Abstract
The Bcl-2 family regulates apoptosis by controlling mitochondrial integrity. To clarify whether its prosurvival members function by sequestering their Bcl-2 homology 3 (BH3)–only ligands or their multidomain relatives Bak and Bax, we analyzed whether four prosurvival proteins differing in their ability to bind specific BH3 peptides or Bak could protect isolated mitochondria. Most BH3 peptides could induce temperature-dependent cytochrome c release, but permeabilization was prevented by Bcl-xl, Bcl-w, Mcl-1, or BHRF1. However, their protection correlated with the ability to bind Bak rather than the added BH3 peptide and could be overcome only by BH3 peptides that bind directly to the appropriate prosurvival member. Mitochondria protected by both Bcl-xl–like and Mcl-1 proteins were disrupted only by BH3 peptides that engage both. BH3-only reagents freed Bak from Bcl-xl and Mcl-1 in mitochondrial and cell lysates. The findings support a model for the control of apoptosis in which certain prosurvival proteins sequester Bak/Bax, and BH3-only proteins must neutralize all protective prosurvival proteins to allow Bak/Bax to induce mitochondrial disruption.
Introduction
The crucial regulators of commitment to apoptotic cell death in mammals include three subclasses of the Bcl-2 family of proteins, each characterized by the presence of one or more Bcl-2 homology (BH) domains (Newmeyer and Ferguson-Miller, 2003; Danial and Korsmeyer, 2004; Adams and Cory, 2007). The prosurvival (or antiapoptotic) proteins, which contain three or four of the BH domains, are represented by Bcl-2, Bcl-xl, Bcl-w, Mcl-1, and A1. The proapoptotic BH3-only members, which act as sensors of specific types of cellular stress, include Bid, Bim, Puma, Bad, Noxa, Bmf, Hrk, and Bik (Willis and Adams, 2005). Acting downstream of both of these groups are the proapoptotic multidomain proteins, which contain BH1–3 and are represented by Bax, Bak, and perhaps Bok (Lindsten et al., 2000; Wei et al., 2001). Once activated, Bax and Bak mediate permeability of the mitochondrial outer membrane, releasing proapoptotic factors, particularly cytochrome c, that provoke caspase activation and the resulting rapid packaging of cell fragments for removal (Green, 2005). Although either Bax or Bak is required for apoptosis, their localization in healthy cells differs: Bak is an integral protein of the mitochondrial outer membrane, whereas Bax is predominantly cytosolic or loosely attached to the mitochondrial outer membrane.
Commitment to apoptosis is governed by interactions between members of the Bcl-2 subclasses mediated by the amphipathic α-helical BH3 domain. Structural studies have revealed that BH3 peptides from Bak and Bad as well as the Bim polypeptide behave like ligands in binding to a hydrophobic groove on the surface of Bcl-xl (Sattler et al., 1997; Liu et al., 2003). Indeed, BH3 peptides derived from each of the BH3-only proteins can mimic their full-length parent polypeptides in binding to prosurvival proteins, permeabilizing isolated mitochondria, and inducing apoptosis (Letai et al., 2002; Chen et al., 2005; Kuwana et al., 2005).
The interactions between Bcl-2 family members that allow the critical step of Bax and Bak activation remain poorly understood (Willis and Adams, 2005). One widely discussed model posits that a subclass of BH3-only proteins termed activators, which are proposed to include Bim and Bid (after its truncation by caspases; Letai et al., 2002; Kuwana et al., 2005; Certo et al., 2006) and perhaps Puma (Cartron et al., 2004), can bind not only to the prosurvival proteins but also to Bax and Bak and provoke their activation. On this direct activation model (Fig. 1 A, left), the remaining BH3-only proteins, which are termed sensitizers, simply bind to the prosurvival proteins, lowering their capacity to sequester the activators. However, important aspects of this model remain problematic (see Discussion; Willis and Adams, 2005).
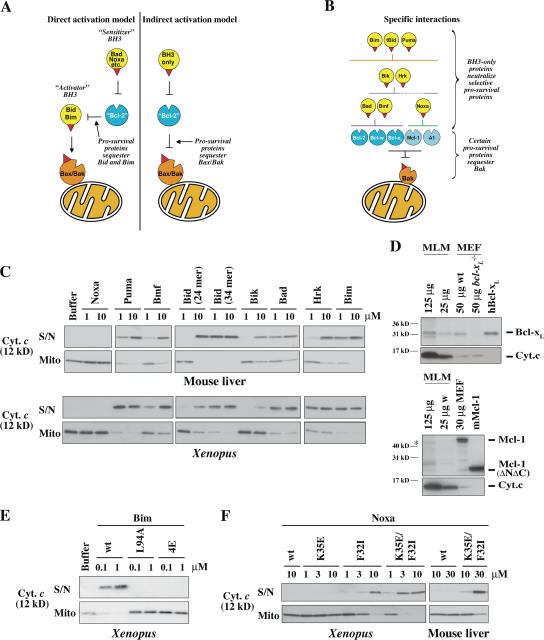
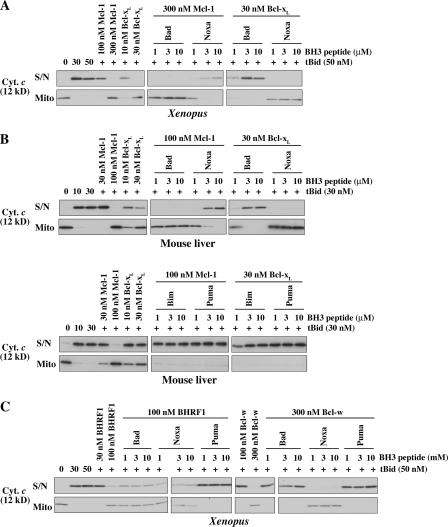
Figure 1.
Specificity of BH3 peptide–induced cytochrome c release. (A) Prosurvival proteins are proposed to block mitochondrial permeability by sequestering BH3-only proteins (direct activation model) or primarily Bax/Bak proteins (indirect activation model). (B) Previously identified specific interactions between members of each Bcl-2 subfamily (Fig. S1 B, available at http://www.jcb.org/cgi/content/full/jcb.200606065/DC1; Chen et al., 2005). (C) Selective permeabilization by BH3 peptides. Mitochondria isolated from mouse liver or from Xenopus eggs were incubated for 2 h at 37 (mouse liver) or 22°C (Xenopus) with peptides derived from the BH3 domains of BH3-only proteins. Equivalent volumes of supernatant (S/N) and mitochondrial pellet (Mito) were then analyzed for cytochrome c by Western blotting. The sequence of each peptide is from the human protein sequence (Fig. S1 A) except for Bmf (from mouse) and Bad (human in top panel and mouse in bottom panel). BH3 peptides derived from mouse and human Bad were equivalent in permeabilizing and binding activity (not depicted) and were used interchangeably. (D) Bcl-xl and traces of Mcl-1 are present in MLM. Bcl-xl and Mcl-1 levels in MLM and mouse embryonic fibroblasts (MEFs) were compared by Western blotting. 0.7 pmol of recombinant Bcl-xL and 0.25 pmol Mcl-1 were included where indicated. Positions of molecular weight markers are indicated. The asterisk denotes a band in MLM that aligns with the bottom band of the Mcl-1 doublet in the mouse embryonic fibroblast. Reprobing the blots for cytochrome c (bottom) provides a comparison of the mitochondrial content of each sample. (E) Mutant Bim peptides lose permeabilizing activity. Mouse BimBH3 peptides with mutations at one (L94A) or four (4E; I89E, L94E, I97E, and F101E) conserved residues were compared with wild-type (wt) peptide for the ability to permeabilize XEM as in C (residue number refers to the mBimL protein). Equivalent results were found with MLM (not depicted). (F) Mutated Noxa peptides with enhanced binding to Bcl-xL gain permeabilizing activity. NoxaBH3 peptides containing substitutions of charge (K35E), hydrophobicity (F32I), or both (K35E and F32I; Fig. S1; Chen et al., 2005) were compared with wild-type peptide for the ability to permeabilize mitochondria as in C.
An alternative view promoted by recent findings (Chen et al., 2005; Willis et al., 2005, 2007) is that the BH3-only proteins exclusively engage the prosurvival proteins, overcoming their sequestration of Bak or Bax (Fig. 1 A, right). Pertinent to this indirect activation model, certain BH3-only proteins interact selectively with subsets of the prosurvival proteins (Fig. 1 B): whereas Bim and Puma bound all five tightly, Noxa instead bound only Mcl-1 and A1, whereas Bad engaged only Bcl-2, Bcl-xL, and Bcl-w (Chen et al., 2005). Importantly, neutralization of multiple prosurvival proteins was required to induce apoptosis (Chen et al., 2005). Furthermore, only certain prosurvival proteins engage Bak (Fig. 1 B): in healthy fibroblasts, both Mcl-1 and Bcl-xL but not Bcl-2 could bind Bak and protect against Bak-mediated permeability (Willis et al., 2005). In accord with the indirect activation model, Bak was freed by BH3-only proteins that bind tightly to both Mcl-1 and Bcl-xL, such as Noxa plus Bad (Willis et al., 2005).
Thus, whether the BH3-only proteins activate Bak and Bax directly or indirectly (or both) remains to be established (Willis and Adams, 2005). Because the Bcl-2 family primarily regulates the integrity of the mitochondrial outer membrane, it is essential to establish which interactions between its subclasses directly control mitochondrial permeability. In this study, we have explored whether the previously identified interactions of prosurvival proteins with Bak or those with BH3 peptides from BH3-only proteins (Chen et al., 2005; Willis et al., 2005) can account for their ability to regulate mitochondrial permeability. Cytochrome c release was assessed on two sources of mitochondria: those from Xenopus laevis eggs (Xenopus egg mitochondria [XEM]) were chosen because they are particularly robust and their permeabilization has been widely studied (Kluck et al., 1999; von Ahsen et al., 2000; Kuwana et al., 2002; Nutt et al., 2005), whereas mouse liver mitochondria (MLM) were selected because they lack Bax and thus exhibit Bak-dependent permeabilization (Letai et al., 2002). We analyze our results both from the perspective of how prosurvival proteins prevent permeabilization and how BH3-only proteins act to permeabilize mitochondria. In accord with the indirect activation model, the findings indicate that prosurvival proteins act by interacting with mitochondrial components such as Bak (and perhaps Bax) and that BH3-only proteins must engage multiple prosurvival proteins to induce efficient permeabilization.
Results
Most BH3 peptides can permeabilize mitochondria isolated from mouse liver and Xenopus eggs
To explore whether selective binding of BH3-only domains to prosurvival proteins could regulate mitochondrial integrity, we first established whether each of eight BH3 peptides could permeabilize mitochondria. We used long BH3 peptides (24–26 mers) with known affinities for the prosurvival proteins (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200606065/DC1; Chen et al., 2005). When MLM were incubated with each peptide for 2 h at 37°C, specificity was observed in that most of the peptides (Puma, Bmf, Bid, Bik, Bad, Hrk, and Bim), if added at 10 μm, promoted Bak oligomerization and cytochrome c release, whereas the human NoxaBH3 was inactive (Fig. 1 C and Fig. S2), as were peptides from both the BH3 domains of mouse Noxa (not depicted). The BH3 peptides exhibited similar specificity on XEM after a 2-h incubation at room temperature, although certain ones were up to 10-fold more active (Fig. 1 C). Notably, under our conditions, several BH3 peptides termed sensitizers (Puma, Bmf, Bik, Bad, and Hrk) promoted cytochrome c release essentially as effectively as the putative activators Bid and Bim (see Discussion).
Each of the BH3 peptides active in this assay exhibits high affinity for Bcl-xL and Bcl-w, whereas the only inactive peptide, Noxa, binds only to Mcl-1 and A1 (Figs. 1 B and S1 B; Chen et al., 2005; Willis et al., 2005). This suggests that the endogenous prosurvival proteins protecting both XEM and MLM are principally Bcl-xL–like rather than Mcl-1–like. Indeed, by Western blot analysis, MLM contained readily detectable levels of Bcl-xL, albeit undetectable Bcl-2, Bcl-w, or A1 and very little Mcl-1 (Fig. 1 D and Fig. S3, A–C; available at http://www.jcb.org/cgi/content/full/jcb.200606065/DC1). Nevertheless, even these low levels of Mcl-1 seem to be functionally relevant because the Bad peptide, which does not bind Mcl-1 or A1, gave a less complete permeabilization of MLM than the Bim or Bid BH3 peptides. For the XEM, appropriate antibodies are not yet available for determining which of the five known Xenopus prosurvival homologues (Aouacheria et al., 2005) are present, but Bcl-xL–like proteins probably predominate because BadBH3 but not NoxaBH3 permeabilized the XEM.
Importantly, the permeabilizing activities of Bim and NoxaBH3 peptides were altered by mutations known to affect both their binding to prosurvival molecules and their proapoptotic activities. Thus, BimBH3 mutated at either a single invariant residue (L94A) or, at four conserved residues (4E: I89E, L94E, I97E, and F101E), no longer permeabilized XEM (Fig. 1 E) or MLM (not depicted). These mutations decrease binding to prosurvival proteins at least 50-fold and abrogate proapoptotic activity in mouse embryonic fibroblasts (Hinds et al., 2003; Wilson-Annan et al., 2003; Chen et al., 2005). Conversely, in the case of NoxaBH3, we previously showed that single (K35E or F32I) or double (K35E/F32I) mutations markedly increase binding to Bcl-xL–like prosurvival proteins as well as proapoptotic activity (Fig. S1; Chen et al., 2005). Accordingly, the mutant peptides, unlike the wild-type NoxaBH3, could permeabilize both XEM and MLM (Fig. 1 F). Thus, both ablating the ability of Bim BH3 to bind prosurvival proteins and extending the range bound by NoxaBH3 have effects on mitochondrial permeability that parallel their known proapoptotic activities in cells. These findings argue that the ability to engage specific subsets of prosurvival proteins determines both the mitochondrial permeabilizing activity and proapoptotic behavior of BH3-only proteins.
The permeabilization of MLM is temperature dependent
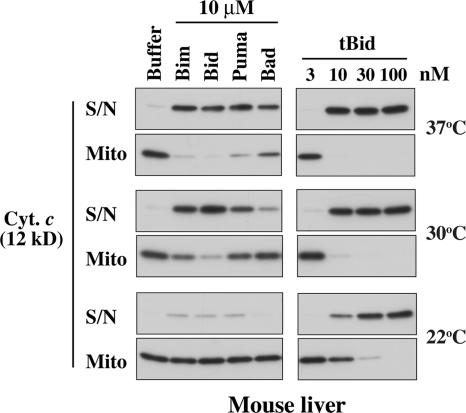
A recent study (Certo et al., 2006) reported results with MLM that differ in important respects from those shown in Fig. 1 C. Specifically, sensitizer BH3 peptides such as Bad and Puma were reported not to permeabilize MLM (Bim and Bid peptides were not tested). Because Certo et al. (2006) apparently incubated the mitochondria at room temperature (Letai et al., 2002), whereas we used 37°C, we tested the effect of temperature on MLM permeabilization elicited by several peptides and by tBid (Fig. 2). Importantly, at room temperature (22°C), Puma and Bad released very little cytochrome c, which is consistent with the study by Certo et al. (2006), but neither did the Bim or Bid peptides. In contrast, all four peptides provoked release at 37°C. At 30 and 37°C, the Puma peptide was essentially as effective as the Bim or Bid peptides, whereas the Bad peptide was weaker, which is consistent with its more restricted binding pattern for prosurvival proteins (Figs. 1 B and S1). Moreover, tBid was several times more active at the higher temperatures than at room temperature. Thus, the release of cytochrome c from MLM involves a temperature-dependent step, but their permeabilization is not confined to Bim and Bid (see Discussion).
Figure 2.
Permeabilization of MLM is temperature dependent. MLM were incubated with the indicated BH3 peptides (left) or tBid (right) for 2 h at different temperatures as indicated. Cytochrome c release was assessed by Western blotting as in Fig. 1 C. S/N, supernatant; Mito, mitochondrial pellet.
Four prosurvival proteins block tBid-induced mitochondrial permeability
We next explored how mitochondrial permeability was affected by the addition of recombinant prosurvival proteins. Bcl-xL, Bcl-w, Mcl-1, and BHRF1 (the Bcl-2 homologue from Epstein-Barr virus) were selected because they have distinct binding profiles for BH3 peptides and/or Bak. Bcl-xL and Bcl-w bind Bad but not Noxa, whereas Mcl-1 binds Noxa but not Bad (Chen et al., 2005), and BHRF1 binds neither Bad nor Noxa (Figs. 1 B and S1 B; unpublished data). Furthermore, whereas both Bcl-xL and Mcl-1 bind Bak, Bcl-w does so only very poorly (Willis et al., 2005). For Bcl-w, Mcl-1, and BHRF1, we had to use forms lacking a portion of the C-terminal hydrophobic segment because the full-length recombinant proteins were poorly soluble, whereas the truncated molecules are well-behaved proteins that retain full BH3 binding (Hinds, M., and P. Czabotar, personal communication; Hinds et al., 2003; Day et al., 2005).
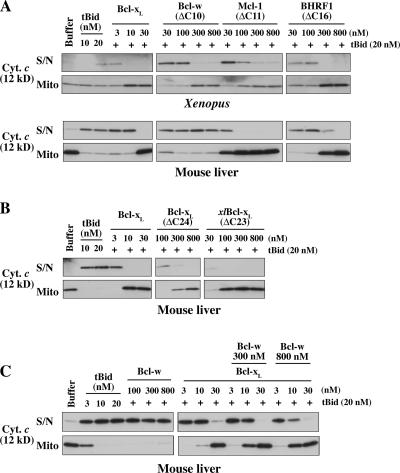
All four prosurvival proteins could block the tBid-induced permeability of XEM, and all except Bcl-w protected MLM (Fig. 3 A). The failure of Bcl-w to protect MLM is consistent with its poor ability to bind to Bak or to protect mouse cells from Bak-mediated apoptosis (Willis et al., 2005). The permeabilization of MLM is Bak dependent, as they contain Bak but no detectable Bax, and MLM from bak −/− mice are insensitive to tBid or BH3 peptides (Fig. S3, D–F; Letai et al., 2002). In contrast, XEM permeability apparently is not mediated solely via Bak because XEM are protected by the addition of either Bcl-w (Fig. 3 A) or Bcl-2 (Kluck et al., 1997), neither of which can bind Bak appreciably or protect cells from Bak-mediated apoptosis (Willis et al., 2005). Because Xenopus possesses homologues of Bax as well as Bak (Aouacheria et al., 2005) and mitochondria of undifferentiated cells can contain high levels of Bax (Polster et al., 2003), we surmise that XEM permeabilization is not solely Bak dependent because of resident Bax (or Bok).
Figure 3.
Four prosurvival proteins block tBid-induced permeabilization of isolated mitochondria. (A) Mitochondria from XEM or MLM were preincubated with recombinant prosurvival proteins at the indicated concentrations and supplemented with tBid for a further 2 h. Supernatant and pellets were examined by Western blotting for cytochrome c (in the MLM experiment, only half of the supernatant samples were loaded compared with the pellets). (B) C-terminal truncation of hBcl-xL decreases potency. MLM were incubated as in A with full-length hBcl-xL, a C-terminally truncated form (hBcl-xLΔC24), or with Xenopus Bcl-xLΔC23. (C) High concentrations of Bcl-w can complement Bcl-xL–mediated protection. MLM were incubated as in A with Bcl-wΔC10, Bcl-xL, or both before exposure to tBid at the indicated concentrations. S/N, supernatant; Mito, mitochondrial pellet.
Although Bcl-xL at low concentrations (∼20 nM) protected both types of mitochondria, 10–30-fold higher concentrations of the other prosurvival proteins were required. Their lower potency can be attributed largely to their C-terminal truncation, as Bcl-xLΔC24 was around 10–30 times less active than full-length Bcl-xL (Fig. 3 B). The cytosolic conformer of the Bcl-w prosurvival protein has its hydrophobic C-terminal domain tucked into the hydrophobic binding groove, but this domain can evert to insert into intracellular membranes (Hinds et al., 2003). Removal of its C-terminal residues facilitates binding of BH3-only proteins to the hydrophobic groove but decreases attachment to mitochondria (Hinds et al., 2003; Wilson-Annan et al., 2003). As each prosurvival protein contains a hydrophobic C-terminal domain, the much stronger protection observed with full-length Bcl-xL (Fig. 3 B) probably reflects its enhanced ability to locate to the mitochondrial outer membrane.
The failure of Bcl-w to protect MLM but not XEM from tBid (Fig. 3 A) may reflect its poor affinity for Bak. Interestingly, high concentrations of Bcl-w did augment the protection from tBid conveyed by Bcl-xL if Bcl-xL was at a concentration that was only partially protective (Fig. 3 C, 10 nM Bcl-xL in the 9th, 12th, and 15th lanes). This limited degree of protection may be caused by Bcl-w sequestration of tBid or by a slight ability of Bcl-w to bind Bak (Willis et al., 2005). Similarly, a recent report that high concentrations of Bcl-w could block the tBid-induced permeabilization of MLM (Certo et al., 2006) might be a result of the sequestration of the low concentration of tBid added (which gave only 50% cytochrome c release). Together, these findings suggest that prosurvival proteins do not protect mitochondria simply by sequestering BH3 ligands (see Discussion). Presumably, Bcl-w can protect the XEM (Fig. 3 A) by engaging a protein present specifically in those mitochondria (perhaps Xenopus Bax).
Mcl-1 and BHRF1 can protect mitochondria without sequestering the challenging BH3 peptide
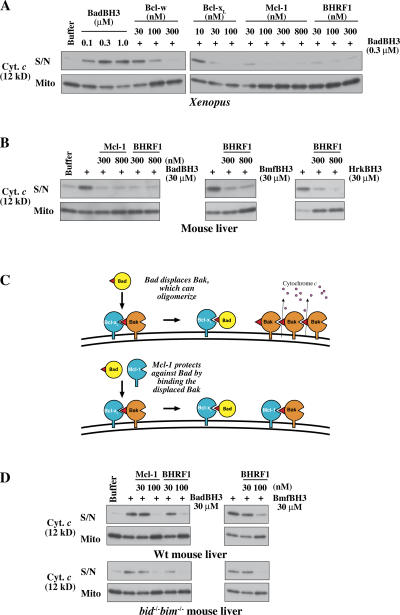
We assessed how the known affinities of these prosurvival proteins for each BH3 peptide (Figs. 1 B and S1 B; Chen et al., 2005) correlated with their ability to block the permeabilization elicited by that peptide. If a prosurvival protein acted solely by sequestering the BH3 peptide, protection would be minimal where interaction with the peptide is weak. To test this, XEM were preincubated with increasing concentrations of a prosurvival protein and were challenged with the BH3 peptides shown (Fig. 1 C) to be active (Fig. 4 A and Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200606065/DC1; and not depicted). Notably, although Mcl-1 does not bind the Bad BH3 peptide (Chen et al., 2005), it efficiently blocked BadBH3-induced permeability. Likewise, despite the poor binding affinity of BHRF1 for the Bad, Bmf, and Hrk BH3 peptides (Fig. S1 B), BHRF1 efficiently blocked cytochrome c release from XEM by each of them (Figs. 4 A and S4). Mcl-1 and BHRF1 also blocked the permeabilizing effect of these BH3 peptides on MLM (Fig. 4 B). A model of how MLM are permeabilized by Bad and protected by Mcl-1 is shown in Fig. 4 C.
Figure 4.
Mcl-1 and BHRF1 can even block the action of those BH3 peptides that they cannot bind. (A) Permeability of XEM induced by BadBH3 is efficiently blocked by prosurvival proteins that cannot bind Bad. XEM were preincubated for 20 min with the indicated concentrations of Mcl-1ΔC11 or BHRF1ΔC16 and were supplemented with mBadBH3 peptide for a further 2 h. Supernatant and pellets were then examined for cytochrome c by Western blotting. Similar experiments involving Mcl-1 and BHRF1-mediated protection from hBad, Bmf, Hrk, Bim, and Puma BH3 peptides are shown in Fig. S4 (available at http://www.jcb.org/cgi/content/full/jcb.200606065/DC1. (B) Permeability of MLM induced by mBad, Bmf, and Hrk BH3 peptides is efficiently blocked by Mcl-1 and BHRF1. MLM were incubated as in A with the indicated combinations of prosurvival proteins and BH3 peptides. (C) Model of how MLM are permeabilized by Bad (top) and protected by Mcl-1 (bottom). For simplicity, all of the Bak in the healthy cell is depicted as bound to Bcl-xL, but the indirect activation model postulates that only a proportion of the Bak molecules is in that form. (D) Permeabilization of mitochondria lacking both Bim and Bid by the sensitizer BH3 peptides Bad and Bmf. In an experiment like that in B, the absence of both of these putative activators does not prevent cytochrome c release or affect the protection conveyed by Mcl-1 and BHRF1, which do not bind Bad or Bmf. S/N, supernatant; Mito, mitochondrial pellet; Wt, wild type.
In these cases, Mcl-1 and BHRF1 cannot protect mitochondria merely by sequestering the added BH3 peptide. Indeed, close examination of further results with XEM (Fig. S4) suggests that Mcl-1 and BHRF1 are actually more effective at blocking the activity of BH3 peptides that they cannot bind. For example, Mcl-1 protected XEM better from the Bad than from the Bim or Puma peptides, and BHRF1 protected XEM better from the Bad, Bmf, or Hrk peptides than from the Bim or Puma peptides. Together, these findings demonstrate that prosurvival function does not rely on the sequestration of BH3 ligands. Instead, the peptides that bind the prosurvival protein simply reduce its ability to bind its true target. As argued above for Bcl-xL and Bcl-w (Fig. 3), Mcl-1 and BHRF1 must be acting on a component of the isolated mitochondria. Because both Mcl-1 and Bcl-xL can bind Bak (Cuconati et al., 2003; Willis et al., 2005) and both can prevent Bak-mediated apoptosis (Nijhawan et al., 2003; Willis et al., 2005), Bak is almost certainly their MLM target. How BHRF1 protects from Bak-mediated apoptosis is currently uncertain.
A proponent of the direct activation model might argue that the sensitizer peptides induce permeabilization in these experiments by releasing endogenous Bim or tBid putatively bound to prosurvival proteins on the mitochondria. Contrary to that notion, we have reported recently that sensitizer BH3 peptides can efficiently induce the permeabilization of mitochondria derived from bim −/− bid −/− mice (Willis et al., 2007). Moreover, Fig. 4 D shows that the absence of both Bim and Bid did not affect the ability of prosurvival proteins to protect the mitochondria from sensitizer peptides. In accord with this result, sensitive Western blots have failed to reveal any form of Bim, Bid, or Puma in wild-type MLM (Fig. S3, G–I). Thus, none of the putative activator BH3-only proteins appears to be required to induce permeabilization.
Binding of NoxaBH3 to Mcl-1 or of BadBH3 to Bcl-xl permeabilizes mitochondria
To explore whether the binding profiles of BH3 peptides to prosurvival proteins regulate mitochondrial permeability, we established mitochondrial incubations in which the dominant acting prosurvival protein was either Bcl-xL or Mcl-1 and exposed the mitochondria to either the BadBH3 peptide (to neutralize Bcl-xL) or the Noxa one (to neutralize Mcl-1). We reasoned that only BH3 peptides that could bind to the governing prosurvival protein and, thus, prevent it from engaging its mitochondrial target would induce permeability. Bcl-xL–protected mitochondria were provided by XEM supplemented with tBid and Bcl-xL, whereas the Mcl-1–protected mitochondria were XEM supplemented with tBid and Mcl-1. Titrations showed that in these mixtures, either 30 nM Bcl-xL or 300 nM Mcl-1 protected against at least 20 nM tBid (Fig. 5 A). Both types of supplemented XEM remained intact unless specific BH3 peptides were added.
Figure 5.
Specific binding of BH3 peptides to prosurvival proteins results in mitochondrial permeability. (A and B) Mcl-1– or Bcl-xL–mediated protection can be overcome only by BH3 peptides that bind that prosurvival protein. XEM (A) or MLM (B) were preincubated with either no addition or with Mcl-1ΔC11 or Bcl-xL and were supplemented with tBid together with Bad (human in A and mouse in B), Noxa, Bim, or Puma peptides as indicated for a further 2 h. (C) Specific binding of BH3 peptides to BHRF1 and Bcl-w results in mitochondrial permeability. XEM were preincubated with no addition, BHRF1ΔC16, or Bcl-wΔC10 and supplemented with tBid together with hBad, Noxa, or Puma peptides as indicated for a further 2 h. Supernatant and pellets were examined by Western blotting for cytochrome c. S/N, supernatant; Mito, mitochondrial pellet.
Notably, although NoxaBH3 did not permeabilize unsupplemented XEM (Fig. 1 C), it readily sensitized the Mcl-1–protected XEM to tBid (Fig. 5 A). Conversely, BadBH3 could permeabilize normal XEM but not the Mcl-1–protected XEM (Fig. 5 A). Thus, in these experiments, NoxaBH3 initiated permeability by directly binding and inactivating Mcl-1, whereas BadBH3 was inactive because it cannot bind Mcl-1. Conversely, the Bcl-xL–protected XEM were susceptible to BadBH3 but not NoxaBH3. Furthermore, MLM supplemented in the same way as XEM gave equivalent results: Noxa but not Bad sensitized MLM protected by Mcl-1 to tBid, whereas Bad but not Noxa could disrupt MLM guarded by Bcl-xL (Fig. 5 B) or by Bcl-xLΔC24 (not depicted). In contrast to Bad and Noxa, the Bim and Puma peptides, which can bind to both Mcl-1 and Bcl-xL, induced permeability in MLM protected by either Mcl-1 or Bcl-xL (Fig. 5 B, bottom).
Thus, the contrasting behavior of the Bad and NoxaBH3 peptides with either XEM or MLM confirms that binding of BH3 ligands to specific prosurvival proteins is the major route to the disruption of mitochondria. In these experiments, it appears unlikely that tBid is acting as a direct activator of Bak because Fig. S5 (available at http://www.jcb.org/cgi/content/full/jcb.200606065/DC1) shows that the BadBH3 peptide, which does not associate with Bak, could substitute for tBid in an experiment equivalent to that in Fig. 5 A. Instead, tBid probably is required to permeabilize mitochondria in Fig. 5 because it, together with the added BH3 peptide, can target all of the relevant prosurvival proteins, whether endogenous or added.
BH3 peptides that can bind Bcl-w or BHRF1 overcome their protection
Because only particular BH3 peptides could neutralize Mcl-1 and Bcl-xL, we asked whether Bcl-w and BHRF1 behaved similarly by establishing mitochondrial mixtures in which each was the dominant prosurvival protein. Again, only specific BH3 peptides triggered permeabilization (Fig. 5 C). Because Bad or Puma but not Noxa can bind to Bcl-w (Figs. 1 B and S1 B), Bcl-w–mediated protection was overcome only by the Bad or Puma peptide. Similarly, BHRF1-mediated protection was overcome by the Puma but not the Bad or Noxa peptides, which is consistent with the ability of Puma but not Bad or Noxa to bind to BHRF1 (Figs. 1 B and S1 B). Thus, the binding of specific BH3 peptides to each prosurvival protein was required to overcome the mitochondrial protection bestowed by that prosurvival protein.
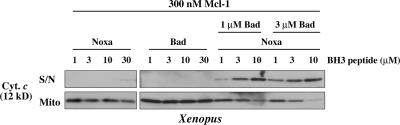
Noxa and Bad provide complementary permeability function
Complementation between Noxa and BadBH3 domains has been observed in killing assays with cells (mouse embryonic fibroblasts) that express both Bcl-xL–like and Mcl-1–like proteins (Chen et al., 2005; Willis et al., 2005). Our aforementioned findings suggested that mitochondria protected by both types of prosurvival proteins would require both the Noxa and Bad peptides to induce permeability. To test their complementarity, we established mitochondria protected by both Bcl-xL–like and Mcl-1 proteins. We reasoned that XEM predominantly contained only Bcl-xL–like prosurvival proteins because BadBH3 readily induced their permeabilization (Figs. 1 C and 4 A). Thus, by simply supplementing XEM with Mcl-1, we generated dual-protected mitochondria.
As predicted, neither the Bad nor Noxa peptide alone could permeabilize the dual-protected mitochondria even at high concentrations (30 μM), whereas the combination was active even at 1 μM (Fig. 6). Presumably, BadBH3 bound the endogenous Bcl-xL–like proteins (as in Figs. 1 C and 4 A), whereas NoxaBH3 neutralized the exogenous Mcl-1 (as in Fig. 5 A). As the permeabilization in these experiments did not involve the addition of tBid (or indeed any other component), it appears that Bax/Bak activity can be triggered simply by inactivating both the Mcl-1 and Bcl-xL–like proteins. It is also noteworthy that the endogenous Bcl-xL–like proteins present in XEM behaved similarly to added recombinant Bcl-xL (Fig. 5), supporting the physiological relevance of those experiments. Thus, the permeabilization of mitochondria protected by both Bcl-xL–like and Mcl-1–like prosurvival proteins requires the neutralization of both types of guardians, as found for apoptosis in cells (Willis et al., 2005, 2007).
Figure 6.
Complementation between Bad and Noxa BH3 peptides is needed to permeabilize mitochondria protected by both Mcl-1 and Bcl-xL–like prosurvival proteins. XEM (which functional experiments indicate contain predominantly Bcl-xL–like prosurvival proteins) were preincubated with 300 nM Mcl-1ΔC11 to produce dual-protected mitochondria. Noxa and mBadBH3 peptides were then added alone or in combination as indicated. Supernatant and pellets were examined by Western blotting for cytochrome c. Similar results were obtained with MLM (not depicted). Note that unlike in Fig. 5, tBid is not included. S/N, supernatant; Mito, mitochondrial pellet.
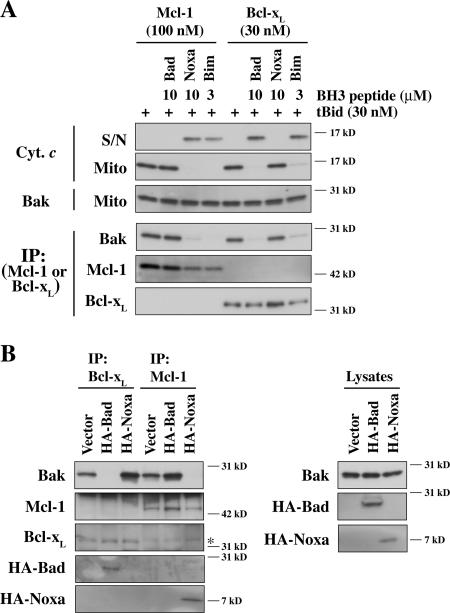
Bak association with Mcl-1 and Bcl-xL is disrupted by the specific binding of BH3-only reagents
We next examined whether BH3 peptide–initiated mitochondrial permeabilization was caused by the disruption of Bak binding to prosurvival proteins. As in Fig. 5 B, MLM were incubated with tBid and either Mcl-1 or Bcl-xL together with Bad, Noxa, or BimBH3 peptides (Fig. 7 A). The mitochondrial pellets were then examined for the binding of Bak to Mcl-1 or Bcl-xL by coimmunoprecipitation. In the absence of BH3 peptides, Bak immunoprecipitated with Mcl-1 and Bcl-xL, whereas in the presence of Bim peptide, Bak failed to associate with either Mcl-1 or Bcl-xL. In contrast, Noxa specifically interfered with Bak binding to Mcl-1, whereas Bad interfered with its binding to Bcl-xL. Importantly, in each case, the failure of Bak to bind its guardian correlated with the mitochondrial release of cytochrome c. Thus, in these mitochondrial incubations, specific binding of BH3 peptides to prosurvival proteins blocks the sequestration of Bak, allowing it to permeabilize mitochondria.
Figure 7.
Specific binding of BH3 ligands to Mcl-1 and Bcl-xL disrupts their binding of Bak. (A) The ability of BH3 peptides to permeabilize MLM corresponds with their disruption of Bak binding to Mcl-1 or Bcl-xL. MLM were treated as in Fig. 5 B, and aliquots of the supernatant and pellet fractions were analyzed by Western blotting for cytochrome c and Bak (top three panels). The remaining mitochondrial pellet fractions underwent immunoprecipitation for Mcl-1 or Bcl-xL before Western blotting for Bak, Mcl-1, and Bcl-xL (bottom three panels). The Noxa BH3 peptide used was K35E. (B) Bad and Noxa expressed in HeLa cells compete with Bak for association with Mcl-1 and Bcl-xL. Mcl-1 or Bcl-xL was immunoprecipitated from HeLa cells stably expressing HA-tagged Bad or HA-tagged Noxa. Immunoprecipitates and cell lysates were examined by Western blotting for Bak, Mcl-1, Bcl-xL, and HA. The asterisk marks the IgG light chain, which runs just above Bcl-xL. IP, immunoprecipitation; S/N, supernatant; Mito, mitochondrial pellet.
Interestingly, the binding of Noxa or Bim to Mcl-1 (which lacks 11 hydrophobic C-terminal residues) reduced its association with mitochondria, as indicated by the lower Mcl-1 levels immunoprecipitated from the mitochondrial pellet (Fig. 7 A, compare the third and fourth lanes with the first and second lanes). The Bad or Bim peptide did not reduce the association of full-length Bcl-xL with mitochondria, presumably because its hydrophobic C terminus can insert into the mitochondrial outer membrane independent of binding to Bak.
The specific effects of Bad and Noxa on Bak association with prosurvival proteins were also observed in whole cell lysates (Fig. 7 B). HA-tagged Bad or Noxa were stably expressed in HeLa cells, which contain functionally relevant levels of the two Bak guards Mcl-1 and Bcl-xL. As previously observed for fibroblasts (Chen et al., 2005; Willis et al., 2005), the cells remained viable because either Mcl-1 or Bcl-xL is sufficient to sequester Bak. Notably, in the Bad-expressing cells, Bcl-xL could bind Bad but could no longer bind Bak (Fig. 7 B, second lane), whereas in Noxa-expressing cells, Mcl-1 could bind Noxa but could not bind Bak (Fig. 7 B, sixth lane). Interestingly, the inability of one prosurvival guardian to bind Bak resulted in higher levels of Bak associating with the other guardian: for example, Noxa targeting of Mcl-1 lead to a higher Bak association with Bcl-xL (Fig. 7 B, third lane) and vice versa for Bad (Fig. 7 B, fifth lane). This suggests that the Bak released from either of its two guards is bound by the other guard.
Discussion
We have explored how interactions between members of the Bcl-2 family affect the integrity of the mitochondrial outer membrane. By relying on the Bak/Bax present in isolated mitochondria, we avoided introducing supraphysiological levels of these proteins, and, by studying MLM, which only contain Bak, we identified Bak-specific pathways. We analyzed how the known binding interactions of four prosurvival proteins with Bak (Willis et al., 2005) or the BH3 peptides from eight BH3-only proteins (Chen et al., 2005) correlate with mitochondrial permeabilization. The impact of each BH3 reagent on permeability correlated well with its known effect on apoptosis in intact cells. Notably, certain prosurvival proteins could prevent permeabilization by BH3 peptides that they do not bind. This suggests that prosurvival proteins convey protection primarily by associating with mitochondrial components such as Bak. However, specific interactions between BH3-only reagents and prosurvival proteins did result in mitochondrial permeability. The findings support a model of apoptotic mitochondrial permeability (Willis et al., 2005) in which BH3-only proteins interact with specific subsets of prosurvival proteins, blocking their binding to endogenous mitochondrial targets: Bak in the MLM but perhaps also Bax in the XEM (Fig. 1 A, right).
Prosurvival proteins protect mitochondria primarily by engaging Bak and perhaps Bax
In accord with a previous study showing that added Bcl-2 could protect XEM (Kluck et al., 1997), each of the four prosurvival proteins tested here protected these mitochondria from proapoptotic permeability. In several cases, the protection must be mediated by interactions with a mitochondrial component rather than by simply sequestering the challenging BH3 reagent. For example, Mcl-1 and BHRF1 could each block permeabilization by BH3 peptides that they do not bind (Figs. 4 and S4). In MLM, the target for Mcl-1 as well as Bcl-xL is almost certainly Bak, as both bind Bak and both protect cells from Bak-mediated apoptosis (Nijhawan et al., 2003; Willis et al., 2005). Because BHRF1 exhibited a similar ability to protect MLM (Fig. 3), it probably also targets Bak, although whether this involves direct interaction is under investigation. On the other hand, Bcl-w may protect largely via its ability to associate with Bax because it could efficiently protect XEM from tBid and Bid, Bad, Hrk, and Bmf BH3 peptides (Figs. 3 A, 4 A, and 5 C; and not depicted). It also efficiently binds to Bax (Willis et al., 2007) but was highly inefficient in blocking the Bak-mediated permeability of MLM (Fig. 3) and does not bind appreciably to Bak (Willis et al., 2005).
Further evidence that Bak is the MLM component guarded by Mcl-1 and Bcl-xL was obtained from coimmunoprecipitation experiments. In lysates of MLM, Bak associated with both prosurvival guardians, but, in the presence of BH3 peptides that could engage the prosurvival guardian, Bak was not bound, and MLM became permeabilized (Fig. 7 A). The disruption of Bak binding to prosurvival proteins was also observed in lysates of HeLa cells expressing either Bad or Noxa (Fig. 7 B). It should be noted that in these experiments, Triton X-100 that was included in the lysis buffer to solubilize the membrane-integrated Bak could change Bak conformation and increase Bak binding to prosurvival proteins (our unpublished data), as observed for Bax (Hsu and Youle, 1998). Thus, in situ, a much smaller fraction of Bak may be bound to Mcl-1 and Bcl-xL. This appears to be true for HeLa cells because in the presence of the zwitterionic detergent CHAPS, less Bak coimmunoprecipitated with Mcl-1 and Bcl-xL (unpublished data). The issue of what proportion of Bak molecules associates with the prosurvival proteins will require more detailed study, including an analysis of how the conformation of Bak and its association with prosurvival proteins is affected by detergents. Conceivably, a small proportion of Bak (or Bax) is already primed in healthy cells sequestered by prosurvival proteins. Alternatively, Bak/Bax become primed at an early stage of apoptotic signaling by changes such as posttranslational modification rather than by direct binding of BH3-only proteins and initiate permeabilization of the mitochondria if their prosurvival antagonists have been engaged by the BH3-only proteins.
Interactions between specific BH3 peptides and prosurvival proteins induce mitochondrial permeability
By establishing mitochondrial incubations in which the governing prosurvival protein was Bcl-xL, Mcl-1, Bcl-w, or BHRF1, we showed that permeabilization of these mitochondria correlated with the ability of the challenging BH3 peptide to bind that guardian. This was most evident in the case of interactions of the BadBH3 peptide with Bcl-xL or Bcl-w and of the Noxa BH3 peptide with Mcl-1 (Figs. 5 and 7 A), but a correlation was observed with every BH3-only reagent. Indeed, both Bad and NoxaBH3 peptides were required to permeabilize mitochondria protected by both the Bcl-xL and Mcl-1 subsets of prosurvival proteins (Fig. 6).
Thus, our findings suggest that the predominant consequence of binding between a BH3-only and a prosurvival protein is not sequestration of the BH3-only protein but inactivation of the prosurvival protein, preventing its interaction with its critical target. As proposed from studies on the apoptosis of fibroblasts (Chen et al., 2005; Willis et al., 2005), the experiments involving MLM demonstrated that engagement of Mcl-1– and Bcl-xL–like proteins initiates Bak-mediated mitochondrial permeability (Figs. 5 B and 7 A). The same may well hold for Bax-mediated permeability, as BH3 ligands of Bcl-w (such as Bad) permeabilized XEM (Fig. 5 C), a process likely mediated by Bax as well as Bak (see Results). Thus, each of the tested prosurvival proteins, including the viral BHRF1 protein, conferred upon mitochondria a highly specific pattern of susceptibility to permeabilization by BH3 reagents. The permeabilization of unsupplemented MLM by Bad, for example, did not require Noxa, as might have been expected from the need for both to kill mouse embryonic fibroblasts (Willis et al., 2005). We think that difference is caused by a far lower level of the Noxa target Mcl-1 in the MLM than in the fibroblasts (Fig. 1 D). Our conclusion that the MLM are protected primarily by Bcl-xL is consistent with the finding that conditional deletion of the bcl-xL gene in hepatocytes provokes chronic spontaneous apoptosis (Takehara et al., 2004).
Recently, another study reported that sensitizer BH3 peptides such as Bad and Puma were unable to induce cytochrome c release from MLM (Certo et al., 2006), whereas we have reproducibly found the permeabilization of MLM by five such peptides (Bad, Puma, Bmf, Bik, and Hrk) as well as by Bim and Bid (Figs. 1 C, 2, 4 B, and S2). These apparent discrepancies probably have two sources. First, most of the peptides used in that study were 20 mers, whereas those used here were 24–26 residues long. Longer peptides (with a few exceptions) bind more tightly to the prosurvival proteins, as can be seen with the two Bid peptides we have tested (Fig. S1). Indeed, the BimBH3 26 mer used here binds as avidly to Bcl-w as a much longer Bim polypeptide (Wilson-Annan et al., 2003), which is consistent with evidence from the Bim–Bcl-xL 3D structure that the BH3 domain is at least 26 residues long (Liu et al., 2003). Second, and perhaps more importantly, the mitochondria in that study were incubated for only 45 min at room temperature, whereas the MLM permeabilization in the current study involved a 2-h incubation at 37°C. For MLM, we showed that the higher temperature was critical for the release of cytochrome c by the peptides and notably enhanced that by tBid (Fig. 2). Because the permeabilization probably involves multiple steps—binding of the BH3 domain to the resident prosurvival proteins, release of Bak from them, oligomerization of Bak, alteration of the membrane, and egress of cytochrome c—it is not surprising that one or more of these steps would be temperature dependent.
MLM permeabilization does not rely on Bid, Bim, or Puma
In the direct activation model for the control of apoptosis (Fig. 1 A), the activation of Bax and Bak is proposed to require their direct engagement by Bid or Bim (Letai et al., 2002; Kuwana et al., 2005; Certo et al., 2006; Kim et al., 2006). However, compared with the high affinity binding of BH3 domains to prosurvival proteins (Chen et al., 2005), the evidence for such associations with Bax/Bak remains tenuous (Willis and Adams, 2005). In this model, Bid, Bim, or Puma (Cartron et al., 2004) would be sequestered in the mitochondria by prosurvival family members until freed to activate Bax/Bak by a sensitizer BH3 domain. However, Bid, Bim, or Puma does not appear to play a direct activator role in our MLM experiments, as Western blot experiments did not reveal any form of Bid, Bim, or Puma in wild-type MLM (Fig. S3), and MLM isolated from mice lacking both Bim and Bid are permeabilized by sensitizer BH3 peptides (Willis et al., 2007). Furthermore, despite the high affinity of Bcl-w for each of these three BH3 ligands, its inability to protect MLM (Fig. 3) argues that none of these three BH3-only proteins are required for Bak activation. Finally, Bid, Bim, and Puma do not appear to bind Bak or be required for Bak-mediated apoptosis (Willis et al., 2007). Together, these results appear inconsistent with the direct activation model (Fig. 1 A, left), at least for Bak.
In conclusion, our results favor the view that BH3-only proteins function primarily, if not exclusively, as antagonists of specific subsets of their prosurvival relatives and that these death ligands induce apoptosis by unleashing Bak or Bax from control by the prosurvival proteins rather than by direct engagement of Bak/Bax (Chen et al., 2005; Willis et al., 2005, 2007). However, further studies are required to establish what proportion of Bak and Bax are bound to the prosurvival proteins within healthy cells and to clarify what induces their changed conformation and oligomerization (Adams and Cory, 2007).
Materials and methods
Materials
The BH3 peptides, most of which were described previously (Chen et al., 2005), were 24–34 residues long (Fig. S1 A). Caspase-8–cleaved human Bid (tBid) and human Bcl-xL were produced as previously described (Kluck et al., 1999). Human Bcl-wA128EΔC10 and mouse Mcl-1ΔN151ΔC11, which were also produced as described previously (Hinds et al., 2003; Day et al., 2005), contained five additional N-terminal residues (GPLGS) as a result of cloning. Recombinant BHRF1ΔC16 with N-terminal Flag tag was overexpressed in Escherichia coli BL21-Codon Plus cells from a pET11a vector (Novagen) and the protein purified from cell lysates using Q-Sepharose ion-exchange chromatography followed by ammonium sulfate precipitation and Superdex G-75 gel filtration chromatography. Human Bcl-xLΔC24 was a gift from P. Czabotar (The Walter and Eliza Hall Institute, Melbourne, Victoria, Australia; produced as described previously [Chen et al., 2005]). Xenopus Bcl-xLΔC23 (xR11; a gift from J.R. Tata, National Institute for Medical Research, London, UK) was subcloned into pGEX-6P-3, and the protein was produced as described previously (Hinds et al., 2003; Day et al., 2005).
Isolation of mitochondria and incubation with BH3-only and prosurvival reagents
Heavy membrane fractions enriched for mitochondria were obtained from Xenopus eggs or from wild-type and bim −/− bid −/− mouse liver as previously described (Uren et al., 2005). For simplicity, these heavy membrane fractions are referred to here as XEM and MLM. For incubations with Bcl-2 family proteins or peptides, 1 mg/ml XEM and MLM were each suspended in buffer (100 mM KCl, 2.5 mM MgCl2, 100 mM sucrose, 20 mM Hepes/KOH, pH 7.5, and 1 mM DTT). Recombinant prosurvival proteins were added, and samples were incubated for 20 min at 22°C for XEM and at 37°C for MLM before the addition of BH3-only reagents and further incubation for 2 h (MLM were also incubated at 22 and 30°C in Fig. 2 as indicated). After centrifugation at 10,000 g for 5 min at 4°C, the supernatant and pellet fractions were carefully separated. To assess mitochondrial permeability, each fraction was combined with loading buffer, and equivalent amounts (except as noted in Fig. 3 B) were analyzed by SDS-PAGE and Western blotting for cytochrome c (clone 7H8.2C12; BD Biosciences). For ease of viewing, the supernatant and pellet fractions were generally run on separate gels, which were then processed as matching pairs during each stage of electrophoresis, blotting, and detection and with no digital adjustment of the scanned images.
Western blot analysis of Bcl-xL and Mcl-1 present in MLM
Aliquots of MLM (125 or 25 μg of protein) and whole cell lysates of mouse embryonic fibroblasts (30 or 50 μg of protein) were analyzed by Western blotting for the presence of Bcl-xL and Mcl-1 using antibodies raised against Bcl-xL (rabbit polyclonal; BD Biosciences) and mMcl-1 (rat monoclonal clone 19C4-15) followed by incubation with anti–rabbit and anti–rat HRP-labeled secondary antibodies (Southern Biotechnology Associates, Inc.). The proteins were detected using ECL (GE Healthcare).
Immunoprecipitation
HeLa cells stably expressing Bad or Noxa were provided by J. Fletcher (The Walter and Eliza Hall Institute, Melbourne, Victoria, Australia). MLM and HeLa cell lysates were prepared in lysis buffer (20 mM Tris, pH 7.4, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, and 10% glycerol) containing 1% Triton X-100 and supplemented with Complete protease inhibitors (Roche). Immunoprecipitation was performed using anti–Mcl-1 (rat monoclonal clone 14CH-20) and anti–Bcl-xL (rat monoclonal clone 1C2; Willis et al., 2005), and immune complexes were captured with protein G–Sepharose. Proteins were resolved by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and detected by antibodies directed against Bak (rabbit polyclonal clone B5929; Sigma-Aldrich), Mcl-1 (rat monoclonal clone 19C4-15), anti–Bcl-xL (mouse monoclonal clone 2H12; BD Biosciences), or HA (mouse monoclonal clone 16B12; BAbCO). Secondary antibodies used were anti–rabbit and anti–rat HRP-labeled antibodies (Southern Biotechnology Associates, Inc.) for Bak and Mcl-1, respectively, whereas Bcl-xL and HA were detected using a goat anti–mouse IgG Fcγ fragment–specific HRP conjugate (Jackson ImmunoResearch Laboratories).
Online supplemental material
Fig. S1 shows the sequence of each BH3 peptide used in this study and their binding affinities for four prosurvival proteins. Fig. S2 shows that MLM permeabilization by sensitizer BH3 peptides correlates with Bak cross-linking. In Fig. S3, Western blots show that MLM contain readily detectable levels of Bak, whereas Bax, Bcl-2, Bcl-w, A1, Bid, Bim, and Puma are undetectable. In Fig. S4, Mcl-1 and BHRF1 are most efficient at blocking the BH3 peptides for which they have least affinity. Fig. S5 shows that specific binding of BH3 peptides to Mcl-1 and Bcl-xL results in mitochondrial permeabilization (as in Fig. 5 A), even if BadBH3 replaces tBid as the initial permeabilizing agent. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200606065/DC1.
Supplementary Material
Acknowledgments
We thank Andrew Wei for sharing unpublished results and Tobias Kratina for technical assistance.
Our work is supported by the Australian National Health and Medical Research Council (NHMRC; program grant 257502), the US National Cancer Institute (grant CA80188), and the Leukemia and Lymphoma Society (Specialized Center of Research grant 7015-02). D.C.S. Huang and J.M. Adams are supported by NHMRC fellowships, G. Dewson is supported by an Australian Research Council postdoctoral fellowship, and R.M. Kluck is supported by a Wellcome Trust Overseas Senior Research Fellowship.
R.T. Uren and G. Dewson contributed equally to this paper.
R.T. Uren's present address is Genes to Cognition Programme, Wellcome Trust, Sanger Institute, Cambridge CB10 1SA, UK.
Abbreviations used in this paper: BH, Bcl-2 homology; MLM, mouse liver mitochondria; XEM, Xenopus egg mitochondria.
References
- Adams, J.M., and S. Cory. 2007. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 26:1324–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouacheria, A., F. Brunet, and M. Gouy. 2005. Phylogenomics of life-or-death switches in multicellular animals: Bcl-2, BH3-only and BNip families of apoptotic regulators. Mol. Biol. Evol. 22:2395–2416. [DOI] [PubMed] [Google Scholar]
- Cartron, P.F., T. Gallenne, G. Bougras, F. Gautier, F. Manero, P. Vusio, K. Meflah, F.M. Vallette, and P. Juin. 2004. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol. Cell. 16:807–818. [DOI] [PubMed] [Google Scholar]
- Certo, M., G. Moore Vdel, M. Nishino, G. Wei, S. Korsmeyer, S.A. Armstrong, and A. Letai. 2006. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 9:351–365. [DOI] [PubMed] [Google Scholar]
- Chen, L., S.N. Willis, A. Wei, B.J. Smith, J.I. Fletcher, M.G. Hinds, P.M. Colman, C.L. Day, J.M. Adams, and D.C.S. Huang. 2005. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell. 17:393–403. [DOI] [PubMed] [Google Scholar]
- Cuconati, A., C. Mukherjee, D. Perez, and E. White. 2003. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 17:2922–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial, N.N., and S.J. Korsmeyer. 2004. Cell death: critical control points. Cell. 116:205–219. [DOI] [PubMed] [Google Scholar]
- Day, C.L., L. Chen, S.J. Richardson, P.J. Harrison, D.C.S. Huang, and M.G. Hinds. 2005. Solution structure of prosurvival Mcl-1 and characterization of its binding by proapoptotic BH3-only ligands. J. Biol. Chem. 280:4738–4744. [DOI] [PubMed] [Google Scholar]
- Green, D.R. 2005. Apoptotic pathways: ten minutes to dead. Cell. 121:671–674. [DOI] [PubMed] [Google Scholar]
- Hinds, M.G., M. Lackmann, G.L. Skea, P.J. Harrison, D.C.S. Huang, and C.L. Day. 2003. The structure of Bcl-w reveals a role for the C-terminal residues in modulating biological activity. EMBO J. 22:1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, Y.-T., and R.J. Youle. 1998. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 273:10777–10783. [DOI] [PubMed] [Google Scholar]
- Kim, H., M. Rafiuddin-Shah, H.C. Tu, J.R. Jeffers, G.P. Zambetti, J.J. Hsieh, and E.H. Cheng. 2006. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 8:1348–1358. [DOI] [PubMed] [Google Scholar]
- Kluck, R.M., E. Bossy-Wetzel, D.R. Green, and D.D. Newmeyer. 1997. The release of cytochrome c from mitochondria—a primary site for Bcl-2 regulation of apoptosis. Science. 275:1132–1136. [DOI] [PubMed] [Google Scholar]
- Kluck, R.M., M.D. Esposti, G. Perkins, C. Renken, T. Kuwana, E. Bossy-Wetzel, M. Goldberg, T. Allen, M.J. Barber, D.R. Green, and D.D. Newmeyer. 1999. The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J. Cell Biol. 147:809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana, T., M.R. Mackey, G. Perkins, M.H. Ellisman, M. Latterich, R. Schneiter, D.R. Green, and D.D. Newmeyer. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 111:331–342. [DOI] [PubMed] [Google Scholar]
- Kuwana, T., L. Bouchier-Hayes, J.E. Chipuk, C. Bonzon, B.A. Sullivan, D.R. Green, and D.D. Newmeyer. 2005. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 17:525–535. [DOI] [PubMed] [Google Scholar]
- Letai, A., M. Bassik, L. Walensky, M. Sorcinelli, S. Weiler, and S. Korsmeyer. 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2:183–192. [DOI] [PubMed] [Google Scholar]
- Lindsten, T., A.J. Ross, A. King, W. Zong, J.C. Rathmell, H.A. Shiels, E. Ulrich, K.G. Waymire, P. Mahar, K. Frauwirth, et al. 2000. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell. 6:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., S. Dai, Y. Zhu, P. Marrack, and J.W. Kappler. 2003. The structure of a Bcl-xL/Bim fragment complex: Implications for Bim function. Immunity. 19:341–352. [DOI] [PubMed] [Google Scholar]
- Newmeyer, D.D., and S. Ferguson-Miller. 2003. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 112:481–490. [DOI] [PubMed] [Google Scholar]
- Nijhawan, D., M. Fang, E. Traer, Q. Zhong, W. Gao, F. Du, and X. Wang. 2003. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 17:1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt, L.K., S.S. Margolis, M. Jensen, C.E. Herman, W.G. Dunphy, J.C. Rathmell, and S. Kornbluth. 2005. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell. 123:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster, B.M., C.L. Robertson, C.J. Bucci, M. Suzuki, and G. Fiskum. 2003. Postnatal brain development and neural cell differentiation modulate mitochondrial Bax and BH3 peptide-induced cytochrome c release. Cell Death Differ. 10:365–370. [DOI] [PubMed] [Google Scholar]
- Sattler, M., H. Liang, D. Nettesheim, R.P. Meadows, J.E. Harlan, M. Eberstadt, H.S. Yoon, S.B. Shuker, B.S. Chang, A.J. Minn, et al. 1997. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 275:983–986. [DOI] [PubMed] [Google Scholar]
- Takehara, T., T. Tatsumi, T. Suzuki, E.B. Rucker III, L. Hennighausen, M. Jinushi, T. Miyagi, Y. Kanazawa, and N. Hayashi. 2004. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 127:1189–1197. [DOI] [PubMed] [Google Scholar]
- Uren, R.T., G. Dewson, C. Bonzon, T. Lithgow, D.D. Newmeyer, and R.M. Kluck. 2005. Mitochondrial release of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. J. Biol. Chem. 280:2266–2274. [DOI] [PubMed] [Google Scholar]
- von Ahsen, O., C. Renken, G. Perkins, R.M. Kluck, E. Bossy-Wetzel, and D.D. Newmeyer. 2000. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J. Cell Biol. 150:1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, M.C., W.X. Zong, E.H. Cheng, T. Lindsten, V. Panoutsakopoulou, A.J. Ross, K.A. Roth, G.R. MacGregor, C.B. Thompson, and S.J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 292:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, S.N., and J.M. Adams. 2005. Life in the balance: how BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 17:617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, S.N., L. Chen, G. Dewson, A. Wei, E. Naik, J.I. Fletcher, J.M. Adams, and D.C. Huang. 2005. Pro-apoptotic Bak is sequestered by Mc1-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19:1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, S.N., J.I. Fletcher, T. Kaufmann, M.F. van Delft, L. Chen, P.E. Czabotar, H. Ierino, E.F. Lee, W.D. Fairlie, P. Bouillet, et al. 2007. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 315:856–859. [DOI] [PubMed] [Google Scholar]
- Wilson-Annan, J., L.A. O'Reilly, S.A. Crawford, G. Hausmann, J.G. Beaumont, L.P. Parma, L. Chen, M. Lackmann, T. Lithgow, M.G. Hinds, et al. 2003. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J. Cell Biol. 162:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.