Abstract
The intracellular sorting of peptide hormone precursors to the dense core secretory granules (DCSGs) is essential for their bioactivation. Despite the fundamental importance of this cellular process, the nature of the sorting signals for entry of proteins into DCSGs remains a source of vigorous debate. This review highlights recent discoveries that are consistent with a model in which several protein domains, acting in a cell-specific fashion and at different steps in the sorting process, act in concert to regulate the entry of proteins into DCSGs.
The accurate sorting of proteins to their cellular destinations is of fundamental importance in biology and must occur with high precision in the context of a highly concentrated and extremely complex mixture of proteins. The identification of the “codes” carried by proteins that ensure their proper intracellular sorting has been a topic of intense and fruitful research for >40 yr. As a result, most introductory textbooks now include descriptions of the canonical signals that direct the sorting of proteins to the secretory pathway, mitochondria, nucleus, and lysosomes, as well as the signals for ER or Golgi retention and endocytosis, to mention but a few. However, a similar statement cannot be made for the protein signals required to direct proteins to dense core secretory granules (DCSGs). These cytoplasmic organelles, which are present in endocrine and neuroendocrine cells, store hormones, proteases, and signaling molecules until the cell receives a signal for their release. As such, they are the key component in the regulated secretory pathway. Why has the identification of DCSG sorting signals been such an elusive goal?
Three truths and three postulates
There has been a lot of debate not only about how DCSG sorting occurs but also about exactly where in the cell this triage takes place. All cells have the capacity to rapidly secrete proteins after their transit through the constitutive secretory pathway. A great deal of evidence supports the view that in the appropriate cell type, DCSG sorting signals can redirect proteins from the constitutive secretory pathway to DCSGs, thus confirming that it is not a default secretory pathway. Some groups have proposed that DCSG sorting occurs through the action of a sorting “receptor” present in the TGN that latches onto granule-destined proteins at sites where nascent granules will bud (Chung et al., 1989; Cool et al., 1997). This has been referred to as the “sorting by entry” model. On the other hand, convincing evidence has been presented that in cells that generate DCSGs, all of the contents of the TGN are initially encapsulated into the nascent granules (Arvan and Castle, 1998). This “sorting by retention” model proposes that those proteins destined to be secreted constitutively are progressively extruded in low-density vesicles as the granule matures, ultimately leaving only the correct cargo protein in the mature DCSG. The first truth is that, regardless of the site at which sorting occurs, a mechanism has to exist that establishes and then maintains the segregation of DCSG cargo proteins from those that are constitutively secreted. Thus, it is a reasonable postulate that some mechanism exists to anchor the appropriate cargo proteins to the DCSG as it forms or matures.
A second truth is that the sorting of proteins to DCSGs is a prerequisite for certain posttranslational processing steps in hormone and protease activation. For example, the conversion of proinsulin to active insulin, the conversion of proopiomelanocortin (POMC) to its many peptides, including ACTH, and the proteolytic activation of prorenin to renin all occur only after the precursors are encapsulated in the nascent secretory granules (Orci et al., 1986; Taugner et al., 1987; Schmidt and Moore, 1995). This makes sense for the organism because it ensures that the secretion of the active hormones or proteases is under appropriate physiological control. However, for granule-restricted activation to occur, it is necessary that both the protein precursors and the appropriate processing enzymes end up in the same DCSG. In the case of proinsulin, this means that the proprotein convertases PC1/3 and PC2, as well as carboxypeptidase E (CPE), all of which are required for generation of active insulin, have to be cotargeted with proinsulin in the budding granules. Thus, a second postulate is that a mechanism exists to ensure efficient cotargeting of protein precursors and their processing enzymes in the same organelle.
DCSGs also share, by definition, the distinguishing trait of a core that appears dark or dense in electron micrographs. However, in spite of this common appearance, there may be important functional and mechanistic differences in DCSGs. For example, the gonadotropes of the pituitary store luteinizing hormone and follicle-stimulating hormone in separate DCSGs, and their release is independently controlled (for review see Dannies, 2001). Likewise, there are two types of DCSG in chromaffin cells containing either epinephrine or norepinephrine, and these are morphologically distinct (Hendy et al., 2006). The signals for targeting proteins to DCSGs also show tissue-specific variations; removal of 90 amino acids from the C terminus of the granin chromogranin A (CgA) prevents its sorting to DCSGs in pituitary GH4 cells, but has no effect on DCSG sorting in sympathoadrenal PC12 cells (Cowley et al., 2000). Likewise, POMC is efficiently stored in DCSGs when transfected into cultured pituitary cells, but not in sympathetic neurons (Marx et al., 1999). Thus, a third truth is that not all DCSGs are alike, and it is a reasonable postulate that DCSGs can be assembled, even within the same cell, through more than one mechanism.
Could some of these truths explain the difficulties in reaching consensus on the protein signals necessary for DCSG targeting?
A plethora of signals, a paucity of consensus
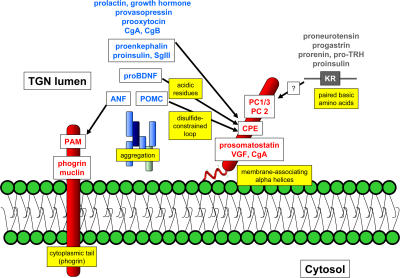
There has been no shortage in the variety of DCSG sorting mechanisms proposed in the last 20 yr; these include protein domains that interact with or that traverse membranes and that may or may not interact with additional proteins on the cytoplasmic side of the DCSG, proteins proposed to be a “master switch” for granule formation, universal granule cargo receptors, protein domains that mediate aggregation in the late TGN, certain paired basic protease cleavage sites or α helices in secretory proteins, disulfide-constrained loops, acidifying proton pumps, and other mechanisms. As a result, investigators have become progressively entrenched in defending their favorite mechanisms and commonly use the descriptors “controversial” and “difficult to repeat” to describe the work of others in their publications. Nevertheless, it is possible to accommodate most of these findings in a model that subdivides targeting function into three components (Fig. 1): membrane associated (or traversing) tethers, tether-associated cargo, and aggregation.
Figure 1.
Proteins sorted to DCSGs can be functionally subdivided into three groups. Tethers (red) either traverse or associate with membranes. Many DCSG cargo proteins also aggregate to form the dense core (blue), and these aggregates may contain more than one protein. Some DCSG proteins associate with membrane tethers (arrows). The yellow boxes indicate the various protein domains or mechanisms that have been implicated in DCSG sorting. Note that some proteins (such as insulin) may have multiple DCSG sorting mechanisms. See text for details.
Peptidyl-α-amidating monooxygenase (PAM), phogrin, and muclin are all type 1 membrane-spanning proteins that are targeted to DCSGs (Bell-Parikh et al., 2001; Wasmeier et al., 2002; Boulatnikov and De Lisle, 2004). In the case of phogrin, the granule sorting domain is located in the cytoplasmic tail of the protein, and although the exact nature of the signal is still debated, it appears that this domain can bind the clathrin adaptor proteins AP-1 and -2 in vitro (Wasmeier et al., 2005; Torii et al., 2005). Such interactions might provide a means of communication between the granule cargo proteins and the membrane domains or cytoplasmic proteins that will define the budding DCSGs. The membrane-binding domains of the granule-resident protein CPE (CPE; Dhanvantari et al., 2002) and the prohormone convertases PC1/3 (Jutras et al., 2000; Arnaoutova et al., 2003) and PC2 (Assadi et al., 2004) are also key for their targeting to DSCGs, and there is agreement that the granule sorting is mediated by short α helical domains. An α helical domain has also been shown to be important for targeting prosomatostatin (Mouchantaf et al., 2001), CgA (Taupenot et al., 2002), and VGF (Garcia et al., 2005) to DCSGs. Recent results suggest that α helices with the ability to direct granule sorting in secretory proteins share the characteristic of charge segregation from a hydrophobic patch, which is consistent with a shallow membrane interaction (Dikeakos et al., 2007). This first group of DCSG proteins could therefore be said to be tethered to the membranes of the TGN or the maturing granule.
A second group of granule sorting domains may act by binding cargo proteins to these granule-tethered proteins. For example, CPE has been proposed to interact with several granule cargo proteins including proenkephalin, proinsulin, POMC (Cool and Loh, 1998), brain-derived neurotropic factor (proBDNF; Lou et al., 2005), and secretogranin III (SgIII; Hosaka et al., 2005) to promote their retention in secretory granules, even though some of these are not enzymatic substrates of CPE. No common mechanism for interaction of these cargo proteins with CPE has yet emerged, although undefined residues within an N-terminal disulfide-constrained hydrophobic loop in POMC (Loh et al., 2002) and acidic residues in proBDNF (Lou et al., 2005) have been reported, and both seem to be important for sorting the respective proteins to DCSGs (Cool et al., 1995; Lou et al., 2005). Paired basic amino acids have also been reported to direct DCSG sorting in some proteins, including proneurotensin (Feliciangeli et al., 2001), prorenin (Brechler et al., 1996), prothyrotropin-releasing hormone (Mulcahy et al., 2005), and progastrin (Bundgaard et al., 2004), and to increase the sorting efficiency of proinsulin (Kuliawat et al., 2000). In the analyses performed to date, it appears that these paired basic amino acids must constitute a cleavage site for one of the granule-resident prohormone convertases (PC1/3 or PC2) to function as a granule sorting domain because changing the cleavage site to one recognized by furin (another member of the family that cleaves its substrates in the early secretory pathway) causes the proteins to be secreted through the default constitutive pathway (Brechler et al., 1996). These results raise the possibility that certain DCSG-targeted proteases can act as sorting chaperones for their substrates, in addition to being processing proteases. Muclin has also been suggested to act as a granule cargo receptor in pancreatic cells through its binding of sulfate groups on O-linked glycosylated proteins (Boulatnikov and De Lisle, 2004). Atrial natriuretic factor (ANF) has also been shown to be tightly bound to the membranes of atrial myocyte secretory granules through its interaction with PAM (O'Donnell et al., 2003), although it is not a substrate of PAM. Thus, a variety of interactions with “tethers” may serve to target proteins to secretory granules. Notably, if this mechanism is correct, it would, in some cases, provide a means to ensure that processing enzymes and their substrates end up in the same DCSGs.
A third category of granule-targeting mechanisms involves formation of high molecular weight protein complexes or aggregates. Indeed, many granule-targeted cargo proteins have the ability to multimerize or aggregate, leading, in most cases, to the formation of electron-dense cores. A direct correlation between the ability to aggregate in vitro and to be sorted to secretory granules in transfected cells has been reported for rat pro-ANF (Canaff et al., 1996) and CgA (Jain et al., 2002). Because granins are acidic proteins that cluster in the slightly acidic environment present in DCSGs (for review see Dannies, 2001) it has been suggested that aggregation may serve to prevent their extrusion from the maturing granule. Indeed, Taupenot et al. (2005) showed that treatment of PC12 cells with bafilomycin A1, which is a specific inhibitor of vacuolar H-ATPase, resulted in a decrease in regulated secretion of CgA with a concomitant decrease in visible DCSGs, suggesting that regulated secretion of CgA and dense core formation are linked in DCSGs. Kim et al. (2001) also showed that silencing CgA expression in PC12 cells results in a loss of visible DCSGs, leading the authors to the striking conclusion that CgA is not only a component of the dense core, but that it is also a “master regulator” of DCSG biogenesis. Further work by Malosio et al. (2004) suggests that the role of CgA in this process may not be that simple; they found no correlation between DSCG content and CgA expression in isolated clonal lines of PC12 cells, suggesting that other proteins could be contributing to DCSG appearance. In fact, expression of several other DCSG cargo proteins, including provasopressin, prooxytocin, POMC, secretogranin II, and chromogranin B, is sufficient to induce aggregate-containing cytoplasmic vesicles, even in cells with no regulated secretory pathway (Beuret et al., 2004), although these probably do not display all of the functional characteristics of DCSGs (Meldolesi et al., 2004). Regulating the formation of the aggregate may also be physiologically important. Knoch et al. (2004) recently reported that a polypyrimidine-binding protein (PTB), which is up-regulated under conditions of high insulin demand, stabilizes messenger RNAs of many of these same DCSG cargo proteins in insulin-producing cells and leads to increased granule formation. CgA has also been reported to induce the expression of PN-1, which is a serine protease inhibitor that slows the turnover of several DCSG cargo proteins (Kim and Loh, 2006) that could provide an additional mechanism for increasing DCSG aggregate formation. Because CgA binds to another granin partner, SgIII (Hosaka et al., 2004), which, in turn, can associate with cholesterol (Hosaka et al., 2004) and CPE (Hosaka et al., 2005), aggregation may synergize with protein–protein and protein–membrane interactions to improve the retention of cargo proteins in the maturing granule and their regulated secretion.
In mus veritas
In spite of the compelling arguments presented for these various DCSG sorting mechanisms, their translation to the whole animal has been anything but simple. One example of this difficulty is the proposed role of CgA as a master regulator of granule formation. Although down-regulation of CgA expression was reported to result in the loss of detectable DCSGs in cultured PC12 cells (Kim et al., 2001), CgA gene inactivation in mice leads to either a “reduction” (Mahapatra et al., 2005) or no discernable effect (Hendy et al., 2006) on DCSG formation in the CgA-rich adrenal chromaffin cells in two independent studies. In spite of the differences in the effects on DCSG morphology, both groups report a similar and dramatic effect on catecholamine secretion in the CgA-deficient mice, proving that CgA deficiency is not entirely without consequence. How can these apparent differences in the requirement for CgA be explained? One obvious possibility is that in vivo, other DCSG cargo proteins can complement the function of CgA in the formation of the dense core, but cannot compensate for its absence in catecholamine storage and secretion. In support of this possibility, the group that saw no effect of CgA inactivation on DCSG formation reported an up-regulation of CgB and SgII in the adrenal glands of the engineered mice (Hendy et al., 2006). Thus, although CgA may affect DCSG formation in some cultured cells, this particular function can obviously be replaced in vivo. Nevertheless, although experiments to date have not identified a master regulator of DCSG formation, the concept may not be entirely wrong in specific cell types; ANF inactivation in mice leads to a complete loss of visible DCSGs in the cardiac atrium (John et al., 1995), and inactivation of the renin gene leads to a complete disappearance of DCSGs in the juxtaglomerular cells of the kidney (Clark et al., 1997). It's important to note, however, that regulated secretion can occur in the absence of a dense core as it does in many neurosecretory vesicles. In the case of the ANF and renin-deficient mice, it will be intriguing to determine if the remaining cargo proteins are still packaged in such vesicles in the absence of the aggregating partner.
A similar conundrum exists with CPE as a sorting receptor for a variety of DCSG cargo proteins. Cool et al. (1997) originally proposed CPE as the regulated secretory pathway sorting receptor because they observed endocrine disorders in the Cpe fat/fat mouse that harbors a mutation in the CPE gene. Proinsulin and POMC are among the several proteins that were shown to bind to CPE and that were proposed to enter DCSGs by this association (Cool and Loh, 1998). However, recent results demonstrate that both proinsulin and POMC are correctly targeted to DCSGs in CPE fat/fat mice (Irminger et al., 1997; Hosaka et al., 2005). What are we missing in this picture?
Synergy and diversity in granule sorting mechanisms
Although there may be many reasons why it has been hard to derive a consensus for the mechanisms and components of the DCSG sorting machinery, the most intuitive is that we are the victims of our own scientific reductionism, i.e., that in our search for a simple canonical sorting mechanism we have developed a grossly oversimplified view of the way in which proteins enter DCSGs. Nearly 100% of the proinsulin produced in pancreatic β enters DCSGs (Rhodes and Halban, 1987), whereas only about 25% of the prorenin in the secretory pathway of kidney juxtaglomerular cells is sorted to DCSGs (Pratt et al., 1987). What can explain these differences? Proinsulin contains numerous potential DCSG sorting domains, such as a binding domain for CPE (Cool and Loh, 1998), two paired basic amino acid protease cleavage sites (Steiner et al., 1996), and the ability to hexamerize and subsequently aggregate (Quinn et al., 1991), whereas prorenin only contains a single DCSG sorting domain: a paired basic amino acid protease cleavage site (Brechler et al., 1996). In the case of prorenin, changing even a single one of these basic amino acids completely eliminates DCSG targeting in tissue culture cells (Brechler et al., 1996). In contrast, neither the mutation of the protease cleavage sites (Halban and Irminger, 2003) nor the hexamerization domain (Quinn et al., 1991) of proinsulin appears to affect its DCSG sorting. Combined with the finding that proinsulin is still efficiently sorted to DCSG in CPE-deficient mice (Irminger et al., 1997), it has been tempting to dismiss the function of these putative sorting signals. However, another possible explanation is that, with its many DCSG sorting signals, proinsulin might be able to compensate for the loss of any single sorting domain. There is, in fact, some evidence to support the view that DCSG sorting signals can synergize; duplicating the disulfide-constrained loop DCSG sorting signal normally found at the N terminus of CgB results in a greater sorting efficiency to DCSG than the native protein (Glombik et al., 1999). Furthermore, combination of α helical and paired basic amino acid sorting domains on either the same protein or on two proteins capable of dimerizing led to a dramatic increase in DCSG sorting over proteins containing either individual domain (Lacombe et al., 2005). Thus, diverse sorting signals may be able to functionally complement each other even through protein–protein interactions. Complementarity in cellular sorting machineries may also occur. Hosaka et al. (2005) also reported that pituitaries of the Cpe fat/fat mouse contain elevated levels of both SgIII and CgA that might compensate for the loss of CPE in targeting POMC to DCSGs.
All of these cases are consistent with the existence of multiple sorting mechanisms, each of which can contribute to the overall efficiency of protein sorting or retention in DCSGs. Cell types and the nature and/or the number of the sorting domains contained in the cargo protein would ultimately determine the extent to which each mechanism is active. Multimerization and aggregation could add synergy between mechanisms used by other DCSG cargo proteins in the aggregate. With such a model, it's also easy to imagine how changing conditions within the cell could alter DCSG sorting efficiency of a protein, which is a potential control point that has important implications for hormone secretion but has received little attention to date.
Conclusion
The past 20 yr have been marked by many interesting discoveries in DCSG targeting. Work to date has largely supported the three previously outlined postulates: (a) proteins exist that could explain the anchoring of DCSG cargo to the granule membrane, (b) protein complexes between processing enzymes and their substrates have been proposed that could explain how these end up in the same granules, and (c) the diversity of sorting mechanisms helps to understand how there could be distinct DCSGs even within single cells and how there could be such a lack of agreement on the mechanism of DCSG sorting. Unfortunately, this lack of simple consensus has limited progress, primarily because of attempts to determine the role of single targeting motifs in given proteins in various cell culture models that may or may not be entirely appropriate. Although this approach has certainly not been without worth, a better understanding of the complexity of this important cellular event may help to design experiments that will help to significantly advance this field of research. Several important questions remain. How does the DCSG protein cargo identify the membrane patches that will make up the mature granule? Does this occur in the lumen of the TGN? Are specific lipids involved? How do these complexes communicate with the cytoplasmic accessory proteins that are necessary for the formation of the budding DCSGs? In fact, although it has been difficult to explain the entry of proteins into DCSGs, we can expect that describing the assembly of proteins on the surface of the DCSGs that are necessary for their cytoplasmic transport, docking at the membrane, and exocytosis will be an equally daunting challenge. A recent report on the components of the functionally related synaptic vesicle identified >400 associated proteins (Takamori et al., 2006). What is abundantly clear is that in the characterization of this unique organelle, there is still a lot to sort out.
Abbreviations used in this paper: ANF, atrial natriuretic factor; CGA, chromogranin A; CPE, carboxypeptidase E; DCSG, dense core secretory granule; PAM, peptidyl-α-amidating monooxygenase; POMC, proopiomelanocortin; PTB, polypyrimidine-binding protein.
References
- Arnaoutova, I., A.M. Smith, L.C. Coates, J.C. Sharpe, S. Dhanvantari, C.R. Snell, N.P. Birch, and Y.P. Loh. 2003. The prohormone processing enzyme PC3 is a lipid raft-associated transmembrane protein. Biochemistry. 42:10445–10455. [DOI] [PubMed] [Google Scholar]
- Arvan, P., and D. Castle. 1998. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem. J. 332:593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assadi, M., J.C. Sharpe, C. Snell, and Y.P. Loh. 2004. The C-terminus of prohormone convertase 2 is sufficient and necessary for Raft association and sorting to the regulated secretory pathway. Biochemistry. 43:7798–7807. [DOI] [PubMed] [Google Scholar]
- Bell-Parikh, L.C., B.A. Eipper, and R.E. Mains. 2001. Response of an integral granule membrane protein to changes in pH. J. Biol. Chem. 276:29854–29863. [DOI] [PubMed] [Google Scholar]
- Beuret, N., H. Stettler, A. Renold, J. Rutishauser, and M. Spiess. 2004. Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J. Biol. Chem. 279:20242–20249. [DOI] [PubMed] [Google Scholar]
- Boulatnikov, I., and R.C. De Lisle. 2004. Binding of the Golgi sorting receptor muclin to pancreatic zymogens through sulfated O-linked oligosaccharides. J. Biol. Chem. 279:40918–40926. [DOI] [PubMed] [Google Scholar]
- Brechler, V., W.N. Chu, J.D. Baxter, G. Thibault, and T.L. Reudelhuber. 1996. A protease processing site is essential for prorenin sorting to the regulated secretory pathway. J. Biol. Chem. 271:20636–20640. [DOI] [PubMed] [Google Scholar]
- Bundgaard, J.R., H. Birkedal, and J.F. Rehfeld. 2004. Progastrin is directed to the regulated secretory pathway by synergistically acting basic and acidic motifs. J. Biol. Chem. 279:5488–5493. [DOI] [PubMed] [Google Scholar]
- Canaff, L., V. Brechler, T.L. Reudelhuber, and G. Thibault. 1996. Secretory granule targeting of atrial natriuretic peptide correlates with its calcium-mediated aggregation. Proc. Natl. Acad. Sci. USA. 93:9483–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, K.N., P. Walter, G.W. Aponte, and H.P. Moore. 1989. Molecular sorting in the secretory pathway. Science. 243:192–197. [DOI] [PubMed] [Google Scholar]
- Clark, A.F., M.G. Sharp, S.D. Morley, S. Fleming, J. Peters, and J.J. Mullins. 1997. Renin-1 is essential for normal renal juxtaglomerular cell granulation and macula densa morphology. J. Biol. Chem. 272:18185–18190. [DOI] [PubMed] [Google Scholar]
- Cool, D.R., and Y.P. Loh. 1998. Carboxypeptidase E is a sorting receptor for prohormones: binding and kinetic studies. Mol. Cell. Endocrinol. 139:7–13. [DOI] [PubMed] [Google Scholar]
- Cool, D.R., M. Fenger, C.R. Snell, and Y.P. Loh. 1995. Identification of the sorting signal motif within pro-opiomelanocortin for the regulated secretory pathway. J. Biol. Chem. 270:8723–8729. [DOI] [PubMed] [Google Scholar]
- Cool, D.R., E. Normant, F. Shen, H.C. Chen, L. Pannell, Y. Zhang, and Y.P. Loh. 1997. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 88:73–83. [DOI] [PubMed] [Google Scholar]
- Cowley, D.J., Y.R. Moore, D.S. Darling, P.B. Joyce, and S.U. Gorr. 2000. N- and C-terminal domains direct cell type-specific sorting of chromogranin A to secretory granules. J. Biol. Chem. 275:7743–7748. [DOI] [PubMed] [Google Scholar]
- Dannies, P.S. 2001. Concentrating hormones into secretory granules: layers of control. Mol. Cell. Endocrinol. 177:87–93. [DOI] [PubMed] [Google Scholar]
- Dhanvantari, S., I. Arnaoutova, C.R. Snell, P.J. Steinbach, K. Hammond, G.A. Caputo, E. London, and Y.P. Loh. 2002. Carboxypeptidase E, a prohormone sorting receptor, is anchored to secretory granules via a C-terminal transmembrane insertion. Biochemistry. 41:52–60. [DOI] [PubMed] [Google Scholar]
- Dikeakos, J.D., M.J. Lacombe, C. Mercure, M. Mireuta, and T.L. Reudelhuber. 2007. A hydrophobic patch in a charged alpha-helix is sufficient to target proteins to dense core secretory granules. J. Biol. Chem. 282:1136–1143. [DOI] [PubMed] [Google Scholar]
- Feliciangeli, S., P. Kitabgi, and J.N. Bidard. 2001. The role of dibasic residues in prohormone sorting to the regulated secretory pathway. A study with proneurotensin. J. Biol. Chem. 276:6140–6150. [DOI] [PubMed] [Google Scholar]
- Garcia, A.L., S.K. Han, W.G. Janssen, Z.Z. Khaing, T. Ito, M.J. Glucksman, D.L. Benson, and S.R. Salton. 2005. A prohormone convertase cleavage site within a predicted alpha-helix mediates sorting of the neuronal and endocrine polypeptide VGF into the regulated secretory pathway. J. Biol. Chem. 280:41595–41608. [DOI] [PubMed] [Google Scholar]
- Glombik, M.M., A. Kromer, T. Salm, W.B. Huttner, and H.H. Gerdes. 1999. The disulfide-bonded loop of chromogranin B mediates membrane binding and directs sorting from the trans-Golgi network to secretory granules. EMBO J. 18:1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban, P.A., and J.C. Irminger. 2003. Mutant proinsulin that cannot be converted is secreted efficiently from primary rat beta-cells via the regulated pathway. Mol. Biol. Cell. 14:1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendy, G.N., T. Li, M. Girard, R.C. Feldstein, S. Mulay, R. Desjardins, R. Day, A.C. Karaplis, M.L. Tremblay, and L. Canaff. 2006. Targeted ablation of the chromogranin a (Chga) gene: normal neuroendocrine dense-core secretory granules and increased expression of other granins. Mol. Endocrinol. 20:1935–1947. [DOI] [PubMed] [Google Scholar]
- Hosaka, M., M. Suda, Y. Sakai, T. Izumi, T. Watanabe, and T. Takeuchi. 2004. Secretogranin III binds to cholesterol in the secretory granule membrane as an adapter for chromogranin A. J. Biol. Chem. 279:3627–3634. [DOI] [PubMed] [Google Scholar]
- Hosaka, M., T. Watanabe, Y. Sakai, T. Kato, and T. Takeuchi. 2005. Interaction between secretogranin III and carboxypeptidase E facilitates prohormone sorting within secretory granules. J. Cell Sci. 118:4785–4795. [DOI] [PubMed] [Google Scholar]
- Irminger, J.C., C.B. Verchere, K. Meyer, and P.A. Halban. 1997. Proinsulin targeting to the regulated pathway is not impaired in carboxypeptidase E- deficient Cpefat/Cpefat mice. J. Biol. Chem. 272:27532–27534. [DOI] [PubMed] [Google Scholar]
- Jain, R.K., W.T. Chang, C. Geetha, P.B. Joyce, and S.U. Gorr. 2002. In vitro aggregation of the regulated secretory protein chromogranin A. Biochem. J. 368:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, S.W., J.H. Krege, P.M. Oliver, J.R. Hagaman, J.B. Hodgin, S.C. Pang, T.G. Flynn, and O. Smithies. 1995. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 267:679–681. [DOI] [PubMed] [Google Scholar]
- Jutras, I., N.G. Seidah, and T.L. Reudelhuber. 2000. A predicted alpha-helix mediates targeting of the proprotein convertase PC1 to the regulated secretory pathway. J. Biol. Chem. 275:40337–40343. [DOI] [PubMed] [Google Scholar]
- Kim, T., and Y.P. Loh. 2006. Protease nexin-1 promotes secretory granule biogenesis by preventing granule protein degradation. Mol. Biol. Cell. 17:789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T., J.H. Tao-Cheng, L.E. Eiden, and Y.P. Loh. 2001. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 106:499–509. [DOI] [PubMed] [Google Scholar]
- Knoch, K.P., H. Bergert, B. Borgonovo, H.D. Saeger, A. Altkruger, P. Verkade, and M. Solimena. 2004. Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis. Nat. Cell Biol. 6:207–214. [DOI] [PubMed] [Google Scholar]
- Kuliawat, R., D. Prabakaran, and P. Arvan. 2000. Proinsulin endoproteolysis confers enhanced targeting of processed insulin to the regulated secretory pathway. Mol. Biol. Cell. 11:1959–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe, M.J., C. Mercure, J.D. Dikeakos, and T.L. Reudelhuber. 2005. Modulation of secretory granule-targeting efficiency by cis and trans compounding of sorting signals. J. Biol. Chem. 280:4803–4807. [DOI] [PubMed] [Google Scholar]
- Loh, Y.P., A. Maldonado, C. Zhang, W.H. Tam, and N. Cawley. 2002. Mechanism of sorting proopiomelanocortin and proenkephalin to the regulated secretory pathway of neuroendocrine cells. Ann. N. Y. Acad. Sci. 971:416–425. [DOI] [PubMed] [Google Scholar]
- Lou, H., S.K. Kim, E. Zaitsev, C.R. Snell, B. Lu, and Y.P. Loh. 2005. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 45:245–255. [DOI] [PubMed] [Google Scholar]
- Mahapatra, N.R., D.T. O'Connor, S.M. Vaingankar, A.P. Hikim, M. Mahata, S. Ray, E. Staite, H. Wu, Y. Gu, N. Dalton, et al. 2005. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J. Clin. Invest. 115:1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malosio, M.L., T. Giordano, A. Laslop, and J. Meldolesi. 2004. Dense-core granules: a specific hallmark of the neuronal/neurosecretory cell phenotype. J. Cell Sci. 117:743–749. [DOI] [PubMed] [Google Scholar]
- Marx, R., M.R. El, D.C. Johns, and R.E. Mains. 1999. Differences in the ways sympathetic neurons and endocrine cells process, store, and secrete exogenous neuropeptides and peptide-processing enzymes. J. Neurosci. 19:8300–8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi, J., E. Chieregatti, and M.M. Luisa. 2004. Requirements for the identification of dense-core granules. Trends Cell Biol. 14:13–19. [DOI] [PubMed] [Google Scholar]
- Mouchantaf, R., U. Kumar, T. Sulea, and Y.C. Patel. 2001. A conserved alpha-helix at the amino terminus of prosomatostatin serves as a sorting signal for the regulated secretory pathway. J. Biol. Chem. 276:26308–26316. [DOI] [PubMed] [Google Scholar]
- Mulcahy, L.R., C.A. Vaslet, and E.A. Nillni. 2005. Prohormone-convertase 1 processing enhances post-Golgi sorting of prothyrotropin-releasing hormone-derived peptides. J. Biol. Chem. 280:39818–39826. [DOI] [PubMed] [Google Scholar]
- O'Donnell, P.J., W.J. Driscoll, N. Back, E. Muth, and G.P. Mueller. 2003. Peptidylglycine-alpha-amidating monooxygenase and pro-atrial natriuretic peptide constitute the major membrane-associated proteins of rat atrial secretory granules. J. Mol. Cell. Cardiol. 35:915–922. [DOI] [PubMed] [Google Scholar]
- Orci, L., M. Ravazzola, M. Amherdt, O. Madsen, A. Perrelet, J.D. Vassalli, and R.G. Anderson. 1986. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J. Cell Biol. 103:2273–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt, R.E., J.E. Carleton, J.P. Richie, C. Heusser, and V.J. Dzau. 1987. Human renin biosynthesis and secretion in normal and ischemic kidneys. Proc. Natl. Acad. Sci. USA. 84:7837–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, D., L. Orci, M. Ravazzola, and H.P. Moore. 1991. Intracellular transport and sorting of mutant human proinsulins that fail to form hexamers. J. Cell Biol. 113:987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes, C.J., and P.A. Halban. 1987. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J. Cell Biol. 105:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, W.K., and H.P. Moore. 1995. Ionic milieu controls the compartment-specific activation of pro-opiomelanocortin processing in AtT-20 cells. Mol. Biol. Cell. 6:1271–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner, D.F., Y. Rouille, Q. Gong, S. Martin, R. Carroll, and S.J. Chan. 1996. The role of prohormone convertases in insulin biosynthesis: evidence for inherited defects in their action in man and experimental animals. Diabetes Metab. 22:94–104. [PubMed] [Google Scholar]
- Takamori, S., M. Holt, K. Stenius, E.A. Lemke, M. Gronborg, D. Riedel, H. Urlaub, S. Schenck, B. Brugger, P. Ringler, et al. 2006. Molecular anatomy of a trafficking organelle. Cell. 127:831–846. [DOI] [PubMed] [Google Scholar]
- Taugner, R., S.J. Kim, K. Murakami, and R. Waldherr. 1987. The fate of prorenin during granulopoiesis in epithelioid cells. Immunocytochemical experiments with antisera against renin and different portions of the renin prosegment. Histochemistry. 86:249–253. [DOI] [PubMed] [Google Scholar]
- Taupenot, L., K.L. Harper, N.R. Mahapatra, R.J. Parmer, S.K. Mahata, and D.T. O'Connor. 2002. Identification of a novel sorting determinant for the regulated pathway in the secretory protein chromogranin A. J. Cell Sci. 115:4827–4841. [DOI] [PubMed] [Google Scholar]
- Taupenot, L., K.L. Harper, and D.T. O'Connor. 2005. Role of H+-ATPase-mediated acidification in sorting and release of the regulated secretory protein chromogranin A: evidence for a vesiculogenic function. J. Biol. Chem. 280:3885–3897. [DOI] [PubMed] [Google Scholar]
- Torii, S., N. Saito, A. Kawano, S. Zhao, T. Izumi, and T. Takeuchi. 2005. Cytoplasmic transport signal is involved in phogrin targeting and localization to secretory granules. Traffic. 6:1213–1224. [DOI] [PubMed] [Google Scholar]
- Wasmeier, C., N.A. Bright, and J.C. Hutton. 2002. The lumenal domain of the integral membrane protein phogrin mediates targeting to secretory granules. Traffic. 3:654–665. [DOI] [PubMed] [Google Scholar]
- Wasmeier, C., P.V. Burgos, T. Trudeau, H.W. Davidson, and J.C. Hutton. 2005. An extended tyrosine-targeting motif for endocytosis and recycling of the dense-core vesicle membrane protein phogrin. Traffic. 6:474–487. [DOI] [PubMed] [Google Scholar]