Abstract
We have shown that muscle-derived stem cells (MDSCs) transplanted into dystrophic (mdx) mice efficiently regenerate skeletal muscle. However, MDSC populations exhibit heterogeneity in marker profiles and variability in regeneration abilities. We show here that cell sex is a variable that considerably influences MDSCs' regeneration abilities. We found that the female MDSCs (F-MDSCs) regenerated skeletal muscle more efficiently. Despite using additional isolation techniques and cell cloning, we could not obtain a male subfraction with a regeneration capacity similar to that of their female counterparts. Rather than being directly hormonal or caused by host immune response, this difference in MDSCs' regeneration potential may arise from innate sex-related differences in the cells' stress responses. In comparison with F-MDSCs, male MDSCs have increased differentiation after exposure to oxidative stress induced by hydrogen peroxide, which may lead to in vivo donor cell depletion, and a proliferative advantage for F-MDSCs that eventually increases muscle regeneration. These findings should persuade researchers to report cell sex, which is a largely unexplored variable, and consider the implications of relying on cells of one sex.
Introduction
We previously reported that the transplantation of muscle-derived stem cells (MDSCs) into diseased muscle results in a large number of regenerated myofibers (Qu-Petersen et al., 2002; Deasy et al., 2005). These studies were initiated to understand the broad heterogeneity in phenotype and performance that has been reported for MDSCs and other muscle stem cell populations (Molnar et al., 1996; Schultz, 1996; Zammit and Beauchamp, 2001; Mueller et al., 2002; Deasy et al., 2004; Collins et al., 2005; Mitchell et al., 2005; Wagers and Conboy, 2005). In our laboratory, we also have observed a high degree of variability in the ability of different populations of MDSCs to regenerate skeletal muscle. While investigating MDSC population heterogeneity and characteristics that define efficient in vivo skeletal muscle regeneration, we have found that cell sex, a rarely considered variable, has a considerable effect on in vivo outcome. Compared with the transplantation of male MDSCs (M-MDSCs), the transplantation of female MDSCs (F-MDSCs) leads to substantially more regeneration of the diseased skeletal muscle of mdx mice, which model Duchenne muscular dystrophy.
More than 2,000 yr ago, Aristotle speculated that sexual dimorphism existed at the earliest stage of embryonic growth; he believed that male embryos became animated 40 d after conception, whereas female animation required 90 d (Aristotle, 350 BC). Recent studies support this notion (Avery et al., 1992; Xu et al., 1992; Pergament et al., 1994; Kochhar et al., 2003;). Male embryos created by in vitro fertilization grow faster before implantation than female embryos, and these findings support a genetic cellular difference between the sexes that exists before the induction of hormonal stimulation (Avery et al., 1992; Xu et al., 1992; Pergament et al., 1994; Kochhar et al., 2003). Sex-related differences in the expression of genes during early embryo development have also been observed: females exhibit higher mRNA levels of glucose-6-phosphate dehydrogenase and hypoxanthine phosphoribosyl transferase, which are two genes involved in the detoxification of reactive oxygen species (ROS; Gutierrez-Adan et al., 2000; Peippo et al., 2002). Male and female embryonic neurons (isolated from rats before gonad differentiation or hormonal stimulation) also display different cellular responses. The female cells are more sensitive to apoptosis-inducing agents, whereas male neurons are more sensitive to ischemia and nitrosative stress, and they cannot maintain the proper level of glutathione, which regulates ROS levels (Du et al., 2004).
Few studies have investigated whether sex-related differences affect tissue or organ regeneration by progenitor cells. Blankenhorn et al. (2003) demonstrated that the regrowth of cartilage, skin, and hair follicles in an ear pinna wound occurred faster and more completely in female mice as compared with male mice. Another study has shown that female mice modeling living donor liver transplantation with ischemia and reperfusion exhibit more efficient liver regeneration than their male counterparts (Harada et al., 2003). Similarly, male rats show more tissue growth than female rats after nephroctomy (Mulroney et al., 1999). Chau et al. (2002) found that blocking antiapoptotic activity in male animals resulted in sex-related differences in the animals' survival after endotoxic stress; the males recovered, but there was no advantage for females. Finally, in a study of human hematopoietic stem cell transplantations, superior survival was observed with maternally donated recipients as compared with recipients of paternal transplantations (Tamaki et al., 2001).
Several theories have been designed to unify cell and tissue aging with the overall aging of organisms and, thereby, explain females' longer life spans. Telomeres, whose length is believed to act as the mitotic clock of cells, are shorter in older males than in older females, which suggests that male cells undergo more rounds of division than female cells (Aviv et al., 2005; Vina et al., 2005). This finding supports a possible link between growth rate and aging (Rollo, 2002). Stindl (2004) has proposed that cells that have finite replicative lifespans must undergo more rounds of division to build the larger bodies of male organisms. Other research has shown that estrogen stimulates telomerase, which slows the rate of telomere attrition (for review see Aviv, 2002). This finding has ties with the free radical theory of cell aging, according to which ROS causes aging by damaging DNA, lipids, and proteins. Estrogens also up-regulate pathways that induce the expression of antioxidants (such as glutathione peroxidase) that reduce damage by ROS (for example, the MAPK pathway; Vina et al., 2005). Together, these findings suggest that sex-related differences in the health of the stem cell compartment could partially explain different rates of aging and disease. Stem and progenitor cells are believed to persist throughout life and contribute to the repair and maintenance of tissue. Therefore, investigations of sex-related differences shown by stem cells, as presented in this study, could lead to an improved understanding of sex-related differences in aging and disease.
Results
MDSC isolation and in vitro characterization
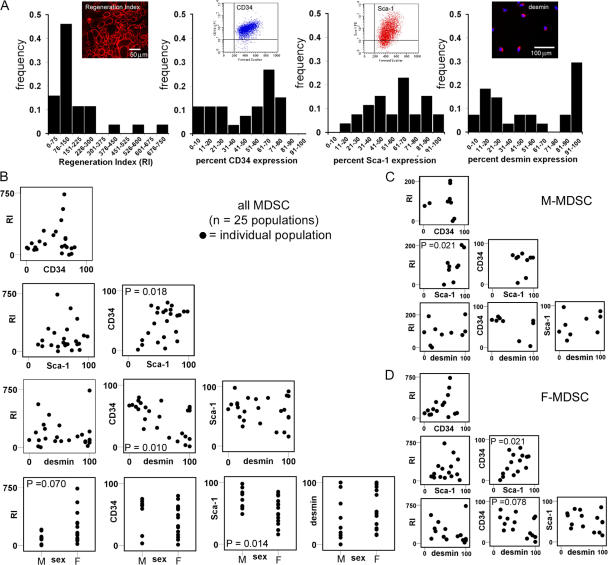
To determine whether any of the standard markers for MDSC characterization are predictive of high in vivo muscle regeneration, we examined 25 populations of MDSCs in terms of five variables: in vivo muscle regeneration efficiency, expression of CD34, expression of Sca-1, expression of desmin, and cell sex. Analysis of these variables revealed a large degree of heterogeneity in the MDSC populations. The distribution of the populations' regeneration indexes (RIs) and CD34, Sca-1, and desmin expression are shown in Fig. 1 A. The RI is a measure of how efficiently stem cells participate in skeletal muscle regeneration (Jankowski et al., 2002; Deasy et al., 2005), whereas CD34 and Sca-1 are stem cell markers, and desmin is a myogenic marker.
Figure 1.
Variability among muscle-derived cell populations. (A) We examined 25 populations of MDSCs for five variables: in vivo muscle regeneration efficiency (RI), CD34 expression, Sca-1 expression, desmin expression, and cell sex. Histograms show the distribution of each parameter for the 25 different populations. In vivo regeneration was quantified as the number of dystrophin-positive myofibers (red) present in the skeletal muscle of dystrophin-deficient mdx mice after the transplantation of MDSCs. Flow plots are shown for CD34 and Sca-1 expression, and immunofluorescence is shown for desmin (desmin, red; nuclei, blue). (B) We performed correlation analysis to identify significant relationships between variables. The scatter plots are shown. Each population is represented as a dot on the scatter plots. P-values are shown for the correlation coefficient when a linear relationship was found to exist between the two given variables. Only cell sex correlated with in vivo regeneration efficiency (R = 0.367; P = 0.070; M, male; F, female); our subsequent t test comparison of the regeneration efficiency of M-MDSCs with that of F-MDSCs revealed a significant difference (P = 0.035). We also detected a significant correlation between Sca-1 expression and cell sex (R = −0.490; P = 0.014), with significantly more M- than F-MDSCs expressing Sca-1 (P = 0.001; t test). (C and D) Examination of the correlation matrices for M- (C) and F-MDSCs (D) again revealed the heterogeneity of the populations. In particular, the higher level of Sca-1 expression by M-MDSCs correlated positively with a higher RI (P = 0.021), which suggests that the significantly higher levels of Sca-1 expression by M-MDSCs were not directly related to their low in vivo RI (see plot of RI vs. Sca-1 for M-MDSCs in D).
We performed a Pearson product moment correlation analysis to identify significant relationships between any two variables and, in particular, to determine whether any of the markers correlate with high in vivo muscle regeneration. Fig. 1 B shows the correlation scatter matrix comprising scatter plots for all possible combinations of variables. Only cell sex correlated with the RI (P = 0.070); M-MDSC populations had a significantly lower RI on average than F-MDSC populations (P = 0.035; t test). We also detected a significant correlation between cell sex and Sca-1 expression (P = 0.014): the mean expression of Sca-1 for all M-MDSC populations was significantly higher (M-MDSCs, 73 ± 16%; F-MDSCs, 52 ± 22%; mean ± SD; P = 0.001; t test). We next separated the correlation matrices on the basis of sex; Fig. 1 C shows the scatter plots for M-MDSCs (10 populations are shown in each plot), and Fig. 1 D shows the scatter plots for F-MDSCs (15 populations are shown in each plot). The scatter plot of the RI versus Sca-1 for M-MDSCs indicates that a higher level of Sca-1 expression by M-MDSCs positively correlated with a higher RI (P = 0.021; Fig. 1 C). This demonstrates that the significantly higher levels of Sca-1 expression by M-MDSCs (as compared with F-MDSCs) are not directly related to their low RIs (as compared with the RIs of F-MDSCs). The scatter plots of the F-MDSC populations did not reveal such a relationship (Fig. 1 D). Significant correlations between variables other than RIs are also indicated in Fig. 1 (C and D), and correlation coefficients are listed in Table S1 (available at http://www.jcb.org/cgi/content/full/jcb.200612094/DC1). We focused our subsequent analysis on the sex-related differences exhibited by MDSCs because cell sex was the only variable that correlated with the RI.
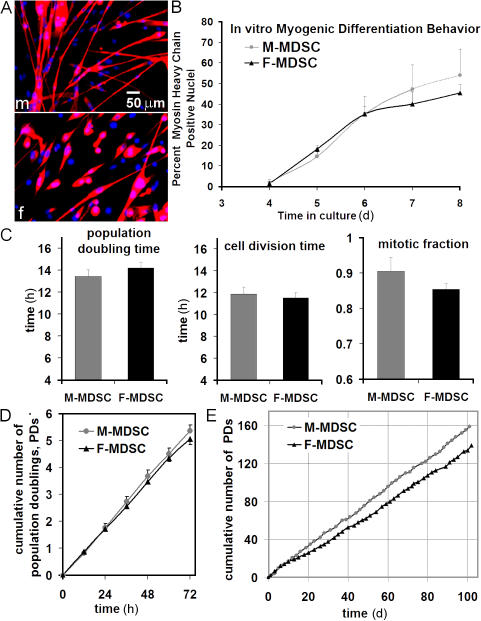
We used identical methods to isolate M- and F-MDSCs, and both exhibited stem cell characteristics in vitro (Fig. 2 and Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200612094/DC1). Morphological comparison of the M- and F-MDSC populations revealed no significant differences in cell size or cell shape (Fig. S1, A and B). Analysis of the cells' multilineage differentiation potential showed that with appropriate stimulation in vitro, both male and female cells expressed markers of myogenic (Fig. 2 A), osteogenic (Fig. S1 C), and adipogenic lineages (Fig. S1 C), although they did so at different rates and to different extents. On average, there was no significant difference in the mean amount of fast myosin heavy chain (MyHC) in M- versus F-MDSCs (Fig. 2 B).
Figure 2.
M- and F-MDSCs demonstrate similar in vitro stem cell characteristics. (A) M- and F-MDSCs underwent myogenic differentiation, as shown by MyHC expression (red; m, M-MDSC; f, F-MDSC). (B) We did not detect a significant difference in the extent or rate of myogenic differentiation as measured by the percentage of nuclei that colocalized with MyHC in low serum–containing medium (P > 0.05). (C) PDT, cell division time, and mitotic fraction were similar for M- and F-MDSCs. (D) Short-term proliferation kinetics were similar for M- and F-MDSCs. (E) Long-term proliferation kinetics showed that both M- and F-MDSCs can be expanded for >150 PDs in vitro. However, we observed a trend toward faster proliferation by M-MDSCs cultured for longer periods of time. Error bars represent SD.
Our analysis of short-term kinetics showed that M-MDSC and F-MDSC populations had similar population doubling (PD) times (PDTs; 13 ± 1.4 h and 14.2 ± 3.1 h, respectively), cell cycle times (12 ± 1.3 h and 12 ± 1.3 h, respectively), and mitotic fractions (0.91 ± 0.10 and 0.85 ± 0.08, respectively; P = 0.28; t test) over a 3-d period (mean ± SD; Fig. 2, C and D). However, after moderate expansion over 14 d or extended expansion over 3 mo (>150 PDs), M-MDSC populations exhibited higher growth rates than F-MDSC populations (M-MDSCs: PDT = ∼13 h and mitotic fraction = 0.87–0.97; F-MDSCs: PDT = ∼15 h and mitotic fraction = 0.81–0.87; Fig. 2 E and not depicted). After extended expansion, there was no significant difference in telomere length (Fig. S1 D) or telomerase activity (Fig. S1 E) as measured by flow cytometry. In addition, we observed a normal modal chromosome number for M- and F-MDSCs that were expanded to 90 PDs (unpublished data); this is consistent with our previous study of F-MDSCs (Deasy et al., 2005).
In vivo skeletal muscle regeneration
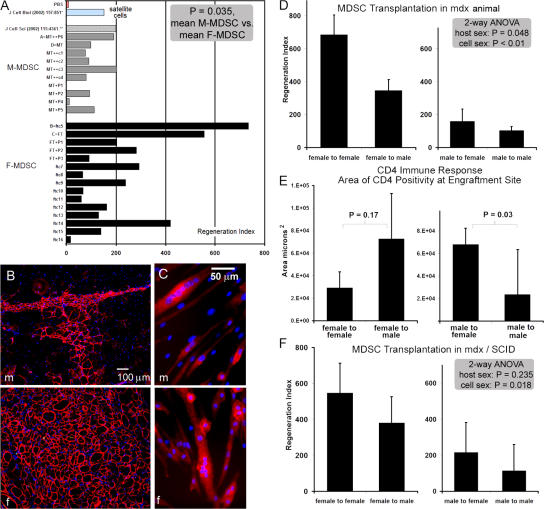
After transplanting M- and F-MDSCs into the skeletal muscle of dystrophic mdx mice (i.e., mice that lack dystrophin at the sarcolemma of muscle fibers), we investigated the cells' abilities to regenerate dystrophin-expressing myofibers. Sex-matched experiments involving several cell populations obtained from various isolations revealed that the implantation of F-MDSC populations led to significantly better skeletal muscle regeneration in vivo, as determined by calculating each population's RI (the ratio of dystrophin+ fibers per 105 donor cells; Fig. 3 A). Female cell populations regenerated significantly more dystrophin-positive myofibers (n = 15 F-MDSC populations; two to six muscles per population; RI = 230 ± 52; mean ± SEM) as compared with male populations (n = 10 M-MDSC populations; two to six muscles per population; RI = 95 ± 20; P = 0.035). Representative images of dystrophin-expressing myofibers within the mdx muscle tissue are shown in Fig. 3 B. Although the RIs of both M- and F-MDSC populations varied, no M-MDSCs exhibited high regeneration efficiency (i.e., RI > 203). In vitro immunostaining of myotubes after MDSC differentiation demonstrated that both M- and F-MDSCs are able to express dystrophin in myotubes containing a similar number of nuclei (Fig. 3 C).
Figure 3.
F-MDSCs induce more efficient skeletal muscle regeneration than M-MDSCs. (A) Quantitation of engraftment in terms of the RI (RI = number of dystrophin-positive fibers per 105 donor cells). The overall mean RI is significantly higher for F-MDSCs (black bars; RI = 230 ± 52; n = 15 F-MDSC populations; two to six muscles per population) than for M-MDSCs (gray bars; RI = 109 ± 18; mean ± SEM; n = 10 M-MDSC populations; two to six muscles per population; P = 0.035; t test). Sham-injected muscles (PBS; red bar) and RI values from previous studies of myoblasts (Qu-Petersen et al., 2002) and male myogenic progenitor cells (Jankowski et al., 2002) are also shown. (B) Representative engraftments of transplanted cells to mouse mdx skeletal muscle show dystrophin-positive fibers (red) within dystrophic muscle (nuclei stained with Hoechst). M-MDSCs produced fewer dystrophin-positive fibers than did F-MDSCs. (C) Dystrophin expression in vitro confirms the ability of both M- and F-MDSCs to express the dystrophin gene after cell fusion. (D)We performed sex-matched and sex-crossed transplantations. Two-way parametric ANOVA indicated significant differences in the RI associated with the sex of the donor cells (P < 0.001) and the sex of the host (P = 0.048). Mean RI and SEM (error bars) are shown (female to female, RI = 686 ± 120; female to male, RI = 347 ± 69; male to female, RI = 160 ± 75; male to male, RI = 105 ± 25; n = 6–12 per group). (E) Immune response at the site of transplantation in immune-competent mdx mice was quantified as the cross-sectional area (micrometers squared) of CD4 expression in tissue sections. There was significantly more CD4 positivity after male to female cross transplantation than after sex-matched male to male transplantation (mean ± SEM; t test; n = 4). (F) Transplantation of MDSCs into mdx/SCID mice. Two-way nonparametric ANOVA revealed no effect on the RI as a result of the sex of the host (P = 0.235), but there was a significant effect as a result of the sex of the cells (P = 0.018). RIs are reported as mean ± SEM (female to female, RI = 546 ± 166; female to male, RI = 381 ± 145; male to female, RI = 216 ± 39; male to male, RI = 115 ± 11; n = 4 per group).
We performed both sex-matched and sex-mismatched transplantations to determine whether the sex of the host tissue might play a role in the cells' differing regeneration abilities. We used two F- and two M-MDSC populations that had similar expression profiles for CD34, Sca-1, and desmin and exhibited similar short-term proliferation characteristics. Regardless of host sex, M-MDSC transplantation resulted in a low RI (160 ± 75 in female hosts and 105 ± 25 in male hosts; Fig. 3 D). Two-way analysis of variance (ANOVA) confirmed these findings and revealed both a significant difference as a result of host sex (P = 0.048) and a significant effect as a result of cell sex (P < 0.001). These results are consistent with those observed in previous studies using sorted populations of M-MDSCs (Jankowski et al., 2002; Jankowski and Huard, 2004a,b) or using male hosts (Mueller et al., 2002). M-MDSCs had a significantly lower RI than F-MDSCs (P < 0.05), and there was a significantly lower RI with the male host as compared with the female host (P < 0.01).
The difference caused by host sex suggests that the female microenvironment might be a more receptive environment for skeletal muscle regeneration by MDSCs and might play a role in RI variability. Hormonal differences between male and female hosts may influence the regeneration process; previous studies have described such differences in the responses of progenitor and stem cells to hormones (Jilka et al., 1992; Steinlein et al., 1995). We first tested the possibility that the low RI of M-MDSCs implanted in female mdx hosts (as compared with female to female transplantations) might be caused by the immune rejection of the M-MDSCs as a result of H-Y antigenicity. We examined the immune response at the site of cell delivery by measuring the total cross-sectional area of CD4 expression after sex-matched and sex-crossed transplantations. We observed a larger area containing CD4-positive cells after sex-crossed transplantations (Fig. 3 E).
We next investigated the effect of host sex in the absence of an immune response by performing sex-crossed and sex-matched transplantations in immune-compromised animals. M-MDSC to female mdx/severe combined immunodeficiency (SCID) mouse transplantations again resulted in a low level of skeletal muscle regeneration (RI = 216 ± 39), which is similar to what we observed in the female mdx host. We observed no significant difference as a result of host sex (P = 0.235), yet the significant difference as a result of cell sex remained (P = 0.018; two-way ANOVA; Fig. 3 F).
Hormonal stimulation
To determine whether estrogen stimulation improves the in vivo regeneration efficiency of MDSCs, we stimulated M- and F-MDSCs with two doses of 17-β-estradiol (10 or 100 nM; physiologic range of 10–100 nM; Maeda et al., 2000). We confirmed the expression of estrogen receptors in M- and F-MDSCs by immunocytochemistry (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200612094/DC1) and by microarray analysis (not depicted). After 2 wk in vitro with estradiol, M-MDSCs displayed lower proliferation rates than unstimulated M-MDSCs (although the decrease was not significant). Consistent with other results, comparison of the proliferation rates of the unstimulated cells showed that F- MDSCs proliferated more slowly than unstimulated M-MDSCs at all time points after 7 d (9 d, P = 0.070; 12 and 14 d, P < 0.05; t test; Fig. S2 B). We compared the in vivo regeneration efficiency of stimulated and unstimulated M- and F-MDSCs. 2 wk after transplantation, F-MDSCs cultured in control conditions of 0 nM estradiol had a higher RI than similarly cultured M-MDSCs (557 ± 229 vs. 115 ± 36; P = 0.06). Stimulation with estradiol had no significant effect on the RIs of M-MDSCs; however a trend toward a decreased RI was detected in F-MDSCs stimulated with 10 or 100 nM estradiol (P = 0.09 and P = 0.07; n = 3; t test; Fig. S2 C).
Exploring alternate M-MDSC donor sources
Based on cell adhesion characteristics, the preplate technique or variations of this technique have been used to isolate cells from adult skeletal muscle tissue (Rando and Blau, 1994; Qu et al., 1998). These cells have demonstrated multilineage differentiation potential ( Lee et al., 2000; Winitsky et al., 2005; Sarig et al., 2006; Nomura et al., 2007). To determine whether M-MDSCs are a rare subpopulation with particular adhesion or isolation characteristics, we attempted to isolate potent M- MDSCs from three other methods: (1) other preplates or other subfractions of myogenic progenitors, (2) a defined FACS subfraction expressing the stem cell marker CD34, and (3) clonal populations.
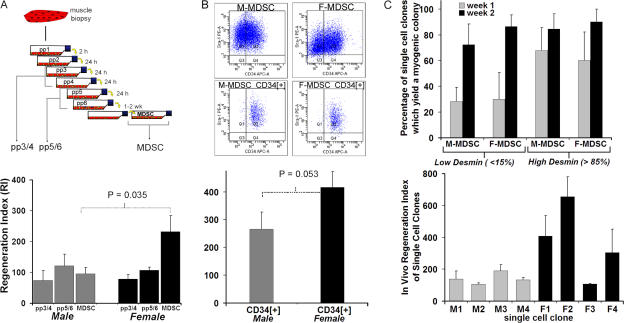
The preplate technique used to isolate MDSCs from a skeletal muscle biopsy involves serial preplating of low-adherence cells found within the primary medium supernatant to separate these cells from cells adhered to the collagenated surface of the flask (Fig. 4 A). Usually, myogenic precursors with characteristics of myoblasts or satellite cells are found within the early preplates (pp3–6), whereas cells isolated from the later preplates (≥pp6) have stem cell characteristics. We compared the in vivo regeneration efficiency (RI) of M-MDSCs with that of male myoblasts obtained from pp3–6 but identified no significant differences (Fig. 4 A).
Figure 4.
Examination of alternate M-MDSC sources. (A) The preplate technique to isolate subfractions of muscle-derived cells is based on their adhesion characteristics from muscle biopsy. Male myoblast subfractions also demonstrated low regeneration efficiency. There was no significant difference between the RIs of male pp3/4 (74 ± 32; n = 4) and pp5/6 (122 ± 38; n = 3) when compared with the RI of M-MDSCs (109 ± 18; n = 10 for all M-MDSC populations shown in Fig. 2). There also was no significant difference between the RIs of male myoblasts and female myoblasts (female pp3/4 RI = 79 ± 14, n = 4; female pp5/6 RI = 107 ± 10, n = 3; P > 0.05). The bars for the M- and F-MDSCs represent the mean RIs for all male and female populations shown in Fig. 2 (95 ± 21 [n = 10] and 230 ± 52 [n = 15], respectively). (B) M- and F-MDSCs were sorted by FACS to obtain a subpopulation expressing the stem cell marker CD34. The presorted population had similar expression levels for desmin/Sca-1 and CD34. Cells were purified to contain >96% CD34+ cells only. Transplantation of purified M-MDSCs expressing CD34 resulted in a significantly lower RI (266 ± 61) than did transplantation of F-MDSCs expressing CD34 (417 ± 56; P = 0.052; t test). All RIs are reported as means ± SEM (error bars), and p-values are for t test comparisons. (C, top) 2 wk after single-cell cloning, there was no significant difference in the percentage of single-cell clones derived from M- or F-MDSC populations that yielded myogenic colonies as defined by myotube formation (73–90%; P = 0.776). We detected no effect as a result of the cell sex (P = 0.756). Parent populations were either low in desmin expression (<15% positive cells within the population) or high in desmin expression (>85% positive). (C, bottom) We did not observe any male clone that exhibited a higher RI as compared with the parent population. We transplanted four M-MDSC clonal populations (M1, M2, M3, and M4) and four F-MDSC clonal populations (F1, F2, F3, and F4; mean ± SEM is shown; n = 3 muscle transplantations per clone). In contrast, we found that three of the four F-MDSCs display a high-regenerating potential as observed with their parent population.
To determine the effect of the expression of CD34 on regeneration efficiency, we used FACS to purify M- and F-MDSCs and obtain CD34+ fractions. The expression of Sca-1 and CD34 by unsorted M- and F-MDSCs was comparable (Fig. 4 B), and, in both populations, <15% of the cells were desmin positive. Our comparison of the RI of CD34-positive F-MDSCs (RI = 417 ± 56) with that of similarly sorted M-MDSCs (RI = 266 ± 61) revealed that the latter RI was significantly lower (P = 0.053; Fig. 4 B).
To test whether there are fewer cells within the M-MDSC populations that are capable of contributing to muscle regeneration, we performed in vitro and in vivo clonal analysis of myogenic behavior. We used FACS to single-cell sort and obtain clones to determine the percentage of clones with myogenic potential in the M- and F-MDSC populations. Because some populations already had a high level of expression of the myogenic marker desmin, we analyzed the clones from these populations separately. At 2 wk after single-cell cloning, there was no significant difference in the percentage of clones among male or female cells derived from MDSC populations (with high or low desmin expression), which yielded myogenic colonies as defined by myotube formation (P = 0.776; one-way ANOVA; Fig. 4 C). When we compared all three factors (cell sex, desmin expression, and week of analysis), we detected a significant effect (P = 0.066, P = 0.003, and P = 0.756, respectively) as a result of the desmin level and week of myogenic analysis but no effect as a result of the cell sex (three-way ANOVA). We transplanted eight representative clonal populations of MDSCs (four male and four female clones) into mdx animals to determine whether a clonal population could be identified within the M-MDSC populations that demonstrated a high RI. We confirmed that the overall trend we observe in Fig. 2 (using 25 populations) is consistent with results obtained from clonal populations (Fig. 4 C). Although we did observe an F-MDSC clone that exhibited a lower RI as compared with its parent population (F3 vs. C parent population), we did not observe any male clone that exhibited a higher RI as compared with the parent population.
Microarray analysis and cell stress response
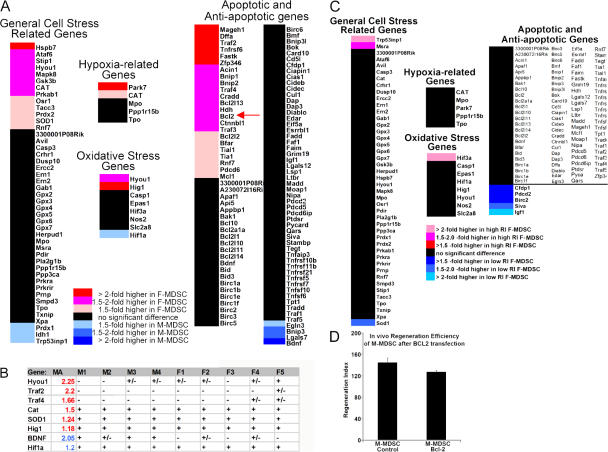
We next performed microarray gene ontology analysis of M- and F-MDSCs to explore global gene expression differences between the populations. We identified the expression of more general cell stress–related genes, including oxidative stress and antiapoptotic genes, in F-MDSCs than in M-MDSCs (Fig. 5 A) using both gene ontology based on the NetAffx annotation database and GeneSifter. Of 45 general cellular stress–related genes, 29% of the genes were significantly increased in females, 7% were significantly higher in males (P < 0.05), and 64% showed no significant difference. There were also sexual dimorphisms in genes that were involved in responses to hypoxia and oxidative stress between M- and F-MDSCs. Of 99 apoptosis-related genes, 23% of the genes were significantly increased in females, 4% were higher in males, and 72% showed no significant difference. We confirmed the sex-related differences in the expression levels of several of these genes using RT-PCR (Fig. 5 B). We also compared microarray data of F-MDSCs that yielded a low level of regeneration with F-MDSCs that yielded a high level of regeneration. We found two stress-related genes (TRP53inp1 and SOD1) that showed significant trends in a direction opposite to what we expected. For example, SOD1 was significantly higher in F-MDSCs as compared with M-MDSCs; however, when we explored the data of F-MDSCs with low RIs versus those with high RIs, we observed that SOD1 was higher in F-MDSCs that had only a low level of regeneration (P < 0.05). Similarly, TRP53Inp1 was significantly higher in the low-regenerating M-MDSCs, but, when we examined the low- versus high-regenerating F-MDSCs, we observed significantly higher levels of TRP53Inp1 in the female populations with the best in vivo regeneration (Fig. 5 C). Although these trends are interesting, this analysis did not provide any clear indicators for genes of importance.
Figure 5.
Differences in hypoxic and oxidative stress genes. (A) Microarray gene ontology analysis of M- and F-MDSCs. An increase in the expression of cell stress–related genes, including oxidative stress and antiapoptotic genes, was identified for F-MDSCs in comparison with M-MDSCs (colored bars represent significant differences; P < 0.05). Of 45 general cellular stress–related genes, 29% of the genes were significantly increased in F-MDSCs, 7% were significantly higher in M-MDSCs, and 64% showed no significant difference. Of 99 apoptosis-related genes, 23% of the genes were significantly increased in F-MDSCs, 4% were higher in M-MDSCs, and 72% showed no significant difference. (B) Gene levels of several key genes were examined using RT-PCR analysis. The results of several rounds of RT-PCR showing the gene symbol (Gene), the increase as seen by microarray analysis (MA; blue indicates that it is higher in M-MDSCs, and red indicates that it is higher in F-MDSCs), and the results of nine screened populations (four male populations, M1–M4, and five female populations, F1–F5; + indicates detection at 25 or 28 cycles, +/− denotes genes that were only observed at 30 cycles, and – represents genes that were not observed at 30 cycles). (C) We also examined the microarray data for differences in F-MDSCs that yielded a high level of muscle regeneration (high RI) as compared with F-MDSCs that yielded a low level of regeneration (low RI). High RIs are shown as red bars, and low RIs are shown blue bars. P < 0.05. (D) We found that the antiapoptotic factor Bcl2 was twofold higher in F-MDSCs than in M-MDSCs (A, arrow). We hypothesized that low Bcl2 expression may be related to reduced survival after cell transplantation, and we transfected M-MDSCs to overexpress Bcl2 (M-MDSC–Bcl2). However, we did not observe a significant difference in the RI of M-MDSC–Bcl2 (RI = 128 ± 17) as compared with the M-MDSC control (RI = 145 ± 48) at 2 wk after transplantation into dystrophic muscle (P = 0.415).
In particular, we found that the antiapoptotic factor Bcl2 was twofold higher in F-MDSCs than in M-MDSCs by microarray analysis (Fig. 5 A, arrow) and Western blotting (not depicted). To test whether the overexpression of Bcl2 in M-MDSCs could provide a gain of function in terms of in vivo skeletal muscle regeneration, we transfected the M-MDSCs with a Bcl2 plasmid. Western blot analysis and quantification showed higher levels of Bcl2 in M-MDSCs that were transfected to overexpress Bcl2 as compared with control M- or F-MDSCs (unpublished data). However, we did not observe a change in the RI of Bcl2-engineered M-MDSCs as compared with M-MDSC controls (Fig. 5 D).
Examination of cell fate at early time points
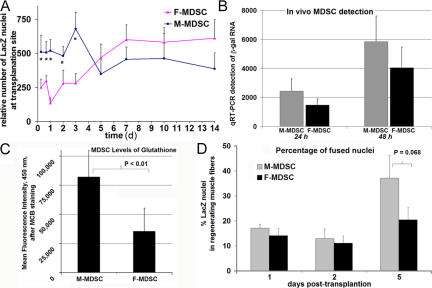
We transplanted M- and F-MDSCs, which were transduced with a lacZ-encoding retroviral vector, into the gastrocnemius muscles of sex-matched mice, and we harvested at several time points between 16 h and 14 d. We quantified the number of lacZ-expressing nuclei that were detected at the transplantation site. At 16, 24, 48, and 72 h, there were significantly more lacZ nuclei detected in muscles of M-MDSC transplantations as compared with F-MDSC transplantations (P < 0.05; Fig. 6 A). In separate experiments, we digested the skeletal muscle 24 and 48 h after the transplantation of M- and F-MDSCs, and we quantified the amount of lacZ gene by quantitative RT-PCR. Similar to the histological analysis, we found more lacZ in muscles transplanted with M-MDSCs as compared with F-MDSC transplantations, although this was not statistically significant (P > 0.05; Fig. 6 B). In support of the finding of more M-MDSCs after transplantation, we detected higher levels of reduced glutathione, an antioxidant peptide whose levels are reported to be maximal in mitotic cells (Li and Oberley, 1998). We used flow cytometry and monochlorobimane labeling to detect glutathione levels and found significantly more glutathione in M-MDSCs as compared with F-MDSCs (P < 0.01; Fig. 6 C).
Figure 6.
Examination of the transplantation site at early time points. (A) M- and F-MDSCs were transduced with the retroviral lacZ gene and transplanted into the gastrocnemius muscles of sex-matched mice, and the muscles were harvested at several time points between 16 h and 14 d. We quantified the number of lacZ nuclei that were detected at the transplantation site. At 16, 24, 48, and 72 h, there were significantly more nuclei detected in M-MDSC transplantations as compared with F-MDSC transplantations (*, P < 0.05 at all time points). (B) We digested the skeletal muscle after 24 and 48 h after transplantation, and we quantified the amount of lacZ gene by RT-PCR to support the histological staining results. Similar to the histological analysis, we detected more β-gal transcripts in muscles transplanted with M-MDSCs as compared with F-MDSC transplantations. (C) We stained M- and F-MDSCs using monochlorobimane (MCB) and found significantly more intracellular glutathione in M-MDSCs as compared with F-MDSCs (P < 0.01). (D) We quantified the percentage of lacZ + donor cells that are located within regenerating muscle fibers at 1, 2, and 5 d after MDSC transplantation. At 5 d after transplantation, we observed a trend toward significantly more donor cell fusion or differentiation with M-MDSCs as compared with F-MDSC transplantations to mdx muscle (P = 0.068). Error bars represent SD.
We also examined the amount of donor cell fusion or differentiation at the engraftment site by quantifying the percentage of lacZ nuclei that were located within muscle fibers. We examined the transplantation sites at 1, 2, and 5 d after cell injection. At 5 d after transplantation, we observed a trend toward significantly more donor cell fusion or differentiation with M-MDSCs as compared with F-MDSC transplantation to mdx muscle (P = 0.068; Fig. 6 D).
Response to cell stress
To evaluate the effect of low oxygen conditions and oxidative stress on M- and F-MDSCs, we examined MDSC viability, phenotype, and myogenic differentiation under these conditions. We hypothesize that after MDSC transplantation, the transplanted cells will experience conditions of low physiologic oxygen environment and oxidative stress. Previous studies have demonstrated that inflammation occurs at the local microenvironment after cell transplantation (Guerette et al., 1997a; Qu et al., 1998; Bottino et al., 2002; Suzuki et al., 2004) and that oxidative stress occurs after an inflammation response and in the presence of low oxygen (Gute et al., 1998; Dhalla et al., 1999).
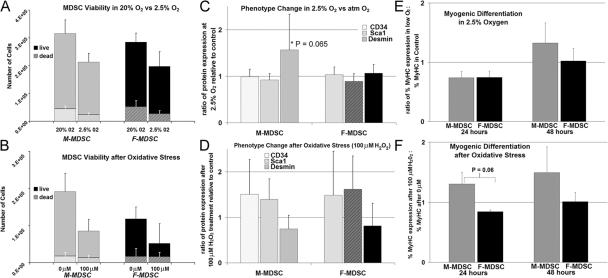
After exposure to physiologic oxygen for 24 h (2.5% O2), we observed a trend toward reduced proliferation in both M-MDSCs and F-MDSCs as compared with their controls (P = 0.18 and P = 0.079, respectively; Fig. 7 A). There was no significant difference in the amount of cell viability or cell death in M-MDSCs as compared with F-MDSCs under conditions of low oxygen (P = 0.652; Fig. 7 A). After exposure to oxidative stress (100 μM H2O2), we observed a significant decrease in cell numbers of both M-MDSCs and F-MDSCs (P = 0.039 and P = 0.001, respectively; Fig. 7 B). However, when we compared the response of M- with F-MDSCs in terms of cell proliferation or total cell numbers after stress, we observed no difference between the populations; there was no difference in the percent change in cell viability for M- vs. F-MDSCs after oxidative stress (41 vs. 55% decrease; P = 0.325; Fig. 7 B).
Figure 7.
Behavioral response to low oxygen and oxidative stress. (A) After exposure to physiologic oxygen for 24 h (2.5% O2), we observed a trend toward reduced cell numbers in both M-MDSCs and F-MDSCs (P = 0.18 and P = 0.079, respectively). There was no significant difference in the amount of cell viability or cell death in M-MDSCs as compared with F-MDSCs (ratio of the percentage of cells in low oxygen to atmospheric oxygen; P = 0.652). (B) After exposure to oxidative stress (100 μM H2O2), we observed a significant decrease in cell numbers of both M-MDSCs and F-MDSCs (P = 0.039 and P = 0.001, respectively). There was no difference in the percent change in cell viability for M-MDSCs vs. F-MDSCs after oxidative stress (ratio of the percentage of cells in 100 μM to atmospheric oxygen; P = 0.325). Bars are means ± SEM (error bars). (C) We examined the expression of CD34, Sca-1, and desmin in the populations after exposure to low oxygen. We did not observe a change in CD34 or Sca-1 expression in M- or F-MDSCs that were exposed to 2.5% O2 as compared with cells cultured in atmospheric O2. We observed a trend in increased desmin expression in M-MDSCs that had initially low levels of desmin (P = 0.065). There was no similar change in desmin expression in a comparable population of F-MDSCs. (D) After treatment with hydrogen peroxide, we observed trends toward an increase in CD34 and Sca-1 in all populations; however, there was no significant difference in the phenotype of M-MDSCs as compared with F-MDSCs (P > 0.05). Bars are means ± SD. (E) We induced myogenic differentiation and subsequently exposed cells to 2.5% oxygen for 24 and 48 h. We quantified the percentage of nuclei that colocalized with cells expressing the myogenic marker fast MyHC. We observed a trend toward more myogenic differentiation with M-MDSCs as compared with F-MDSCs. (F) We observed an increase in myogenic differentiation with M-MDSCs that were treated with 100 μM H2O2 as compared with their untreated controls at both 24 and 48 h. In comparison with their female counterparts, this change was significant at 24 h (P = 0.06). Bars are means ± SEM.
We examined the expression of CD34, Sca-1, and desmin in the populations after exposure to cell stress. We did not observe a change in CD34 or Sca-1 expression in M- or F-MDSCs that were exposed to 2.5% O2 as compared with cells cultured in atmospheric O2. We did observe a trend in increased desmin expression in M-MDSCs that had initially low levels of desmin (P = 0.065; Fig. 7 C). There was no similar change in desmin expression in a comparable population of F-MDSCs. After treatment with hydrogen peroxide, we observed trends toward an increase in CD34 and Sca-1 in all populations; however, after oxidative stress, there was no significant difference in the phenotype of M-MDSCs as compared with F-MDSCs (Fig. 7 D).
Next, we tested the ability of the cells to undergo myogenic differentiation after exposure to low oxygen or hydrogen peroxide. We plated M- and F-MDSCs at high density. After 4 d in culture, we transferred cells to either low oxygen or applied 100 μM H202 growth media. Control MDSCs were maintained at atmospheric oxygen with no H202. After 24 and 48 h, we performed immunochemistry for fast MyHC expression.
Under control conditions, there was no significant difference in the percentage of nuclei that colocalized with MyHC in M- or F-MDSC populations at 24 or 48 h (27–45% of the nuclei colocalized with MyHC). We observed a trend toward increased myogenic differentiation in M-MDSCs as compared with F-MDSCs after 48 h in low oxygen (Fig. 7 E). The sex-related difference in the differentiation response was also observed after exposure to hydrogen peroxide for 24 (P = 0.060) and 48 h (Fig. 7 F). In comparison with F-MDSCs, we observed an increase in myogenic differentiation with M-MDSCs as compared with their untreated controls (Fig. 7 F).
Discussion
We show here that the sex of MDSCs influences their ability to promote skeletal muscle regeneration. These studies were initiated to understand the broad heterogeneity that has been reported for MDSCs and other muscle stem cell populations (Molnar et al., 1996; Schultz, 1996; Zammit and Beauchamp, 2001; Mueller et al., 2002; Deasy et al., 2004; Collins et al., 2005; Mitchell et al., 2005; Wagers and Conboy, 2005). The use of different isolation techniques, culture techniques, and tools for stem cell characterization complicates cross comparisons among cell populations isolated in different research laboratories. This study identifies sex-related differences as a factor in MDSC variability in skeletal muscle regeneration.
The M- and F-MDSC populations isolated by the preplate technique shared stem cell characteristics; however, extensive in vivo screening showed that only 2/10 male populations had an in vivo RI near 200. In comparison, 60% of the 15 female populations had an RI higher than the mean RI of M-MDSCs (RI = 95), and 40% of the F-MDSCs had an RI higher than the maximal male RI (RI = 203). After transplantation into the skeletal muscle of dystrophic mice, F-MDSCs transplanted into hosts of either sex consistently regenerated more dystrophin-positive myofibers than did M-MDSCs transplanted into hosts of either sex.
Searching for a robust M-MDSC
To determine whether the M-MDSC population is more elusive than the F-MDSC population, we attempted to isolate cells with the M-MDSC phenotype from other isolation subfractions. First, we investigated whether preplate isolation might result in the inadvertent removal of potent M-MDSCs. To determine whether viable M-MDSCs reside in an earlier preplate passage than previously believed, we transplanted male pp3, 4, 5, and 6 cells into dystrophic mice. However, the RIs of these male cells were no higher than that of their parent M-MDSCs. We examined clones of M- and F-MDSCs and did not observe any male clones that participated in muscle regeneration to a level similar to that of F-MDSCs. We also compared M- and F-MDSC populations sorted on the basis of their expression of CD34, an established muscle stem cell marker whose expression decreases as cells differentiate. In side by side experiments, male CD34+ cells led to substantially less skeletal muscle regeneration than did female CD34+ cells.
A retrospective analysis of our previous work with muscle-derived cells provided further evidence that M-MDSCs exhibit lower regeneration efficiency than F-MDSCs (Jankowski et al., 2002; Jankowski and Huard, 2004a,b). Two previous studies performed by members of our laboratory involved the use of Y-chromosome in situ hybridization to track male cells injected into female hosts (before our notion of sex-related differences; Jankowski et al., 2002; Jankowski and Huard, 2004b). The authors of both studies calculated RIs to assess the efficiency with which the donor cells regenerated dystrophin-positive fibers in dystrophic mice. These studies found that male populations, which were sorted for CD34 expression by magnetic antibody cell sorting (Jankowski et al., 2002) or by FACS (Jankowski and Huard, 2004b), exhibited RIs <200, which is a level consistent with the RIs reported in this study. In addition, because those studies used freshly isolated populations, the authors performed >20 separate isolations from male animals to generate a sufficient sample size; none of the male populations had an RI >200 (the authors scaled their RI to the percentage of myogenic [desmin+] donor cells; we have adjusted for the authors' reported values for desmin expression). A third study using female cells obtained from rats demonstrated a high RI (>200) after transplantation of the F-MDSCs into mdx/SCID mice (RI = 392 ± 55 dystrophin-positive fibers/105 myogenic donor cells; Jankowski and Huard, 2004a). In a study performed by another group, Mueller et al. (2002) used MDSCs derived from our group and found a low efficiency of muscle regeneration when the MDSCs were transplanted to male hosts, as we observe here. Collectively, these findings provide compelling evidence that MDSCs and host animals exhibit sex-related differences.
We tested whether hormones play a role in this process. We failed to observe increased regeneration by normal M-MDSCs transplanted into female mice or M-MDSCs prestimulated with physiologic estrogen levels. This finding parallels the results of other studies indicating that sex-related differences might not be exclusively hormonal (Xu et al., 1992; Gutierrez-Adan et al., 2000; Du et al., 2004). Indeed, other dosing regimes and other sex steroids such as progesterone or testosterone should be considered in conjunction with studying the various receptor isoforms to fully evaluate the potential role of hormones in the sex-related differences exhibited by MDSCs.
Sex-related differences in cell stress response
By microarray analysis, we observed trends in sex differences in genes related to apoptosis, hypoxia, oxidative stress, and general cell stress response. In particular, the RNA and protein levels of the antiapoptotic gene Bcl2 were lower in M-MDSCs as compared with F-MDSCs. The rapid cell death in muscle cell transplantation has been previously reported (Beauchamp et al., 1994, 1999; Fan et al., 1996). Therefore, we attempted a gain of function in M-MDSCs by the overexpression of Bcl2. We did not, in fact, observe an increase in muscle regeneration after Bcl2 gene transfer. Indeed, subsequent experiments surprisingly demonstrated that there were more M-MDSCs than F-MDSCs at the transplantation site up to 3 d after transplantation. We also detected higher levels of reduced glutathione in the M-MDSC than in the F-MDSC. Glutathione's role in protecting cells from antioxidants could explain a better survival of M-MDSCs after transplantation. In addition, it has also been shown that glutathione levels are elevated during mitosis (Li and Oberley, 1998; Menon et al., 2003). This finding, along with the finding of differences in the mitotic fraction of M- and F-MDSCs, could also suggest that the M-MDSCs were more mitotic than the F-MDSCs after transplantation.
We also tested the cells' abilities to undergo myogenic differentiation after exposure to low oxygen environment or oxidative stress, two conditions that are expected to be present in the microenvironment after transplantation. We found an increase in the percentage of cells that expressed the myogenic marker desmin in M-MDSCs grown in low oxygen. We also found more MyHC expression by M-MDSCs after exposure to oxidative stress for 24 h and a similar trend after 48 h.
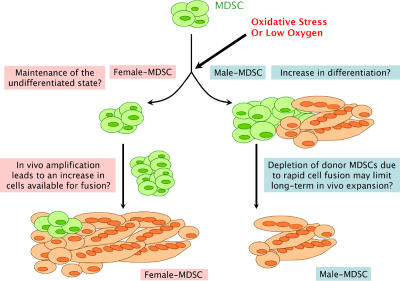
We hypothesize that the effect of cell stress on myogenic differentiation leads to in vivo sex-related differences in skeletal muscle regeneration (Fig. 8). F-MDSCs respond to low oxygen or oxidative stress by maintaining a low level of proliferation. In a myogenic environment with low oxygen or oxidative stress, F-MDSCs do not readily differentiate. Conversely, M-MDSCs demonstrate increased myogenic differentiation in the presence of low oxygen or oxidative stress. In vivo, we hypothesize that the increased differentiation will result in the rapid depletion of donor M-MDSCs as cells undergo myogenic differentiation, including transient amplification and fusion to form terminally differentiated multinucleated myotubes/fibers. F-MDSCs may be less proliferative, or we speculate that they may enter quiescence in response to oxidative stress at early time points after implantation. Subsequently, F-MDSCs demonstrate an increase in cell numbers starting at 3 d after implantation, a point at which acute inflammation subsides (Guerette et al., 1997a,b). Ultimately, the tendency to maintain the undifferentiated phenotype allows for in vivo expansion of F-MDSCs that are consequently available at later time points for muscle regeneration. These results both strongly support the notion that MDSCs exhibit sex-related differences at the cellular level and suggest that pathways involved with cellular responses to stress may differ in M- and F-MDSCs.
Figure 8.
Schematic of proposed sex-related differences in response to cell stress and role in skeletal muscle regeneration. Sex-related differences affect the response of MDSCs to low oxygen or oxidative stress. F-MDSCs may respond by maintaining a low level of proliferation. In a myogenic environment with low oxygen or oxidative stress, F-MDSCs do not appear to readily differentiate. Conversely, M-MDSCs demonstrate increased myogenic differentiation in the presence of low oxygen or oxidative stress. In vivo, we hypothesize that the increased differentiation will result in the rapid depletion of donor M-MDSCs as cells fuse to form terminally differentiated multinucleated myotubes/fibers. F-MDSCs may be less proliferative at early time points after implantation and demonstrate an increase in cell numbers starting at 3 d after implantation. Ultimately, the tendency to maintain the undifferentiated phenotype or resist differentiation would be a mechanism that allows for in vivo expansion of F-MDSCs that are subsequently available at later time points for muscle regeneration.
It will be interesting to determine whether other stem cell types also exhibit sex-related differences. In addition, stem cell experiments could have skewed results if male cells are selectively used for the purpose of in vivo cell tracking via the Y chromosome. This evidence that innate cellular sex differences may influence stem and progenitor biology should influence how researchers report and investigate the use of progenitor cells for regenerative medicine and the treatment of diseases.
Materials and methods
MDSC isolation and in vitro characterization
A modified preplate technique was used to isolate a low-adhering fraction of MDSCs from skeletal muscle biopsies obtained from 3-wk-old normal C57BL mice (Rando and Blau, 1994; Qu-Petersen et al., 2002). Six isolations were performed using anatomically sexed animals, and cell sex was later confirmed by FISH analysis. All populations were negative for CD45 expression (<0.1% of cells). Both M- and F-MDSCs were cultured in normal growth medium (GM): DME (supplemented with 10% FBS; Invitrogen), 10% horse serum, 1% penicillin/streptomycin, and 0.5% chick embryo extract (Accurate Chemical).
Cell populations were characterized by flow cytometry for CD34 and Sca-1 expression. MDSCs were labeled with rat anti–mouse Sca-1 (phycoerythrin) and CD34 (biotin) mAbs (BD Biosciences). A separate portion of cells was treated with equivalent amounts of isotype control antibodies. Both fractions were washed and labeled with streptavidin-allophycocyanin. We added 7-amino-actinomycin D to exclude nonviable cells from the analysis. Sca-1 and CD34 expression was determined by flow cytometry with a cell sorter (FACStar Plus or FACSAria; Becton Dickinson). Desmin expression was assessed via immunocytochemistry. After cold methanol fixation, cells were blocked in 5% horse serum and were incubated with mouse monoclonal IgG desmin (1:250; Sigma-Aldrich), secondary biotinylated IgG (1:250; Vector Laboratories), and streptavidin-Cy3 (1:500; Sigma-Aldrich) to fluorescently label the cells and determine the percentage of myogenic cells within the population. Cell sex was confirmed by FISH. Degenerate oligonucleotide-primed PCR-labeled Y probes were used to determine cell sex. Populations were screened for their in vivo regeneration efficiency after transplantation into skeletal muscle (see Cell transplantation to skeletal muscle).
In vitro characterization
To measure morphology, phase-contrast light microscopy images were acquired for several M- and F-MDSC isolations. Northern Eclipse software (Sun Systems) was used to collect morphometric data by outlining or tracing individual cells. A rank sum test was performed to compare quantitative measurements of F- and M-MDSC area (number of pixels calibrated to micrometers squared), cell diameter (maximal length in micrometers), cell elongation (ratio of the major axis to the minor axis), and cell roundness (perimeter squared/4 × π × area).
To examine multipotency, MDSCs were induced to undergo myogenic, osteogenic, and adipogenic differentiation. To promote myogenic differentiation, MDSCs were plated at high confluence (1,500 cells/cm2) in GM for 3 d, transferred to low serum–containing medium (2% horse serum/FBS in DME) for 4 d, and examined for myotube formation by MyHC expression (1:250; Sigma-Aldrich), biotinylated IgG (1:250; Vector Laboratories), and streptavidin-Cy3 (1:500) or dystrophin immunocytochemistry. Osteogenic differentiation was induced by stimulation with 10 ng/ml of bone morphogenetic protein 4 for 10 d and was evaluated by AP and von Kossa staining (Lee et al., 2000). Adipogenic differentiation was induced after cells reached confluency by adding adipogenic medium to the cells (dexamethasone, insulin, indomethacin, and 3-isobutyl-1-methyl-xanthinine; Cambrex) as previously described (Pittenger et al., 1999). Lipid vacuole formation was assessed by Oil Red O staining.
To examine short-term growth kinetics, the division time, PDT, and mitotic or dividing fraction were determined from time-lapsed microscopy as previously described (Sherley et al., 1995; Deasy et al., 2003). To examine long-term proliferation potential, M- and F-MDSCs were plated in 25-cm2 collagen-coated flasks, and routine cell passaging was performed every 2–3 d (Deasy et al., 2005). At each passage, cells were replated to a density of 225 cells/cm2. The number of PDs for each subculturing was calculated as the log2 (N/N0). This process was repeated for >150 PDs. Karyotyping was performed as previously described (Deasy et al., 2005) for the two M-MDSC populations (A and D) and two F-MDSC populations (B and C).
Acquisition and processing of images.
Immunofluorescent images of dystrophin, desmin, and MyHC were acquired at RT on a microscope (DM IRB; Leica) using a 20× NA 0.04 objective (CorrPh1 α/0–2/C; Leica). No imaging medium was used, and the fluorochromes used are as indicated. Digital images were acquired using a camera (Regita; QImaging) and Northern Eclipse software (version 6.0; Sun Systems). Images were acquired at exposures that were based on unstained controls. The final presentation of images was prepared in Photoshop versions 5.0–7.0 (Adobe), and, in some cases, only uniform brightness or contrast adjustments were performed.
Telomerase activity and telomere length
Telomerase activity was determined using the TeloTAGGG Telomerase PCR ELISA PLUS kit (Roche) according to the manufacturer's protocol. 2 × 105 cells were lysed in 200 μl of lysis reagent, and 10 μl of the lysate was used for the telomere repeat amplification protocol reaction. The experiment was performed in triplicate, and relative telomerase activity was recorded and plotted. For the flow cytometry–based measurement of telomere length, telomeres in M- and F-MDSCs were detected with the PNA Kit for Flow Cytometry (DakoCytomation) according to the manufacturer's protocol. 2 × 106 male or female cells were divided into four 1.5-ml tubes. The cellular DNA was denatured for 10 min at 82°C and hybridized with the telomere PNA probe (FITC) at RT overnight. The cells were then analyzed by flow cytometry, and the relative telomere length was calculated.
Cell transplantation to skeletal muscle
The use of animals and the surgical procedures performed in this study were approved by the Institutional Animal Care and Use Committee of the Children's Hospital of Pittsburgh (University of Pittsburgh Medical Center). mdx mice (C57BL/10ScSn-Dmdmdx) were obtained from The Jackson Laboratory or bred at the institution's animal facility. mdx/SCID mice were bred by crossing C57BL/10ScSn-Dmdmdx and C57BL/6J-Prkdcscid/SzJ mice.
For all experiments involving the transplantation of MDSCs, satellite cells, or CD34-sorted populations, 1–2 × 105 cells were transplanted into the gastrocnemius muscles of male or female mice as indicated and harvested after 2 wk. For sex-mismatched experiments, two M-MDSCs (populations A and D) were transplanted into female hosts, and two F-MDSCs (populations B and C) were transplanted into male hosts. The populations had similar phenotypes: populations A and C (CD34 [60–80% positive], Sca-1 [70–100% positive], and desmin [>95% positive]), populations B and D (CD34 [60–80% positive] and Sca-1 [40–80% positive], and desmin [<15% positive]). For CD34-sorting experiments, populations A and C were used.
Tissues were harvested and snap frozen in liquid nitrogen–cooled 2-methyl butane and cryosectioned at 10 μm. Either the MOM (mouse on mouse) kit (Vector Laboratories) with DYS2 antibody (1:50; Novocastra) or a donkey anti–rabbit dystrophin (1:300; Abcam) antibody was used to stain tissue sections for dystrophin. For the DYS2 antibody, the sections were fixed with cold methanol, and the MOM kit protocol was followed (DYS2 at 1:50, biotinylated anti–mouse IgG at 1:250, and streptavidin-Cy3 T at 1:500; Sigma-Aldrich). For anti–rabbit dystrophin, sections were fixed with 5% formalin, blocked with donkey serum, incubated with primary antibody, and incubated with AlexaFluor594 donkey anti–rabbit (1:500; Invitrogen). For CD4 staining, tissue was blocked with goat serum and was incubated with rat anti–mouse CD4 (1:100; BD Biosciences), biotin goat anti–rat (1:400; Vector Laboratories), and peroxidase-streptavidin (1:800; DakoCytomation).
A previously described technique (Jankowski et al., 2002; Deasy et al., 2005) was used to measure the cells' RIs. The RI is the ratio of the number of dystrophin-positive fibers per 105 donor cells (RI is scored at the cross section of maximal engraftment). The large majority of the >150 muscle transplantations of this study were quantitated for the RI in a blind manner by more than one investigator.
The RIs or areas containing CD4-positive cells were compared by two-way ANOVA or a t test to assess the effects of cell sex and host sex. For transplantations of lacZ MDSCs, cells were first labeled with retrovirus encoding for the lacZ gene as previously described (Lee et al., 2000). We performed X-gal staining to visualize β-gal activity and quantified the total number of these lacZ-positive nuclei and the number that were located within muscle fibers.
Single-cell cloning and clonal analysis
We used FACS to obtain single-cell clones (FACSAria and visual confirmation) of M- and F-MDSCs (populations A–D) on 96-well plates. Clones were cultured in 50 μl GM. At 1 wk after sorting, we identified viable colonies and scored these clones as myogenic or not myogenic based on the presence of distinct multinucleated myotubes. At 2 wk after cloning, we again determined the cumulative number of myogenic colonies based on the presence of distinct myotubes. For transplantation experiments using clonal populations, we similarly obtained single-cell clones from M- and F-MDSCs (populations A–D). After culture expansion, we selected eight representative populations and performed transplantations as described above (Cell transplantation to skeletal muscle) to determine the RI of each clone.
Estrogen stimulation
M- and F-MDSCs (populations A–D) were cultured in GM without phenol red and supplemented with 0, 10, or 100 nM 17-β-estradiol (Sigma-Aldrich). Cells were replated in fresh medium every 2–3 d for 14 d. After in vitro stimulation, 105 cells per muscle were transplanted into the gastrocnemius of female mdx/SCID mice (n = 3 muscles per group). 2 wk after transplantation, the muscles were harvested, sectioned, and immunostained for dystrophin, and the RI was analyzed as described above (Cell transplantation to skeletal muscle).
Microarray analysis
RNA was isolated from five M- and five F-MDSC populations using an RNeasy kit (QIAGEN) and analyzed on a GeneChip Mouse Genome 430A 2.0 array (Affymetrix, Inc.). Fold increase was determined using previously established techniques (Perez-Iratxeta et al., 2005). Gene ontology was determined using the NetAffx (Affymetrix, Inc.) annotation database (Liu et al., 2003; Harris et al., 2004). All results were confirmed using GeneSifter (VizX Labs), a certified GeneChip-compatible web-based analysis tool. The resulting data on fold increase for each population were analyzed using a t test to determine significant differences (P < 0.05) between the M- and F-MDSC populations.
RT-PCR and real-time quantitative PCR
Several genes of interest from the microarray were confirmed with RT-PCR analysis. Total RNA was extracted from 5 × 105 cells using the Nucleospin RNA kit (CLONTECH Laboratories, Inc.). cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions. PCR was performed with Taq polymerase (Invitrogen) according to the manufacturer's instructions for 25, 28, and 30 cycles at 58°C annealing temperature, and PCR products were separated by electrophoresis on 1% agarose gels. The primers used are listed in Table S2 (available at http://www.jcb.org/cgi/content/full/jcb.200612094/DC1).
For quantitative PCR, we used 50 ng cDNA. cDNA and β-gal primers (forward, ACAGTACGCGTAG; reverse, CCATCAATCCGGTAGGTTTTCCGG) were added to SYBR green PCR master mix (Applied Biosystems) according to the manufacturer's instructions. All lacZ data were normalized to 18S, which was used as the internal control.
Overexpression of Bcl2
M- and F-MDSCs were plated at 2,500 cells/9.6 cm2 in GM. The following day, the medium was replaced with 500 μl DME (without serum or antibiotics) containing 4.0 μg Bcl2 plasmid, which also encoded for the neomycin resistance gene, and 5 μl LipofectAMINE 2000 reagent (Invitrogen). MDSCs were incubated at 37°C for 6 h in this medium, and then medium was removed and replaced with normal GM. After 5 d in culture, we began a selection process. For the next 2 wk, MDSCs were cultured in GM containing 1.5 mg/ml G418 sulfate (Cellgro). Controls were also transfected with GFP plasmid to confirm transfection. Cells were maintained in GM and transplanted to male mdx/SCID mice as described above (Cell transplantation to skeletal muscle). Western blot analysis was performed using standard molecular biological techniques. Cells were lysed in Laemmli sample buffer (Bio-Rad Laboratories) and resolved on 4–20% precast gradient gels. Mouse Bcl2 was detected with anti-Bcl2 (1:1,000; BD Biosciences) with goat anti–rabbit HRP (1:2,500; BD Biosciences) and imaged using SuperSignal (Pierce Chemical Co.). β-actin levels were detected with anti–β-actin (1:5,000; Sigma-Aldrich) and goat anti–mouse HRP (1:5,000; Pierce Chemical Co.).
Response to cell stress
Viability.
For the low oxygen environment, M- and F-MDSCs (populations A–D) were plated at a density of 500 cells/cm2 on collagen-coated flasks with normal GM. After cell adherence (4–6 h), MDSCs were incubated for 24 h in either atmospheric (∼20%) or 2.5% O2 conditions (HERAcell 150 incubator; MidAtlantic Diagnostics). For oxidative stress, M- and F-MDSCs (populations A–D) were plated at a density of 700 cells/cm2. After cell adherence, MDSCs were incubated for 24 h with either 0 or 100 μM H2O2 (under atmospheric oxygen). After 24 h in low O2 or after 24 h with 100 μM H2O2, MDSCs from the supernatant and adherent cells were collected (0.25% trypsin-EDTA). Cells were counted using a Neubauer hemacytometer (Fisher Scientific), and trypan blue staining was used to identify the live and dead cells.
Phenotype after cell stress.
We examined CD34, Sca-1, and desmin expression as described above (MDSC isolation and in vitro characterization).
Myogenic differentiation after cell stress.
The ability of MDSCs to form myotubes under low oxygen conditions or after exposure to hydrogen peroxide was determined by fast MyHC staining. M- and F-MDSCs (populations A–D) were plated at a density of 1,000 cells/cm2 on collagen-coated plates with normal GM. For low oxygen stress, after 4 d of growth in atmospheric conditions, growth media was refreshed, and cells were transferred to a 2.5% oxygen incubator. Control flasks were maintained in atmospheric O2 incubators. For oxidative stress, after 4 d of growth in atmospheric conditions with normal media, growth media was refreshed (control), or MDSCs received media with 100 μM H2O2. After 24 and 48 h of exposure to low oxygen or incubation with H2O2, MDSC cultures were fixed and immunostained for MyHC as described above (In vitro characterization).
Reduced glutathione levels were determined for M- and F-MDSC populations by flow cytometry. Cells were plated at 1,600 cells/cm2 on collagen-coated flasks with normal GM. After 24 h, cells were incubated in 5 μM monochlorobimane (Invitrogen) in normal growth media for 20 min at 37°C. The cells were then washed twice with PBS and harvested in 0.25% trypsin-EDTA. Intracellular glutathion levels were determined with a FACSAria machine (monochlorobimane excitation of 380 nm and emission of 461 nm).
Online supplemental material
Fig. S1 shows M- and F-MDSC similarities in cell morphology comparisons, multilineage marker expression, telomerase activity, and telomere length. Fig. S2 shows the in vitro and in vivo effects of estrogen stimulation on M- and F-MDSCs. Table S1 presents the correlation coefficients for relations between variables. Table S2 presents the primers for RT-PCR. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200612094/DC1.
Supplementary Material
Acknowledgments
We would like to thank Samantha Sanford, Seiji Kubo, Michele Jones, Michael Mentzer, Patrick Blake, Chris Scelfo, Michelle Wiit, and Maria Branca for technical assistance, Ryan Sauder for excellent editorial assistance, and Dr. Bruno Péault (University of Pittsburgh, Pittsburgh, PA) and Dr. Art Levine (University of Pittsburgh School of Medicine, Pittsburgh, PA) for insightful discussions.
This work was supported by the Jesse's Journey Foundation, the Muscular Dystrophy Association, the National Institutes of Health (grant R01 AR49684-01), the William F. and Jean W. Donaldson Chair at the Children's Hospital of Pittsburgh, and the Henry J. Mankin Chair at the University of Pittsburgh.
Abbreviations used in this paper: ANOVA, analysis of variance; F-MDSC, female MDSC; GM, growth medium; MDSC, muscle-derived stem cell; M-MDSC, male MDSC; MyHC, myosin heavy chain; PD, population doubling; PDT, PD time; RI, regeneration index; ROS, reactive oxygen species; SCID, severe combined immunodeficiency.
References
- Aristotle. 350 BC. Historia Animalium: Books VII–X. 1991. edition. D.M. Balme, editor. Harvard University Press, Cambridge, MA. 435–437.
- Avery, B., C.B. Jorgensen, V. Madison, and T. Greve. 1992. Morphological development and sex of bovine in vitro-fertilized embryos. Mol. Reprod. Dev. 32:265–270. [DOI] [PubMed] [Google Scholar]
- Aviv, A. 2002. Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J. Mol. Med. 80:689–695. [DOI] [PubMed] [Google Scholar]
- Aviv, A., J. Shay, K. Christensen, and W. Wright. 2005. The longevity gender gap: are telomeres the explanation? Sci. Aging Knowledge Environ. 2005:pe16. [DOI] [PubMed]
- Beauchamp, J.R., J.E. Morgan, C.N. Pagel, and T.A. Partridge. 1994. Quantitative studies of efficacy of myoblast transplantation. Muscle Nerve. 1:S261. [Google Scholar]
- Beauchamp, J.R., J.E. Morgan, C.N. Pagel, and T.A. Partridge. 1999. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J. Cell Biol. 144:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenhorn, E.P., S. Troutman, L.D. Clark, X.M. Zhang, P. Chen, and E. Heber-Katz. 2003. Sexually dimorphic genes regulate healing and regeneration in MRL mice. Mamm. Genome. 14:250–260. [DOI] [PubMed] [Google Scholar]
- Bottino, R., A.N. Balamurugan, S. Bertera, M. Pietropaolo, M. Trucco, and J.D. Piganelli. 2002. Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes. 51:2561–2567. [DOI] [PubMed] [Google Scholar]
- Chau, B.N., H.L. Borges, T.T. Chen, A. Masselli, I.C. Hunton, and J.Y. Wang. 2002. Signal-dependent protection from apoptosis in mice expressing caspase-resistant Rb. Nat. Cell Biol. 4:757–765. [DOI] [PubMed] [Google Scholar]
- Collins, C.A., I. Olsen, P.S. Zammit, L. Heslop, A. Petrie, T.A. Partridge, and J.E. Morgan. 2005. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 122:289–301. [DOI] [PubMed] [Google Scholar]
- Deasy, B.M., R.J. Jankowski, T.R. Payne, B. Cao, J.P. Goff, J.S. Greenberger, and J. Huard. 2003. Modeling stem cell population growth: incorporating terms for proliferative heterogeneity. Stem Cells. 21:536–545. [DOI] [PubMed] [Google Scholar]
- Deasy, B.M., Y. Li, and J. Huard. 2004. Tissue engineering with muscle-derived stem cells. Curr. Opin. Biotechnol. 15:419–423. [DOI] [PubMed] [Google Scholar]
- Deasy, B.M., B.M. Gharaibeh, J.B. Pollett, M.M. Jones, M.A. Lucas, Y. Kanda, and J. Huard. 2005. Long-term self-renewal of postnatal muscle-derived stem cells. Mol. Biol. Cell. 16:3323–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla, N.S., L. Golfman, S. Takeda, N. Takeda, and M. Nagano. 1999. Evidence for the role of oxidative stress in acute ischemic heart disease: a brief review. Can. J. Cardiol. 15:587–593. [PubMed] [Google Scholar]
- Du, L., H. Bayir, Y. Lai, X. Zhang, P.M. Kochanek, S.C. Watkins, S.H. Graham, and R.S. Clark. 2004. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J. Biol. Chem. 279:38563–38570. [DOI] [PubMed] [Google Scholar]
- Fan, Y., M. Maley, M. Beilharz, and M. Grounds. 1996. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 19:853–860. [DOI] [PubMed] [Google Scholar]
- Guerette, B., I. Asselin, D. Skuk, M. Entman, and J.P. Tremblay. 1997. a. Control of inflammatory damage by anti-LFA-1: increase success of myoblast transplantation. Cell Transplant. 6:101–107. [DOI] [PubMed] [Google Scholar]
- Guerette, B., D. Skuk, F. Celestin, C. Huard, F. Tardif, I. Asselin, B. Roy, M. Goulet, R. Roy, M. Entman, and J.P. Tremblay. 1997. b. Prevention by anti-LFA-1 of acute myoblast death following transplantation. J. Immunol. 159:2522–2531. [PubMed] [Google Scholar]
- Gute, D.C., T. Ishida, K. Yarimizu, and R.J. Korthuis. 1998. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol. Cell. Biochem. 179:169–187. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Adan, A., M. Oter, B. Martinez-Madrid, B. Pintado, and J. De La Fuente. 2000. Differential expression of two genes located on the X chromosome between male and female in vitro-produced bovine embryos at the blastocyst stage. Mol. Reprod. Dev. 55:146–151. [DOI] [PubMed] [Google Scholar]
- Harada, H., K.P. Pavlick, I.N. Hines, D.J. Lefer, J.M. Hoffman, S. Bharwani, R.E. Wolf, and M.B. Grisham. 2003. Sexual dimorphism in reduced-size liver ischemia and reperfusion injury in mice: role of endothelial cell nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 100:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, T.W., N. Chen, F. Cunningham, M. Tello-Ruiz, I. Antoshechkin, C. Bastiani, T. Bieri, D. Blasiar, K. Bradnam, J. Chan, et al. 2004. WormBase: a multi-species resource for nematode biology and genomics. Nucleic Acids Res. 32:D411–D417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski, R.J., and J. Huard. 2004. a. Establishing reliable criteria for isolating myogenic cell fractions with stem cell properties and enhanced regenerative capacity. Blood Cells Mol. Dis. 32:24–33. [DOI] [PubMed] [Google Scholar]
- Jankowski, R.J., and J. Huard. 2004. b. Myogenic cellular transplantation and regeneration: sorting through progenitor heterogeneity. Panminerva Med. 46:81–91. [PubMed] [Google Scholar]
- Jankowski, R.J., B.M. Deasy, B. Cao, C. Gates, and J. Huard. 2002. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J. Cell Sci. 115:4361–4374. [DOI] [PubMed] [Google Scholar]
- Jilka, R.L., G. Hangoc, G. Girasole, G. Passeri, D.C. Williams, J.S. Abrams, B. Boyce, H. Broxmeyer, and S.C. Manolagas. 1992. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 257:88–91. [DOI] [PubMed] [Google Scholar]
- Kochhar, H.S., K.P. Kochhar, P.K. Basrur, and W.A. King. 2003. Influence of the duration of gamete interaction on cleavage, growth rate and sex distribution of in vitro produced bovine embryos. Anim. Reprod. Sci. 77:33–49. [DOI] [PubMed] [Google Scholar]
- Lee, J.Y., Z. Qu-Petersen, B. Cao, S. Kimura, R. Jankowski, J. Cummins, A. Usas, C. Gates, P. Robbins, A. Wernig, and J. Huard. 2000. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J. Cell Biol. 150:1085–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N., and T.D. Oberley. 1998. Modulation of antioxidant enzymes, reactive oxygen species, and glutathione levels in manganese superoxide dismutase-overexpressing NIH/3T3 fibroblasts during the cell cycle. J. Cell. Physiol. 177:148–160. [DOI] [PubMed] [Google Scholar]
- Liu, G., A.E. Loraine, R. Shigeta, M. Cline, J. Cheng, V. Valmeekam, S. Sun, D. Kulp, and M.A. Siani-Rose. 2003. NetAffx: Affymetrix probesets and annotations. Nucleic Acids Res. 31:82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, K.-I., S. Ohkura, and H. Tsukamura. 2000. Physiology of reproduction. In The Laboratory Rat: the Handbook of Experimental Animals. G.J. Krinke, editor. Academic Press, San Diego. 756 pp.
- Menon, S.G., E.H. Sarsour, D.R. Spitz, R. Higashikubo, M. Sturm, H. Zhang, and P.C. Goswami. 2003. Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res. 63:2109–2117. [PubMed] [Google Scholar]
- Mitchell, P.O., T. Mills, R.S. O'Connor, T. Graubert, E. Dzierzak, and G.K. Pavlath. 2005. Sca-1 negatively regulates proliferation and differentiation of muscle cells. Dev. Biol. 283:240–252. [DOI] [PubMed] [Google Scholar]
- Molnar, G., M.L. Ho, and N.A. Schroedl. 1996. Evidence for multiple satellite cell populations and a non-myogenic cell type that is regulated differently in regenerating and growing skeletal muscle. Tissue Cell. 28:547–556. [DOI] [PubMed] [Google Scholar]
- Mueller, G.M., T. O'Day, J.F. Watchko, and M. Ontell. 2002. Effect of injecting primary myoblasts versus putative muscle-derived stem cells on mass and force generation in mdx mice. Hum. Gene Ther. 13:1081–1090. [DOI] [PubMed] [Google Scholar]
- Mulroney, S.E., C. Woda, M. Johnson, and C. Pesce. 1999. Gender differences in renal growth and function after uninephrectomy in adult rats. Kidney Int. 56:944–953. [DOI] [PubMed] [Google Scholar]
- Nomura, T., E. Ashihara, K. Tateishi, S. Asada, T. Ueyama, T. Takahashi, H. Matsubara, and H. Oh. 2007. Skeletal myosphere-derived progenitor cell transplantation promotes neovascularization in delta-sarcoglycan knockdown cardiomyopathy. Biochem. Biophys. Res. Commun. 352:668–674. [DOI] [PubMed] [Google Scholar]
- Peippo, J., A. Farazmand, M. Kurkilahti, M. Markkula, P.K. Basrur, and W.A. King. 2002. Sex-chromosome linked gene expression in in-vitro produced bovine embryos. Mol. Hum. Reprod. 8:923–929. [DOI] [PubMed] [Google Scholar]
- Perez-Iratxeta, C., G. Palidwor, C.J. Porter, N.A. Sanche, M.R. Huska, B.P. Suomela, E.M. Muro, P.M. Krzyzanowski, E. Hughes, P.A. Campbell, et al. 2005. Study of stem cell function using microarray experiments. FEBS Lett. 579:1795–1801. [DOI] [PubMed] [Google Scholar]
- Pergament, E., M. Fiddler, N. Cho, D. Johnson, and W.J. Holmgren. 1994. Sexual differentiation and preimplantation cell growth. Hum. Reprod. 9:1730–1732. [DOI] [PubMed] [Google Scholar]
- Pittenger, M.F., A.M. Mackay, S.C. Beck, R.K. Jaiswal, R. Douglas, J.D. Mosca, M.A. Moorman, D.W. Simonetti, S. Craig, and D.R. Marshak. 1999. Multilineage potential of adult human mesenchymal stem cells. Science. 284:143–147. [DOI] [PubMed] [Google Scholar]
- Qu, Z., L. Balkir, J.C. van Deutekom, P.D. Robbins, R. Pruchnic, and J. Huard. 1998. Development of approaches to improve cell survival in myoblast transfer therapy. J. Cell Biol. 142:1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu-Petersen, Z., B. Deasy, R. Jankowski, M. Ikezawa, J. Cummins, R. Pruchnic, J. Mytinger, B. Cao, C. Gates, A. Wernig, and J. Huard. 2002. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J. Cell Biol. 157:851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando, T.A., and H.M. Blau. 1994. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125:1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollo, C.D. 2002. Growth negatively impacts the life span of mammals. Evol. Dev. 4:55–61. [DOI] [PubMed] [Google Scholar]
- Sarig, R., Z. Baruchi, O. Fuchs, U. Nudel, and D. Yaffe. 2006. Regeneration and transdifferentiation potential of muscle-derived stem cells propagated as myospheres. Stem Cells. 24:1769–1778. [DOI] [PubMed] [Google Scholar]
- Schultz, E. 1996. Satellite cell proliferative compartments in growing skeletal muscles. Dev. Biol. 175:84–94. [DOI] [PubMed] [Google Scholar]
- Sherley, J.L., P.B. Stadler, and J.S. Stadler. 1995. A quantitative method for the analysis of mammalian cell proliferation in culture in terms of dividing and non-dividing cells. Cell Prolif. 28:137–144. [DOI] [PubMed] [Google Scholar]
- Steinlein, P., O. Wessely, S. Meyer, E.M. Deiner, M.J. Hayman, and H. Beug. 1995. Primary, self-renewing erythroid progenitors develop through activation of both tyrosine kinase and steroid hormone receptors. Curr. Biol. 5:191–204. [DOI] [PubMed] [Google Scholar]
- Stindl, R. 2004. Tying it all together: telomeres, sexual size dimorphism and the gender gap in life expectancy. Med. Hypotheses. 62:151–154. [DOI] [PubMed] [Google Scholar]
- Suzuki, K., B. Murtuza, J.R. Beauchamp, R.T. Smolenski, A. Varela-Carver, S. Fukushima, S.R. Coppen, T.A. Partridge, and M.H. Yacoub. 2004. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J. 18:1153–1155. [DOI] [PubMed] [Google Scholar]
- Tamaki, S., T. Ichinohe, K. Matsuo, N. Hamajima, N. Hirabayashi, and H. Dohy. 2001. Superior survival of blood and marrow stem cell recipients given maternal grafts over recipients given paternal grafts. Bone Marrow Transplant. 28:375–380. [DOI] [PubMed] [Google Scholar]
- Vina, J., C. Borras, J. Gambini, J. Sastre, and F.V. Pallardo. 2005. Why females live longer than males: control of longevity by sex hormones. Sci. Aging Knowledge Environ. 2005:pe17. [DOI] [PubMed]
- Wagers, A.J., and I.M. Conboy. 2005. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 122:659–667. [DOI] [PubMed] [Google Scholar]
- Winitsky, S.O., T.V. Gopal, S. Hassanzadeh, H. Takahashi, D. Gryder, M.A. Rogawski, K. Takeda, Z.X. Yu, Y.H. Xu, and N.D. Epstein. 2005. Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro. PLoS Biol. 3:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, K.P., B.R. Yadav, W.A. King, and K.J. Betteridge. 1992. Sex-related differences in developmental rates of bovine embryos produced and cultured in vitro. Mol. Reprod. Dev. 31:249–252. [DOI] [PubMed] [Google Scholar]
- Zammit, P., and J. Beauchamp. 2001. The skeletal muscle satellite cell: stem cell or son of stem cell? Differentiation. 68:193–204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.