Abstract
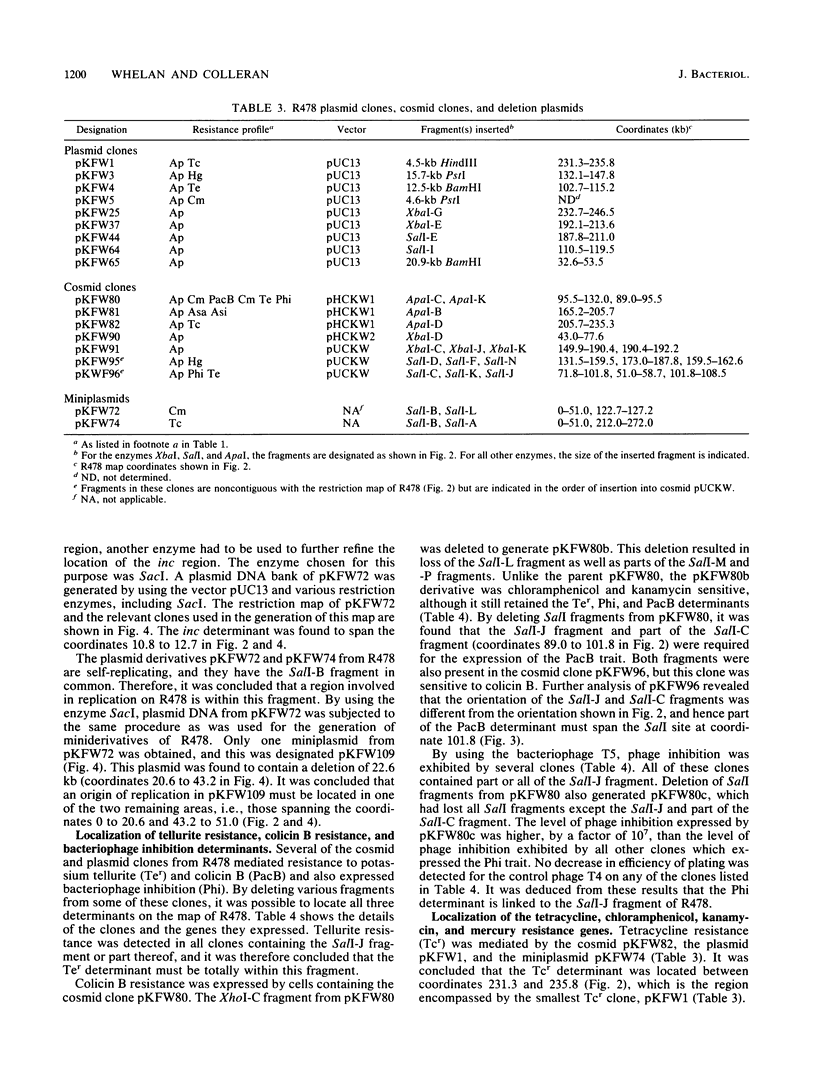
A restriction map of the 272-kb IncHI2 plasmid R478 was constructed by using the enzymes ApaI, XbaI, SalI, and XhoI. The map was derived from cloned restriction fragments from R478 inserted into cosmid and plasmid vectors as well as from double-digestion analysis of R478 and R478 miniplasmids. All previously known resistance determinants were cloned from R478, and their positions were located on the restriction map. A region involved in incompatibility was cloned and mapped. The location of a previously unreported arsenite resistance gene was also determined. The genes encoding tellurite resistance, colicin B resistance, and phage inhibition were found to be associated with a 6.7-kb SalI fragment of R478.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S., Smith H. R. Chloramphenicol resistance in the typhoid bacillus. Br Med J. 1972 Aug 5;3(5822):329–331. doi: 10.1136/bmj.3.5822.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. S. The problem and implications of chloramphenicol resistance in the typhoid bacillus. J Hyg (Lond) 1975 Apr;74(2):289–299. doi: 10.1017/s0022172400024360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E., Hughes V. M., Richards H., Datta N. R plasmids of a new incompatibility group determine constitutive production of H pili. Plasmid. 1982 May;7(3):230–238. doi: 10.1016/0147-619x(82)90004-x. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. The unique conjugation system of IncHI3 plasmid MIP233. Plasmid. 1986 Jul;16(1):63–71. doi: 10.1016/0147-619x(86)90080-6. [DOI] [PubMed] [Google Scholar]
- Chen C. M., Misra T. K., Silver S., Rosen B. P. Nucleotide sequence of the structural genes for an anion pump. The plasmid-encoded arsenical resistance operon. J Biol Chem. 1986 Nov 15;261(32):15030–15038. [PubMed] [Google Scholar]
- Couturier M., Bex F., Bergquist P. L., Maas W. K. Identification and classification of bacterial plasmids. Microbiol Rev. 1988 Sep;52(3):375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983 Sep;47(3):361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Baumberg S. Resistance to arsenic compounds conferred by a plasmid transmissible between strains of Escherichia coli. J Bacteriol. 1973 Jul;115(1):459–460. doi: 10.1128/jb.115.1.459-460.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Rodriguez-Lemoine V., Datta N. R factors from Serratia marcescens. J Gen Microbiol. 1975 Jan;86(1):88–92. doi: 10.1099/00221287-86-1-88. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Jobling M. G., Ritchie D. A. Genetic and physical analysis of plasmid genes expressing inducible resistance of tellurite in Escherichia coli. Mol Gen Genet. 1987 Jun;208(1-2):288–293. doi: 10.1007/BF00330455. [DOI] [PubMed] [Google Scholar]
- Newnham P. J., Taylor D. E. Genetic analysis of transfer and incompatibility functions within the IncHI plasmid R27. Plasmid. 1990 Mar;23(2):107–118. doi: 10.1016/0147-619x(90)90029-c. [DOI] [PubMed] [Google Scholar]
- Praszkier J., Wei T., Siemering K., Pittard J. Comparative analysis of the replication regions of IncB, IncK, and IncZ plasmids. J Bacteriol. 1991 Apr;173(7):2393–2397. doi: 10.1128/jb.173.7.2393-2397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Lemoine V., Jacob A. E., Hedges R. W., Datta N. Thermosensitive production of their transfer systems by group S plasmids. J Gen Microbiol. 1975 Jan;86(1):111–114. doi: 10.1099/00221287-86-1-111. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., Weigel U., Karkaria C., Gangola P. Molecular characterization of an anion pump. The arsA gene product is an arsenite(antimonate)-stimulated ATPase. J Biol Chem. 1988 Mar 5;263(7):3067–3070. [PubMed] [Google Scholar]
- Roussel A. F., Chabbert Y. A. Taxonomy and epidemiology of gram-negative bacterial plasmids studied by DNA-DNA filter hybridization in formamide. J Gen Microbiol. 1978 Feb;104(2):269–276. doi: 10.1099/00221287-104-2-269. [DOI] [PubMed] [Google Scholar]
- Schottel J., Mandal A., Clark D., Silver S., Hedges R. W. Volatilisation of mercury and organomercurials determined by inducible R-factor systems in enteric bacteria. Nature. 1974 Sep 27;251(5473):335–337. doi: 10.1038/251335a0. [DOI] [PubMed] [Google Scholar]
- Silver S., Budd K., Leahy K. M., Shaw W. V., Hammond D., Novick R. P., Willsky G. R., Malamy M. H., Rosenberg H. Inducible plasmid-determined resistance to arsenate, arsenite, and antimony (III) in escherichia coli and Staphylococcus aureus. J Bacteriol. 1981 Jun;146(3):983–996. doi: 10.1128/jb.146.3.983-996.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Jacoby G. A. Plasmid-determined resistance to tellurium compounds. J Bacteriol. 1977 Jan;129(1):276–281. doi: 10.1128/jb.129.1.276-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Brose E. C., Kwan S., Yan W. Mapping of transfer regions within incompatibility group HI plasmid R27. J Bacteriol. 1985 Jun;162(3):1221–1226. doi: 10.1128/jb.162.3.1221-1226.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Brose E. C. Restriction endonuclease mapping of R27 (TP117), an incompatibility group HI subgroup 1 plasmid from Salmonella typhimurium. Plasmid. 1985 Jan;13(1):75–77. doi: 10.1016/0147-619x(85)90058-7. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Levine J. G. Studies of temperature-sensitive transfer and maintenance of H incompatibility group plasmids. J Gen Microbiol. 1980 Feb;116(2):475–484. doi: 10.1099/00221287-116-2-475. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Summers A. O. Association of tellurium resistance and bacteriophage inhibition conferred by R plasmids. J Bacteriol. 1979 Mar;137(3):1430–1433. doi: 10.1128/jb.137.3.1430-1433.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walter E. G., Taylor D. E. Comparison of tellurite resistance determinants from the IncP alpha plasmid RP4Ter and the IncHII plasmid pHH1508a. J Bacteriol. 1989 Apr;171(4):2160–2165. doi: 10.1128/jb.171.4.2160-2165.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter E. G., Weiner J. H., Taylor D. E. Nucleotide sequence and overexpression of the tellurite-resistance determinant from the IncHII plasmid pHH1508a. Gene. 1991 May 15;101(1):1–7. doi: 10.1016/0378-1119(91)90217-y. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Palchaudhuri S. Incompatibility repressor in a RepA-like replicon of the IncFI plasmid ColV2-K94. J Bacteriol. 1986 Jun;166(3):1106–1112. doi: 10.1128/jb.166.3.1106-1112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M., Taylor D. E. Identification of DNA homologies among H incompatibility group plasmids by restriction enzyme digestion and Southern transfer hybridization. Antimicrob Agents Chemother. 1983 Aug;24(2):194–200. doi: 10.1128/aac.24.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble D. D., Dong X., Wu R. P., Luckow V. A., Martinez A. F., Rownd R. H. IncFII plasmid incompatibility product and its target are both RNA transcripts. J Bacteriol. 1984 Oct;160(1):28–35. doi: 10.1128/jb.160.1.28-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Taylor D. E. Characterization of transfer regions within the HII incompatibility group plasmid pHH1508a. J Bacteriol. 1987 Jun;169(6):2866–2868. doi: 10.1128/jb.169.6.2866-2868.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



