Abstract
Unlike adherens junctions, synapses are asymmetric connections, usually between axons and dendrites, that rely on various cell adhesion molecules for structural stability and function. Two cell types of adhesion molecules found at adherens junctions, cadherins and nectins, are thought to mediate homophilic interaction between neighboring cells. In this issue, Togashi et al. (see p. 141) demonstrate that the differential localization of two heterophilic interacting nectins mediates the selective attraction of axons and dendrites in cooperation with cadherins.
The selective initiation and stabilization of synapses, which is, in part, controlled by adherence between neuronal processes, is thought to underlie the formation of neural circuitry (Fig. 1 A; Yamagata et al., 2003). A functional synapse is inherently asymmetric with the synthesis and release of neurotransmitters occurring presynaptically, typically within axons, and receipt and response to neurotransmitter release occurring postsynaptically, typically within dendrites (Fig. 1, A and B). Thus, except in limited classes of cells, contacts between axons and dendrites are preferentially stabilized. Consistent with a role in synaptogenesis, cadherins, which are Ca2+-dependent homophilic cell adhesion molecules, have been implicated in the formation and stabilization of new synaptic contacts (Yamagata et al., 2003; Takeichi and Abe, 2005). However, because of their homophilic binding properties, cadherins alone are incapable of mediating preferential interactions between dendrites and axons. Togashi et al. (2006) describe the differential distribution between axons and dendrites of two nectins, which are Ca2+-independent immunoglobulin-type cell adhesion molecules, that interact preferentially via heterophilic binding and collaborate with cadherins to form adherens junctions (Takai and Nakanishi, 2003).
Figure 1.
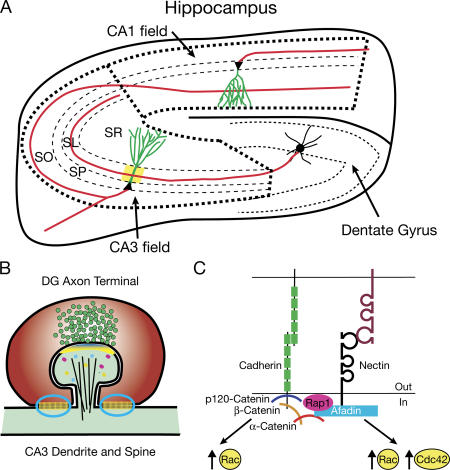
Cellular and molecular role of cadherins and nectins in the hippocampus. (A) Simplified schematic diagram of the hippocampus depicting the major divisions, including the dentate gyrus, CA1 field, and CA3 field, and the major three-cell circuit. CA3 and CA1 pyramidal cell bodies are located within the stratum pyramidale (SP). A dentate gyrus granule cell extends an axon and synapses (area in yellow) onto a CA3 pyramidal neuron's proximal, apical dendrite within the stratum lucidum (SL). A CA3 pyramidal neuron extends an axon through the stratum oriens (SO) and synapses onto dendrites of a CA1 pyramidal neuron within the stratum radiatum (SR). Based on Togashi et al. (2006), axons, likely nectin-1+, are colored red, and dendrites, likely nectin-3+, are colored green. (B) A dentate gyrus (DG) granule cell synapses onto a CA3 pyramidal spine with puncta adherentia junctions (circled in blue) on the CA3 dendrite on either side. (C) Cadherin and nectin interactions at adherens junctions and puncta adherentia junctions are described in more detail in the text.
Colocalization of nectins and cadherins at adherens junctions is mediated by their intracellular interactors (Fig. 1 C; Takai and Nakanishi, 2003). Classic cadherin intracellular domains interact with the family of catenins (Gumbiner, 2005). The juxtamembrane region binds one p120-catenin family member (p120, δ-catenin, ARVCF, or p0071), and the C-terminus binds β- or γ-catenin (plakoglobin), which, in turn, recruits an α-catenin (types E, N, and T; Gumbiner, 2005; Hatzfeld, 2005; Takeichi and Abe, 2005). A principle intracellular interactor of nectins is afadin (AF-6). This association is mediated by the C-terminal four amino acids of each nectin and the PDZ domain in afadin (Takai and Nakanishi, 2003). Afadin and α-catenin can bind each other directly, although this interaction is weak and may require additional modifications or proteins for stabilization (Takai and Nakanishi, 2003). Both cadherins and nectins impinge upon the actin cytoskeleton either through activation of Rho family GTPases, including Rac and Cdc42, or through their intracellular protein complexes, as α-catenin and afadin can interact with F-actin and additional F-actin–binding proteins (Fig. 1 C; Hoshino et al., 2004; Drees et al., 2005; Gumbiner, 2005; Sato et al., 2006). In addition, nectin ligation results in the activation of the Ras family GTPase Rap1, which, in turn, binds afadin as an effector (Sato et al., 2006).
Although cadherins and nectins are both found at adherens junctions, nectins likely initiate the formation of adherens junctions and then recruit cadherins (Takai and Nakanishi, 2003). Kinetic analysis in cells and thermodynamic data in vitro demonstrate that the nectin–nectin trans-interaction is faster and more stable than the cadherin–cadherin trans-interaction (Takai and Nakanishi, 2003; Hoshino et al., 2004; Sato et al., 2006). Additionally, blocking nectin trans-interactions in cells significantly slows adherens junction formation (Hoshino et al., 2004). Recent studies investigating the cross talk between nectins and cadherins in cultured cells suggest two mechanisms through which nectins control cadherin ligation: inhibition of the endocytosis of cadherins and alteration of the conformation of the cadherin extracellular domain to potentiate binding to cadherins on neighboring cells (Hoshino et al., 2005; Sato et al., 2006). Both functions are predicted to occur via the activation of Rap1, subsequent Rap1 localization to the downstream effector afadin, and Rap1-afadin acting upon cadherin-associated p120-catenin (Hoshino et al., 2005; Sato et al., 2006).
Unlike cadherins, nectins are involved in both homophilic and heterophilic interactions. Two nectins that interact heterophilically, nectin-1 and -3, are colocalized within the stratum lucidum of the hippocampus, the site of synapses between axons of dentate gyrus granule cells and dendrites of CA3 pyramidal neurons (Fig. 1 A; Takai and Nakanishi, 2003; Togashi et al., 2006). These particular synapses are highly structured, with flanking sites of symmetric adhesion (puncta adherentia junctions) that resemble adherens junctions in epithelia (Fig. 1 B; Takai and Nakanishi, 2003; Takeichi and Abe, 2005). Using immunoelectron microscopy, nectin-1 was shown to be localized to the axonal side of the puncta adherentia junction, whereas nectin-3 is localized to the dendritic side, suggesting that their heterophilic interaction may occur in vivo (Fig. 1 A; Takai and Nakanishi, 2003). In younger animals, before the formation of the puncta adherentia junctions, both nectins are found at the synaptic junction, suggesting that nectins may play a role in synapse formation (Takai and Nakanishi, 2003). Intriguingly, in the hippocampus, the loss of expression of either nectin causes a dramatic reduction in the localization of the other (Honda et al., 2006). In addition, the heterophilic interaction between nectin-1 and -3 is important for ciliary body development in the eye, where their association mediates contact between the pigment and nonpigment cell layers (Inagaki et al., 2005). Importantly, the nectin-1–nectin-3 interaction is stronger than either homophilic interaction, as determined by cell separation force measurements (Martinez-Rico et al., 2005).
Togashi et al. (2006) used overexpression and loss of expression analysis to probe the role of nectin-1 and -3 in cultured hippocampal neurons. In hippocampal cultures, they found that both endogenous and exogenous nectin-1 are primarily localized to axons, although the overexpression of nectin-1 decreases this specificity. Nectin-3 is expressed more generally, being detected in both axons and dendrites. Based upon domain swapping and deletion analysis, the intracellular domain of nectin-1 appears to be responsible for its axonal localization. However, the exact nature of the sorting signal in nectin-1 has not been identified.
Overexpression of nectin-1 but not nectin-3 induces a significant increase in axodendritic and dendrodendritic interactions within the transfected cells (Togashi et al., 2006). Interactions through the nectin-1 cytoplasmic domain are required for this effect. Additional analyses in which nectin-1 and -3 are expressed in HEK293 cells and/or hippocampal neurons demonstrated the unique ability of both nectins to potently induce adhesion between cells. When HEK293 cells expressing nectin-1 are mixed with HEK293 cells expressing nectin-3, adhesion is induced by their heterophilic interaction. Interestingly, adhesion is reduced or lost between HEK293 cells expressing the same nectin.
To test the role of endogenous nectin-1, Togashi et al. (2006) examined interneuronal connections and synapses in hippocampal cultures derived from nectin-1–null mice. In this context, they observed a reduction in spine head width and an increase in spine length, suggesting that postsynaptic spines are more immature in the absence of nectin-1. Moreover, they found reduced or nonexistent synaptotagmin staining apposed to the spine heads, suggesting that the adhesive connection between the presynaptic terminal and the postsynaptic spine is significantly weakened (Togashi et al., 2006). In the adult hippocampus, constitutive loss of nectin-1 or -3 results in a dramatic reduction in the number of puncta adherentia junctions, demonstrating reduced axodendritic adhesion (Fig. 1 B; Honda et al., 2006). In addition, dentate gyrus granule cell axons are misrouted around the CA3 region, aberrantly entering the stratum oriens layer, which again correlates with reduced axodendritic affinity (Fig. 1 A; Honda et al., 2006). In contrast, analysis of synaptic structure in adult nectin-1– and -3–null mice revealed no significant reductions in the number or size of synapses in the CA3 region of the hippocampus (Honda et al., 2006). The lack of correlation between the in vivo and in vitro results may be caused by the expression of multiple nectin isoforms or possible compensation by alternative mechanisms seen in mature animals. Prior localization studies in vivo have focused on nectin expression at the dentate gyrus–CA3 synapse in juvenile and adult animals (Takai and Nakanishi, 2003). The studies in vitro use embryonic hippocampal cultures consisting of mostly CA1 and CA3 pyramidal neurons, as most of the dentate gyrus granule cells have not been born yet (Bayer, 1980). Togashi et al. (2006) clearly demonstrate endogenous expression of nectin-1 and -3 in these cultures, which is in agreement with in situ hybridization data (nectin-1 is Pvrl1, and nectin-3 is Pvrl3; Magdaleno et al., 2006). Although additional immunohistochemistry for the nectins will be informative, the interaction of axonal nectin-1 with -3 appears to be a mechanism mediating axodendritic adhesion in multiple regions of the hippocampus and perhaps throughout the nervous system (Fig. 1 A).
Consistent with cadherin adhesion being involved in nectin-induced interactions, Togashi et al. (2006) found that β-catenin staining is localized to sites of nectin-1–nectin-3 interaction. In addition, there was reduced localization of β-catenin to synapses in nectin-1–null cultures. Importantly, nectin-1 cannot induce an increase in neurite adhesion without its PDZ-binding motif or in the absence of αN-catenin. Previous analysis has shown that the loss of αN-catenin causes an increase in spine length and spine dynamics, which is strikingly similar to the effect of nectin-1 depletion in cultured hippocampal neurons (Takeichi and Abe, 2005; Togashi et al., 2006). Thus, the heterophilic interaction between nectin-1 and -3, which is likely through an afadin–αN-catenin interaction, requires the recruitment of cadherins to promote interneurite adhesion. Togashi et al. (2006) showed that the overexpression of either nectin results in the up-regulation of N-cadherin protein levels. However, up-regulation of N-cadherin alone is not sufficient to induce aberrant neurite patterning (Togashi et al., 2006).
In summary, selective axodendritic adhesion is mediated, in part, by the localization of nectin-1 to axons (Fig. 1 A). As a result of the selective potency of the heterophilic nectin-1– nectin-3 interaction, the adhesion between neurites expressing the same nectin is not maintained. The interaction of axonal nectin-1 with dendritic nectin-3 subsequently recruits cadherins to this burgeoning junction through an afadin–αN-catenin connection. Intriguingly, besides binding nectins, the PDZ domain in afadin can alternatively bind EphA- and EphB-type receptors as well as neurexins, suggesting that other cell surface receptors akin to nectins may recruit and activate cadherins (Takai and Nakanishi, 2003; Zhou et al., 2005). Additional work is needed to unveil how the various cell surface receptors collaborate to specify connections.
References
- Bayer, S.A. 1980. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J. Comp. Neurol. 190:87–114. [DOI] [PubMed] [Google Scholar]
- Drees, F., S. Pokutta, S. Yamada, W.J. Nelson, and W.I. Weis. 2005. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 123:903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner, B.M. 2005. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6:622–634. [DOI] [PubMed] [Google Scholar]
- Hatzfeld, M. 2005. The p120 family of cell adhesion molecules. Eur. J. Cell Biol. 84:205–214. [DOI] [PubMed] [Google Scholar]
- Honda, T., T. Sakisaka, T. Yamada, N. Kumazawa, T. Hoshino, M. Kajita, T. Kayahara, H. Ishizaki, M. Tanaka-Okamoto, A. Mizoguchi, et al. 2006. Involvement of nectins in the formation of puncta adherentia junctions and the mossy fiber trajectory in the mouse hippocampus. Mol. Cell. Neurosci. 31:315–325. [DOI] [PubMed] [Google Scholar]
- Hoshino, T., K. Shimizu, T. Honda, T. Kawakatsu, T. Fukuyama, T. Nakamura, M. Matsuda, and Y. Takai. 2004. A novel role of nectins in inhibition of the E-cadherin-induced activation of Rac and formation of cell-cell adherens junctions. Mol. Biol. Cell. 15:1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino, T., T. Sakisaka, T. Baba, T. Yamada, T. Kimura, and Y. Takai. 2005. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J. Biol. Chem. 280:24095–24103. [DOI] [PubMed] [Google Scholar]
- Inagaki, M., K. Irie, H. Ishizaki, M. Tanaka-Okamoto, K. Morimoto, E. Inoue, T. Ohtsuka, J. Miyoshi, and Y. Takai. 2005. Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development. 132:1525–1537. [DOI] [PubMed] [Google Scholar]
- Magdaleno, S., P. Jensen, C.L. Brumwell, A. Seal, K. Lehman, A. Asbury, T. Cheung, T. Cornelius, D.M. Batten, C. Eden, et al. 2006. BGEM: an in situ hybridization database of gene expression in the embryonic and adult mouse nervous system. PLoS. Biol. 10.1371/journal.pbio.0040086. [DOI] [PMC free article] [PubMed]
- Martinez-Rico, C., F. Pincet, E. Perez, J.P. Thiery, K. Shimizu, Y. Takai, and S. Dufour. 2005. Separation force measurements reveal different types of modulation of E-cadherin-based adhesion by nectin-1 and -3. J. Biol. Chem. 280:4753–4760. [DOI] [PubMed] [Google Scholar]
- Sato, T., N. Fujita, A. Yamada, T. Ooshio, R. Okamoto, K. Irie, and Y. Takai. 2006. Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 281:5288–5299. [DOI] [PubMed] [Google Scholar]
- Takai, Y., and H. Nakanishi. 2003. Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 116:17–27. [DOI] [PubMed] [Google Scholar]
- Takeichi, M., and K. Abe. 2005. Synaptic contact dynamics controlled by cadherin and catenins. Trends Cell Biol. 15:216–221. [DOI] [PubMed] [Google Scholar]
- Togashi, H., J. Miyoshi, T. Honda, T. Sakisaka, Y. Takai, and M. Takeichi. 2006. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J. Cell Biol. 174:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata, M., J.R. Sanes, and J.A. Weiner. 2003. Synaptic adhesion molecules. Curr. Opin. Cell Biol. 15:621–632. [DOI] [PubMed] [Google Scholar]
- Zhou, H., Y. Xu, Y. Yang, A. Huang, J. Wu, and Y. Shi. 2005. Solution structure of AF-6 PDZ domain and its interaction with the C-terminal peptides from Neurexin and Bcr. J. Biol. Chem. 280:13841–13847. [DOI] [PubMed] [Google Scholar]