrane (4). At the ER membrane, Herp performs ER stress–resistance functions, one of which is the restriction of further calcium release, probably through an interaction with a calcium channel (5). A candidate for a Herp-regulated calcium channel is presenilin, with which Herp interacts (Sai et al., 2002, 2003). In agreement with this hypothesis, presenilins were recently shown to form calcium leak channels mediating the bulk of the passive ER to cytosol calcium flow (Tu et al., 2006).
Figure 8.
Proposed model for the POSH-dependent regulation of Herp. See Discussion for details.
The assumption that the elevation of cytosolic calcium directly triggers the activation of Herp relocalization is based on the findings that the relocalization of Herp is rapidly induced by treatment of cells with Tpg and is independent of new protein synthesis. These findings also exclude a role for the ER stress response–induced transcriptional program. Independence of the ER stress response is further supported by our kinetic analysis, indicating that relocalization of Herp occurs well before any elevation in Herp protein level as part of the ER stress response (Fig. 4 B; Ma and Hendershot, 2004). In addition, the observed mobilization of exogenous Herp (Fig. 5 B) cannot result from ER stress response transcriptional up-regulation, as it is under the control of a cytomegalovirus promoter, which does not express an ER stress response element. It has been reported that in yeast, accumulation of unfolded proteins in the ER stimulates calcium influx through the plasma membrane (Bonilla et al., 2002). Similarly, unfolded proteins may activate calcium influx in mammalian cells, which would explain our observation that treatment with Tm, which causes unfolded protein accumulation, also rapidly induces Herp redistribution (Fig. S2).
The proposed model for the regulation of Herp traffic requires a mechanism that couples the transient increase in cytosolic calcium with the activation of POSH. One possibility is that Herp is activated by calcium and, in turn, activates POSH. This model is supported by our findings that Herp is a POSH activator. Nevertheless, we have thus far not found evidence that calcium stimulates the association of Herp with POSH or further stimulates POSH activity in the presence of Herp in vitro. This is not unexpected, and, because neither POSH nor Herp express a known calcium-sensing domain, calcium regulation likely involves a calcium-sensing molecule. Evidence for a possible calcium sensor was recently provided by the findings that in Drosophila, POSH interacts directly in a calcium-dependent fashion with the calcium-binding protein ALG2 (Tsuda et al., 2006). Whether ALG2 is the molecule that confers calcium regulation on POSH or whether it is mediated by another molecule that activates POSH (either directly or through Herp) such as a TGN resident calcium channel is currently under investigation.
In conclusion, we provide evidence for a novel spatial regulation of Herp by a calcium-activated Ub-mediated mechanism. Therefore, we propose that recruitment of Herp to the ER through POSH-mediated ubiquitination plays an essential role in the subsequent resistance of cells to ER stress.
Materials and methods
Plasmids, siRNA, and cell lines
pCMV-Herp-FLAG was constructed by adding a C-terminal FLAG tag to IMAGE clone 5575914 (IMAGE Consortium; obtained from the UK Human Genome Mapping Project Resource Center). pCMV-POSH-V5 was described previously (Alroy et al., 2005). The siRNA duplex used to knock down Herp (5′-AAGGGAAGUUCUUCGGAACCUdTdT-3′ and 5′-AGGUUCCGAAGAACUUCCCdTdT-3′) was synthesized by Dharmacon. The H310 and H314 cell lines that stably express POSH-specific and control small hairpin RNA (shRNA) are matching clones of the previously described H153 and H187 cell lines, respectively (Alroy et al., 2005).
Antibodies
Anti-Herp 25B was produced in rabbits immunized with affinity-purified Herp1–272 (tHerp). PT1 mouse anti-POSH was produced in mice immunized with affinity-purified POSH731–888, and rabbit anti-POSH was previously described (Alroy et al., 2005). Both protein antigens were produced in bacteria as GST fusions and were used for immunization after removal of the GST portion. The following primary antibodies were used for immunofluorescence: anticalnexin (Santa Cruz Biotechnology, Inc.), anti-V5 (Invitrogen), anti-Flag (Sigma-Aldrich), and sheep anti-TGN46 (Serotec). Secondary Cy-conjugated antibodies were purchased from Jackson ImmunoResearch Laboratories.
Plasmids.
The plasmid encoding UbG76V-GFP was a gift from M. Masucci (Karolinska Institute, Stockholm, Sweden). pEF1-AMFR-GFP was constructed as follows: full-length AMFR (AF124145) was amplified by RT-PCR from HeLa poly-A RNA (Sigma-Aldrich) and cloned into pEF1/V5-HisA (Invitrogen). Then, sgGFP (Quantum Biotechnologies) was ligated in frame downstream of AMFR.
Cells.
HeLa SS6 was grown in DME containing 10% FCS and transfected using LipofectAMINE 2000 transfection reagent (Invitrogen).
Recombinant proteins
POSH was produced in bacteria as an MBP fusion protein (molecular mass of the fusion protein is ∼150 kD; Alroy et al., 2005). tHerp and tHerpΔUbl were produced by PCR amplification of Herp codons 1–272 (tHerp) or codons 85–272 (tHerpΔUbl) followed by a FLAG tag cloned into pGEX-6P-2 (GE Healthcare). tHerpK15-78R was constructed by two-step PCR mutagenesis mutating all of the lysine residues within the Ubl domain (positions 15, 38, 61, 75, and 78) to arginines. Proteins were expressed in Escherichia coli BL21 by IPTG induction. Proteins were purified by glutathione-agarose affinity chromatography followed by removal of GST by PreScission protease (Ge Healthcare) and Q-Sepharose chromatography.
Yeast two-hybrid screen
A yeast two-hybrid screen was performed using Matchmaker system 3 (CLONTECH Laboratories, Inc.). Bait plasmid was constructed by cloning hPOSHΔRING (amino acids 53–888) into pGBK-T7. Yeast AH109 cells containing pGBK-hPOSHΔRING were mated with Y187 yeast cells containing a pretransformed HeLa cDNA library (CLONTECH Laboratories, Inc.). Colonies were selected on defined media lacking tryptophan, leucine, and histidine and containing 2 mM 3-aminotriazol. Colonies that grew on the selective media were tested for β-galactosidase activity, and positive clones were rescued from yeast that was sequenced and reintroduced into Y187 to confirm interaction with bait plasmid. One of the clones was identified as a novel splice variant of Herp containing the first 250 amino acids of Herp and a unique C-terminal region (GenBank/EMBL/DDBJ accession no. DQ837586).
Immunofluorescence microscopy
Fluorescence microscopy was performed as previously described (Alroy et al., 2005). In brief, the imaging medium in all cases was PBS. Images were taken in a confocal microscope (LSM 510; Carl Zeiss MicroImaging, Inc.) with 40× NA 1.6 objective lenses using the LSM 510 acquisition software (Carl Zeiss MicroImaging, Inc.). Fluorochromes used in this study and their respective emission wavelengths are as follows: Cy2 and Fluo-4, 488 nm; Cy3, 588 nm; and Cy5, 633 nm. For colocalization experiments, 10 optical horizontal sections with intervals of 1 μm were taken through each preparation (z stack). A single median section is shown in Figs. 1 (C and D), 4 A, and 5 B.
Immunoprecipitation and immunoblotting
Cells were lysed in buffer A containing 50 mM Hepes-NaOH, pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 1.5 mM MgCl2, 0.5 mM DTT, and protease inhibitor cocktail (1:100; Sigma-Aldrich). Immunoprecipitation was performed using protein A–Sepharose beads coated with the antibodies indicated in the figures. The detergent extracts were incubated at 4°C for 2 h. After precipitation, the beads were washed with buffer A containing 0.1% Triton X-100 and without MgCl2. Bound proteins were eluted with SDS sample buffer and subjected to Western blot analysis by standard procedures. Immunoreactive proteins were visualized by ECL.
Ub conjugation assays
50 nM of purified recombinant E1, 0.3 μM UbcH5c or Ubc13/Uev1, 13 nM of bacterially expressed POSH, and Herp (where indicated) were incubated in a final volume of 20 μl containing 40 mM Tris-HCl, pH 7.5, 1 mM DTT, 2 mM ATP, 5 mM MgCl2, 5 × 10−3% (vol/vol) Tween 20, and 5 μg Ub. After incubation for 30 min at 30°C, reactions were resolved by 7.5% SDS-PAGE and subjected to immunoblot analysis with PT1 and anti-Flag for the detection of POSH and Herp ubiquitination, respectively.
Glycerol gradient density centrifugation
7 pmol POSH and 10 pmol tHerp were incubated either alone or together in a final volume of 50 μl containing 40 mM Tris-HCl, pH 7.5, 0.5 mM DTT, and 5 × 10−3% (vol/vol) Tween 20. After incubation for 30 min at 30°C, the mixture was applied to a 10–50% glycerol gradient (2 ml) in the same buffer. Gradients were centrifuged at 250,000 g for 3 h at 4°C in a 55Ti rotor (Beckman Coulter). Fractions of 200 μl were collected, and aliquots were analyzed by immunoblotting or POSH ubiquitination assays.
Homogeneous time-resolved fluorescence–based ubiquitination assay
For measurement of POSH self-ubiquitination activity in the glycerol density gradient fractions (Fig. 3 B), we used the homogeneous time-resolved fluorescence method (Xu et al., 2003a). In solution, energy transfer between a donor fluorophore coupled to Ub and an acceptor fluorophore coupled to anti-POSH occurs only if Ub is conjugated to POSH. Consequently, aliquots of the glycerol gradient fractions were incubated with 3 nM E1 and 20 nM Ubc13/Uev1a and a mixture of 90 nM Ub and 10 nM Eu3+-cryptate-Ub (Cisbio) in an in vitro ubiquitination reaction as described in Ub conjugation assays. After incubation at 30°C for 30 min, Herp was immunoprecipitated with anti-Flag (Herp) in radioimmunoprecipitation buffer containing 1% SDS for 2 h at 4°C. The mixtures were then briefly centrifuged, and a 10-μl aliquot from the supernatant was removed and further incubated for 2 h at room temperature in a 96-well plate with XL665-PT1 (anti-POSH; Cisbio). Emission at 665 nm was subsequently determined in a homogeneous time-resolved fluorescence reader (RUBYstar; BMG Labtech) and expressed as δ F (function of the 665/620-nm emission ratio). Complete removal of Herp-Ub conjugates was confirmed in a parallel assay that determined the lack of energy transfer between Eu3+-cryptate-Ub and XL665-conjugated anti-Flag.
Measurement of intracellular calcium (calcium imaging)
Cells were grown on glass-bottom microwell dishes coated with poly- l-lysine (MatTek). Intracellular calcium was measured by determining emission at 533 nm using the Fluo-4 Calcium Assay kit (Invitrogen) according to the manufacturer's instructions. The cell culture was visualized in a confocal microscope (LSM 510; Carl Zeiss MicroImaging, Inc.), and an image was taken at individual time points (Fig. S3). Calcium concentration was subsequently determined as the intensity of Fluo-4 emission by the ImageJ program (National Institutes of Health; http://rsb.info.nih.gov/ij/index.html) using the mean gray value measurement option and is expressed as arbitrary emission units.
Online supplemental material
Fig. S1 shows the localization of Herp in cells immunostained in the presence of a mild detergent. Fig. S2 shows the time course of Herp protein induction and Herp redistribution in the presence of Tm. Fig. S3 shows that calcium induced the redistribution of Herp in PC3 and SW480 cell lines. Fig. S4 shows calcium imaging of the time course of calcium release in cells expressing POSH and dominant-negative POSH. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200611036/DC1.
Supplementary Material
Acknowledgments
We thank Dr. Mark Hochstrasser for critical reading of the manuscript.
S. Tuvia's present address is Bioline Innovations Jerusalem, Jerusalem 91450, Israel.
I. Alroy's present address is Pharmos Ltd., Kiryat Weizmann, Rehovot 76326, Israel.
M. Dori-Bachash's present address is Dept. of Plant Pathology and Microbiology, Faculty of Agricultural, Food, and Environmental Quality Sciences, Hebrew University of Jerusalem, Rehovot 76100, Israel.
Abbreviations used in this paper: AMFR, autocrine motility factor receptor; CHX, cycloheximide; ERAD, ER-associated degradation; MBP, maltose-binding protein; NRIF, neurotrophin receptor–interacting factor; shRNA, small hairpin RNA; tHerp, truncated Herp; Tm, tunicamycin; Tpg, thapsigargin; TRAF, TNF receptor–associated factor; Ub, ubiquitin.
References
- Alroy, I., S. Tuvia, T. Greener, D. Gordon, H.M. Barr, D. Taglicht, R. Mandil-Levin, D. Ben-Avraham, D. Konforty, A. Nir, et al. 2005. The trans-Golgi network-associated human ubiquitin-protein ligase POSH is essential for HIV type 1 production. Proc. Natl. Acad. Sci. USA. 102:1478–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla, M., K.K. Nastase, and K.W. Cunningham. 2002. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 21:2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, S.L., W. Fu, P. Zhang, A. Cheng, J. Lee, K. Kokame, and M.P. Mattson. 2004. Herp stabilizes neuronal Ca2+ homeostasis and mitochondrial function during endoplasmic reticulum stress. J. Biol. Chem. 279:28733–28743. [DOI] [PubMed] [Google Scholar]
- Dantuma, N.P., S. Heessen, K. Lindsten, M. Jellne, and M.G. Masucci. 2000. Inhibition of proteasomal degradation by the Gly-Ala repeat of Epstein-Barr virus is influenced by the length of the repeat and the strength of the degradation signal. Proc. Natl. Acad. Sci. USA. 97:8381–8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ea, C.K., L. Sun, J. Inoue, and Z.J. Chen. 2004. TIFA activates IkappaB kinase (IKK) by promoting oligomerization and ubiquitination of TRAF6. Proc. Natl. Acad. Sci. USA. 101:15318–15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, S., M. Ferrone, C. Yang, J.P. Jensen, S. Tiwari, and A.M. Weissman. 2001. The tumor autocrine motility factor receptor, gp78, is a ubiquitin protein ligase implicated in degradation from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 98:14422–14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa, C., S. Tarras, J. Taylor, and A.B. Vojtek. 2003. Akt2 negatively regulates assembly of the POSH-MLK-JNK signaling complex. J. Biol. Chem. 278:47922–47927. [DOI] [PubMed] [Google Scholar]
- Geetha, T., R.S. Kenchappa, M.W. Wooten, and B.D. Carter. 2005. TRAF6-mediated ubiquitination regulates nuclear translocation of NRIF, the p75 receptor interactor. EMBO J. 24:3859–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425–479. [DOI] [PubMed] [Google Scholar]
- Hofmann, R.M., and C.M. Pickart. 2001. In vitro assembly and recognition of Lys-63 polyubiquitin chains. J. Biol. Chem. 276:27936–27943. [DOI] [PubMed] [Google Scholar]
- Hori, O., F. Ichinoda, A. Yamaguchi, T. Tamatani, M. Taniguchi, Y. Koyama, T. Katayama, M. Tohyama, D.M. Stern, K. Ozawa, et al. 2004. Role of Herp in the endoplasmic reticulum stress response. Genes Cells. 9:457–469. [DOI] [PubMed] [Google Scholar]
- Kim, G.H., E. Park, and J.K. Han. 2005. a. The assembly of POSH-JNK regulates Xenopus anterior neural development. Dev. Biol. 286:256–269. [DOI] [PubMed] [Google Scholar]
- Kim, G.H., E. Park, Y.Y. Kong, and J.K. Han. 2006. Novel function of POSH, a JNK scaffold, as an E3 ubiquitin ligase for the Hrs stability on early endosomes. Cell. Signal. 18:553–563. [DOI] [PubMed] [Google Scholar]
- Kokame, K., K.L. Agarwala, H. Kato, and T. Miyata. 2000. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J. Biol. Chem. 275:32846–32853. [DOI] [PubMed] [Google Scholar]
- Ma, Y., and L.M. Hendershot. 2004. Herp is dually regulated by both the endoplasmic reticulum stress-specific branch of the unfolded protein response and a branch that is shared with other cellular stress pathways. J. Biol. Chem. 279:13792–13799. [DOI] [PubMed] [Google Scholar]
- Pickart, C.M. 2001. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70:503–533. [DOI] [PubMed] [Google Scholar]
- Pickart, C.M., and D. Fushman. 2004. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 8:610–616. [DOI] [PubMed] [Google Scholar]
- Rabouille, C., and J. Klumperman. 2005. Opinion: the maturing role of COPI vesicles in intra-Golgi transport. Nat. Rev. Mol. Cell Biol. 6:812–817. [DOI] [PubMed] [Google Scholar]
- Rechards, M., W. Xia, V.M. Oorschot, D.J. Selkoe, and J. Klumperman. 2003. Presenilin-1 exists in both pre- and post-Golgi compartments and recycles via COPI-coated membranes. Traffic. 4:553–565. [DOI] [PubMed] [Google Scholar]
- Sai, X., Y. Kawamura, K. Kokame, H. Yamaguchi, H. Shiraishi, R. Suzuki, T. Suzuki, M. Kawaichi, T. Miyata, T. Kitamura, et al. 2002. Endoplasmic reticulum stress-inducible protein, Herp, enhances presenilin-mediated generation of amyloid beta-protein. J. Biol. Chem. 277:12915–12920. [DOI] [PubMed] [Google Scholar]
- Sai, X., K. Kokame, H. Shiraishi, Y. Kawamura, T. Miyata, K. Yanagisawa, and H. Komano. 2003. The ubiquitin-like domain of Herp is involved in Herp degradation, but not necessary for its enhancement of amyloid beta-protein generation. FEBS Lett. 553:151–156. [DOI] [PubMed] [Google Scholar]
- Schulze, A., S. Standera, E. Buerger, M. Kikkert, S. van Voorden, E. Wiertz, F. Koning, P.M. Kloetzel, and M. Seeger. 2005. The ubiquitin-domain protein HERP forms a complex with components of the endoplasmic reticulum associated degradation pathway. J. Mol. Biol. 354:1021–1027. [DOI] [PubMed] [Google Scholar]
- Seong, K.H., T. Matsuo, Y. Fuyama, and T. Aigaki. 2001. Neural-specific overexpression of Drosophila plenty of SH3s (DPOSH) extends the longevity of adult flies. Biogerontology. 2:271–281. [DOI] [PubMed] [Google Scholar]
- Sun, L., L. Deng, C.K. Ea, Z.P. Xia, and Z.J. Chen. 2004. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell. 14:289–301. [DOI] [PubMed] [Google Scholar]
- Tapon, N., K. Nagata, N. Lamarche, and A. Hall. 1998. A new rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-kappaB signalling pathways. EMBO J. 17:1395–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda, M., C. Langmann, N. Harden, and T. Aigaki. 2005. The RING-finger scaffold protein plenty of SH3s targets TAK1 to control immunity signalling in Drosophila. EMBO Rep. 6:1082–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda, M., K.H. Seong, and T. Aigaki. 2006. POSH, a scaffold protein for JNK signaling, binds to ALG-2 and ALIX in Drosophila. FEBS Lett. 580:3296–3300. [DOI] [PubMed] [Google Scholar]
- Tu, H., O. Nelson, A. Bezprozvanny, Z. Wang, S.F. Lee, Y.H. Hao, L. Serneels, B. De Strooper, G. Yu, and I. Bezprozvanny. 2006. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 126:981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, K., C. Belunis, W. Chu, D. Weber, F. Podlaski, K.S. Huang, S.I. Reed, and L.T. Vassilev. 2003. a. Protein-protein interactions involved in the recognition of p27 by E3 ubiquitin ligase. Biochem. J. 371:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z., N.V. Kukekov, and L.A. Greene. 2003. b. POSH acts as a scaffold for a multiprotein complex that mediates JNK activation in apoptosis. EMBO J. 22:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z., A. Sproul, W. Wang, N. Kukekov, and L.A. Greene. 2006. Siah1 interacts with the scaffold protein POSH to promote JNK activation and apoptosis. J. Biol. Chem. 281:303–312. [DOI] [PubMed] [Google Scholar]
- Zhang, Q.G., R.M. Wang, X.H. Yin, J. Pan, T.L. Xu, and G.Y. Zhang. 2005. Knock-down of POSH expression is neuroprotective through down-regulating activation of the MLK3-MKK4-JNK pathway following cerebral ischaemia in the rat hippocampal CA1 subfield. J. Neurochem. 95:784–795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.